非酒精性脂肪肝病(non-alcoholic fatty liver disease,NAFLD)是由非酒精消耗或其他原因引起的肝脏脂质过量,造成肝细胞中脂滴积聚,肝脏呈现肿大的现象,会对人体健康产生较大的影响[1]。目前,NAFLD的药物治疗主要是通过预防肝细胞的氧化、减少肝细胞的促炎因子及血脂水平等方式进行[2-3],但其存在一定的副作用[4]。通过膳食干预也能有效改善肝脏的脂肪变性和炎症反应,并具有良好的益生作用[5],因此,膳食干预是人们进行辅助治疗NAFLD的重要途径。
益生菌及其发酵乳制品不仅可以通过调节肠道微生态平衡等来增强机体的抗氧化能力和免疫能力[6-7],还可以通过降低肝细胞的甘油三酯(triglyceride,TG)、总胆固醇(total cholesterol,TC)含量以及调节脂肪变性和炎症因子水平等来改善机体的NAFLD症状[8-10]。而临床研究也发现,益生菌可以通过改善患者血清中的天门冬氨酸氨基转移酶(aspartate aminotransferase,AST)、肿瘤坏死因子-α(tumor necrosis factor,TNF-α)、白细胞介素-6(interleukin-6,IL-6)水平及降低脂肪肝指数等来达到辅助治疗NAFLD的效果[11-14],但具体的作用机制尚不明确。
目前,NAFLD的机制研究大多数采用动物和肝癌细胞株HepG2来进行[15],但动物模型造模周期长、成本高、稳定性较差;同时,肝癌细胞株HepG2与人肝细胞存在较大的差距[16]。与之相比,人肝细胞系L-02具有造模周期短、个体差异小等优势[17]。来源于广西巴马长寿人群的鼠李糖乳杆菌 hsryfm 1301发酵乳对由高血脂症引起的大鼠肝脏细胞脂肪变性具有良好的改善作用[18],但对于人肝细胞的作用效应和机制尚不明确。研究显示,将样品在大鼠体内经过一系列的生物转化,再作用于细胞的研究结果较样品直接添加到细胞中更为准确[19]。本文利用油酸和棕榈酸的混合物游离脂肪酸(free fat acid,FFA)建立体外人肝细胞L-02 NAFLD模型,探讨鼠李糖乳杆菌 hsryfm 1301发酵乳干预大鼠后的血清在模型中对脂肪变性的人肝细胞L-02的益生作用,并建立一种更接近于人肝细胞的体外NAFLD细胞变性模型。
1 材料与方法
1.1 材料与试剂
Wistar雄性大鼠,6周龄,体重(200±20) g,扬州大学比较医学中心,动物生产许可证号SCXK(苏)2017-0007,实验动物使用许可证号SYXK(苏)2017-0044;基础饲料(面粉、米粉、玉米、鼓皮、豆料、鱼粉及骨粉的质量分数分别为20%、10%、20%、26%、20%、2%、2%),江苏省协同医药生物工程有限责任公司;鼠李糖乳杆菌(Lactobacillus rhamnosus)hsryfm 1301,江苏省乳品生物技术与安全控制重点实验室;人肝细胞株L-02,苏州北纳创联生物技术有限公司。
