发酵是一种通过微生物或酶的作用改变植物基质的理化结构并潜在地提高其生物活性的有效途径[1]。利用微生物对薏米进行发酵,可改善薏米的组织结构,提高制品营养、风味及食用品质,增强产品功能活性。以红曲霉发酵薏米,可获得112.649 mg/kg的洛伐他汀,同时红曲薏米制品具有抗氧化及降血脂作用[2-3]。YIN等[4]以植物乳杆菌(Lactobacillus plantarum) NCU137发酵薏米,发现多种营养成分含量增加,风味独特,黏度特性稳定。WANG等[5]研究发现枯草芽孢杆菌[Bacillus subtilis (natto)]发酵薏米制品改善了高血脂仓鼠的脂质代谢、抗氧化状态及肠道菌群。课题组前期研究发现,B.subtilis BJ3-2发酵薏米中游离氨基酸、γ-氨基丁酸、总多酚、黄酮、总三萜和薏苡酯含量较原料显著增加,同时还产生新化合物——川芎嗪[6-7]。
薏米被誉为“世界禾本科植物之王”和药食同源的“粮药”之一[8]。薏苡籽粒(薏仁谷),最外层为坚韧的薏米壳,壳下为种皮层,加工脱壳后即得糙薏米,再精碾去皮抛光即为精薏米,同时产生碎薏米和薏米糠[9]。我国薏米加工技术仍停留在初级阶段,多数企业仅限于薏米原料销售及简单烘焙制粉;碎薏米作为薏米加工过程中的主要副产物,其主要用于饲料生产[10]。因此,薏米精深加工产品亟待开发。课题组前期借鉴日本纳豆研发思路,基于B.subtilis BJ3-2发酵精薏米可高产川芎嗪前期成果,拟以贵州兴仁薏米(精薏米、糙薏米和碎薏米)为原料,采用B.subtilis BJ3-2为发酵菌株分别对3种薏米进行不添加前体物质的单一基质发酵,通过单因素及Box-Behnken试验构建高产川芎嗪和溶纤酶的薏米发酵体系,为薏米高值化加工提供科学依据。
1 材料与方法
1.1 材料与试剂
薏米,贵州兴仁农业发展有限公司;黄豆,合力超市;B.subtilis BJ3-2,贵州大学吴拥军教授赠送;川芎嗪、尿激酶,Sigma;其余相关生理生化试验所用试剂均为分析纯。
1.2 仪器与设备
H2-16KR台式高速冷冻离心机,湖南可成仪器设备有限公司;FD-1P冷冻干燥机,上海田枫实业有限公司;1260高效液相色谱仪,美国安捷伦公司。
1.3 B.subtilis BJ3-2发酵薏米的工艺流程
B.subtilis BJ3-2发酵薏米工艺流程具体如下:
薏米挑选![]() 清洗
清洗![]() 浸泡
浸泡![]() 蒸煮
蒸煮![]() 接种
接种![]() 发酵
发酵![]() 成品
成品
1.4 B.subtilis BJ3-2发酵薏米的工艺单因素试验
以川芎嗪含量和溶纤酶酶活为指标,考察发酵方式、料水比(g∶mL)(薏米∶水)=1∶0.8、1∶1、1∶1.2、1∶1.4、1∶1.6)、装载量(10%、14%、18%、22%和26%)、蒸煮时间(10、20、30、40、50 min)、初始pH(5.5、6、6.5、7、7.5)、接种量(1%、3%、5%、7%、9%)、发酵温度(28、31、34、37、40 ℃)和发酵时间(48、60、72、84、96 h)等因素对发酵效果的影响。采用控制变量法,其发酵的初始条件为料水比1∶1(g∶mL)、装载量18%、蒸煮时间20 min、pH 7.0、接种量5%、发酵温度37 ℃和发酵时间72 h。
1.5 B.subtilis BJ3-2发酵薏米的Box-Behnken优化试验
在单因素试验基础上,确定发酵方式、料水比、装载量、蒸煮时间、pH、接种量、发酵温度和发酵时间的取值范围,采用Box-Behnken试验设计3因素3水平的响应面分析,以川芎嗪含量和溶纤酶酶活为响应值,得出B.subtilis发酵薏米的最佳发酵工艺。响应面试验因素与水平设计见表1。B.subtilis BJ3-2发酵黄豆和CICC 20637发酵薏米按B.subtilis BJ3-2发酵糙薏米的优化条件制备。
1.6 分析方法
川芎嗪待测样液制备:取3.00 g干燥粉末,加入15 mL 80%乙醇溶液,超声处理30 min,功率500 W,温度30 ℃;超声完成后以8 000 r/min离心15 min,取上清液。按以上步骤再提取2次,收集上清液并定容至50 mL,过0.22 μm膜,得供试液。川芎嗪测定采用HPLC测定[6]。
