植物雌激素是从植物中发现或源自植物前体的多酚类物质,结构与内源性雌激素类似,具有雌激素和抗雌激素功能[1]。根据其化学结构的不同,主要分为异黄酮类、香豆雌酚类、木酚素类、二苯乙烯类、鞣花丹宁类[2]。其中木酚素是一类由两分子苯丙素衍生物(C6-C3)聚合而成的化合物[3],按其来源可分为植物木酚素和哺乳动物木酚素。植物中含量最多的植物木酚素是开环异落叶松树脂酚(secoisolariciresinol,SECO)及其前体开环异落叶松树脂酚二葡萄糖苷(secoisolariciresinol diglucoside,SDG)、罗汉松脂酚(matairesinol,MAT)。另外,松脂酚(pinoresinol,PIN)、皮树脂醇(medioresinol,MED)、丁香脂素(syringaresinol,SYR)、异落叶松脂素(isolariciresinol,ISOLAR)、羟基罗汉松脂酚(hydroxymatairesinol,HYMAT)、落叶松树脂醇(lariciresinol,LARI)、牛蒡子苷(arctiin,ART)、牛蒡苷元(arctigenin,ARG)、脱水开环异落叶松脂酚(anhydrosecoisolariciresinol,AHS)、芝麻素酚(sesaminol,SES)及前体芝麻素酚三糖苷(sesaminol triglucoside,STG)也是在植物中发现的木酚素[1]。植物木酚素因具有抗真菌、抗细菌、抗病毒甚至杀虫的特性,可以保护植物免受病原体和害虫侵害,如MAT及其相关代谢物可抑制云杉真菌生长[4]。哺乳动物木酚素也叫肠道木酚素,由植物木酚素经过肠道微生物的作用形成,主要是肠二醇(enterodiol,END)和肠内酯(enterolactone,ENL),存在于血清、血浆、尿液和粪便中。
木酚素因在预防结肠癌、乳腺癌、前列腺癌、心血管疾病和更年期综合症[1]等疾病中发挥作用,近年来越来越受到关注,然而植物木酚素由于其生物利用度低而具有有限的生物学特性[5-6],必须将其转化为肠道木酚素才能在人体内发挥最大有益作用。尽管已经有了化学合成END的方法[7],但因其使用的化学试剂价格昂贵、步骤复杂且对环境有污染,实际生产中越来越倾向于生物转化法,生物转化法具有操作简单、对设备要求低、反应快速等优点,具有重要的应用前景以及巨大的经济价值和现实意义。因此,本文将主要综述木酚素的结构、种类和分布、木酚素肠道微生物代谢机制,以及体外木酚素微生物和酶法转化的研究进展。
1 木酚素生理功能
END和ENL与雌二醇化学结构相似,进入机体后与雌激素竞争性结合雌激素受体,产生一系列生理活性[8]。体内外试验表明,肠道木酚素是哺乳动物芳香酶抑制剂,它通过与竞争底物雌烯二酮的结合、抑制雄性激素的合成、与性甾结合蛋白竞争性结合、促进性激素结合蛋白的合成、抑制二氢睾酮与促进性激素结合蛋白的结合及抑制人组织中5α-还原酶和17β-羟基类固醇脱氢酶的活性,进而降低血浆中雌二醇和睾丸激素的水平达到防治性激素依赖性疾病的目的。现有研究表明,肠道木酚素还具有内源性洋地黄样及降低冠心病发作的生理活性[9]。除此之外,肠道木酚素能影响结肠、前列腺和乳腺的体外培养细胞的增殖,在体外比木酚素前体具有更强的抗氧化性能[10]。
2 木酚素分布
木酚素主要存在于植物中,在根、茎、叶、种子及果实中都能发现,软木树的结中存在大量的木酚素,主要是以HYMAT的形式[3]存在,如挪威云杉结中含有高达20%的木酚素(干重),其中HYMAT为主要成分。亚麻籽是最广泛的植物木酚素来源,含量约300 mg/100 g鲜重,以SECO的糖苷形式SDG为主[11](表1)。谷物类如黑麦、小麦、荞麦和燕麦含有中等到高等含量的木酚素,全麦亚麻籽面包和杂粮面包中含量也较高,羽衣甘蓝、卷心菜、芦笋、花椰菜、大蒜、杏、柚子和枣等蔬菜水果都含有适量的木酚素,红茶、绿茶、咖啡、啤酒等常见饮料中也有少量木酚素[12-16]。
表1 植物性食品中植物木酚素的含量 单位:μg/100 g(湿重)
Table 1 Lignan content in plant foods
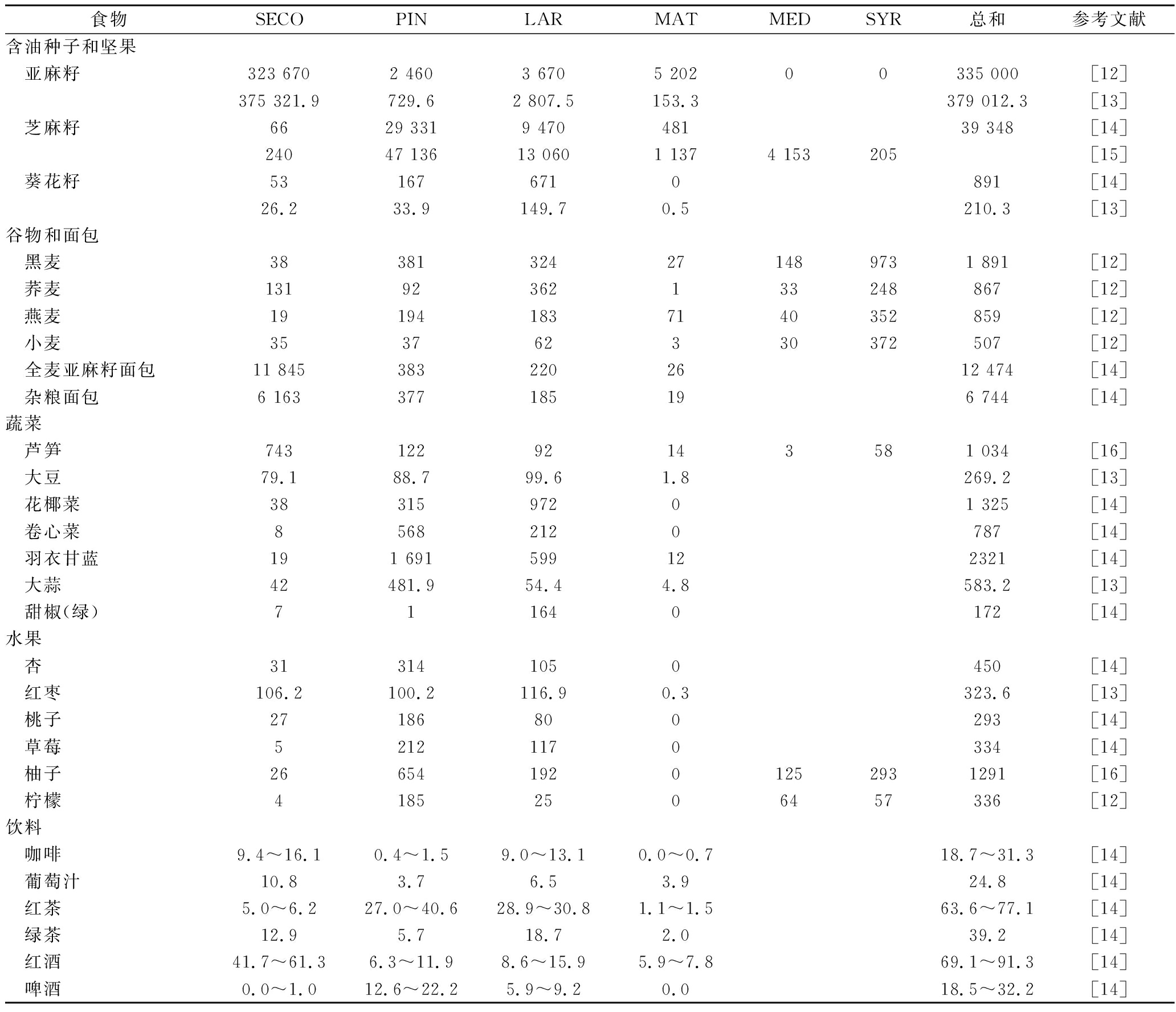