胎牛血清,杭州四季青生物有限公司;RPMI-1640培养液,美国Hyclone公司;无脂肪酸牛血清白蛋白(bovine serum albumin,BSA),德国Ruibio公司;细胞冻存液、胰酶消化液(质量分数为0.25%)、RIPA裂解液,上海碧云天生物技术有限公司;油酸钠(oleic acid,OA)、油红O试剂盒、AV/PI凋亡试剂盒,生工生物工程(上海)股份有限公司;棕榈酸钠,美国Sigma公司;CCK8(Cell Counting Kit-8)细胞增殖-毒性检测试剂盒,日本同仁公司;甘油三酯(triglyceride,TG)、总胆固醇(total cholesterol,TC)、天门冬氨酸氨基转移酶(aspartate aminotransferase,AST)、丙氨酸氨基转移酶(alanine aminotransferase,ALT)试剂盒,美康生物科技股份有限公司。
1.2 仪器与设备
HERAcell 150CO2培养箱、Multiskan Sky 1510全波长酶标仪,美国ThermoFisher科技有限公司;IX2-ILL100荧光倒置显微镜,日本Olympus公司;7020全自动生化分析仪,日本日立公司;5804R高速冷冻离心机,德国Eppendorf股份公司;LSRFortessa流式细胞仪,美国BD公司。
1.3 试验方法
1.3.1 细胞培养
将复苏的人肝细胞L-02置于完全培养液中(RPMI-1640培养液和胎牛血清的体积分数分别为90% 和10%),于37 ℃,体积分数为5% CO2的培养箱中培养,每24 h更换1次培养液,待细胞生长至对数期时(细胞面积>80%),用胰酶消化液消化传代,取5代后的细胞进行后续试验。
1.3.2 NAFLD细胞模型中FFA的添加浓度
1.3.2.1 不同FFA浓度对细胞存活率的影响
取100 μL浓度为5×104 个/mL的人肝细胞L-02悬液接种于96孔培养板中,在37 ℃,体积分数为5% CO2的培养箱中培养24 h后弃去培养液;利用含质量分数为1%的BSA完全培养液将5 mmol/L FFA分别稀释至0、0.25、0.5、0.75、1、1.5和2 mmol/L;以未添加FFA为对照组,添加不同浓度的FFA为模型组,每组3个复孔,CO2培养箱培养24 h后加入10 μL CCK8试剂,37 ℃孵育1 h后于490 nm处测定OD值,按公式(1)计算细胞存活率:
细胞存活率![]()
(1)
1.3.2.2 不同FFA浓度对细胞脂滴的影响
取1 mL浓度为5×105 个/mL的人肝细胞L-02悬液接种于6孔培养板中,在37 ℃,体积分数为5% CO2的培养箱中培养24 h后弃去培养液,以未添加FFA为对照组,添加1.3.2.1小节中不同浓度的FFA为模型组,每组3个复孔,CO2培养箱培养24 h后,釆用油红O染色法观察细胞内脂滴变化情况。
1.3.3 NAFLD细胞模型的评价
1.3.3.1 细胞内TG、TC含量的测定
取1 mL浓度为5×105 个/mL的人肝细胞L-02悬液接种于6孔培养板中,以不添加FFA为对照组,添加最佳浓度的FFA为模型组,每组3个复孔,在37 ℃,体积分数为5% CO2的培养箱中培养24 h后弃去培养液,胰酶消化液消化3 min后,用完全培养液终止消化,1 000×g离心5 min,PBS溶液清洗2遍,每管加入1 mL RIPA细胞裂解液,吹打均匀,置于冰上裂解4 h后,12 000×g离心10 min取上清液,采用全自动生化分析仪测定上清液中TG和TC含量。
1.3.3.2 细胞培养液中AST、ALT酶活力的测定