溶纤酶待测样制备:取25.00 g薏米发酵鲜样于50 mL灭菌的生理盐水中浸泡24 h,再以4 000 r/min离心30 min,取上清液待测。溶纤酶酶活采用纤维蛋白平板法测定[11]。
1.7 统计分析
每个样品至少3次重复数据,实验结果以平均值±标准差表示,采用SPSS 17.0软件进行统计方差分析,图标采用Origin绘制。显著性水平为P<0.05。
2 结果与分析
2.1 发酵方式对B.subtilis BJ3-2发酵薏米中川芎嗪产量和溶纤酶酶活的影响
由图1可知,静态发酵中川芎嗪产量和溶纤酶酶活显著高于动态发酵(P<0.05)。静态发酵中,B.subtilis BJ3-2发酵糙薏米的川芎嗪含量和溶纤酶酶活分别为1.88 mg/g干基(dry weight,DW)和854.37 U/g;B.subtilis BJ3-2发酵精薏米中分别为2.97 mg/g DW和720.84 U/g;B.subtilis BJ3-2发酵碎薏米中分别为0.61 mg/g DW和501.22 U/g。固态发酵具有缺少游离水的流动,供氧足和消耗能量低等优势;动态发酵具有发酵完全和生产周期短等优点[12]。薏米呈颗粒状,动态发酵不利于B.subtilis BJ3-2附着在薏米表面,限制其生长繁殖;静态发酵过程中B.subtilis BJ3-2可深入薏米基料中并穿入基质细胞内,利于B.subtilis BJ3-2生长、繁殖及代谢。吴晶晶[13]也发现红曲霉发酵固态大米培养基中川芎嗪的含量比液体培养基高3倍。因此,其以静态发酵方式进行下一步试验。
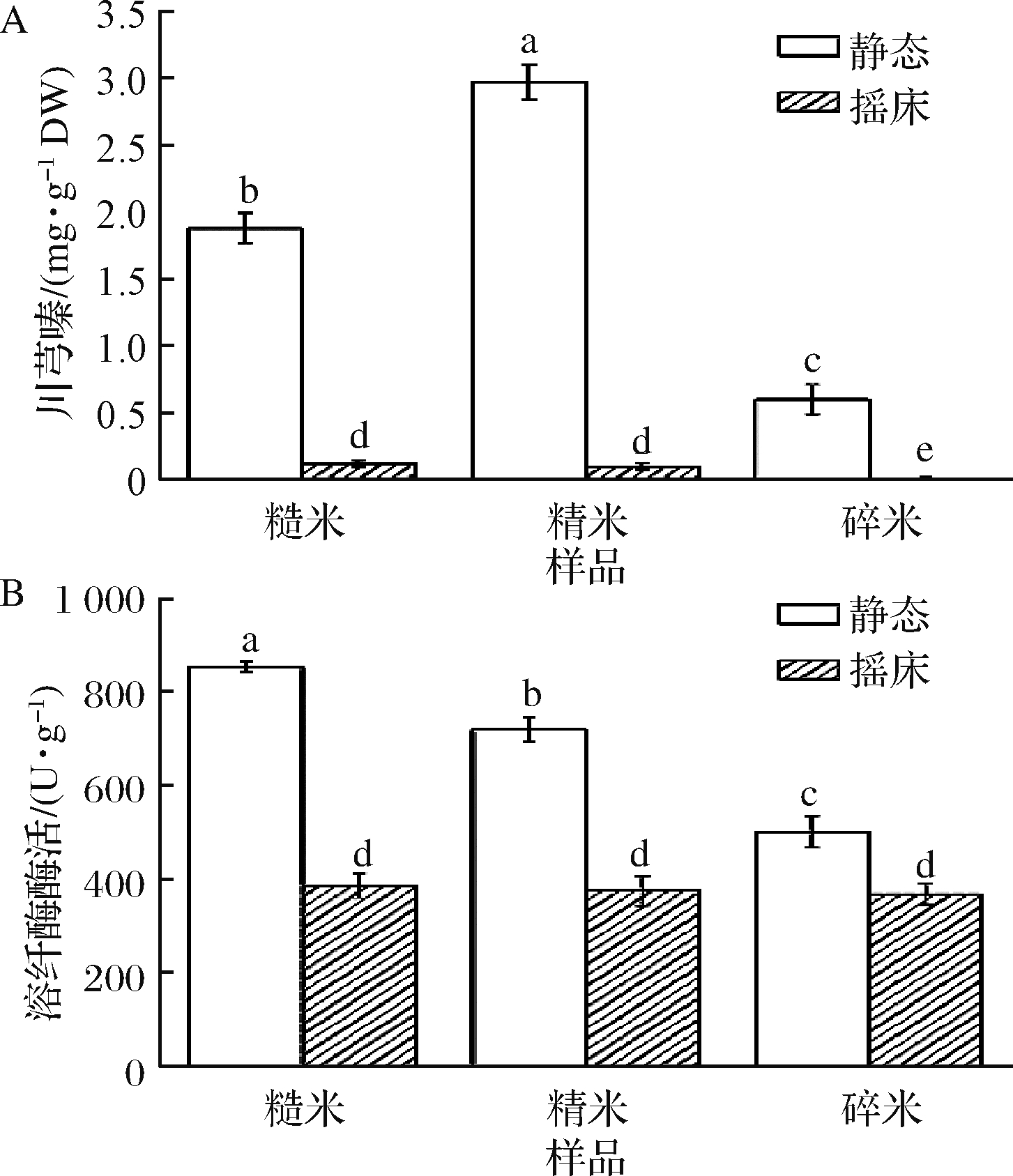
A-川芎嗪产量;B-溶纤酶酶活
图1 发酵方式对B.subtilis发酵薏米中川芎嗪产量和溶纤酶酶活的影响
Fig.1 Effect of fermentation methods on the tetramethylpyrazine yield and fibrinolytic enzyme activity in B.subtilis-fermented adlay
2.2 料水比、装载量和蒸煮时间对B.subtilis发酵薏米中川芎嗪产量和溶纤酶酶活的影响
随着料水比、装载量和蒸煮时间的增加,薏米中川芎嗪产量和溶纤酶酶活呈先增加后减少的趋势(图2)。料水比为1∶1.2(g∶mL)时,B.subtilis BJ3-2发酵糙薏米中川芎嗪产量和溶纤酶酶活都达到最大值;在B.subtilis BJ3-2发酵精薏米中,料水比为1∶1.2(g∶mL)时川芎嗪产量达最大值,但溶纤酶酶活在料水比为1∶1.4(g∶mL)时达最大值;B.subtilis BJ3-2发酵碎薏米中,川芎嗪产量和溶纤酶酶活均在料水比为1∶1.4(g∶mL)时达最大值。装载量为14%时,B.subtilis BJ3-2发酵糙薏米中川芎嗪产量和溶纤酶酶活达到最大值;B.subtilis BJ3-2发酵精薏米和碎薏米中,装载量为10%时川芎嗪产量最高,而溶纤酶酶活在装载量为14%时最高。蒸煮时间为30 min时,B.subtilis BJ3-2发酵糙薏米中川芎嗪产量达最大值,而溶纤酶酶活在蒸煮时间为40 min时达到最大值;B.subtilis BJ3-2发酵精薏米中,川芎嗪产量和溶纤酶酶活均在30 min时达最大值;B.subtilis BJ3-2发酵碎薏米中,川芎嗪产量和溶纤酶酶活均在蒸煮时间为40 min时达到最大值。料水比较低,水分不能满足菌体生长繁殖所需水分;料水比过高,样品易发黏结块致使供氧不足[14]。同时,装载量过少导致底物被消耗快且水分蒸发快;装载量过高致使发酵过程中溶氧较少且菌体分布不均匀[15]。此外,蒸煮时间较短,薏米颗粒质地硬且松散;当蒸煮时间过长,薏米易发黏结块致使溶氧量少[16]。因此,料水比、装载量和蒸煮时间选择为1∶1.4(g∶mL)、14%和30 min。

A、D、G-B.subtilis BJ3-2发酵糙薏米;B、E、H-B.subtilis BJ3-2发酵精薏米;C、F、I-B.subtilis BJ3-2发酵碎薏米
图2 料水比、装载量和蒸煮时间对B.subtilis BJ3-2发酵薏米中川芎嗪产量和溶纤酶酶活的影响
