玉米赤霉烯酮(zearalenone, ZEN)是由镰刀菌属真菌产生的一种毒素[1],广泛存在于玉米、小麦、大米等谷物、饲料和动物制品中。ZEN具有类雌激素作用,进而影响人体和动物的生殖系统[2-3]。此外,ZEN还具有免疫毒性、肝肾毒性及致癌性[4-5]。我国国标规定小麦及玉米中ZEN含量不超过60 μg /kg,饲料中ZEN含量不超过500 μg/kg。目前,食品中ZEN的检测方法有高效液相色谱法(high performance liquid chromatography,HPLC)[6-10]、免疫法[11-14]、生物传感器法[15-18]等。高效液相色谱法准确度和稳定性较高,但需要复杂的样品前处理及昂贵的仪器设备,不能满足食品安全快速检测的要求。免疫分析法具有高效、快速的特点,但其会出现假阳性问题,并且抗体的制备周期较长、稳定性差,限制了该方法的应用。
适配体是一种单链DNA或RNA,通过指数富集(systematic evolution of ligands by exponential enrichment,SELEX)系统筛选产生,能够与靶标进行特异性结合,稳定且易制备,在4 ℃条件下能保存半年甚至更久,不会影响其结构[19-21],目前已广泛应用于食品安全检测及疾病诊断方面。HE等[22]利用适配体的特异性构建基于内滤效应的荧光比率分析法检测ZEN和伏马毒素,该方法选择性高,实际样品加标回收率在89.9%~106.6%。ZHANG等[23]通过SELEX法筛选出ZEN适配体,并建立基于纳米金的无标记可视化方法检测ZEN。HE等[24]构建基于纳米材料和适配体的高灵敏的电化学传感方法成功用于ZEN检测,其中纳米材料(CoSe2/AuNRs和DNA-PtNi@Co-MOF)的主要作用是放大检测信号,该方法的检出限为1.37 fg/mL。另有新型3D樱花状金属有机框架材料也可用于电化学信号放大,该方法检出限可达0.45 fg/mL[25]。本课题组也开发了多种适配体荧光传感器检测黄曲霉毒素、展青霉毒素等真菌毒素[26-28]。