从1.3.3.1小节培养24 h后的6孔培养板中取出细胞上清液,以不添加FFA为对照组,4 000×g离心5 min,测定培养液中AST和ALT的含量。
1.3.3.3 细胞凋亡率的测定
取1 mL浓度为5×106 个/mL的人肝细胞L-02悬液接种于6孔培养板中,在37 ℃,体积分数为5% CO2的培养箱中培养24 h,对照组加入含1%的BSA的完全培养液,模型组加入最佳浓度的FFA,每组3个复孔,培养24 h后加入500 μL胰酶消化液消化3 min后,加入完全培养液终止消化,1 000×g离心5 min,去上清液收集细胞;用100 μL 1×buffer轻轻吹打细胞后,分别加入4 μL 异硫氰酸荧光素和4 μL 碘化丙啶,轻轻涡旋混匀,室温避光孵育15 min后每管加入400 μL 1×buffer,混匀,测定细胞凋亡率。
1.3.4 鼠李糖乳杆菌 hsryfm 1301发酵乳的制备
将在MRS液体培养基中活化2代后的鼠李糖乳杆菌 hsryfm 1301按3%的接种量接种至热处理后质量分数为12%的脱脂乳中,42 ℃发酵至活菌数为109 CFU/mL,进行活菌计数,4 ℃贮藏备用。
1.3.5 大鼠血清的制备
试验期间保持动物房通风、透光,室温为(23±1) ℃,湿度为(50±5)%,22只Wistar雄性大鼠用基础饲料正常饲喂1周后,按各组间平均体重无显著差异分为对照组和益生菌干预组,每组11只。干预组喂食基础饲料,并按1 mL/100 g的量灌胃鼠李糖乳杆菌hsryfm 1301发酵乳,对照组喂食基础饲料及等量的质量分数为0.9%的生理盐水,每天上午灌胃1次,记录每天的进食量及每周的体重,以便调整灌胃量。4周后眼球取血,4 ℃孵育30 min,3 500×g离心20 min,合并同组大鼠血清,56 ℃灭活30 min除去血清中的杂抗体等物质,并利用0.22 μm滤孔过滤除菌,避免细胞受到污染[17,20],-80 ℃保存备用。所有实验程序严格按照《实验动物的护理和使用指南》进行。
1.3.6 鼠李糖乳杆菌 hsryfm 1301发酵乳干预后的大鼠血清对模型中细胞的益生作用
利用RPMI-1640培养液分别将对照组和干预组的大鼠血清稀释至10%[17],然后取1 mL 加至细胞模型中,每组3个复孔,于37 ℃,体积分数为5% CO2的培养箱中培养24 h,PBS溶液清洗2遍;观察各组细胞内脂滴的变化,并测定细胞中TG、TC含量、细胞凋亡率以及血清细胞培养液中AST和ALT的含量。
1.4 数据处理与分析
数据均采用Sigmaplot 10.0、SPSS 21.0软件进行统计和分析,结果以均值±标准差(Mean±SD)表示,以单因素方差分析进行显著性检验,P<0.05表示差异具有统计学意义。
2 结果与分析
2.1 NAFLD细胞模型中FFA的添加浓度
通过测定模型中人肝细胞L-02的存活率及观察细胞内脂滴变化情况来确定模型中FFA的最佳添加浓度,结果如表1和图1所示。
表1 不同浓度的FFA对细胞存活率的影响(n=3)
Table 1 Effect of different concentration of FFA on the survival rate of cells
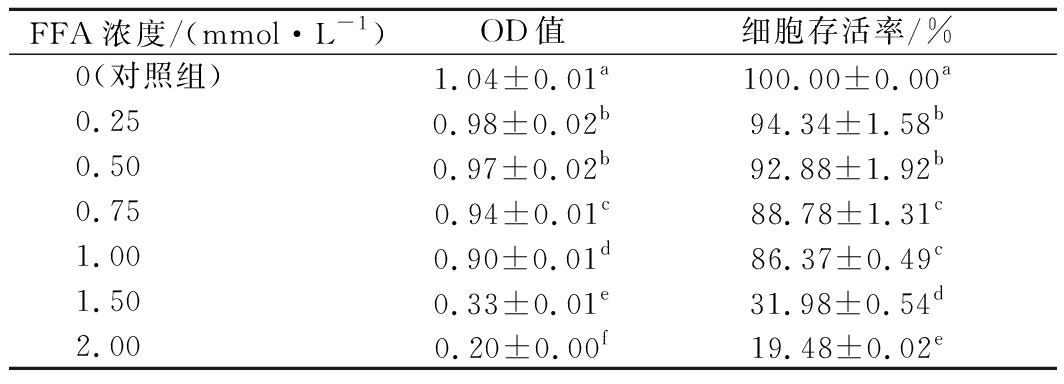