Fig.2 Effect of cooking time on the tetramethylpyrazine yield and fibrinolytic enzyme activity in B.subtilis BJ3-2-fermented adlay
注:图中不同小写字母表示差异显著(P<0.05)(下同)
2.3 初始pH对B.subtilis BJ3-2发酵薏米中川芎嗪产量和溶纤酶酶活的影响
随着初始pH的增加,川芎嗪产量和溶纤酶酶活先增加后减少(图3)。初始pH值为7.0时,B.subtilis BJ3-2发酵糙薏米和精薏米中川芎嗪产量和溶纤酶酶活达最大值。B.subtilis BJ3-2发酵碎薏米中,川芎嗪产量在初始pH值为7时达到最大值,但溶纤酶酶活却在pH值为6.5时达最大值。弱酸环境有利于B.subtilis BJ3-2生长繁殖及代谢合成乙偶姻(川芎嗪的前体物),但过酸的环境条件下,菌株生长缓慢,延迟期较长[17]。ZHU等[18]利用两阶段pH值控制培养法来获取高产量川芎嗪,即发酵48 h前pH值控制为5.5、48 h后pH值调控为7.0,获得7.43 g/L的川芎嗪。但纳豆激酶最适pH值为8.0,而碱性条件下川芎嗪不稳定[19]。因此,pH 7.0是B.subtilis BJ3-2生长繁殖并同时获得较高水平的川芎嗪和溶纤酶的保证。
2.4 接种量、发酵温度和发酵时间对B.subtilis BJ3-2发酵薏米中川芎嗪产量和溶纤酶酶活的影响
随着接种量、发酵时间和发酵温度的增加,川芎嗪产量和溶纤酶酶活先增加后减少(图4)。接种量为9%时,B.subtilis BJ3-2发酵糙薏米和碎薏米中川芎嗪产量和溶纤酶酶活达最大值;在B.subtilis BJ3-2发酵精薏米中,川芎嗪产量在接种量为9%时达到最大值,而溶纤酶酶活在接种量为7%时达到最大值。发酵温度为37 ℃时,B.subtilis BJ3-2发酵糙薏米中川芎嗪产量和溶纤酶酶活达最大值。B.subtilis BJ3-2发酵精薏米和碎薏米中,川芎嗪产量在发酵温度为37 ℃时为最大值,但溶纤酶酶活在40 ℃时为最大值。川芎嗪产量在发酵时间为96 h时为最大值;而发酵时间为84 h时,B.subtilis BJ3-2发酵糙薏米、精薏米和碎薏米中溶纤酶酶活达最大值。接种量较少,致使菌体发酵周期延长及菌体活力低诱导次级代谢产物产生的酶少;接种量过大,菌体生长旺盛并较快达稳定期,后迅速老化自溶,次级产物生成的时间短[20]。朱兵峰[21]建立了多阶段转速偶联温度控制发酵策略:0~48 h时,控制培养温度37 ℃;48~120 h,控制培养温度55 ℃,川芎嗪的产量达7.12 g/L。
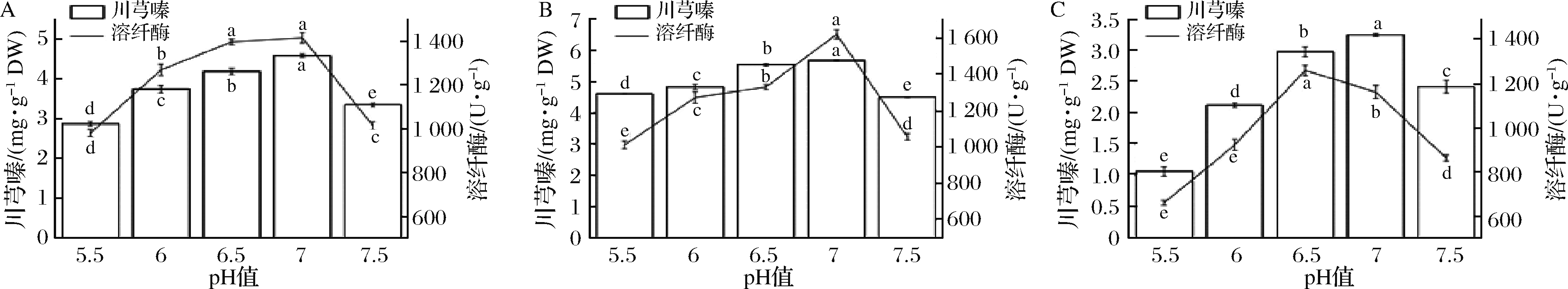
A-B.subtilis BJ3-2发酵糙薏米;B-B.subtilis BJ3-2发酵精薏米;C-B.subtilis BJ3-2发酵碎薏米
图3 初始pH值对B.subtilis BJ3-2发酵薏米中川芎嗪产量和溶纤酶酶活的影响
Fig.3 Effect of initial pH on the tetramethylpyrazine yield and fibrinolytic enzyme activity in B.subtilis BJ3-2-fermented adlay

A、D、G-B.subtilis BJ3-2发酵糙薏米;B、E、H-B.subtilis BJ3-2发酵精薏米;C、F、I-B.subtilis BJ3-2发酵碎薏米
图4 接种量、发酵温度和发酵时间对B.subtilis BJ3-2发酵薏米中川芎嗪产量和溶纤酶酶活的影响
Fig.4 Effect of fermentation time on the tetramethylpyrazine and fibrinolytic enzyme activity in B.subtilis BJ3-2-fermented adlay
2.5 B.subtilis BJ3-2发酵薏米的Box-Behnken优化结果
为确定B.subtilis BJ3-2发酵薏米最佳发酵条件,选择接种量、发酵温度和发酵时间3个因素,以川芎嗪产量和溶纤酶酶活为评价指标进行响应面分析,Box-Behnken优化试验结果如表1所示。如表2~表4所示,在B.subtilis BJ3-2发酵糙薏米、精薏米和碎薏米中,回归模型极显著(P<0.01),失项拟合不显著(P>0.05),说明该回归模型拟合度良好,实验误差小。
