L-岩藻糖(L-fucose)是一种六碳糖,也可视为一种甲基戊糖(图1),又称6-脱氧-L-半乳糖,分子式为C6H12O5,为白色结晶粉末,易吸潮,亲水性弱于其他单糖,熔点为138.0~142.0 ℃。L-岩藻糖是岩藻糖在自然界中主要的存在形式,常见于植物或微生物多糖中,而D-岩藻糖较少地存在于一些糖苷类化合物中[1-2]。L-岩藻糖特殊化学结构,在哺乳动物细胞中可作为癌症生物标记物或靶标的重要的生物功能特征[3-5],在智能纳米载药系统实现抗肿瘤药物的靶向递送[6-7]、减少皮肤受损或延缓皮肤老化[8]等多领域发挥重要的生理活性。
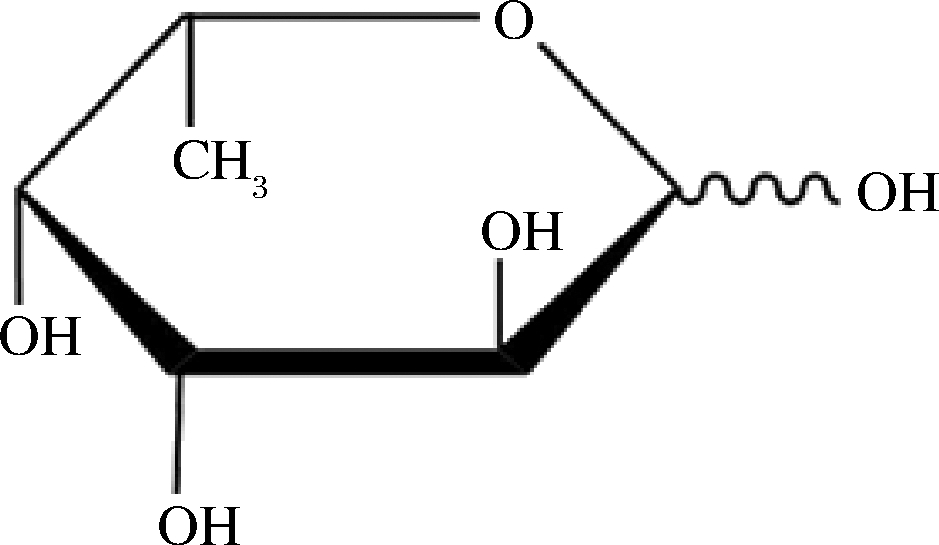
图1 L-岩藻糖结构
Fig.1 Structure of L-fucose
已知,L-岩藻糖的生产主要有天然植物水解(提取)法、化学合成法及微生物发酵法。其中天然植物提取法主要从藻类植物中提取L-岩藻糖,国内外常用的提取方法为溶剂萃取法、酶萃取法等[9-10];而化学合成法制备L-岩藻糖主要是以L-阿拉伯糖、D-葡萄糖等单糖为起始物通过多步化学反应得到L-岩藻糖[11]。综合现有植物提取或化学合成方法普遍存在高污染和含量低的问题,而微生物发酵法是一种可持续的L-岩藻糖生产方法,具有不受季节、环境影响、生产周期短、发酵过程容易操作控制、可利用的发酵底物类型多等多种优点,本文围绕微生物发酵法生产L-岩藻糖的研究进展进行介绍。
1 微生物发酵法
L-岩藻糖常常作为单糖成分存在于微生物胞外岩藻糖基化产物中,微生物发酵法可以利用此特性,以葡萄糖或廉价原料生产富含L-岩藻糖的岩藻糖基化产物或者岩藻胞外多糖(fucose-containing exopolysaccharide, FucoPol),再从岩藻多糖中水解制备L-岩藻糖。目前常见发酵法生产L-岩藻糖的菌株为肠杆菌属(Enterobacter sp.)、克雷伯菌属(Klebsiella sp.)、棒形杆菌属(Clavibacter sp.)等[12]。为了提升岩藻糖基化产物产量,通常会采用诱变育种或者基因工程技术构建高产工程菌株的方法,如黄宜兰等[12]使用紫外诱变和NTG结合的方法获得高产菌株肠杆菌(Enterobacter sp.SIPI-N79),结果显示L-岩藻糖的含量达到1.28 g/L,比出发菌株提高了81%。
2 发酵工艺优化