Genefinder染料是一种新型花青素类核酸染料,与双链DNA作用后荧光会增强800~1 000倍,毒性低、灵敏度高[29-30],可以有效保护实验人员和环境,常用于电泳中核酸染色[30-31],在检测方面的研究较少。因此,本研究利用核酸适配体的特异性,结合荧光染料Genefinder特殊的荧光特性构建荧光传感体系,用于简单、高灵敏、快速检测ZEN。
1 材料与方法
1.1 仪器与试剂
UV-2450紫外分光光度计,日本岛津公司;F-2500荧光分光光度计,日本日立公司;台式高速离心机,德国Eppendorf公司。
ZEN适配体(5′-TCATCTATCTATGGTACATTACTATCTGTAATGTGATATG-3′)[32],互补链(5′- TCAAATTAAAGATAATAATGTATTATAGAT-3′),生工生物工程(上海)有限公司;染料Genefinder,合肥博美生物科技有限责任公司;ZEN、黄曲霉毒素B1(aflatoxin B1,AFB1)、黄曲霉毒素G1(aflatoxin G1,AFG1)、赭曲霉毒素A(ochratoxin A,OTA)、单端孢霉烯(T-2)毒素标准品,美国Sigma公司;玉米粉购于本地超市。
1.2 试验方法
1.2.1 DNA浓度测定
DNA浓度以单链浓度表示,利用紫外-可见分光光度计测定DNA在260 nm处的吸光度,依据朗伯比尔定律(A=εbc)计算得DNA的浓度,于-20 ℃备用。
1.2.2 荧光传感器对ZEN的检测
DNA备用溶液解冻后,水浴90 ℃处理10 min,逐渐冷却至室温备用。将ZEN适配体与等体积等浓度互补链混合,再加入一定浓度ZEN孵育一段时间后,加入2 μL Genefinder100×,测定荧光光谱(激发波长500 nm)。
1.2.3 实际样品中ZEN的检测