FFA浓度/(mmol·L-1)OD值细胞存活率/%0(对照组)1.04±0.01a100.00±0.00a0.250.98±0.02b94.34±1.58b0.500.97±0.02b92.88±1.92b0.750.94±0.01c88.78±1.31c1.000.90±0.01d86.37±0.49c1.500.33±0.01e31.98±0.54d2.000.20±0.00f19.48±0.02e
注:不同字母表示具有显著性差异(P<0.05)(下同)

a-0 mmol/L (对照组);b-0.25 mmol/L;c-0.5 mmol/L;
d-0.75 mmol/L;e-1 mmol/L;f-1.5 mmol/L;g-2 mmol/L
图1 不同浓度的FFA对细胞脂滴的影响(×400)
Fig.1 The effect of different concentration of FFA on the lipid droplets in cells
由表1可知,当模型中FFA的浓度分别为0.25、0.50和0.75 mmol/L时,人肝细胞L-02的存活率较高,均大于88.78%;而随着FFA浓度的不断增加,细胞的存活率随之下降,当FFA浓度大于1 mmol/L时,细胞的存活率显著下降(P<0.05),由1 mmol/L时的86.37%分别下降至1.5 mmol/L时的31.98%和2 mmol/L时的19.48%。
由图1可知,人肝细胞L-02经油红O染色后,未添加FFA的细胞边缘清晰,核膜完整,细胞内未见红色脂滴;而随着FFA浓度的增加,细胞内脂滴数量呈逐渐增多,且体积呈逐渐增大的趋势。当FFA浓度大于1 mmol/L时,肉眼可见的细胞数量大幅度减少,细胞形状发生变化,表明当添加的FFA超过一定浓度时,会严重损伤细胞;但当浓度低于1 mmol/L时,脂肪变性不充分,脂滴数量较少;当FFA浓度为1 mmol/L时,细胞脂肪变性充分,脂滴数量较多,结合细胞存活率的试验结果,选取1 mmol/L作为模型中FFA的最佳添加浓度。
2.2 NAFLD细胞模型
由表2可知,添加了1 mmol/L FFA的模型组人肝细胞L-02培养24 h后,细胞内TG含量为5.73 mmol/L,显著高于对照组(P<0.05);同时,培养液中AST含量为113.50 U/L,显著高于对照组的73.67 U/L(P<0.05),表明1 mmol/L的FFA造成了细胞的脂肪变性,与NAFLD大鼠模型特征较为一致[21]。
表2 脂肪变性细胞内TG、TC及细胞培养液中
AST和ALT 的含量(n=3)
Table 2 Content of TG,TC in the steatosis cells and AST,ALT content in the cell culture medium
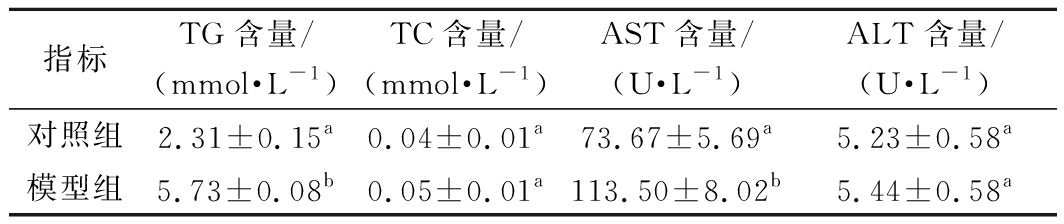
指标TG含量/(mmol·L-1)TC含量/(mmol·L-1)AST含量/(U·L-1)ALT含量/(U·L-1)对照组2.31±0.15a0.04±0.01a73.67±5.69a5.23±0.58a模型组5.73±0.08b0.05±0.01a113.50±8.02b5.44±0.58a
