植物多糖作为一种高分子化合物,不仅参与细胞的各种生理生化反应,同时也具有免疫调节[1]、抗肿瘤[2]、降血糖等生物活性[3-4]。与此同时,也是天然抗氧化剂的重要来源[5-6]。川明参是我国特有的伞形科多年生草本植物,主要分布于四川与湖北等地,富含多糖、香豆素、氨基酸及脂肪酸等物质[7-10]。川明参作为一种传统中药,被广泛应用于药膳制作,具有深远的食用历史。多糖作为川明参中主要的活性成分,具有较好的增强免疫[11-12]、抗病毒[13]、抗氧化[14-15]等性质。
研究表明,川明参多糖能在RAW 264.7巨噬细胞模型、小鼠脾脏细胞模型及环磷酰胺诱导的免疫力低下小鼠模型中表现出较好的免疫活性[16-18];硫化川明参多糖可通过阻断鸭肠炎病毒吸附宿主细胞来抵抗鸭肠炎病毒[13];川明参多糖以及硒化川明参多糖可作为佐剂来提高口蹄疫病毒疫苗及乙肝病毒疫苗的免疫应答[19-20]。另外,体外抗氧化实验表明,川明参多糖具有较好的ABTS阳离子自由基以及DPPH自由基清除能力[14-15]。此外,FAN等[21]的研究表明,川明参多糖能显著提高D-半乳糖致衰老小鼠的Cu/Zn-超氧化物歧化酶(superoxide dismutase,SOD)、Mn-SOD、Fe-SOD、过氧化氢酶、谷胱甘肽过氧化物酶和重组抗氧化酶的mRNA表达水平,以此来提高这些酶的活性,实现川明参多糖的体内抗氧化作用。最新的研究表明,川明参多糖的抗氧化活性及其免疫活性可能与其α-1,4-GalA有关[12]。
近年来,尽管在川明参多糖的生物活性方面有了大量的报道,但其结构方面的研究还较为浅显。目前,对川明参多糖的结构的研究还处于单糖组成及分子质量等方面的研究,糖苷键组成及连接方式等鲜有报道。本文采用超声辅助水提醇沉法提取川明参粗多糖,并采用离子色谱、凝胶渗透色谱(high-performance gel filter chromatography,HPGPC)、紫外光谱、傅里叶红外光谱(Fourier transform infrared spectroscopy,FTIR)和核磁共振光谱对凝胶柱层析后的主要组分进行结构鉴定。此外,以ABTS阳离子自由基清除能力、DPPH自由基清除能力、·OH清除能力、Fe2+螯合能力及总还原力为指标考察其抗氧化活性。
1 材料与方法
1.1 材料与试剂