向玉米粉样品中加入一定量ZEN,分别配成质量浓度为5、20 μg/L的阳性样本。用90%(体积分数)乙腈水溶液超声提取30 min,离心后取上清液,氮吹吹干后加入缓冲溶液,按照构建的荧光传感体系检测ZEN。
2 结果与分析
2.1 检测原理
本研究利用Genefinder的特性构建荧光传感体系用于快速检测ZEN。检测原理图如图1-A,ZEN适配体与其互补链形成双链结构,Genefinder染料与双链结构DNA作用后产生较强的荧光,当加入毒素ZEN后,适配体优先识别ZEN,双链打开,Genefinder染料荧光猝灭。首先研究方法的可行性,以500 nm作为激发波长,观察不同条件下体系荧光信号的变化情况。图1-B显示单链适配体(a)及互补链(b)与Genefinder作用后显示出较微弱的荧光信号。ZEN适配体与其互补链形成双链结构,Genefinder染料与双链结构DNA结合后荧光信号明显增强(d),加入毒素ZEN后,适配体与ZEN结合,双链打开,Genefinder染料荧光强度降低(e)。根据荧光强度的改变检测目标物ZEN的含量。

a-ZEN适配体+Genefinder;b-互补链+Genefinder;c-40 μg/L ZEN;
d-ZEN适配体+互补链+Genefinder;e-ZEN适配体+互补链+
40 μg/L ZEN+Genefinder
A-传感体系的检测原理图;B-不同条件下荧光光谱
图1 传感体系原理图及可行性分析
Fig.1 Schematic of sensing system and feasibility
2.2 检测条件优化
2.2.1 孵育时间
实验中适配体需要与目标物孵育一段时间才能更好地识别目标物。因此本试验研究孵育时间对荧光信号的影响。如图2所示,随着孵育时间的延长,荧光猝灭量逐渐增强,当孵育时间60 min时,荧光猝灭量达到最大,说明体系中ZEN适配体与ZEN结合已达到饱和。因此,本实验选择60 min为最佳孵育时间。
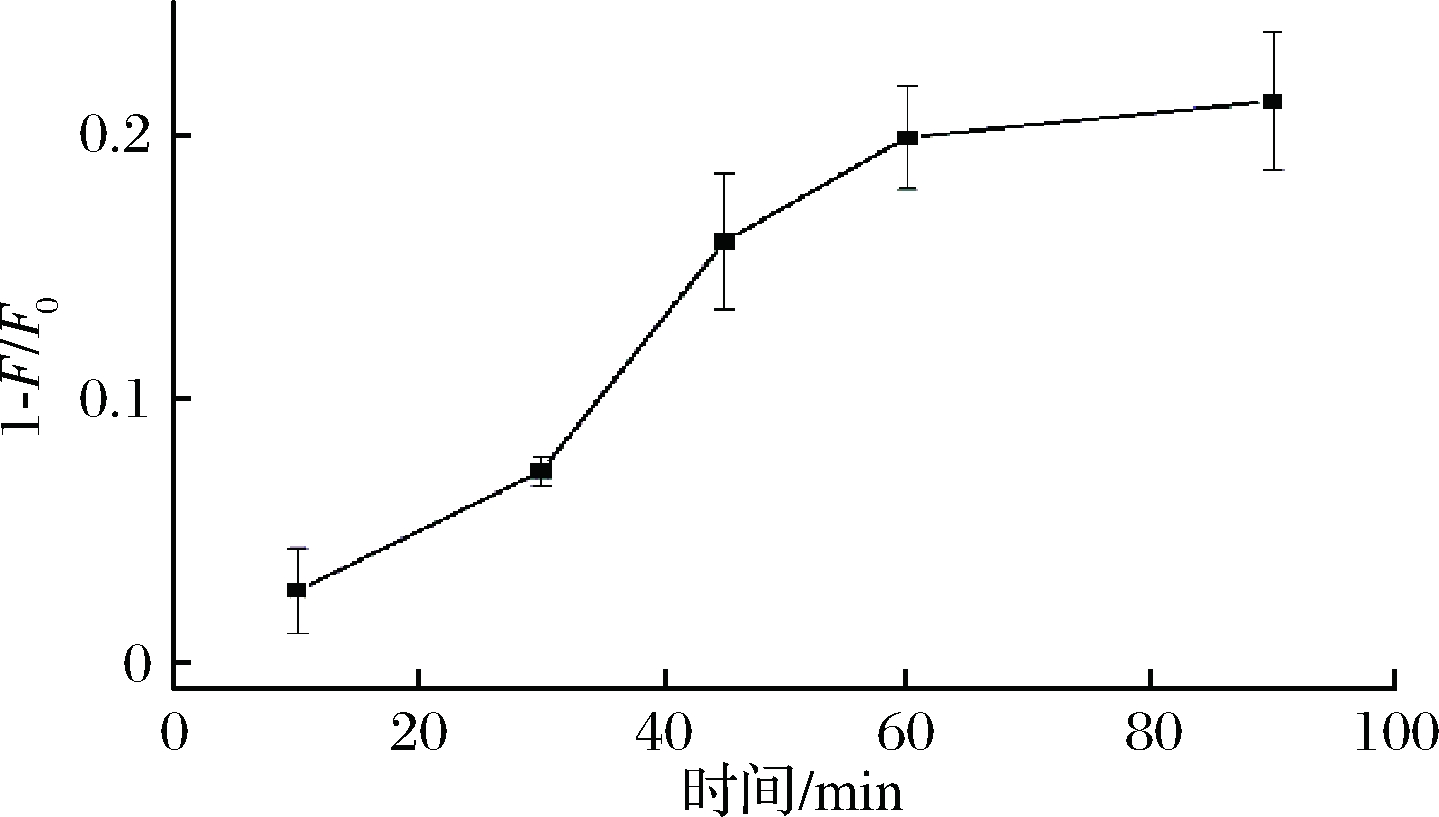
图2 孵育时间对荧光强度的影响