由图2可知,对照组人肝细胞L-02的早期凋亡率为1.82%,晚期凋亡率为1.79%,占总体细胞的3.61%;而模型组细胞早期凋亡率为1.88%,晚期凋亡率为2.14%,占总体细胞的4.02%,两组之间的细胞凋亡率差异较小,表明1 mmol/L的FFA对细胞未造成严重损伤,与机体单纯性脂肪变性特征相符[22]。

a-对照组;b-模型组
图2 脂肪变性对细胞凋亡率的影响
Fig.2 Effect of steatosis on the apoptosis rate of cells
2.3 鼠李糖乳杆菌hsryfm 1301发酵乳干预后的大鼠血清对模型中细胞的益生作用
2.3.1 大鼠血清对细胞脂滴的影响
以未干预菌株发酵乳的大鼠血清作为对照组,研究鼠李糖乳杆菌hsryfm 1301发酵乳干预后的大鼠血清对NAFLD模型中人肝细胞L-02脂滴的影响,结果如图3所示。

a-对照组;b-益生菌干预组
图3 大鼠血清对细胞脂滴的影响(×400)
Fig.3 Effect of rats serum on the lipid droplets in cells
由图3可知,对照组大鼠血清作用脂肪变性的人肝细胞L-02 24 h后,细胞边缘较为明显,核膜完整,胞内出现红色脂滴且体积较大,出现脂肪变性现象;与对照组相比,干预组大鼠血清作用的细胞内胞浆丰富,胞内红色脂滴数量较少、体积较小,表明鼠李糖乳杆菌hsryfm 1301发酵乳干预的大鼠血清能有效改善细胞的脂肪变性。
2.3.2 大鼠血清对细胞TG、TC含量及血清培养液中AST和ALT含量的影响
鼠李糖乳杆菌hsryfm 1301发酵乳干预后的大鼠血清对模型中人肝细胞L-02 胞内TG、TC含量的影响及血清培养液中AST和ALT的含量见图4和图5。

图4 大鼠血清对细胞TG和TC含量的影响(n=3)
Fig.4 The effect of rats serum on the content of TG and TC in cells

图5 大鼠血清细胞培养液中AST和ALT的含量(n=3)
Fig.5 The content of AST and ALT in the cell culture medium of rats serum
由图4可知,干预组的大鼠血清作用于人肝细胞L-02后,细胞内TG含量为1.95 mmol/L,显著低于对照组的2.88 mmol/L(P<0.05);TC含量稍低于对照组,但无显著性差异(P>0.05)。
由图5可知,对照组的人肝细胞L-02培养液中AST的含量为118.67 U/L,干预组的血清细胞培养液中AST的含量为93.33 U/L,显著低于对照组(P<0.05);而ALT的含量分别为5.59 U/L和5.83 U/L,无显著性差异(P>0.05);表明鼠李糖乳杆菌hsryfm 1301发酵乳可通过降低细胞内TG和AST转氨酶的含量来减轻NAFLD对细胞的损伤。
2.3.3 大鼠血清对细胞凋亡率的影响
鼠李糖乳杆菌hsryfm 1301发酵乳干预后的大鼠血清对人肝细胞L-02凋亡率的影响如图6所示。
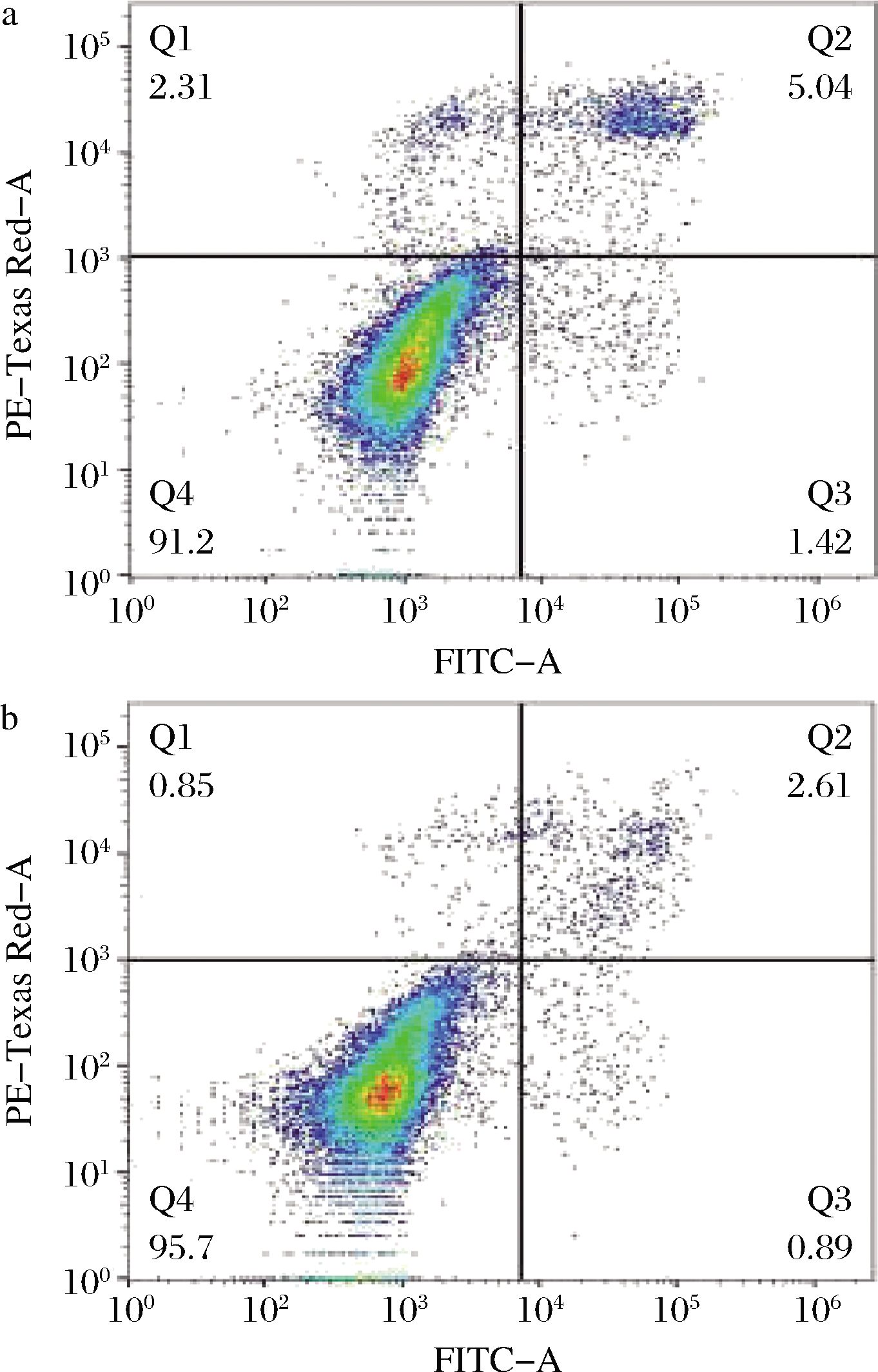
a-对照组;b-益生菌干预组