川明参,重庆市万州区;葡聚糖凝胶G-150和单糖标品(葡萄糖、甘露糖、半乳糖、鼠李糖、岩藻糖、核糖、木糖、阿拉伯糖、半乳糖醛酸、葡萄糖醛酸、甘露糖醛酸),美国Sigma公司。其余均为国产分析纯试剂。
1.2 仪器及设备
UV-2450紫外可见光分光光度计、LC-10A高效液相色谱仪,日本岛津公司;FTIR-650傅里叶变换红外光谱仪,天津港东科技股份有限公司;ALPH1-2/LD-Plus冷冻干燥机,德国CHRIST公司;高压离子色谱仪,美国赛默飞公司;Bruker DRX-400核磁共振波谱仪,德国Bruker公司。
1.3 试验方法
1.3.1 川明参粗多糖的提取
称取一定量川明参粉置于2 000 mL锥形瓶中,按料液比1∶20(g∶mL)加入蒸馏水溶解。在300 Hz、60 ℃条件下,将溶解液放入超声清洗仪中超声提取90 min。超声完成后用4层纱布过滤,然后将滤液浓缩至原体积的1/4,向浓缩液中加入体积分数为80%的乙醇沉淀。静置过夜,离心收集沉淀,沉淀经真空冷冻干燥得到粗多糖粉末。
1.3.2 川明参粗多糖的分离纯化
取一定量粗多糖粉末配制成10 mg/mL溶液,采用Sevag法除去游离蛋白。按3∶1的体积比例向川明参粗多糖溶液加入氯仿-甲醇溶液(V∶V=4∶1),剧烈振荡30 min后离心,利用分液漏斗收集上层液体。重复6次后加入无水乙醇并调节乙醇体积分数为80%。静置过夜,离心收集沉淀并冷冻干燥。将除蛋白后的样品用去离子水配制成质量浓度为10 mg/mL的样品溶液。采用Sephadex G-150 (2.5×60 cm)进行分离纯化。上样量为5 mL,流速1 mL/min,采用自动收集器每10 min收集一管滤出液。再采用苯酚-硫酸法在490 nm处测定滤出液中的多糖含量,并绘制洗脱图。根据洗脱曲线收集主要组分以备后续分析。
1.3.3 川明参多糖主要成分的结构表征
1.3.3.1 单糖组成
色谱条件:DionexTM CarboPacTM PA10色谱柱(250 mm×4.0 mm×10 μm);ICS-5000+型高压离子色谱仪(配备电化学检测器);上样量为20 μL;流速为0.5 mL/min;柱温30 ℃;流动相A:去离子水;流动相B:100 mmol/L的NaOH溶液;采用梯度洗脱模式:0~30 min 2.5% B上升至20 %,30~30.1 min 20% B上升到40 %,30.1~45 min 40% B等度洗脱,45~45.1 min 40% B下降至2.5%,45.1~60 min 2.5% B等度洗脱。