Fig.2 Effect of incubated time on the fluorescence
注:F0和F分别表示没有目标物和添加目标物时的荧光强度(下同)
2.2.2 适配体用量
适配体用量会影响荧光传感体系的灵敏度。图3显示适配体浓度对荧光传感体系的影响。结果表明,随着ZEN适配体用量的增加,荧光猝灭程度迅速增加,当适配体浓度达到100 nmol/L时,荧光猝灭量趋于稳定。因此,适配体用量选择100 nmol/L作为后续实验用量。
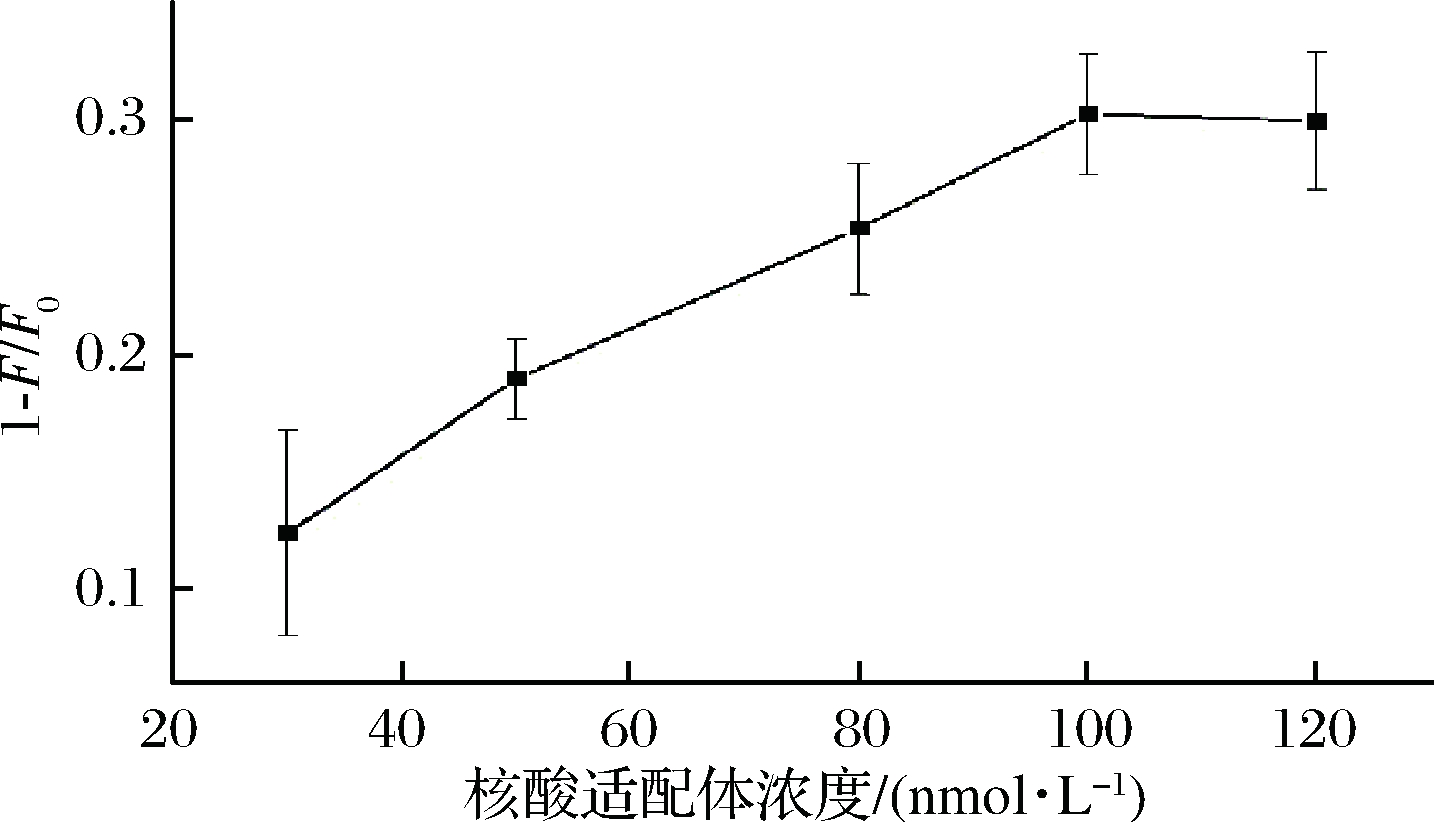
图3 适配体用量对荧光强度的影响
Fig.3 Effect of aptamer on the fluorescence
2.3 荧光传感体系的构建
在上述最佳的实验条件下,以ZEN为目标物构建荧光传感体系。结果如图4-A所示,随着ZEN浓度的增加,传感体系荧光强度逐渐减弱,这是由于ZEN的加入破坏了双链结构,从而造成荧光减弱。然而当ZEN浓度达到一定值时,荧光强度不再明显降低,达到平衡。从图4-B可以看出,1-F/F0与ZEN浓度的对数在0.1~200 μg/L呈线性关系,实际可检测的检出限为0.1 μg/L。结果表明该荧光传感体系具有较高的灵敏度。

a-0 μg/L;b-0.1 μg/L;c-0.5 μg/L;d-1 μg/L;e-5 μg/L;
f-10 μg/L;g-50 μg/L;h-100 μg/L;i-200 μg/L;j-500 μg/L
A-不同浓度ZEN对所构建传感体系荧光响应变化;
B-1-F/F0与ZEN浓度对数的线性关系
图4 传感体系对ZEN的荧光响应
Fig.4 Fluorescence resptnse of sensor with ZEN
2.4 荧光传感体系选择性