表1 B.subtilis BJ3-2发酵糙薏米产川芎嗪和溶纤酶的Box-Behnken优化结果
Table 1 Box-Behnken design matrix of experimental values of the tetramethylpyrazine yield and fibrinolytic enzyme activity in B.subtilis BJ3-2-fermented adlay

试验号X1(接种量)X2(发酵温度)X3(发酵时间)川芎嗪/(mg·g-1 DW)溶纤酶/(U·g-1)BDABPABBABDABPABBA10 (7%)0 (37 ℃)0 (84 h)6.136.155.152 128.541 938.012 064.882-1 (5%)01 (96 h)4.554.022.48832.24648.591 567.2930005.366.564.342 236.191 755.882 021.7540005.385.845.111 805.771 843.931 952.7451(9%)015.794.884.041 902.771 940.241 806.0360005.965.784.591 654.911 987.261 850.84701(40 ℃)14.766.943.082 014.862 142.182 074.62801-1 (72 h)2.945.965.08692.371 985.211 490.2591105.935.784.331 904.422 016.561 610.62100-1 (34 ℃)-13.243.892.13744.86980.22975.26110-114.253.552.49593.1444.881 114.3712-1-104.373.231.49487.51366.521 413.6213-1103.124.973.471 522.911 833.811 768.341410-14.563.143.77657.93642.211 125.7715-10-13.193.583.56808.961 062.552 085.85160005.495.934.471 950.922 047.031 929.42171-103.822.61.76589.5636.48457.92
注:BDA,B.subtilis BJ3-2发酵糙薏米;BPA,B.subtilis BJ3-2发酵精薏米;BBA,B.subtilis BJ3-2发酵碎薏米
为了验证模型预测,并分析川芎嗪产量和纤溶酶酶活的发酵基质(薏米和黄豆)及菌株(B.subtilis BJ3-2和CICC 20637)依懒性。经验证(图5),B.subtilis BJ3-2发酵糙薏米中川芎嗪产量为6.15 mg/g DW,纤溶酶酶活为2 236.47 U/g,比未优化的发酵糙薏米分别增加了3.27和2.64倍;B.subtilis BJ3-2发酵精薏米中川芎嗪产量为6.93 mg/g DW,纤溶酶酶活为2 097.26 U/g,比未优化的发酵糙薏米分别增加了2.34和2.97倍;B.subtilis BJ3-2发酵碎薏米中川芎嗪产量为5.09 mg/g DW,纤溶酶酶活为2 011.56 U/g,比未优化的发酵糙薏米分别增加了8.48和4.01倍。而在B.subtilis BJ3-2发酵黄豆中,溶纤酶酶活(2 674.68 U/g)高于B.subtilis BJ3-2发酵薏米,但川芎嗪产量(0.05 mg/g DW)远低于B.subtilis BJ3-2发酵薏米。在B.subtilis CICC 20637发酵薏米中,川芎嗪产量和溶纤酶酶活均低于B.subtilis BJ3-2发酵薏米。而原料加工过程中,糙薏米通过精碾去皮抛光才获得精薏米,同时产生占总质量5%~10%的碎薏米和5%的薏米糠[21],因此,综合考虑加工成本,糙薏米可作为发酵高产川芎嗪和溶纤酶的最佳原料。
表2 B.subtilis BJ3-2发酵糙薏米的回归模型方差分析