如表1所示,岩藻多糖产生菌株可以利用多种碳源进行发酵培养。FREITAS等[13]以最常用碳源葡萄糖作为发酵碳源,Enterobacter A47(DSM 23139)菌株分批补料培养后得到的L-岩藻糖产量为3.59 g/L。黄宜兰等[12]对肠杆菌(Enterobacter sp.SIPI-N79)的发酵培养基进行优化,得出最佳培养基配方由甘油、胰蛋白胨和碳酸钙组成,采用甘油代替葡萄糖作为碳源,5 L发酵罐中分批补料发酵,L-岩藻糖产量达到4.55 g/L。除了葡萄糖外, 还使用不同食品加工副产物为廉价发酵底物,考察对Enterobacter A47菌株发酵产岩藻多糖及L-岩藻糖含量的影响[13-19]。本课题组以kosakonia sp.CCTCC M2018092菌株于50 L发酵罐中发酵72 h得到L-岩藻糖最终产量为5.19 g/L[20]。
表1 不同微生物菌株多糖及多糖中L-岩藻糖产量
Table 1 L-fucose production in polysaccharides and
polysaccharides of different microbial strains
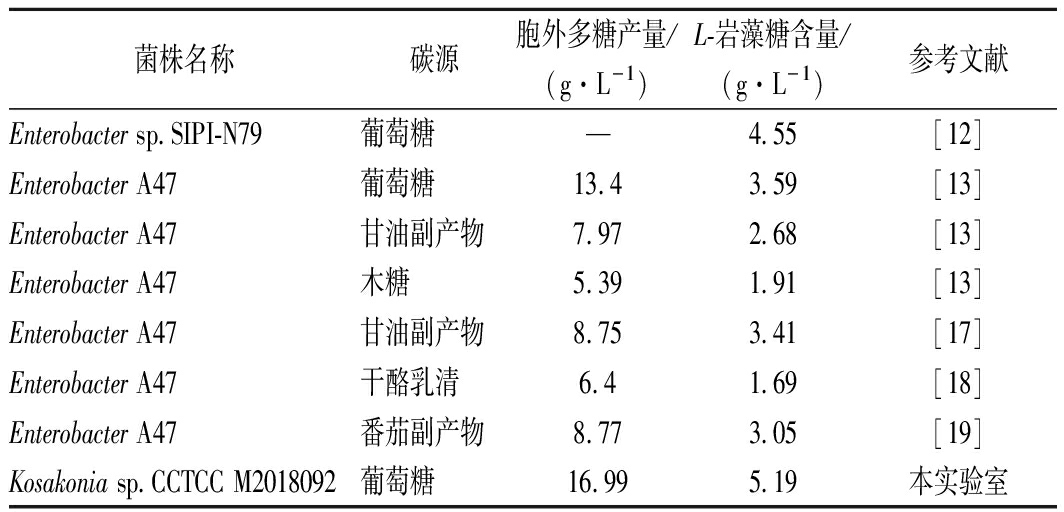
菌株名称碳源胞外多糖产量/(g·L-1)L-岩藻糖含量/(g·L-1)参考文献Enterobacter sp.SIPI-N79葡萄糖—4.55[12]Enterobacter A47葡萄糖13.43.59[13]Enterobacter A47甘油副产物7.972.68[13]Enterobacter A47木糖5.391.91[13]Enterobacter A47甘油副产物8.753.41[17]Enterobacter A47干酪乳清6.4 1.69[18]Enterobacter A47番茄副产物8.773.05[19]Kosakonia sp.CCTCC M2018092葡萄糖16.995.19本实验室
注:-表示文中未提及
利用微生物生产岩藻多糖相比植物提取方法具有生产周期短等多种优点,但这种富含L-岩藻糖的生物多糖发酵普遍具有高黏度的发酵特性。传统搅拌桨组合的传质传气和混合效率并不能满足这种高黏度发酵体系要求,存在气体分散不均匀,传质效率差等不足,影响多糖合成效率[21-22]。通过对不同搅拌桨进行设计与组合可以有效改善高黏度发酵体系的生产效率[23-28],如郑之明等[29]研究黄原胶高黏度发酵系统发现,具有较高剪切力和较大有效搅拌区域的反应器更适合黄原胶发酵。本实验室结合计算流体动力学(computational fluid dynamics,CFD)[30-31]的方法通过对剑叶,四宽折叶,六直叶以及设计加框形桨等4种搅拌桨进行组合优化(图2),筛选搅拌性能改善的搅拌桨组合来进行实际发酵验证,进而优化L-岩藻糖发酵过程中的传质传氧效率。
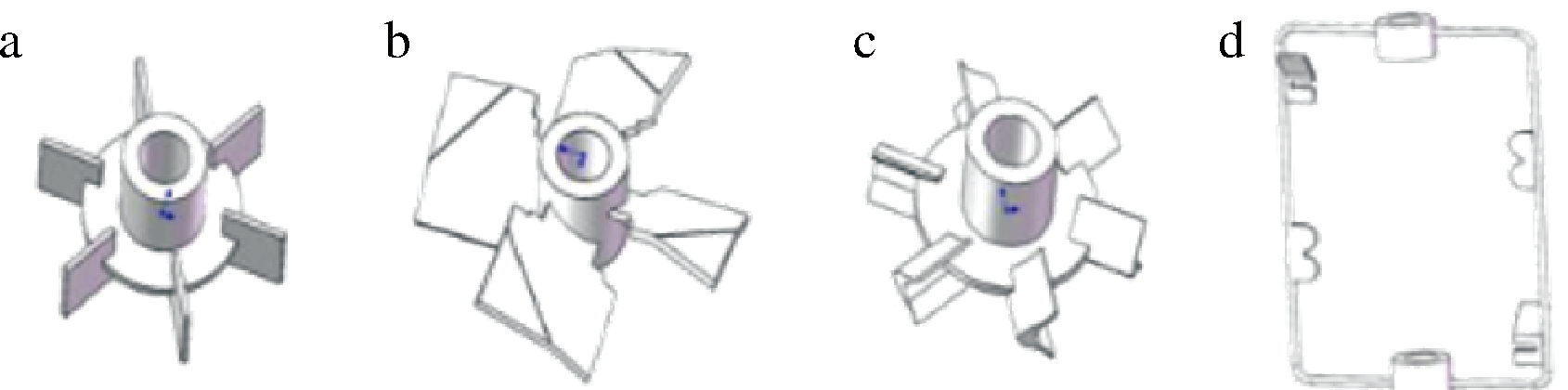
a-六剑叶桨;b-四宽折叶桨;c-六直叶桨;d-框形桨