为了考察荧光传感体系的选择性,本研究以黄曲霉毒素B1(AFB1)、黄曲霉毒素G1(AFG1)、赭曲霉毒素A(OTA)、单端孢霉烯毒素(T-2)为对照毒素研究该传感体系的特异性,结果如图5所示。加入40 μg/L ZEN时,荧光传感体系的荧光强度猝灭量显著增加,而加入同浓度的对照毒素并未造成荧光体系明显的荧光猝灭,这是因为传感体系中使用的适配体与ZEN可以特异性结合,而与其他毒素无明显作用。结果表明该传感体系对ZEN具有较好的选择性。
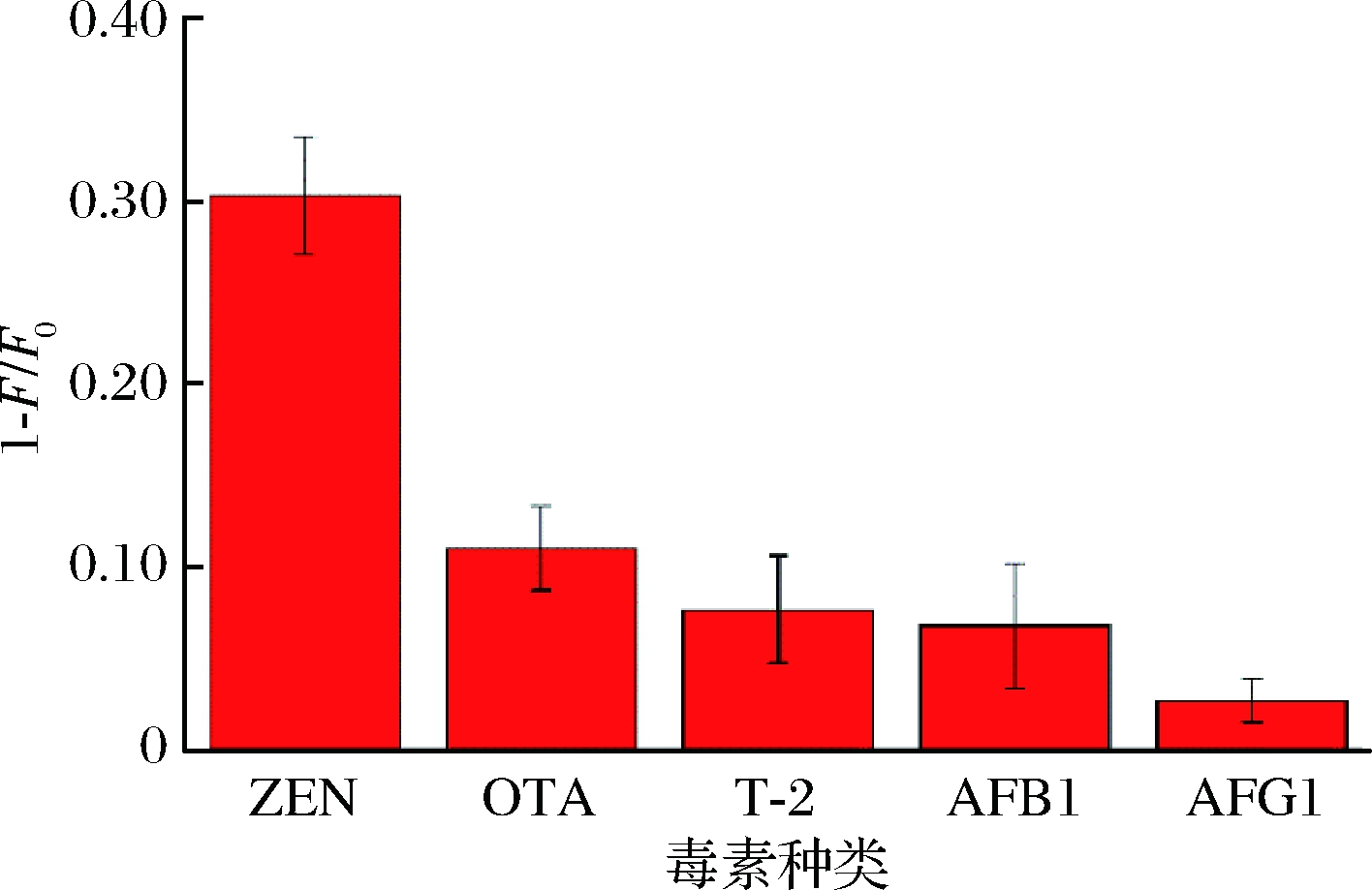
图5 方法选择性
Fig.5 Selectively of sensing system
注:ZEN质量浓度为40 μg/L,其他对照毒素质量浓度均为40 μg/L
2.5 实际样品测定
为了考察传感体系实际测定效果,以玉米粉作为实际阴性样品,研究加标回收情况。结果如表1所示,利用传感体系检测的加标回收率在100.4%~105.8%,这说明该荧光传感体系可用于真实复杂样品中ZEN的测定。
表1 实际样品加标回收率(n=3)
Table 1 Real sample spike recovery (n=3)
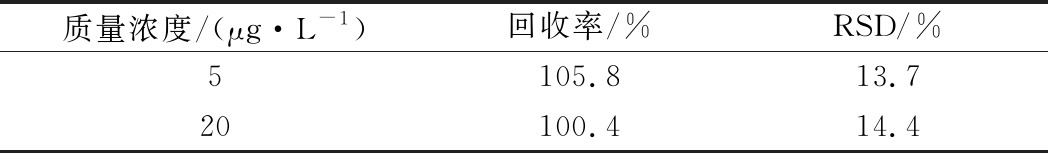
质量浓度/(μg·L-1)回收率/%RSD/%5105.813.720100.414.4
3 结论
本研究构建了一种基于Genefinder和适配体的荧光传感体系检测ZEN,在最优条件下,传感体系的线性范围为0.1~200 μg/L,实际检出限为0.1 μg/L,并成功用于出玉米粉中ZEN的测定,加标回收率在100.4%~105.8%。本方法为开发简单、快速、灵敏的生物传感体系开辟了新的途经。
[1] MALLY A, SOLFRIZZO M, DEGEN G H.Biomonitoring of the mycotoxin zearalenone:current state-of-the art and application to human exposure assessment[J].Archives of Toxicology, 2016, 90(6):1-12.