Table 2 Regression analysis of variance in B.subtilis BJ3-2
fermented dehulled adlay

方差来源df川芎嗪溶纤酶SSMSFPSSMSFP模型914.521.6120.960.000 38.822×1069.802×10519.790.000 4X111.181.1815.110.006 02.492×1052.492×1055.030.059 8X210.110.111.390.277 42.774×1062.774×10656.010.000 1X311.641.6421.010.002 51.478×1061.478×10629.850.000 9X1X211.391.3917.860.003 918 295.2718 295.270.370.562 5X1X310.220.222.770.139 83.731×1053.731×1057.530.028 7X2X310.820.8210.510.014 21.530×1061.530×10630.900.000 9X1212.072.0726.580.001 31.134×1061.134×10622.900.002 0 X2214.014.0151.500.000 23.998×1053.998×1058.070.025 0X3212.152.1527.530.001 26.273×1056.273×10512.670.009 2残差70.550.073.467×10549 525.44失拟项30.0910.030.270.847 21.247×10541 575.630.750.577 0纯误差40.450.112.220×10555 487.80总和1615.079.169×1069.802×105
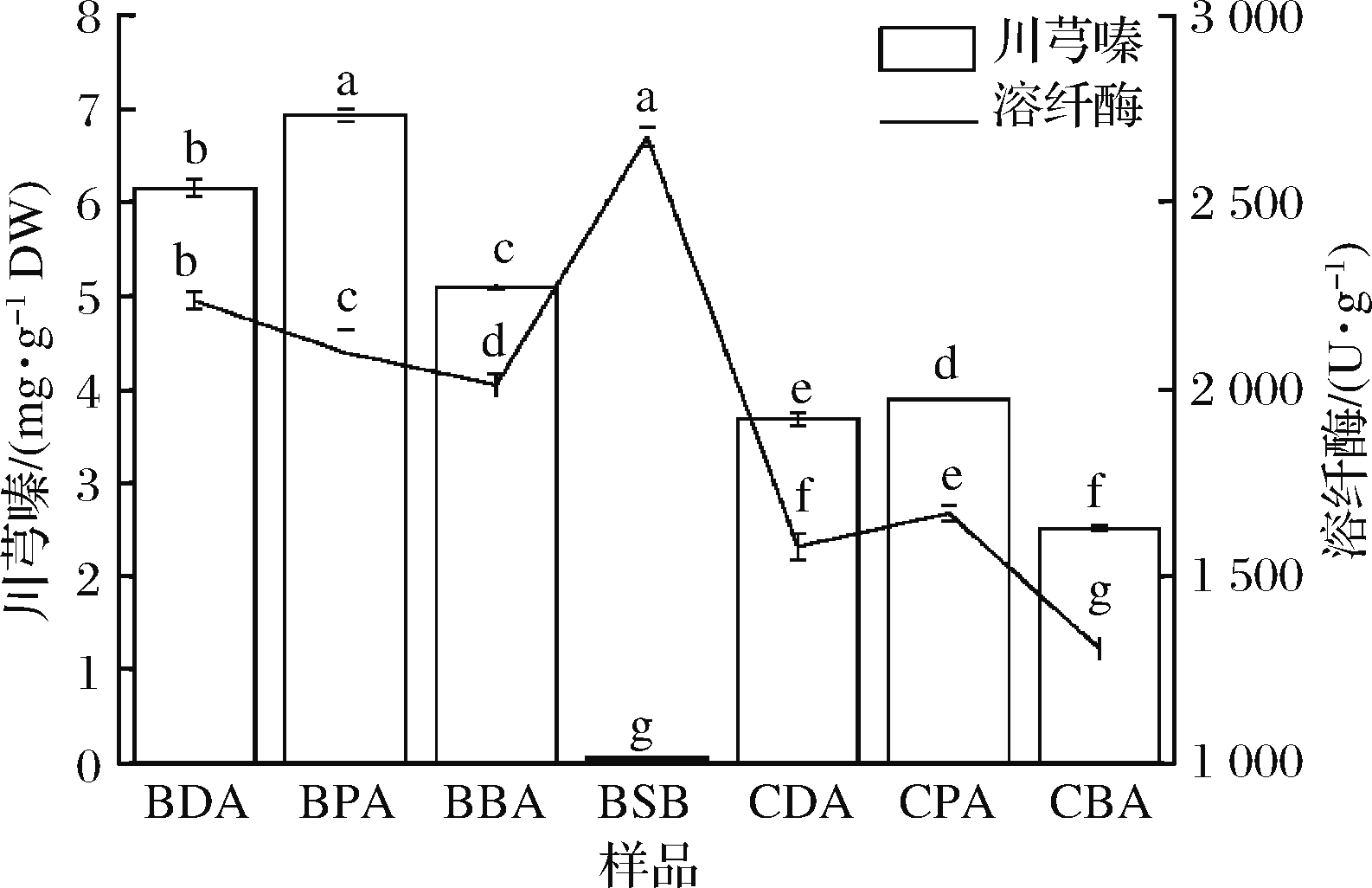
图5 不同发酵基质和发酵菌株中川芎嗪产量和溶纤酶
酶活结果
Fig.5 Results of the tetramethylpyrazine yield and fibrinolytic enzyme activity in different substrate-and bacterial strain-fermentation
注:BDA-B.subtilis BJ3-2发酵糙薏米;BPA-B.subtilis BJ3-2发酵
精薏米;BBA-B.subtilis BJ3-2发酵碎薏米;BSB-B.subtilis BJ3-2发酵
黄豆;CDA-B.subtilis CICC 20637发酵糙薏米;CPA-B.subtilis CICC 20637
发酵精薏米;CBA-B.subtilis CICC 20637发酵碎薏米
川芎嗪是中药川芎的主要生物活性成分,但川芎嗪含量较低(平均含量2.19 μg/g)。在其他发酵产品如醋、白酒和豆豉中,川芎嗪含量分别为0.001~0.131 mg/g[22]、88.70~1 417.59 μg/L[23]和0.511 μg/g[24],其含量均低于B.subtilis BJ3-2发酵薏米中川芎嗪含量。利用微生物高效合成川芎嗪主要集中于筛选优良菌株、添加前体物质、优化培养条件或改造细胞工程等[25],但目前专注于转换培养基基料的相关研究较少。XIAO等[26]研究发现,添加30.1 g/L的乙偶姻和67.7 g/L的磷酸二铵,可得到8.34 g/L的川芎嗪,这是目前研究中的川芎嗪最高产量。本研究以单一薏米为发酵基料,构建了B.subtilis BJ3-2发酵薏米高产川芎嗪的优化模型,其含量分别为B.subtilis BJ3-2发酵黄豆和CICC 20637发酵糙薏米的204和12倍,表明B.subtilis BJ3-2发酵薏米是一种高产川芎嗪的新方法。
表3 B.subtilis BJ3-2发酵精薏米的回归模型方差分析
Table 3 Regression analysis of variance in B.subtilis BJ3-2
fermented polished adlay
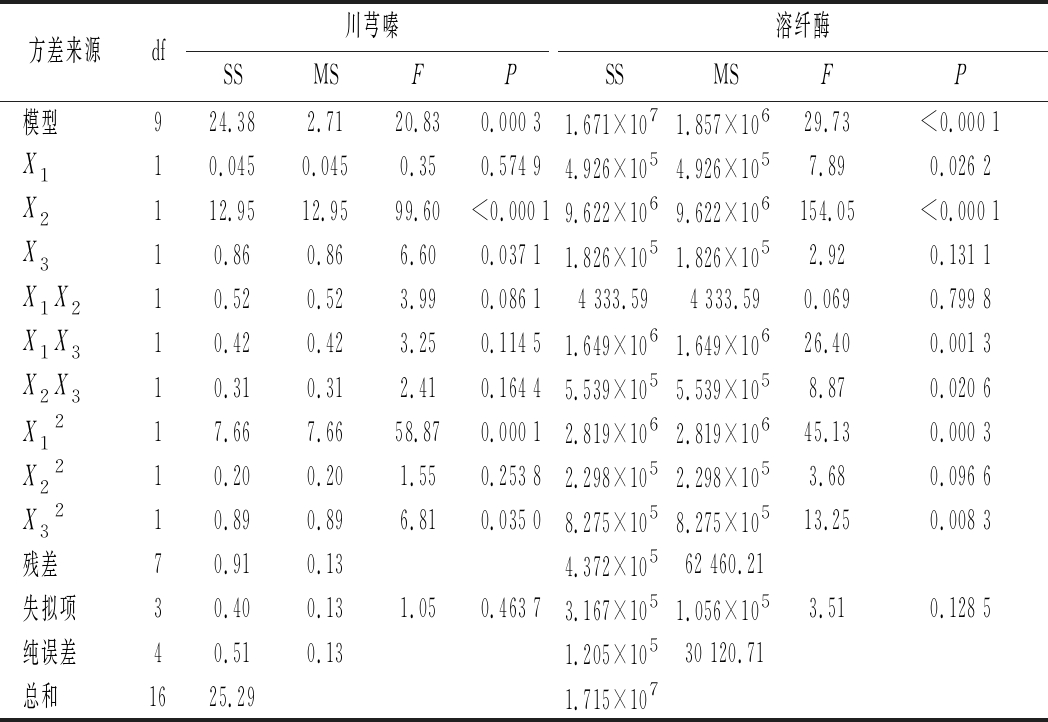