图2 四种搅拌桨示意图
Fig.2 Four types of agitation paddles
新式框式桨的加入降低了搅拌桨尖外的气液混合迟滞区,有效提高罐内流体的混合效率,促进气液混合,岩藻多糖发酵水平得到明显提高。
3 代谢工程改造
岩藻多糖是以二磷酸尿苷葡萄糖(UDP-Glucose)、鸟苷二磷酸-岩藻糖(GDP-fucose)等糖核苷酸分子为底物,在糖基转移酶(glycosyltransferases,GTs)的催化下合成,随后又通过Wzx/Wzy依赖的途径将多糖运输到细胞表面[32]。研究表明合成单元GDP-岩藻糖的产量直接决定了岩藻糖基化产物中L-岩藻糖的含量,因此提高GDP-岩藻糖产量对生产L-岩藻糖具有重要意义[33]。GDP-岩藻糖的生产有2条途径,从头合成途径和补救途径,如图3所示。

图3 GDP-岩藻糖的从头合成途径和补救途径
Fig.3 De novo pathway and salvage pathway of GDP-fucose
从头合成途径的关键酶主要有甘露糖-6-磷酸异构酶(ManA)、磷酸甘露糖变位酶(ManB)、甘露糖-1-磷酸鸟苷酰转移酶(ManC)、GDP-甘露糖-4,6-脱水酶(Gmd)、以及一个双功能酶GDP-4-酮-6-脱氧甘露糖-3,5-变旋酶/4-还原酶(WcaG),在这些酶的参与下葡萄糖被逐渐转变为GDP-岩藻糖;补救途径合成GDP-岩藻糖的关键酶主要有GDP-岩藻糖激酶、GDP-岩藻糖-1-磷酸鸟苷酰转移酶(Fkp)。岩藻糖首先在GDP-岩藻糖激酶的作用下转变成岩藻糖-1-磷酸,然后经过GDP-岩藻糖-1-磷酸鸟苷酰转移酶(Fkp)的催化转变为GDP-岩藻糖。
当前GDP-岩藻糖的代谢工程改造主要围绕合成通路优化、辅因子工程、转录因子调控以及GDP-岩藻糖合成宿主筛选等方面(如表2所示)[34]。LEE等[35]在E.coli中对GDP-岩藻糖关键酶基因(manB,manC,gmd和wcaG/fcl)过表达,产量相比于表达gmd和wcaG(fcl)的对照株提高了4.4倍,表明在进行GDP-岩藻糖合成通路优化时,相比于仅对限速酶进行过表达,通路多基因的过表达更为有效。此外,GDP-岩藻糖合成酶催化GDP-4-酮6-脱氧-D-甘露糖形成GDP-岩藻糖的过程需要还原力NADPH,而磷酸戊糖途径和三羧酸循环途径是两大产生还原力NADPH的主要途径;通过过表达葡萄糖-6-磷酸脱氢酶基因(zwf)使得磷酸戊糖途径得到强化,增强NADPH供应并采用pH稳态的分批发酵控制,最终获得了235.2 mg/L的GDP-岩藻糖[36]。除此之外,GDP-岩藻糖的合成还受到其他因素影响,其中转录因子RcsA就能够调节GDP-岩藻糖合成途径的关键酶,但是温度敏感的ATP依赖型蛋白激酶Lon会快速降解RcsA,HUANG等[37]将温度敏感的ATP依赖型蛋白激酶基因(lon)敲除使得GDP-岩藻糖产量提升,相比于对照株提高了约61%。