1.3.3.2 分子质量测定
采用HPGPC法测定分子质量[23]。精密称取5 mg样品溶于1 mL浓度为0.05 mol/L的NaCl溶液中(超纯水配制),超声溶解后于12 000 r/min离心10 min,取上清液过0.22 μm水系滤膜后进行上机分析。
色谱条件:LC-10A型高效液相色谱(配备RI-502示差检测器);BRT-105-102(8 mm×300 mm)串联色谱柱;上样量为20 L;流速为0.6 mL/min;柱温40 ℃;流动相为0.05 mol/L的氯化钠溶液。
1.3.3.3 紫外及红外光谱扫描
制备不同浓度的多糖溶液于波长200~600 nm处进行紫外光谱扫描。称取1~2 mg多糖粉末,置于玛瑙研钵中,加入150~200 mg干燥后的KBr晶体,研磨均匀后压片,在4 000~400 cm-1记录其红外光谱。
1.3.3.4 核磁分析
称取适量多糖样品溶解于99.9%的D2O中,并使用Bruker DRX-400核磁共振波谱仪测定样品1H谱及13C谱,再测定样品的HSQC,COSY以及HMBC光谱。核磁数据采用MestRanova software进行分析。
1.3.4 川明参多糖主要成分的结构表征
参考文献[24-26]的方法,对多糖样品的ABTS阳离子自由基、DPPH自由基、·OH清除活性、Fe2+螯合能力以及总还原力等体外抗氧化活性指标进行考察。
1.4 数据分析
每组实验重复3次,结果采用X±SD表示。绘图采用Origin 2018软件,采用SPSS 23.0软件并根据Probit回归模型计算半抑制浓度(IC50)。
2 结果与讨论
2.1 川明参多糖的分离纯化
川明参粗多糖经Sevag法除去蛋白后,将其溶于去离子水中,采用Sephadex G-150进行分离纯化。洗脱曲线如图1所示,川明参粗多糖经凝胶柱层析后得到3个组分,将其分别命名为CVPs-I、CVPs-II和CVPs-III。经比较发现,CVPs-III为其主要组分。对其收集后进行后续检测。
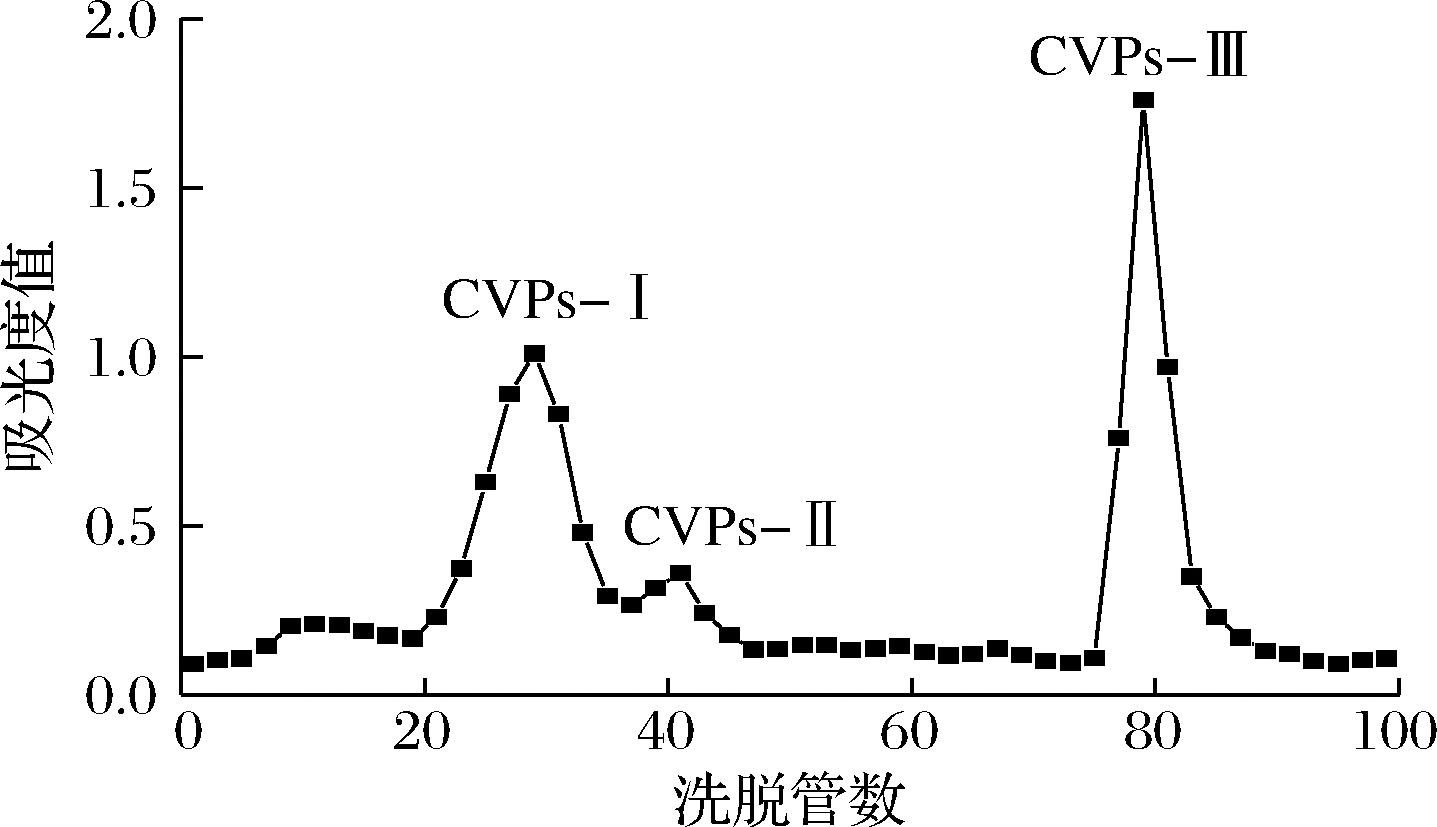