食物SECOPINLARMATMEDSYR总和参考文献含油种子和坚果亚麻籽323 6702 4603 6705 20200335 000[12]375 321.9729.62 807.5153.3379 012.3[13]芝麻籽6629 3319 47048139 348[14]24047 13613 0601 1374 153205[15]葵花籽531676710891[14]26.233.9149.70.5210.3[13]谷物和面包黑麦38381324271489731 891[12]荞麦13192362133248867[12]燕麦191941837140352859[12]小麦353762330372507[12]全麦亚麻籽面包11 8453832202612 474[14]杂粮面包6 163377185196 744[14]蔬菜芦笋74312292143581 034[16]大豆79.188.799.61.8269.2[13]花椰菜3831597201 325[14]卷心菜85682120787[14]羽衣甘蓝191 691599122321[14]大蒜42481.954.44.8583.2[13]甜椒(绿)711640172[14]水果杏313141050450[14]红枣106.2100.2116.90.3323.6[13]桃子27186800293[14]草莓52121170334[14]柚子2665419201252931291[16]柠檬41852506457336[12]饮料咖啡9.4~16.10.4~1.59.0~13.10.0~0.718.7~31.3[14]葡萄汁10.83.76.53.924.8[14]红茶5.0~6.227.0~40.628.9~30.81.1~1.563.6~77.1[14]绿茶12.95.718.72.039.2[14]红酒41.7~61.36.3~11.98.6~15.95.9~7.869.1~91.3[14]啤酒0.0~1.012.6~22.25.9~9.20.018.5~32.2[14]
3 木酚素代谢
SETCHELL等[17]用人粪便菌培养亚麻籽可产生END和ENL,但是无菌粪便培养不能产生,且抗生素处理降低了结肠中END和ENL的产生,表明肠道微生物群负责将植物木酚素转化为肠道木酚素,主要部位是盲肠和结肠。肠道菌群主要包括拟杆菌门、厚壁菌门、放线菌门和变形杆菌门,有超过1 000种不同的细菌种类,肠道菌群扮演着基因编码酶的储存库的角色,这些酶可用于不同的代谢活动,而这是宿主本身所不具备的[2]。植物雌激素的潜在健康益生功能和生物活性很大程度上依赖于肠道微生物群,通过研究人体肠道菌群的代谢活动,可以更好地了解人类肠道菌群代谢木酚素的机制。
表2整理了能使植物木酚素转化为肠道木酚素及其前体的各种特定肠道细菌。因为亚麻籽是木酚素最丰富的来源,大多数实验都是使用亚麻籽为底物,亚麻籽中主要含有SECO,一般以其糖苷形式SDG存在。SDG的生物转化通过肠道菌群中占优势、亚优势组之间复杂的相互作用进行,一般要经过4个步骤(图1),转化的第一步是从SDG中除去葡萄糖基产生SECO,这种生物转化可以通过产β-葡萄糖苷酶的菌株进行[6]。SDG释放葡萄糖基是益生菌增加SECO释放、提高其结肠黏膜吸收的生物利用度、肠道微生物向END和ENL的生物转化的重要特征[18]。水解SDG的细菌有拟杆菌,如吉氏拟杆菌(Bacteroides distasonis),脆弱拟杆菌(B.fragilis),卵形拟杆菌(B.ovatus)和梭菌,如耳蜗形梭菌(Clostridium cocleatum)、多枝梭菌(C.ramosum)、噬糖梭菌(C.saccharogumia)[19-21]。RONCAGLIA等[22]证明了双歧杆菌可将SDG水解为SECO,发现了10株能够水解SDG的双歧杆菌菌株,尤其是假链状双歧杆菌WC401,对水解SDG和其他糖缀合的多酚类物质具有较高的效率。
表2 使植物木酚素转化为肠道木酚素及其前体的各种特定肠道细菌