方差来源df川芎嗪溶纤酶SSMSFPSSMSFP模型924.382.7120.830.000 31.671×1071.857×10629.73<0.000 1X110.0450.0450.350.574 94.926×1054.926×1057.890.026 2X2112.9512.9599.60<0.000 19.622×1069.622×106154.05<0.000 1X310.860.866.600.037 11.826×1051.826×1052.920.131 1X1X210.520.523.990.086 14 333.594 333.590.0690.799 8X1X310.420.423.250.114 51.649×1061.649×10626.400.001 3X2X310.310.312.410.164 45.539×1055.539×1058.870.020 6X1217.667.6658.870.000 12.819×1062.819×10645.130.000 3X2210.200.201.550.253 82.298×1052.298×1053.680.096 6X3210.890.896.810.035 08.275×1058.275×10513.250.008 3残差70.910.134.372×10562 460.21失拟项30.400.131.050.463 73.167×1051.056×1053.510.128 5纯误差40.510.131.205×10530 120.71总和1625.291.715×107
表4 B.subtilis BJ3-2发酵碎薏米的回归模型方差分析
Table 4 Regression analysis of variance in B.subtilis BJ3-2 fermented broken adlay

方差来源df川芎嗪溶纤酶SSMSFPSSMSFP模型921.862.4320.610.000 31.007×1071.119×10614.880.000 9X111.051.058.920.020 39.979×1059.979×10513.270.008 3X218.188.1869.42<0.000 12.642×1062.642×10635.120.000 6X310.750.756.370.039 62.372×1052.372×1053.150.119 0X1X210.080.0870.740.418 63.745×1053.745×1054.980.060 8X1X310.460.463.870.090 09.011×1059.011×10511.980.010 5X2X311.391.3911.820.010 91.672×1051.672×1052.220.179 6X1213.053.0525.880.001 41.038×1061.038×10613.800.007 5X2215.275.2744.700.000 32.922×1062.922×10638.850.000 4X3210.740.746.260.040 93.811×1053.811×1055.070.059 1残差70.820.125.265×10575 216.03失拟项30.260.0880.630.632 81.779×10559 315.230.680.608 4纯误差40.560.143.486×10530 120.71总和1622.691.183×107
3 结论
通过单因素和Box-Behnken试验分析,接种量为9.0%、发酵温度为40 ℃和发酵时间为93 h时,B.subtilis BJ3-2发酵糙薏米中川芎嗪产量和溶纤酶酶活分别为6.15 mg/g DW和2 236.47 U/g,接种量为8.1%、发酵温度为38 ℃和发酵时间为96 h时,发酵精薏米中川芎嗪产量和溶纤酶酶活分别为6.94 mg/g DW和2 142.18 U/g,但碎薏米中川芎嗪产量和溶纤酶酶活均明显偏低。综合考虑加工成本,B.subtilis BJ3-2发酵糙薏米可作为高产川芎嗪产量和溶纤酶的最佳发酵体系。拟在B.subtilis BJ3-2发酵薏米高产川芎嗪工艺优化基础上,将对发酵过程中川芎嗪合成的关键酶及前体物进行关联剖析,并采用转录组学和蛋白组学技术揭示其高产川芎嗪机理等相关研究,为延长薏米加工产业链和开发高值化新产品提供科学依据。