[2] KUIPER G T, SCOTT P M, WATANABE H.Risk assessment of the mycotoxin zearalenone[J].Regulatory Toxicology and Pharmacology, 1987, 7(3):253-306.
[3] WANG J J, WEI Z K, HAN Z, et al.Zearalenone induces estrogen-receptor-independent neutrophil extracellular trap release in vitro[J].Journal of Agricultural and Food Chemistry, 2019, 67(16):4 588-4 594.
[4] BECCI P J, VOSS K A, HESS F G, et al.Long-term carcinogenicity and toxicity study of zearalenone in the rat[J].Journal of Applied Toxicology, 1982, 2(5):247-254.
[5] LIOI M B, SANTORO A, BARBIERI R B, et al.Ochratoxin A and zearalenone:A comparative study on genotoxic effects and cell death induced in bovine lymphocytes[J].Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 2004, 557(1):19-27.
[6] 邵瑞婷, 张丽华, 史娜, 等.免疫亲和净化-超高效液相色谱-串联质谱法测定食品中玉米赤霉烯酮类真菌毒素[J].食品科学, 2017, 38(16):274-279.
SHAO R T, ZHANG L H, SHI N, et al.Determination of zeranols in food by ultra performance liquid chromatography-tandem mass spectrometr[J].Food Sience, 2017, 38(16):274-279.
[7] OK H E, CHOI S W, KIM M, et al.HPLC and UPLC methods for the determination of zearalenone in noodles, cereal snacks and infant formula[J].Food Chemistry, 2014, 163:252-257.
[8] 黎睿, 谢刚, 王松雪.高效液相色谱法同时检测粮食中常见8种真菌毒素的含量[J].食品科学, 2015, 26(6):206-210.
LI R, XIE G, WANG S X.Simultaneous analysis of 8 mycotoxins in grains by high performance liquid chromatography[J].Food Science, 2015, 26(6):206-210.
[9] GIOVANNI D, JAVIER H B, HERRERA A V, et al.Determination of estrogenic compounds in milk and yogurt samples by hollow-fibre liquid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry[J].Analytical and Bioanalytical Chemistry, 2016, 408(26):1-13.
[10] WANG S W, NIU R, YANG Y M, et al.Development and evaluation of rapid immunomagnetic extraction for effective detection of zearalenone in agricultural products[J].Food Control, 2020, 110:106 973-106 978.
[11] 李晓波, 李慧娟, 宋鸽, 等.竞争性酶联免疫快速检测高产奶牛精料补充料中玉米赤霉烯酮[J].食品安全质量检测学报, 2020, 11(1):280-284.
LI X B, LI H J, SONG G, et al.Rapid detection of zearalenone in high-yielding dairy concentrate supplements by competitive enzyme-linked immunosorbent assay[J].Journal of Food Safety Quality, 2020,11(1):280-284.
[12] FANG D D, ZENG B S, ZHANG S P, et al.A self-enhanced electrochemiluminescent ratiometric zearalenone immunoassay based on the use of helical carbon nanotubes[J].Microchimica Acta, 2020, 187(5):303-311.
[13] CHEN Y J, ZHANG S P, HONG Z S, et al.A mimotope peptide-based dual-signal readout competitive enzyme-linked immunoassay for nontoxic detection of zearalenone[J].Journal of Materials Chemistry B, 2019, 7(44):6 972-6 980.
[14] 俞大海, 毛佳甜, 王一铭, 等.可食包装纸原料中玉米赤霉烯酮的快速检测研究[J].包装工程, 2019,40(1):87-92.
YU D H, MAO J T, WANG Y M, et al.Rapid detection of zearalenone in edible packaging materials[J].Packaging Engineering, 2019,40(1):87-92.
[15] 张天磊, 周奕华, 曹晟.碳点荧光探针在农产品快速检测中的应用进展[J].包装工程, 2020,41(3):82-91.
ZHANG T L, ZHOU Y H, CAO S.Advances in application of CDs fluorescent probe in rapid detection of agriculture products[J].Packaging Engineering, 2020,41(3):82-91.