Fig.2 Various specific intestinal bacterias of transforming plant lignans into intestinal lignans and precursors

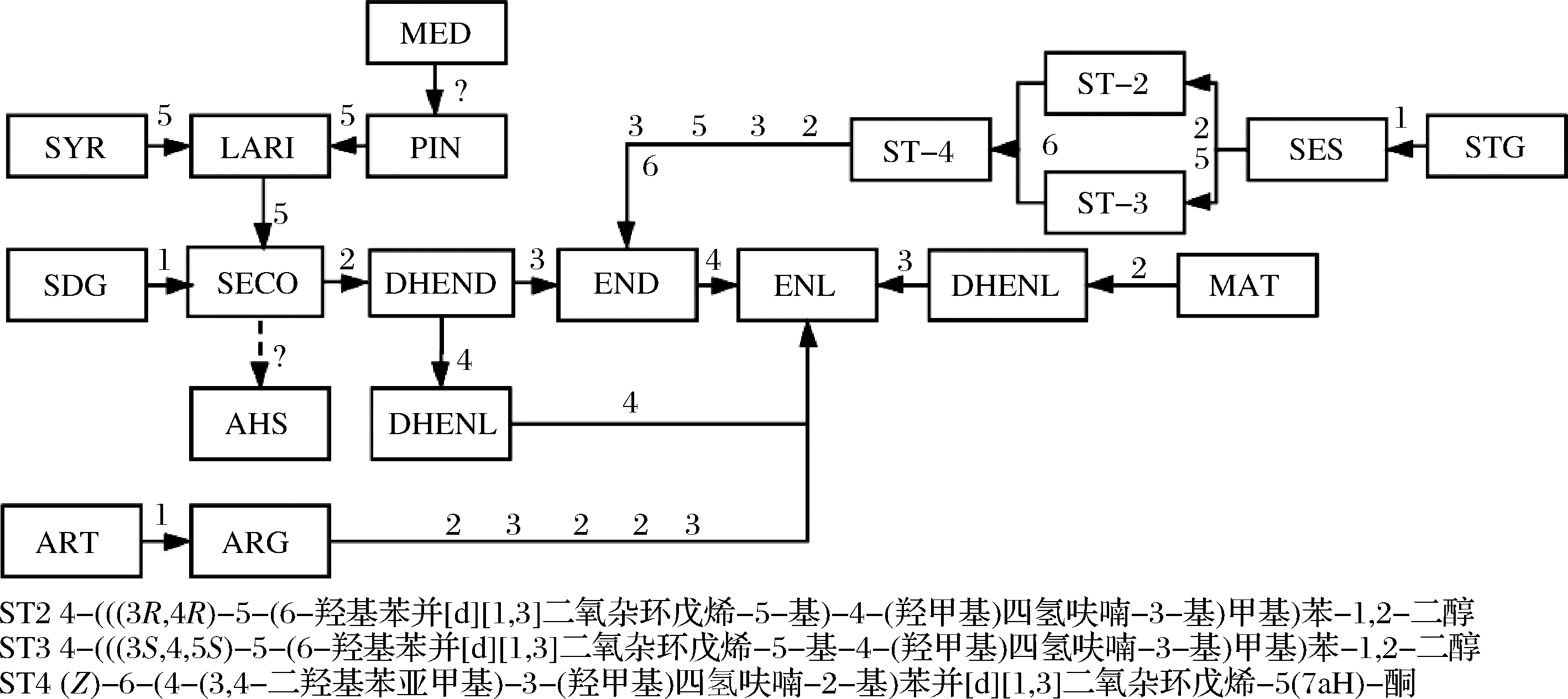
图1 常见的植物木酚素转化为肠道木酚素END和ENL的途径
Fig.1 Conversion pathway of common plant lignans to enterolignans END and ENL
注:实线表示已知路径,虚线表示理论路径;反应为:1-去糖基;2-脱甲基;3-脱羟基;4-脱氢;5-还原;6-氧化;?-未知
转化的第二步是SECO去除甲基,产生二羟基肠二醇(dihydroxyenterodiol,DHEND)。能够使SECO去甲基的菌株有食甲基丁酸杆菌(Butyribacterium methylotrophicum)、卡兰德真杆菌(Eubacterium callanderi)、粘液真杆菌(Eubacterium limosum)、布劳特氏菌(Blautia producta)、消化链球菌(Peptostreptococcus productus)[19-20]。P.productus不仅催化SECO去甲基化,而且还催化LAR、MAT、PIN和其他各种甲基化芳香化合物的去甲基化[19]。Eubacterium. sp.ARC-2可使牛蒡苷元脱甲基为二羟基肠内脂(dihydroxyenterolactone,DHENL),还可以通过去甲基化将SECO转化为DHEND[23]。JIN等[24]发现梭菌科细菌(Clostridiaceae) END-2具有使SECO脱甲基的能力。
转化的第三步是脱羟基,产生END。能去除芳香环上的羟基的细菌有迟缓埃格特菌(Eggerthella lenta)和梭状芽苞杆菌(C.scindens)[20]。Lactonifactor longoviformis可从END脱氢产生ENL[20-21]。这些反应似乎是对映体特异性的,SDG与肠道细菌共孵育后分离出的END和ENL的形式为(+)-END和(+)-ENL。而ART或松脂酚二葡萄糖苷为底物则生成(-)-END和(-)-ENL[25-26]。E.lenta SDG-2可以将(+)-DHEND转化为(+)-END,而不能将(-)-DHEND转化为(-)-END,人类粪便中分离出的ARC-1,不仅能将(-)-DHEND转化为(-)-END,也能将(+)-DHENL转化为(+)-ENL[24]。另外,DHEND可以通过脱氢作用形成内酯环,从而形成中间体DHENL,然后再脱氢生成ENL[27]。
膳食木酚素PIN也通过4种化学反应被肠道微生物转化:首先是经过2次还原反应生成SECO,然后脱去2个甲基成为DHEND,再脱去2个羟基成为END,最后内酯化成为ENL[19,28]。BESS等[29]通过比较基因组学和转录组研究了4种细菌在厌氧环境生长过程中进行每种反应的能力。E.lenta DSM2243T发生催化还原和脱甲基反应,菌株产生的二苄醚还原酶(benzyl ether reductase,ber),由基因ber编码,足够使PIN发生还原和脱甲基反应;B.producta DSM3507把SECO代谢为DHEND,培养过程中表达最高的基因guaiacol lignan methyltransferase(glm);Gordonibacter pamelaea 3C使DHEND脱羟基,生成END,培养过程中catechol lignan dehydroxylase(cldh)基因显著上调;L.longoviformis DSM 17459T催化END的2个羟基内酯化为ENL,在END的存在下,1个包含3个基因的单一基因组位点的基因转录水平显著上调,其中1个基因与主要协助转运蛋白超家族转运体有同源性,另外2个基因与4Fe-4S NADP-依赖型氧化还原酶基和NAD (P)依赖型短链脱氢酶/还原酶基因(END lactonizing enzyme, edl)具有同源性,可能是这些酶基因簇使END发生到ENL的转化。其他分离菌株的基因簇和酶还有待鉴定,虽然目前已经分离并鉴定了大量的木酚素生物转化和产生肠道木酚素的菌株,但对其基因及编码酶的详细研究并不普遍,比较基因组学和转录分析已被证明是鉴定木酚素生物转化过程中所涉及的酶的有效工具。