表2 GDP-岩藻糖的代谢工程改造策略
Table 2 Metabolic engineering transformation
strategy of GDP-fucose
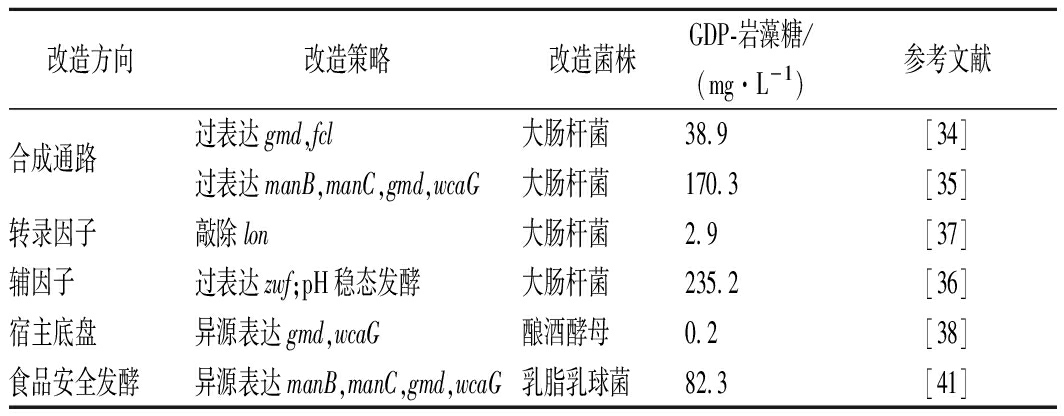
改造方向改造策略改造菌株GDP-岩藻糖/(mg·L-1)参考文献合成通路过表达gmd,fcl大肠杆菌38.9[34]过表达manB,manC,gmd,wcaG大肠杆菌170.3[35]转录因子敲除lon大肠杆菌2.9[37]辅因子过表达zwf;pH稳态发酵大肠杆菌235.2[36]宿主底盘异源表达gmd,wcaG酿酒酵母0.2[38]食品安全发酵异源表达manB,manC,gmd,wcaG乳脂乳球菌82.3[41]
GDP-岩藻糖的生产除以大肠杆菌为底盘外,也可以用酿酒酵母、谷氨酸棒杆菌以及乳酸乳球菌等宿主细胞进行生产。酿酒酵母有着优良的GDP-甘露糖代谢池,因此,MATTILA等[38]将大肠杆菌中合成GDP-岩藻糖的2个关键基因(gmd,wcaG)克隆到酿酒酵母中,并且获得了0.2 mg/L的GDP-岩藻糖。而CHIN等[39]通过将大肠杆菌中的manB,manC,gmd和wcaG联合表达到谷氨酸棒杆菌中获得了5.5 mg/g细胞干重的GDP-岩藻糖。不过单一菌株发酵产量不能满足工业化生产,KOIZUMI等[40]利用谷氨酸棒杆菌将鸟苷一磷酸(GMP)转化为鸟苷三磷酸(GTP)的高转化特性和过表达GDP-岩藻糖合成途径中的关键基因的重组大肠杆菌联合来生产GDP-岩藻糖。利用这2种不同的菌株以GMP和甘露糖作为出发底物,在发酵22 h的时候就获得了高达29 mmol/L(18.4 g/L)的产量,这是目前报道的GDP-岩藻糖的最高产量。LI等[41]用食品安全菌株乳脂乳球菌作为宿主菌株,采用一锅多酶法将合成GDP-岩藻糖的关键基因(manB,manC,gmd和wcaG)克隆到乳脂乳球菌,GDP-岩藻糖产量也达到了0.13 mmol/L(82.3 mg/L)。
当前代谢工程改造主要围绕提升GDP-岩藻糖目标产量,近期LIU等[42]以L-岩藻糖为直接产物进行代谢工程改造,首先改造大肠杆菌产生岩藻糖基乳糖,再通过表达岩藻糖苷酶从岩藻糖基乳糖中释放L-岩藻糖,分批补料发酵使L-岩藻糖产量达到16.7 g/L,为发酵法生产L-岩藻糖提供了新思路。
4 L-岩藻糖分离与纯化