图6 大鼠血清对细胞凋亡率的影响
Fig.6 The effect of rats serum on the apoptosis rate of cells
由图6可知,对照组中人肝细胞L-02的早期凋亡率为1.42%,晚期凋亡率为5.04%,正常细胞数量为91.2%;经干预组的大鼠血清作用后,人肝细胞L-02的早期凋亡率为0.89%,晚期凋亡率为2.61%,正常细胞数量高达95.7%。与对照组相比,干预组的细胞凋亡率相对下调了2.96%,正常细胞数量相对上调了4.5%,说明鼠李糖乳杆菌hsryfm 1301发酵乳干预的大鼠血清能够降低人肝细胞L-02的凋亡率,缓解细胞损伤,对NAFLD具有较好的改善作用。
3 讨论
由于人与动物之间生物代谢表达和活性不同[23],使得在动物试验中肝细胞脂肪变性的稳定性较差[14],因此建立一种理想的体外模拟人肝细胞的NAFLD模型尤为重要。棕榈酸是饮食和血清中最丰富的游离脂肪酸,容易引发肝脏的脂肪变性[24-25],而油酸毒性较小,能够抵消棕榈酸对肝细胞的毒性[26],并具有增强棕榈酸酯化和稳定脂滴的能力[27]。因此,试验采用油酸和棕榈酸混合的FFA建立人肝细胞L-02脂肪变性模型。当1 mmol/L的FFA作用于人肝细胞L-02时,对细胞的存活率影响较小,同时还使得细胞内脂滴数量达到最大值,并显著增加了模型中细胞TG含量及培养液中AST含量(P<0.05),且细胞的凋亡率与对照组无显著性差异(P>0.05),符合NAFLD的特征指标[21]。表明成功建立的NAFLD细胞模型,具有造模周期短、稳定性好等优势[28]。
鼠李糖乳杆菌可以通过调节肠道微生物来改善机体的NAFLD症状[29]。鼠李糖乳杆菌 hsryfm 1301发酵乳能够通过调节与脂质代谢相关的肠道微生物来改善大鼠血清的脂质代谢[18],而本文的研究还发现,鼠李糖乳杆菌hsryfm 1301发酵乳干预后的大鼠血清不仅可以显著降低细胞TG含量,还可以显著降低细胞培养液中AST的含量(P<0.05);CAUSSY等[30]的研究发现,血清中来自肠道微生物的代谢产物在改善NAFLD症状过程中发挥着重要作用;而双歧杆菌、乳杆菌等益生菌可以通过调节机体血清中的脂质、转氨酶、炎症因子及抗氧化物质等代谢产物来降低NAFLD对机体的损伤[11-13,31]。因此,鼠李糖乳杆菌 hsryfm 1301发酵乳可能是通过调节肠道微生物来改善机体血清中脂质、转氨酶等代谢产物,进而发挥其对NAFLD人肝细胞L-02的益生作用。
益生菌还能通过调节 SIRT-1/PGC-1α/SREBP-1、Nrf-2/HO-1和PPAR-α等与血脂、抗氧化物质及胆汁酸代谢途径相关基因的表达来减轻机体的NAFLD症状[8,32-33]。因此,后续将从肠道微生物-血清的代谢产物及其代谢通路等方面入手,进一步阐明鼠李糖乳杆菌hsryfm 1301发酵乳干预的大鼠血清在NAFLD模型中对人肝细胞L-02的益生作用机制。
[1] DAY C,SAKSENA S.Non-alcoholic steatohepatitis:Definitions and pathogenesis[J].Journal of Gastroenterology & Hepatology,2003,17:S377-S384.