[1] YE J, HUANG L, TEREFE N S, et al.Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria [J].Food Chemistry, 2019, 286:616-623.
[2] DING Y, PU L, KAN J.Hypolipidemic effects of lipid-lowering granulated tea preparation from monascus-fermented grains (adlay and barley bran) mixed with lotus leaves on Sprague-Dawley rats fed a high-fat diet[J].Journal of Functional Foods, 2017, 32:80-89.
[3] LI P, TSAI W, CHIEN C.Dietary monascus adlay supplements facilitate suppression of cigarette smoke-induced pulmonary endoplasmic reticulum stress, autophagy, apoptosis and emphysema-related PLGF in the rat[J].Food Chemistry, 2013, 136(2):765-774.
[4] YIN H M, ZHONG Y D, XIA S K, et al.Effects of fermentation with Lactobacillus plantarum NCU137 on nutritional, sensory and stability properties of Coix (Coix lachryma-jobi L.) seed[J].Food Chemistry, 2020,134:126 037.
[5] WANG C, LIN H, WU S.Influence of dietary supplementation with Bacillus-fermented adlay on lipid metabolism, antioxidant status and intestinal microflora in hamsters[J].Journal of the Science of Food and Agriculture, 2011, 91(12):2 271-2 276.
[6] WEN A, XIE C, MAZHAR M, et al.Comparative evaluation of drying methods on kinetics, biocompounds and antioxidant activity of Bacillus subtilis-fermented dehulled adlay[J].Drying Technology, 2020, 38(11):1 505-1 515.
[7] WEN A, QIN L, ZENG H, et al.Comprehensive evaluation of physicochemical properties and antioxidant activity of B.subtilis-fermented polished adlay subjected to different drying methods [J].Food Science & Nutrition, 2020, 8(4):2 124-2 133.
[8] 涂鸿. 贵州薏米原料及加工产品有害重金属污染剖析[D].贵阳:贵州大学, 2016.
TU H.Analysis of the harmful heavy metal pollution in Coix Rice raw material and processing products from Guizhou[D].Guiyang:Guizhou University, 2016.