目前市面上岩藻糖产品大多通过植物源的岩藻多糖水解制备[43],存在岩藻糖含量低、能耗污染大等缺点。利用发酵法生产富含L-岩藻糖的岩藻多糖,可采用酸水解法进行提取。岩藻多糖发酵液由于混有菌体、蛋白以及杂单糖等杂质组分,核心工艺主要考虑通过酸水解降低聚合度和黏度,过滤、离心以及柱层析等方法除去菌体、蛋白等。如GORI等[44]针对肠杆菌科菌株DSM 22227的岩藻多糖提出了一条提取分离L-岩藻糖工艺。首先利用强酸水解降低料液黏度并通过超滤回收脱去盐和酸的部分水解多糖溶液;再利用高温强酸完全水解后使用碱中和溶液,沉淀物CaSO4等通过离心或者过滤去除;最后使用层析色谱或者微生物的方法除去水解液中的其他杂质,使用甲醇或乙醇等有机溶剂结晶从中提取分离L-岩藻糖,最终得到产品收率为75%,纯度为98%的结晶样品。
本实验室建立了岩藻多糖提取、梯度酸水解及水相结晶的高纯度 L-岩藻糖分离提取工艺,以实验室Kosakonia sp.CCTCC M2018092胞外岩藻多糖出发,建立了通过水解岩藻多糖得到高纯度L-岩藻糖的提取方法。并对关键步骤的最佳操作条件进行初步摸索与研究(图4),最终产品结晶L-岩藻糖的纯度达到99%以上[45]。

图4 L-岩藻糖的提取工艺流程初步研究
Fig.4 Preliminary study on extraction process of L-fucose
5 总结与展望
L-岩藻糖具有的独特生理功能,在医药、化妆品、食品等领域具有市场开发潜力。近年来,L-岩藻糖在抗癌药物的靶向研究、延缓皮肤衰老以及特殊人群的营养补充剂等领域研究展现出其特殊的生理活性。最新研究表明,L-岩藻糖还在缓解体内外炎症反应[46-47]等方面具有良好的活性,并在调节肠道菌群、抵抗病原微生物入侵[48]等临床研究方面展现出巨大的应用前景。同时,L-岩藻糖是新型食品添加剂岩藻糖基乳糖的关键底物,L-岩藻糖的生产成本降低将推动酶法合成岩藻糖基乳糖的低成本生物制造。
采用发酵法生产L-岩藻糖,筛选食品安全型高效合成宿主是未来主要的研究方向。当前代谢工程改造工作目前主要围绕GDP-岩藻糖合成代谢通路进行,未来可基于新的宿主系统开展GDP-岩藻糖供体的L-岩藻糖合成元件挖掘以及合成路线优化设计。本实验室以Kosakonia sp.CCTCC M2018092为新的底盘工厂,利用其天然的GDP-岩藻糖高效合成能力,正在建立Kosakonia sp.菌株岩藻多糖、L-岩藻糖及寡糖分子设计等多目标产物生产体系,以期在发酵法生产岩藻糖及衍生物方面取得突破。
[1] 王永胜, 王硕, 张慧林, 等.L-岩藻糖对母乳寡糖(HMOs)合成的意义及其产业化研究进展[J].中国农学通报, 2019, 35(11):127-132.