芝麻种子中最丰富的木酚素为芝麻素酚三葡萄糖苷(sesaminol triglucoside,STG),是一种有亚甲二氧基苯基的四氢呋喃类木酚素,去糖基后生成芝麻素酚(sesaminol,SES),肠道菌群将四氢呋喃木酚素转化为哺乳动物木酚素,涉及到糖苷的水解、亚甲基的去甲基化、二苄基丁二醇氧化为二苄基丁内酯、呋喃环的还原裂解[30]。对转化SES的生物体的研究没有涉及SDG转化的生物体那么广泛,在人类和大鼠体内以及体外培养中,SES也能转化为END和ENL[30]。ART的苷元ARG转化为ENL需要5个反应步骤,包括3个去甲基化反应和2个去羟基化反应[1]。
END和ENL生成后被肠道吸收,出现在血浆中,然后以葡萄糖醛酸盐或硫酸盐偶联物的形式随尿液和胆汁排出体外。QUARTIERI等[6]用5名连续7 d有亚麻籽膳食补充的受试者的粪便接种含SDG的培养物。在粪便培养物中鉴定出了与SECO、MAT和AHS有关的新型中间体,这些代谢物也以天然形式和/或以葡萄糖苷酸盐或硫酸盐复合物的形式随尿液和胆汁排出体外。GAYA等[31]通过肠道菌群分析木酚素的代谢,体外发酵实验采用14例健康成年志愿者的粪便样品,所有受试者中均发现ENL,而END仅出现5例,木酚素代谢中没有发现性别差异。虽然在肠道木酚素的产生中可以观察到一些个体间的差异,但成年人类的肠道微生物在木酚素的代谢中表现出类似的行为。
4 体外木酚素微生物和酶法转化研究进展
体外木酚素的微生物和酶法转化研究以生产应用为目的,表3整理了与体外木酚素代谢有关的微生物和酶。杜木香等[37]利用生物酶水解SDG,结果显示,3 mg/mL来源于绿色木霉的纤维素酶在60 ℃下反应48 h时,对SDG的水解效果最佳,此时产物SECO的得率为43.6%,相较于化学法水解得率更高,且反应后处理更为简便、快捷。HWANG等[38]从人类粪便中分离出一株蜡样芽孢杆菌(Bacillus cereus H62L),可以释放香豆酸葡萄糖苷(coumaric acid glucoside,CAG)、阿魏酸葡萄糖苷(ferulic acid glucoside,FAG)和SDG的糖基,但是其将SDG转换为SECO的过程是有限的。由于蜡样芽孢杆菌H62L葡萄糖苷酶的可逆性,SDG向SECO的生物转化的产率极其有限,影响产率的参数包括SDG的纯度和β-葡萄糖苷酶的可逆性。为了提高肠道木酚素的产量,有研究提出在较高浓度下使用高纯度SDG底物,或使用变构抑制剂延缓SECO向SDG的反向反应,或对蜡样芽孢杆菌株H62L进行转基因处理。之后,HWANG等[39]研究了从臭豆腐水中分离出的高地芽孢杆菌(Bacillus altitudinis HK02)产生的SDG-β-葡萄糖苷酶对亚麻籽糖苷SDG、CAG和FAG 3种糖苷的去糖作用。这些酚类糖苷的脱糖率取决于糖苷元上的糖基数目。3种糖苷的酶解糖效率可达100%左右。脱脂亚麻籽粉可以增强酶的活性,葡萄糖、蔗糖、果糖、甘露醇等碳源对酶活性有明显的抑制作用,纤维素可能是产生β-葡萄糖苷酶的良好诱导剂。ZHOU等[40]发现1株从人类肠道菌群中分离出来的菌株S1,与克雷伯氏菌属(Klebsiella)亲缘关系相近,该菌株可以通过转化脱脂亚麻籽中前体产生SECO。
表3 与体外木酚素代谢有关的微生物和酶
Table 3 List of microbial and enzyme implicated in the metabolism of lignans in vitro