[2] NOBILI V,ALISI A,MOSCAET A,et al.The antioxidant effects of hydroxytyrosol and vitamin E on pediatric nonalcoholic fatty liver disease,in a clinical trial:A new treatment?[J].Antioxidants & Redox Signaling,2019,31(2):127-133.
[3] BRUINSTROOP E,DALAN R,CAO Y,et al.Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD[J].The Journal of Clinical Endocrinology & Metabolism,2018,103(7):2 698-2 706.
[4] SHATTAT F.A review article on hyperlipidemia:Types,treatments and new drugtargets[J].Biomedical & Pharmacology Journal,2015,7(2):399-409.
[5] KENNEALLY S,SIER J H,MOORE J B.Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease:A systematic review[J].Proceedings of the Nutrition Society,2017,4:e000 139.
[6] ZHANG J,ZHAO X,JIANG Y Y,et al.Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir[J].Journal of Dairy Science,2017,100(8):6 025-6 041.
[7] MORI N,KANO M,MASUOKA N,et al.Effect of probiotic and prebiotic fermented milk on skin and intestinal conditions in healthy young female students[J].Bioscience of Microbiota Food and Health,2016,35(3):105-112.
[8] CHEN Y T,LIN Y C,LIN,J S,et al.Sugary kefir strain Lactobacillus mali APS1 ameliorated hepatic steatosis by regulation of SIRT-1/Nrf-2 and gut microbiota in rats[J].Molecular Nutrition & Food Research,2018,62(8):e1 700 903.
[9] AL-MUZAFAR H M,AMIN K A.Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles,leptin,and inflammatory biomarkers[J].BMC Complementary and Alternative Medicine,2017,17:e43.
[10] SHIN H S,PARK S Y,LEE D K,et al.Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats[J].Archives of Pharmacal Research,2010,33(9):1 425-1 431.
[11] MALAGUARNERA M,VACANTE M,ANTIC T,et al.Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis[J].Digestive Diseases & Sciences,2012,57(2):545-553.
[12] ALISI A,BEDOGNI G,BAVIERA G,et al.Randomised clinical trial:The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis[J].Alimentary Pharmacology and Therapeutics,2014,39(11):1 276-1 285.
[13] KOBYLIAK N,ABENAVOLI L,MYKHALCHYSHYN G,et al.A multi-strain probiotic reduces the fatty liver index,cytokines and aminotransferase levels in NAFLD patients:Evidence from a randomized clinical trial[J].Journal of Gastrointestinal & Liver Diseases Jgld,2018,27(1):41-49.
[14] HADI A,MOHAMMADI H,MIRAGHAJANI M,et al.Efficacy of synbiotic supplementation in patients with non-alcoholic fatty liver disease:A systematic review and meta-analysis of clinical trials:Synbiotic supplementation and NAFLD[J].Food Science and Nutrition,2019,59(15):3 341-3 357.
[15] XIA H G,ZHU X Y,ZHANG X Y,et al.Alpha-naphthoflavone attenuates non-alcoholic fatty liver disease in oleic acid-treated HepG2 hepatocytes and in high fat diet-fed mice[J].Biomedicine & Pharmacotherapy,2017,118.DOI:10.1016/j.biopha.2019.109287.
[16] WARE B R,KHETANI S R.Engineered liver platforms for different phases of drug development[J].Trends in Biotechnology,2017,35(3):172-183.