WANG Y S, WANG S, ZHANG H L, et al.L-Fucose:The significance to synthesis of human milk oligosaccharides (HMOs) and its research progress of industrialization[J].Chinese Agricultural Science Bulletin, 2019, 35(11):127-132.
[2] 吴巍, 孙敬, 冯士超.HPLC-ELSD测定岩藻聚糖硫酸酯中L(-)岩藻糖[J].食品研究与开发, 2013, 34(21):90-92.
WU W, SUN J, FENG S C.Determination of L-fucose in fucoidan extract by HPLC-ELSD[J].Food Research and Development, 2013, 34(21):90-92.
[3] LISTINSKY J J, SIEGAL G P, LISTINSKY C M.The emerging importance of α-L-fucose in human breast cancer:A review[J].American Journal of Translational Research, 2011, 3(4):292-322.
[4] LISTINSKY J J, LISTINSKY C M, Alapati V, et al.Cell surface fucose ablation as a therapeutic strategy for malignant neoplasms[J].Anatomic Pathology, 2001, 8(6):330-337.
[5] SHETTY R K S, BHANDARY S K, KALI A.Significance of serum L-fucose glycoprotein as cancer biomarker in head and neck malignancies without distant metastasis[J].Journal of Clinical and Diagnostic Research, 2013, 7(12):2 818-2 820.
[6] KATO J, TAKIMOTO R, OSUGA T,et al.Targeting SN38 delivery to gastrointestinal cancer cells using a fucose-bound nanoparticle approach[C].Proceedings of the 105th Annual Meeting of the American Association for Cancer Research,San Diego:Cancer Research, 2014.
[7] YOSHIDA M, TAKIMOTO R, MURASE K, et al.Targeting anticancer drug delivery to pancreatic cancer cells using a fucose-bound nanoparticle approach[J].PLoS One, 2012, 7(7):e39 545.
[8] FODIL B I, BIZBIZ L, SCHOEVAERT D, et al.Effect of L-fucose and fucose-rich oligo- and polysaccharides (FROP-s) on skin aging:Penetration, skin tissue production and fibrillogenesis[J].Biomed Pharmacother, 2003, 57(5):209-215.
[9] WIJESINGHE W, JEON Y J.Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds:A review[J].Carbohydrate Polymers, 2012, 88(1):13-20.
[10] 余华. 海带多糖提取条件的优化和脱蛋白研究[J].中国食品添加剂, 2006(3):39-43.
YU H.Study on the extracting condition and deproteinization of laminaria japonica aresch polysaccharide[J].China Food Additives, 2006(3):39-43.
[11] VANHOOREN P T,VANDAMME E J.L-Fucose:Occurrence,physiological role,chemical,enzymatic and microbial synthesis[J].Cheminform, 1999, 30(33):479-497.
[12] 黄宜兰, 王岩, 陈少欣.L-岩藻糖的生产菌种选育及发酵工艺优化[J].中国医药工业杂志, 2015, 46(8):823-826.
HUANG Y L, WANG Y, CHEN S X.Strain screening and fermentation production of L-fucose by Enterobacter sp.[J].Chinese Journal of Pharmaceuticals, 2015, 46(8):823-826.
[13] FREITAS F,ALVES V D,GOUVEIA A R, et al.Controlled production of exopolysaccharides from Enterobacter A47 as a function of carbon source with demonstration of their film and emulsifying abilities[J].Applied Biochemistry & Biotechnology, 2014, 172(2):641-657.
[14] ALVES V D, FREITAS F,TORRES C A V, et al.Rheological and morphological characterization of the culture broth during exopolysaccharide production by Enterobacter sp.[J].Carbohydrate Polymers, 2010, 81(4):758-764.