图1 川明参多糖的Sephadex G-150洗脱曲线
Fig.1 The elution curve of C.violaceum polysaccharides with Sephadex G-150
2.2 CVPs-III的结构鉴定
2.2.1 单糖组成及分子质量分析
采用离子色谱法分析CVPs-III的单糖组成。其结果如图2-a所示,CVPs-III主要由葡萄糖组成。根据其单糖组成结果推断CVPs-III可能为葡聚糖。有文献报道川明参多糖多由葡萄糖、半乳糖、甘露糖、阿拉伯糖及木糖组成,与CVPs-III在单糖组成上存在较大差异[12,19-20]。LIN等[12,27]研究表明,不同产地间的川明参多糖的单糖组成不同,这种差异可能是样品的生长环境不同引起的。此外,由于本研究所采用的单糖组成测定方法与已报道的文献所述方法不同,这也可能是单糖组成差异较大的原因。
采用HPGPC法测定CVPs-III的分子质量,其结果如图2-b所示。CVPs-III的重均分子质量(Mw)和分布系数(Mw/Mn)分别为176 kDa和1.05,与文献中报道的川明参多糖的分子质量相似[20]。
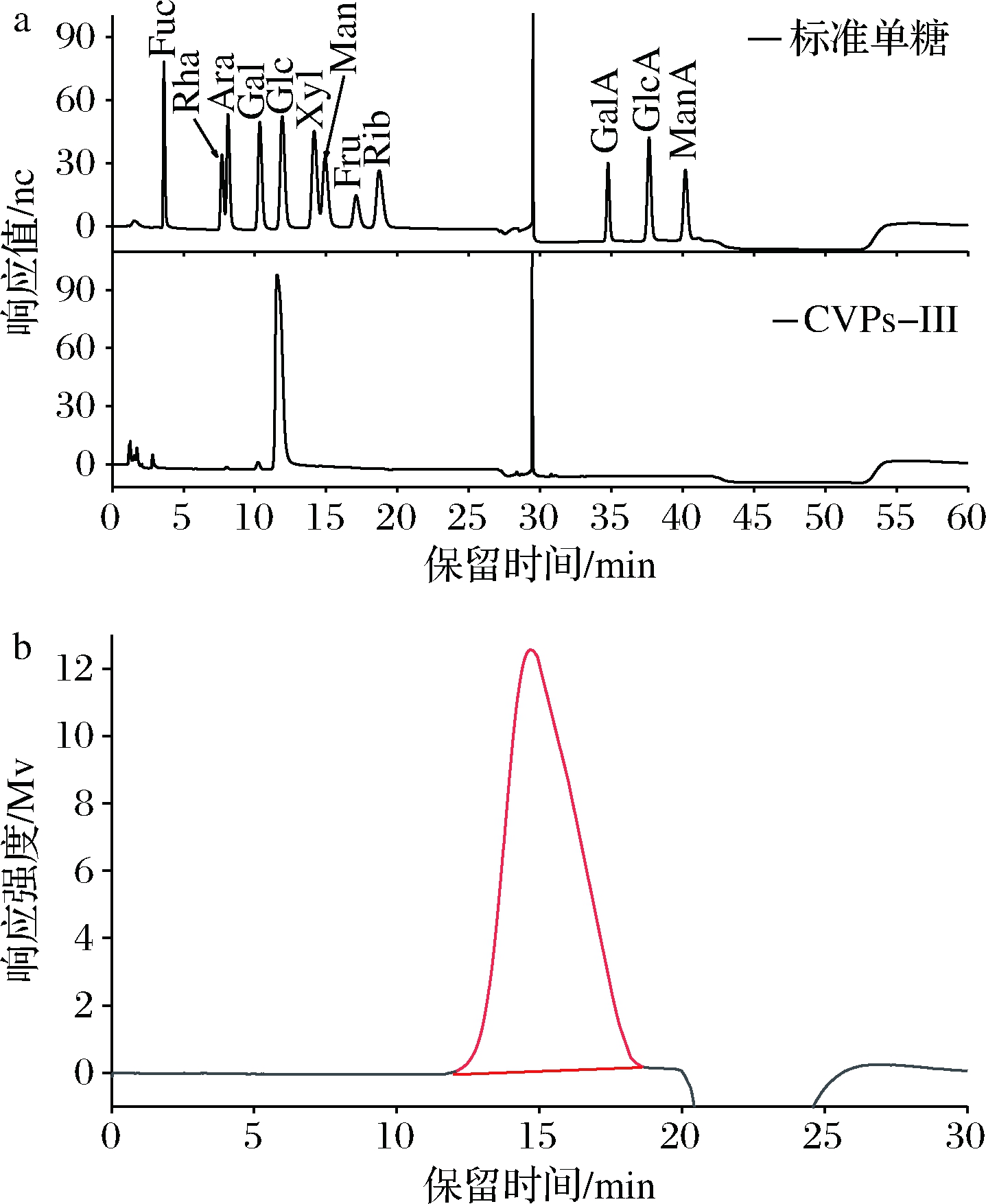
a-单糖组成;b-分子质量
图2 CVPs-III的单糖组成与分子质量
Fig.2 The monosaccharide content and molecular weight of CVPs-III
2.2.2 紫外光谱及红外光谱解析
CVPs-III的红外光谱及紫外光谱如图3所示。紫外光谱中,在260~280 nm处无明显吸收,这表明CVPs-III中不含蛋白质和核酸[28]。红外光谱中,3 405 cm-1处的吸收峰为分子间和分子内的O—H伸缩振动引起的[29];2 925 cm-1处的吸收峰为多糖的C—H伸缩振动峰,此为多糖的特征吸收峰[30];1 640 cm-1处的吸收峰为结合水的特征吸收峰[31];1 417 cm-1处的吸收峰为多糖的C—O伸缩振动[32];1 000~1 200 cm-1 3个吸收峰表明CVPs-III主要由吡喃糖构成[33];932和852 cm-1处的吸收峰表明CVPs-III既含有α-吡喃糖苷键,也有β-吡喃糖苷键[34]。

a-紫外光谱;b-红外光谱
图3 CVPs-III的紫外光谱及红外光谱分析