[16] AZRI F A, EISSA S, ZOUROB M, et al.Electrochemical determination of zearalenone using a label-free competitive aptasensor[J].Microchimica Acta, 2020, 187(5):266-275.
[17] HAN Z, TANG Z M, JIANG K Q, et al.Dual-target electrochemical aptasensor based on co-reduced molybdenum disulfide and AuNPs (rMoS-Au) for multiplex detection of mycotoxins[J].Biosensors and Bioelectronics, 2020, 150:111 894-111 901.
[18] MA L Y, BAI L J, ZHAO M, et al.An electrochemical aptasensor for highly sensitive detection of zearalenone based on PEI-MoS 2-MWCNTs nanocomposite for signal enhancement [J].Analytica Chimica Acta, 2019, 1 060:71-78.
[19] ELLINGTON A D, SZOSTAK J W.In vitro selection of RNA molecules that bind specific ligands[J].Nature, 1990, 346:818-822.
[20] GUO T, LIN X D, LIU Y Q, et al.Target-induced DNA machine amplification strategy for high sensitive and selective detection of biotoxin[J].Sensors and Actuators B:Chemical, 2018, 262:619-624.
[21] TUERK C, GOLD L.Systematic evolution of ligands by exponential enrichment:RNA ligands to bacteriophage T4 DNA polymerase[J].Science, 1990, 249:505-510.
[22] HE D Y, WU Z Z, CUI B, et al.A fluorometric method aptamer-based simultaneous determination of two kinds of the fusarium mycotoxins zearlenone and fumonisin B-1 making use of gold nanorods and upconversion nanoparticles[J].Microchimica Acta, 2020, 187(4):254-261.
[23] ZHANG Y Y, LU T F, WANG Y, et al.Selection of a DNA aptamer against zearalenone and docking analysis for highly sensitive rapid visual detection with label-free aptasensor[J].Journal of Agricultural and Food Chemistry, 2018, 66(5):12 102-12 110.
[24] HE B S, YAN X H.Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@Co-MOF networks for the detection of zearalenone[J].Sensors and Actuators:B chemical, 2020, 306:127 558-127 565.
[25] JI X D, YU C, WEN Y L, et al.Fabrication of pioneering 3D sakura-shaped metal-organic coordination polymers Cu@L-Glu phenomenal for signal amplification in highly sensitive detection of zearalenone[J].Biosensors and Bioelectronics, 2019, 129:139-146.
[26] 郭婷, 林淑凤, 马良, 等.基于磁性纳米材料和适配体的荧光传感器检测牛奶中黄曲霉毒素M1[J].食品与发酵工业, 2019, 45(5):218-223.
GUO T, LIN S F, MA L, et al.A fluorescent biosensor based on magnetic nanoparticles and aptamer for detecting AFM1 in milk[J].Food and Fermentation Industries, 2019, 45(5):218-223.
[27] TAN H X, MA L, GUO T, et al.A novel fluorescence aptasensor based on mesoporous silica nanoparticles for selective and sensitive detection of aflatoxin B1[J].Analytica Chimica Acta, 2019, 1 068:87-95.
[28] MA L, GUO T, PAN S L, et al.A fluorometric aptasensor for patulin based on the use of magnetized graphene oxide and DNase I-assisted target recycling amplification[J].Microchimica Acta, 2018, 185(10):487-492.
[29] ZHAN S S, XU H C, ZHANG D W, et al.Fluorescent detection of Hg2+ and Pb2+ using Genefinder (TM) and an integrated functional nucleic acid[J].Biosensors and Bioelectronics, 2015, 72:95-99.
[30] HONG G H, PARK Y G, JIN H W, et al.Comparison of the clinical performance of Omniplex-HPV and Genefinder HPV for the detection and genotyping of human papillomaviruses in cervical specimens[J].Journal of Medical Microbiology, 2018, 67(9):1 279-1 286.
[31] ALMASI M A, MANESH M E, JAFARY H, et al.Visual detection pZENto leafroll virus by loop-mediated isothermal amplification of DNA with the Genefinder (TM) dye[J].Journal of Virological Methods, 2013, 192(1-2):51-54.
[32] CHEN X J, HUANG Y K, DUAN N, et al.Selection and identification of ssDNA aptamers recognizing zearalenone[J].Analytical and Bioanalytical Chemistry, 2013, 405(20):6 573-6 581.