[15] TORRES C A V,MARQUES R, ANTUNES S, et al.Kinetics of production and characterization of the fucose-containing exopolysaccharide from Enterobacter A47[J].Journal of Biotechnology, 2011, 156(4):261-267.
[16] TORRES C A V, ANTUNES S,RICARDO A R, et al.Study of the interactive effect of temperature and pH on exopolysaccharide production by Enterobacter A47 using multivariate statistical analysis[J].Bioresource Technology, 2012, 119:148-156.
[17] TORRES C A V, MARQUES R,FERREIRA A R V, et al.Impact of glycerol and nitrogen concentration on Enterobacter A47 growth and exopolysaccharide production[J].International Journal of Biological Macromolecules, 2014, 71:81-86.
[18] ANTUNES S,FREITAS F,ALVES V D, et al.Conversion of cheese whey into a fucose-and glucuronic acid-rich extracellular polysaccharide by Enterobacter A47[J].Journal of Biotechnology, 2015, 210:1-7.
[19] ANTUNES S,FREITAS F,SEVRIN C, et al.Production of fucopol by Enterobacter A47 using waste tomato paste by-product as sole carbon source[J].Bioresource Technology, 2017, 227:66-73.
[20] NIU S F, MA W, JIN M Y, et al.Complete genome sequence of Kosakonia sp.strain CCTCC M2018092, a fucose-rich exopolysaccharide producer[J].Microbiology Resource Announcements, 2019, 8(30).DOI:10.1128/MAR.00567-19.
[21] VLAEV S D, STAYKOV P, POPOV R.Pressure distribution at impeller blades of some radial flow impellers in saccharose and xanthan gum solutions-A CFD visualization approach[J].Food and Bioproducts Processing, 2004, 82(C1):13-20.
[22] HUANG J, JIANG S, XU X Q, et al.Effects of carbon/nitrogen ratio, dissolved oxygen and impeller type on gellan gum production in Sphingomonas paucimobilis [J].Ann Microbiol, 2012, 62(1):299-305.
[23] 曾真,彭坤,王为国.发酵罐搅拌轴的优化设计[J].食品与机械, 2010, 26(6):97-100.
ZENG Z, PENG K, WANG W G.Optimum design of the agitating shaft for the fermentation tank[J].Food & Machinery, 2010, 20(6):97-100.
[24] HARWEY P S, GREAVES M.Turbulent flow in an agitated vessel.part I:A predictive model[J].Transactions of the Institution Chemical Engineer, 1982, 60:195-200.
[25] OLDSHUE J Y.Fluid mixing in fermentation processes[J].Industrial & Engineering Chemistry Research, 1960, 2:275-287.
[26] ZHAO H B, FEI X N, ZHANG B L, et al.Controlling the size of fragrance microcapsules using designed agitator paddles:Experiment and CFD simulation[J].Particuology, 2019, 43:38-45.
[27] 张淑华, 李涛, 朱炳辰, 等.三相机械搅拌反应器气液传质[J].化工学报, 2005, 56(2):220-226.
ZHANG S H, LI T, ZHU B C, et al.Gas-liquid mass transfer in three-phase mechanical agitated reactor[J].CIESC Journal, 2005, 56(2):220-226.
[28] 袁海荣, 贾绍义.搅拌桨形式对盐酸四环素发酵的影响[J].西北药学杂志, 2005, 20(6):255-257.
YUAN H R, JIA S Y.The effect of stirring blade on the fermentation of tetracycline hydrochloride[J].Northwest Pharmaceutical Journal, 2005, 20(6):255-257.
[29] 郑之明, 杨守志, 樊永红, 等.黄原胶发酵过程中流变学行为和气液传质的研究[J].天然气化工, 2006(5):27-31.
ZHENG Z M, YANG S Z, FAN Y H, et al.Studies on fluid rheological properties and mass transfer during the fermentation of xanthomonas campestris in a stired reactor[J].Natural Gas Chemical Industry, 2006(5):27-31.
[30] HALFI E, ARAD A, BRENNER A, et al.Development of an oscillation-based technology for the removal of colloidal particles from water:CFD modeling and experiments[J].Engineering Applications of Computational Fluid Mechanics, 2020, 14(1):622-641.