功能微生物/酶SDG、CAG、FAG脱糖基蜡样芽胞杆菌(B.cereus H62L)/β-葡萄糖苷酶[38]、高山芽孢杆菌(B.altitudinis HK02)[39]SDG脱糖基为SECO单行拟杆菌(B.uniformis ZL-I)[44]从脱脂亚麻籽产SECO克雷伯氏菌(Klebsiella sp. strain S1)[40]从脱脂亚麻籽产(+)-SECO拟杆菌(Bacteroides ZL1)[46]从木酚素提取物产生SECO假链状双歧杆菌(B.pseudocatenulatum INIA P946)[43]SECO脱甲基粘液真杆菌(E.limosum ZL-II)[44]脱羟基迟缓埃格特菌(E.lenta ZL-III)[44]从木酚素提取物产END青春双歧杆菌(B.adolescentis INIA P784)[41]、两歧双歧杆菌(B.bifidum INIA P466)[43]、链状双歧杆菌(B.catenulatum INIA P732)[43]、假长双歧杆菌(B.pseudolongum INIA P2)[43]植物原料转化为END和ENL家畜类哺乳动物肠道菌群[48]、人类肠道菌群[49]、梭菌(Clostridium sp.SDG-1020)株、迟缓埃格特菌(Eggertherlla sp.SDG-1110)株、真杆菌(Eubacterium sp.SDG-1220)株、Lactonifactor sp.SDG-4210株的组合[50]从亚麻提取物产END和ENL唾液乳杆菌(L.salivarius INIA P183)[42]、唾液乳杆菌(L.salivarius INIA P448)[42]、格式乳杆菌(L.gasse-ri INIA P508)[42]
GAYA等[41]研究了90种乳酸菌和双歧杆菌中木酚素的代谢,发现大多数菌株有将SDG脱糖基为SECO的能力。还发现在一株青春双歧杆菌(Bifidobacterium adolescentis INIA P784)作用下,能从木酚素提取物中产生1.12~4.18 μmol/L的END。然而,纯木酚素SECO、MAT和SES并不能产生最终产物。BRAVO等[42]分析了70株乳酸菌、乳球菌和肠球菌产END和ENL的情况。在唾液乳杆菌(Lactobacillus salivarius INIA P183、L.salivarius INIA P448)和格氏乳杆菌(L.gasseri INIA P508)中发现存在END和ENL,浓度分别约为46和6 μmol/L,且这些菌株产END的量是阳性对照B.adolescentis INIA P784产END量的10倍左右,这是首次发现单个菌株同时产生END和ENL。在此基础上,PEIROTN等[43]在20种双歧杆菌菌株中发现3种双歧杆菌(Bifidobacterium bifidum INIA P466、B.catenulatum INIA P732和B.pseudolongum INIA P2)能够从木酚素提取物中产生低水平END(2~11 μmol/L),而另一种假链状双歧杆菌(B.pseudocatenulatum INIA P946)则使SECO产生量大大增加。随后,这3种产END的双歧杆菌和另外3种先前被确定能产肠道木酚素的乳杆菌(L.gasseri INIA P508、L.salivarius INIA P448和L.salivarius INIA P183)分别与纯SECO共同培养,得到END和ENL,而没有代谢MAT。其中B.catenulatum P732和L.gasseri INIA P508是转化SECO效率最高的菌株,产生浓度为2 mmol/L以上的ENL。此外,所有菌株的发酵过程中都观察到中间化合物DHEND的形成。这项工作首次证明了双歧杆菌和乳杆菌能够进行完全的肠道木酚素代谢,转化纯木酚素SECO为END和ENL。
ZHU等[44]通过反复传代和平板培养,从活性菌群中分离鉴定出3个形态各异的菌落,分别称为ZL-I、ZL-II和ZL-III,在ZL-(I-II-III)菌株的联合作用下,可直接从脱脂亚麻籽中产生END。3种菌株分别具有去糖基(ZL-I)、去甲基(ZL-II)和脱羟基(ZL-III)的功能。通过16Sr RNA序列分析发现它们分别与单行拟杆菌(B.uniformis)、粘液真杆菌(E.limosum)和迟缓埃格特菌(E.lenda)密切相关,这些物种都常见于人的肠道。用单行拟杆菌(B.uniformis ZL-I)和粘液真杆菌(E.limosum ZL-II)转化亚麻木酚素的试验中,在2 L的发酵体系中于24 h内约59.1%的SDG转化为DHEND,到第6天时,发酵液中DHEND浓度达最大值,此时SDG转化率达94.4%[45]。而与ZL-I菌株将脱脂亚麻籽中SDG转化为SECO的比例(约80%)[46]相比,SDG转化为END的转化率大大提高,达到90%以上。