[9] 遇靓. 红曲固态发酵薏米茶工艺及品质研究[D].贵阳:贵州大学, 2016.
YU L.Study on processing technology and quality of solid fermented tea by monacus[D].Guiyang:Guizhou University, 2016.
[10] WEN A, XIE C, MAZHAR M, et al.Tetramethylpyrazine from adlay (Coix lacryma-jobi) biotransformation by Bacillus subtilis and its quality characteristics[J].Journal of Food Science and Technology, 2020, 57(11):4 092-4 102.
[11] 林榕姗. 细菌型豆豉发酵机理及功能性研究[D].泰安:山东农业大学, 2012.
LIN R S.Studies on the fermentation mechanism and healthy functions of bacteria-fermented of bacteria-fermented douchi[D].Taian:Shandong Agricultural University, 2012.
[12] 郑战伟, 陈菁, 孙娟, 等.利用表面静态法发酵生产果醋的研究进展[J].农产品加工(学刊), 2011(3):24-26.
ZHENG Z W, CHEN J, SUN J, et al.Research progress of fermentation fruit vinegar by surface static method[J].Academic Periodical of Farm Products Processing, 2011(3):24-26.
[13] 吴晶晶. 红曲霉(Monascus spp.)产生川芎嗪的发酵条件研究[D].杭州:浙江大学, 2011.
WU J J.Study on fermentation conditions of Monascus spp. producing tetramethypyrazine[D].Hangzhou:Zhejiang University, 2011.
[14] 郑环宇, 郎松彬, 崔月婷, 等.混菌株固态发酵高温豆粕工艺优化[J].中国食品学报, 2017, 17(9):116-124.
ZHENG H Y, LANG S B, CUI Y T, et al.Process optimization for solid-state fermentation of high temperture soybean meal by mixture strains[J].Journal of Chinese Institute of Food Science and Technology, 2017, 17(9):116-124.
[15] 彭真, 吴振强, 李运南, 等.红曲黄色素固态发酵条件的研究[J].现代食品科技, 2009, 25(6):669-673.
PENG Z, WU Z Q, LI Y N, et al.Study of solid-state fermentation by mohascus ahka for yellow pigment production[J].Modern Food Science and Technology, 2009, 25(6):669-673.
[16] 王辉. 薏仁蒸煮特性及改进技术的研究[D].重庆:西南大学, 2014.
WANG H.Research on cooking characteristics and improved technology of coix seed[D].Chongqing:Southwest University, 2014.
[17] 郝飞, 吴群, 徐岩.枯草芽孢杆菌(Bacillus subtilis)发酵生产乙偶姻的pH调控策略[J].微生物学通报, 2013,40(6):921-927.
HAO F, WU Q, XU Y.Two-stage pH control strategy of acetoin production by Bacillus subtilis CCTCC M208157[J].Microbiology China, 2013,40(6):921-927.
[18] ZHU B, XU Y.Production of tetramethylpyrazine by batch culture of Bacillus subtilis with optimal pH control strategy[J].Journal of Industrial Microbiology & Biotechnology, 2010, 37(8):815-821.
[19] 赵菡. 纳豆激酶热稳定性及pH稳定性的改造研究[D].无锡:江南大学, 2018.
ZHAO H.Study on enhanced thermal and pH stability of nottakinase[D].Wuxi:Jiangnan University, 2018.
[20] 付云, 赵谋明, 卢美杉, 等.枯草芽孢杆菌YA215发酵螺旋藻渣产抑菌活性的工艺[J].食品与发酵工业, 2020, 46(4):146-152.
FU Y, ZHAO M M, LU M S, et al.Fermentation optimization of antimicrobial spirulina residue from Bacillus subtilis YA215[J].Food and Fermentation Industries, 2020, 46(4):146-152.
[21] 朱兵峰. 枯草杆菌两步法生产四甲基吡嗪的调控及机制的研究[D].无锡:江南大学, 2010.
ZHU B F.Fermentation control and mechanism of two-step tetramethylpyrazine production by Bacillus subtilis[D].Wuxi:Jiangnan University, 2010.
[22] CHEN J, CHEN Q, GUO Q, et al.Simultaneous determination of acetoin and tetramethylpyrazine in traditional vinegars by HPLC method[J].Food Chemistry, 2010, 122(4):1 247-1 252.
[23] NIU Y, YAO Z, XIAO Q, et al.Characterization of the key aroma compounds in different light aroma type Chinese liquors by GC-olfactometry, GC-FPD, quantitative measurements, and aroma recombination[J].Food Chemistry, 2017, 233:204-215.
[24] XIAO Z, ZHAO L, TIAN L, et al.GC-FID determination of tetramethylpyrazine and acetoin in vinegars and quantifying the dependence of tetramethylpyrazine on acetoin and ammonium[J].Food Chemistry, 2018, 239:726-732.
[25] 侯孝元, 顾如林, 梁文龙, 等.利用发酵法生产四甲基吡嗪研究进展[J].生物技术通报, 2016, 32(1):58-64.
HOU X Y, GU R L, LIANG W L, et al.Research progress on production of tetramethylpyrazine by fermentation[J].Biotechnology Bulletin, 2016, 32(1):58-64.
[26] XIAO Z, HOU X, LIU X, et al.Accelerated green process of tetramethylpyrazine production from glucose and diammonium phosphate[J].Biotechnology for Biofuels, 2014, 7(1):106.