[17] 殷锦锦,唐外姣,曾璐,等.人肝细胞系L-02细胞单纯肝脂肪变性细胞模型的建立与应用[J].南方医科大学学报,2014,34(6):837-842.
YIN J J,TANG W J,ZENG L,et al.Establishment of a L-02 cell model of hepatic steatosis[J].Journal of Southern Medical University,2014,34(6):837-842.
[18] CHEN D W,YANG Z Q,GU R X,et al.The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model[J].BMC Complementary and Alternative Medicine,2014,14:386-394.
[19] WANG B C,ZHU L C,CHEN Q,et al.Primary study on the application of serum pharmacology in Chinese traditional medicine[J].Colloids and Surfaces B:Biointerfaces,2005,43(3-4):194-197.
[20] 朱晓莹, 李韬,李盛毅,等.肿节风复方含药血清对肝癌HepG2细胞增殖、端粒酶及凋亡的影响[J].中国实验方剂学杂志,2014,20(2):109-112.
ZHU X Y,LI T,LI S Y,et al.Effect of serum containing sarcandrae compound on proliferation,telomerase activity and cellular apoptosis of HepG2 cells[J].Chinese Journal of Experimental Traditional Medical Formulae.2014,20(2):109-112.
[21] MA L L,YUAN Y Y,ZHAO M,et al.Mori Cortex extract ameliorates nonalcoholic fatty liver disease (NAFLD) and insulin resistance in high-fat-diet/streptozotocin induced type 2 diabetes in rats[J].Chinese Journal of Natural Medicines,2018,16(6):411-417.
[22] XIE C F,CHEN Z,ZHANG C F,et al.Dihydromyricetin ameliorates oleic acid-induced lipid accumulation in L02 and HepG2 cells by inhibiting lipogenesis and oxidative stress[J].Life Sciences,2016,157(15):131-139.
[23] MARTIGNONI M,GROOTHUIS G M M,KANTER R D,et al.Species differences between mouse,rat,dog,monkey and human CYP-mediated drug metabolism,inhibition and induction[J].Expert Opinion on Drug Metabolism & Toxicology,2007,2(6):875-894.
[24] LIRUSSI F,MASTROPASQUA E,ORANDO S,et a1.Probiotics for nonalcoholic fatty liver disease and/or steatohepatitis[J].Cochrane Database of Systematic Reviews,2007,24(1):51-65.
[25] CAO J,FENG X X,YAO L,et al.Saturated free fatty acid sodium palmitate-induced lipoapoptosis by targeting glycogen synthase kinase-3β activation in human liver cells[J].Digestive Diseases and Sciences,2014,59(2):346-357.
[26] WEI Y R,WANG D,PAGLIASSOTTI M J,et al.Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells[J].AmericanJournal of Physiology Endocrinology and Metabolism,2006,291(2):275-281.
[27] MORAVCOV A,CERVINKOV Z,KUCERA O,et al.The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture[J].Physiological Research,2015,64(5):627-636.
[28] ANGELICO F,DELBEN M,CONTI R,et al.Insulin resistance,the metabolic syndrome,and non-alcoholic fatty liver disease[J].Journal of Clinical Endocrinology & Metabolism,2005,90(3):1 578-1 582.
[29] RITZE Y,BARDOS G,CLAUS A,et al.Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice[J].PLoS ONE,2014,9(1):e80 169.
[30] CAUSSY C,HSU C,LO M T,et al.Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD[J].Hepatology,2018,68(3):918-932.
[31] PARK E J,LEE Y S,KIM S M,et al.Beneficial effects of Lactobacillus plantarum strains on non-alcoholic fatty liver disease in high fat/high fructose diet-fed rats[J].Nutrients,2020,12(2).DOI:10.3390/nu12020542.
[32] ZHANG Z,ZHOU H,ZHOU X H,et al.Lactobacillus casei YRL577 ameliorates markers of non-alcoholic fatty liver and alters expression of genes within the intestinal bile acid pathway[J].British Journal of Nutrition,2020,28:1-9.
[33] KIM D H,JEONG D,KANG I B,et al.Dual function of Lactobacillus kefiri DH5 in preventing high-fat-diet-induced obesity:Direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue[J].Molecular Nutrition & Food Research,2017,61(11).DOI:10.1002/mnfr.201700252.