MUNOZ等[47]通过完全体外消化全亚麻籽和亚麻籽粉来评估干酪乳杆菌和嗜酸乳杆菌对亚麻木酚素生物可给性(bioaccessibility)的影响。干酪乳杆菌在体外消化过程中提高了亚麻籽中SDG和END的生物可给性。然而,嗜酸乳杆菌在开始的12 h内增加了END的生物可给性,48 h后无增加。2种益生菌对亚麻籽粉的消化均无明显影响。
5 结论
木酚素广泛存在于亚麻籽、黑麦麸皮等各种植物中,是一种植物雌激素,具有预防结肠癌、乳腺癌、前列腺癌、心血管疾病和更年期综合症等作用。植物木酚素需经肠道微生物多步代谢转化为哺乳动物木酚素才能被人体吸收利用,各种木酚素的肠道代谢途径、参与转化的肠道微生物已有许多报道。其中,催化木酚素各转化步骤的微生物酶系仍然有待进一步明确,这将为指导木酚素体外生物转化提供重要理论依据。由于木酚素的生物利用度对肠道菌群健康状况的高度依赖,木酚素体外生物转化,尤其是体外益生菌转化对开发高活性木酚素产品具有重要意义。对植物木酚素分布、生理功能、体内代谢吸收和体外生物转化的研究将推动以亚麻籽粕、麸皮等农副产品为原料开发木酚素高附加值产品的深加工产业发展,实现农业废弃物的资源化利用,具有重要社会经济价值。
[1] LANDETE J M.Plant and mammalian lignans:A review of source,intake,metabolism,intestinal bacteria and health[J].Food Research International,2012,46(1):410-424.
[2] HAMEED A S S,RAWAT P S,MENG X F,et al.Biotransformation of dietary phytoestrogens by gut microbes:A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota[J].Biotechnology Advances,2020,43:107 576.
[3] DURAZZO A,LUCARINI M,CAMILLI E,et al.Dietary lignans:Definition,description and research trends in databases development[J].Molecules,2018,23(12):3 251.
[4] 赵德宝,戴志刚,杨学,等.亚麻木酚素合成及相关基因的研究进展[J].农业科技通讯,2015(7):242-245.
ZHAO D B,DAI Z G,YANG X,et al.Research progress in the synthesis of flax lignans and related genes[J].Bulletin of Agricultural Science and Technology,2015(7):242-245.
[5] LANDETE J M,ARQUES J L,MEDINA M,et al.Bioactivation of phytoestrogens:Intestinal bacteria and health[J].Critical Reviews in Food Science and Nutrition,2016,56(11):1 826-1 843.
[6] QUARTIERI A,GARCIA-VILLALBA R,AMARETTI A,et al.Detection of novel metabolites of flaxseed lignans in vitro and in vivo[J].Molecular Nutrition & Food Research,2016,60(7):1 590-1 601.