[31] YAO M, RAN Z, CHEN T, et al.Numerical simulation studies of agitating paddle dependence on characteristics of the flow field in the mechanical-L-continuous-flow-stirred reactor for flocculation[J].Desalination and Water Treatment, 2020, 183:30-41.
[32] SCHMID J, SIEBER V, REHM B.Bacterial exopolysaccharides:Biosynthesis pathways and engineering strategies[J].Frontiers in Microbiology, 2015, 6:496.
[33] YI W, LIU X W, LI Y H, et al.Remodeling bacterial polysaccharides by metabolic pathway engineering[J].Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(11):4 207-4 212.
[34] BYUN S G, KIM M D, LEE W H, et al.Production of GDP-L-fucose, L-fucose donor for fucosyloligosaccharide synthesis, in recombinant Escherichia coli[J].Applied Microbiology Biotechnology, 2007, 74(4):768-775.
[35] LEE W H, HAN N S, PARK Y C, et al.Modulation of guanosine 5′-diphosphate-D-mannose metabolism in recombinant Escherichia coli for production of guanosine 5′-diphosphate-L-fucose[J].Bioresource Technology, 2009, 100(24):6 143-6 148.
[36] LEE W H, CHIN Y W, HAN N S, et al.Enhanced production of GDP-L-fucose by overexpression of NADPH regenerator in recombinant Escherichia coli[J].Applied Microbiology and Biotechnology, 2011, 91:967-976.
[37] HUANG D, YANG K X, LIU J, et al.Metabolic engineering of Escherichia coli for the production of 2′-fucosyllactose and 3-fucosyllactose through modular pathway enhancement[J].Metabolic Engineering, 2017, 41:23-38.
[38] MATTILA P, RABINA J, HORTLING S, et al.Functional expression of Escherichia coli enzymes synthesizing GDP-L-fucose from inherent GDP-D-mannose in Saccharomyces cerevisiae[J].Glycobiology, 2000, 10(10):1 041-1 047.
[39] CHIN Y W, PARK J B, PARK Y C, et al.Metabolic engineering of Corynebacterium glutamicum to produce GDP-L-fucose from glucose and mannose[J].Bioprocess and Biosystems Engineering, 2013, 36(6):749-756.
[40] KOIZUMI S, ENDO T, TABATA K, et al.Large-scale production of GDP-fucose and Lewis X by bacterial coupling[J].Journal of Industrial Microbiology & Biotechnology, 2000, 25(4):213-217.
[41] LI L, KIM S A, HEO J E, et al.One-pot synthesis of GDP-L-fucose by a four-enzyme cascade expressed in Lactococcus lactis[J].Journal of Biotechnology, 2017, 264:1-7.
[42] LIU J J, LEE J W, YUN E J, et al.L-Fucose production by engineered Escherichia coli[J].Biotechnology and Bioengineering, 2019, 116(4):904-911.
[43] 程仕伟,陈超男,冯志彬,等.海带岩藻多糖的水提制备及其抗氧化活性研究[J].食品科学, 2010, 31(6):101-104.
CHENG S W, CHEN C N, FENG Z B, et al.Water extraction and antioxidant activity of fucoidan from Laminaria japonica[J].Food Science, 2010, 31(6):101-104.
[44] GORI A, BIAGIOLINI S, MANONI M, et al.Process for production of L-fucose:WO/2012/034996A1[P].2012-03-22.
[45] LI S S, XIA H Q, XIE A Q, et al.Structure of a fucose-rich polysaccharide derived from EPS produced by Kosakonia sp.CCTCC M2018092 and its application in antibacterial film[J].International Journal of Biological Macromolecules, 2020, 159:295-303.
[46] HE R H, LI Y, HAN C Q, et al.L-fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-κB activation[J].International Immunopharmacology, 2019, 73:379-388.
[47] WIERICHS R J, ZELCK H, DOERFER C E, et al.Effects of dentifrices differing in fluoride compounds on artificial enamel caries lesions in vitro[J].Odontology, 2017, 105(1):36-45.
[48] DONALDSON G P, LADINSKY M S, YU K B, et al.Gut microbiota utilize immunoglobulin A for mucosal colonization[J].Science, 2018, 360(6 390):795-800.