[7] PAN J Y,CHEN S Z,YANG M H,et al.An update on lignans:Natural products and synthesis[J].Natural Product Reports,2009,26(10):1 251-1 292.
[8] PEIROTEN  ,BRAVO D,LANDETE J M.Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health[J].Critical Reviews in Food Science and Nutrition,2020,60(11):1 922-1 937.
,BRAVO D,LANDETE J M.Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health[J].Critical Reviews in Food Science and Nutrition,2020,60(11):1 922-1 937.
[9] 滕晖,向大位.肠内脂与肠二醇的生理活性研究进展[J].中南药学,2012(3):219-223.
TENG H,XIANG D W.Research progress on the physiological activities of enterodiol and enterolactone[J].Central South Pharmacy,2012(3):219-223.
[10] ELKON I M.Taxonomic and functional characterization of human gut microbes involved in dietary plant lignan metabolism[D].Seattle:University of Washington,2015.
[11] GUTTE K B,SAHOO A K,RANVEER R C.Bioactive components of flaxseed and its health benefits[J].International Journal of Pharmaceutical ences Review and Research,2015,31(1):42-51.
[12] PENALVO J L,HAAJANEN K M,BOTTING N P,et al.Quantification of lignans in food using isotope dilution gas chromatography/mass spectrometry[J].Journal of Agricultural and Food Chemistry,2005,53(24):9 342-9 347.
[13] THOMPSON L U,BOUCHER B A,LIU Z,et al.Phytoestrogen content of foods consumed in Canada,including isoflavones,lignans,and coumestan[J].Nutrition & Cancer,2006,54(2):184-201.
[14] MILDER I E J,ARTS I C W,VAN DE PUTTE B,et al.Lignan contents of dutch plant foods:A database including lariciresinol,pinoresinol,secoisolariciresinol and matairesinol[J].The British Journal of Nutrition,2005,93(3):393-402.
[15] SMEDS A I,EKLUND P C,SJOHOLM R E,et al.Quantification of a broad spectrum of lignans in cereals,oilseeds,and nuts[J].Journal of Agricultural & Food Chemistry,2007,55(4):1 337-1 346.
[16] PENALVO J L,ADLERCREUTZ H,UEHARA M,et al.Lignan content of selected foods from Japan[J].Journal of Agricultural and Food Chemistry,2008,56(2):401-409.
[17] SETCHELL K D R,LAWSON A M,MITCHELL F L,et al.Lignans in man and animal species[J].Nature,1980,287(5 784),740-742.
[18] LANDETE J M,GAYA P,RODRIGUEZ E,et al.Probiotic bacteria for healthier aging:Immunomodulation and metabolism of phytoestrogens[J].BioMed Research International,2017,2017:1-10.
[19] CLAVEL T,BORRMANN D,BRAUNE A,et al.Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans[J].Anaerobe,2006,12(3):140-147.
[20] CLAVEL T,HENDERSON G,ENGST W,et al.Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside[J].FEMS Microbiology Ecology,2006,55(3):471-478.
[21] CLAVEL T,LIPPMAN R,GAVINI F,et al.Clostridium saccharogumia sp.nov.and Lactonifactor longoviformis gen.nov.,sp.nov.,two novel human faecal bacteria involved in the conversion of the dietary phytoestrogen secoisolariciresinol diglucoside[J].Systematic and Applied Microbiology,2007,30(1):16-26.
[22] RONCAGLIA L,AMARETTI A,RAIMONDI S,et al.Role of bifidobacteria in the activation of the lignan secoisolariciresinol diglucoside[J].Applied Microbiology & Biotechnology,2011,92(1):159-168.
[23] JIN J S,ZHAO Y F,NAKAMURA N,et al.Isolation and characterization of a human intestinal bacterium,Eubacterium sp.ARC-2,capable of demethylating arctigenin,in the essential metabolic process to enterolactone[J].Biological & Pharmaceutical Bulletin,2007,30(5):904-911.
[24] JIN J S,KAKIUCHI N,HATTORI M.Enantioselective oxidation of enterodiol to enterolactone by human intestinal bacteria[J].Biological & Pharmaceutical Bulletin,2007,30(11):2 204-2 206.
[25] JIN J S,HATTORI M.Further studies on a human intestinal bacterium Ruminococcus sp.END-1 for transformation of plant lignans to mammalian lignans[J].Journal of Agricultural and Food Chemistry,2009,57(16):7 537-7 542.
[26] YODER S C,LANCASTER S M,HULLAR M A J,et al.Diet-Microbe Interactions in the Gut[M].Amsterdam:Elsevier Inc,2015.
[27] CLAVEL T,DORE J,BLAUT M.Bioavailability of lignans in human subjects[J].Nutrition Research Reviews,2006,19(2):187-196.
[28] WOTING A N,CLAVEL T,LOH G,et al.Bacterial transformation of dietary lignans in gnotobiotic rats[J].FEMS Microbiology Ecology,2010,72(3):507-514.
[29] BESS E N,BISANZ J E,YARZA F,et al.Genetic basis for the cooperative bioactivation of plant lignans by Eggerthella lenta and other human gut bacteria[J].Nature microbiology,2020,5(1):56-66.
[30] JAN K C,HWANG L S,HO C T.Biotransformation of sesaminol triglucoside to mammalian lignans by intestinal microbiota[J].Journal of Agricultural and Food Chemistry,2009,57(14):6 101-6 106.
[31] GAYA P,MEDINA M,SANCHEZ-JIMENEZ A,et al.Phytoestrogen metabolism by adult human gut microbiota[J].Molecules,2016,21(8):1 034-1 051.
[32] JIN J,ZHAO Y,NAKAMURA N,et al.Enantioselective dehydroxylation of enterodiol and enterolactone precursors by human intestinal bacteria[J].Biological & Pharmaceutical Bulletin,2007,30(11):2 113-2 119.
[33] JIN J S,HATTORI M.Human intestinal bacterium,strain END-2 is responsible for demethylation as well as lactonization during plant lignan metabolism[J].Biological & Pharmaceutical Bulletin,2010,33(8):1 443-1 447.
[34] WANG C,MA X,YANG D,et al.Production of enterodiol from defatted flaxseeds through biotransformation by human intestinal bacteria[J].BMC Microbiology,2010,10(1):115.
[35] CORTES C,GAGNON N,BENCHAAR C,et al.In vitro metabolism of flax lignans by ruminal and faecal microbiota of dairy cows[J].Journal of Applied Microbiology,2008,105(5):1 585-1 594.
[36] LIU Z,SAARINEN N M,THOMPSON L U.Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats[J].Journal of Nutrition,2006,136(4):906-912.
[37] 杜木香.亚麻木酚素的纯化、结构修饰及其衍生物的生物活性研究[D].广州:暨南大学,2019.
DU M X.Purification,structural modification of flaxseed lignan and biological activity of its derivatives[D].Guangzhou:Jinan University,2019.
[38] HWANG C F,WANG H E,KER Y B,et al.The limited deglucosylation process of β-glucosidase in Bacillus cereus H62L for biotransforming secoisolariciresinol diglucoside into mammalian lignans[J].Process Biochemistry,2011,46(8):1 632-1 640.
[39] HWANG C F,YIN T C,CHANG N Y,et al.Efficiency of a SDG-β-glucosidase from Bacillus altitudinis HK02 for the deglycation of glycosides from flaxseeds[J].Process Biochemistry,2015,50(12):2 188-2 193.
[40] ZHOU Y,ZHU S,YANG D,et al.Characterization of Klebsiella sp.strain S1:A bacterial producer of secoisolariciresinol through biotransformation[J].Canadian Journal of Microbiology,2017,63(1):1-10.
[41] GAYA P,PEIROTEN A,MEDINA M,et al.Bifidobacterium adolescentis INIA P784:The first probiotic bacterium capable of producing enterodiol from lignan extracts[J].Journal of Functional Foods,2017,29:269-274.
[42] BRAVO D,PEIROTEN A,ALVAREZ I,et al.Phytoestrogen metabolism by lactic acid bacteria:Enterolignan production by Lactobacillus salivarius and Lactobacillus gasseri strains[J].Journal of Functional Foods,2017,37:373-378.
[43] PEIROTEN A,GAYA P,ALVAREZ I,et al.Influence of different lignan compounds on enterolignan production by Bifidobacterium and Lactobacillus strains[J].International Journal of Food Microbiology,2019,289:17-23.
[44] ZHU H Y,LI M X,YANG D H,et al.Biotransformation of the SDG in defatted flaxseed into END co-cultured by three single bacterial colonies[J].Process Biochemistry,2014,49(1):19-24.
[45] 陈家兴,李淼鑫,朱红云,等.人体肠道菌生物转化亚麻木酚素产生4,4′-二羟基肠二醇的条件研究[J].中国科技论文,2014,9(12):1 389-1 392.
CHEN J X,LI M X,ZHU H Y,et al.Biotransformation of defatted flaxseed into 4,4′-dihydroxyenterodiol cultured by human intestinal bacteria[J].China Science Paper,2014,9(12):1 389-1 392.
[46] LI M X,ZHU H Y,YANG D H,et al.Production of secoisolariciresinol from defatted flaxseed by bacterial biotransformation[J].Journal of Applied Microbiology,2012,113(6):1 352-1 361.
[47] MUNOZ O,FUENTEALBA C,AMPUERO D,et al.The effect of Lactobacillus acidophilus and Lactobacillus casei on the in vitro bioaccessibility of flaxseed lignans (Linum usitatissimum L.)[J].Food & Function,2018,9(4):2 426-2 432.
[48] 刘树林.一种肠二醇和/或肠内酯的生物转化方法:中国,CN109588368A[P].2019-04-09.
LIU S L.A bioconversion method for enterodiol and/or enterolactone:China,CN109588368A[P].2019-04-09.
[49] 刘树林. 脱脂亚麻籽生物体内转化高效生成动物木酚素方法:中国,CN108450879A[P].2018-08-28.
LIU S L.Method for high-efficient generation of animal lignanoid through organism internal conversion of degreased linseeds:China,CN108450879A[P].2018-08-28.
[50] FUKUMITSU S,HAZAMA K,TAMURA M.Combination of intestinal bacteria having plant lignan assimilation activity,and use of the same:Japan,JP2015204784A[P].2015-11-19.