Fig.3 The UV and FI-IR spectra of CVPs-III
2.2.3 核磁共振光谱分析
采用一维及二维核磁技术可对多糖的糖残基组成及连接方式进行分析[35-37]。CVPs-III的核磁数据如图4和图5所示。由图4与图5-a中的异头碳氢质子信号可知,CVPs-III可能由5种糖残基组成,其异头碳氢质子信号依次为98.61/5.29、99.99/5.26、98.45/4.86、95.70/4.53和103.16/4.53 ppm,并将其分别标记为残基A、B、C、D和E。此外,根据图5-b和图5-c可对各残基的δ H2/C2-H6/C6进行推断,其结果如表1所示。残基A的C1和C4均向低场方向移动,表明残基A的C1与C4位置均发生了取代。因此,根据文献以及CVPs-III的单糖组成,推断残基A可能为→4)-α-Glcp-(1→[38-42]。依次类推,残基B、C、D和E分别为→4,6)-α-Glcp-(1→[38-42]、α-Glcp-(1→[38-41]、→4)-β-Glcp[40,42]、→3)-β-Glcp-(1→[41]。
由于HMBC光谱上包含异头氢与其他糖残基上碳的耦合信号,异头碳与其他糖残基上氢的耦合信号,故糖苷键连接方式可通过HMBC光谱进行确定。CVPs-III的HMBC谱如图5-c所示,残基A的C1与H4之间存在耦合信号;残基A的H1和C4之间存在耦合信号,故推测残基A之间可能是通过(1→4)糖苷键进行连接。此外,残基A的C1与残基B的H4之间存在耦合信号,残基A的H1与残基B的C4之间存在耦合信号,残基B的C1与残基A的H4之间存在耦合信号,残基B的H1与残基A的C4之间存在耦合信号,故推测残基A与残基B之间也是通过(1→4)糖苷键进行连接。另外,残基A的H1与残基E的C3存在耦合信号,推测残基A可通过(1→3)糖苷键与残基E连接;残基E的C1与残基A的H4之间存在耦合信号,推测残基E 可通过(1→4)糖苷键与残基A连接。综上所述,考虑到各糖残基在核磁图中信号的强弱,并结合文献中报道的葡聚糖的核磁光谱,推测CVPs-III的主链主要由→4)-α-Glcp-(1→4)-α-Glcp-(1→构成,并含有少量的→4)-α-Glcp-(1→3)-β-Glcp-(1→4)-α-Glcp-(1→[38-42]。
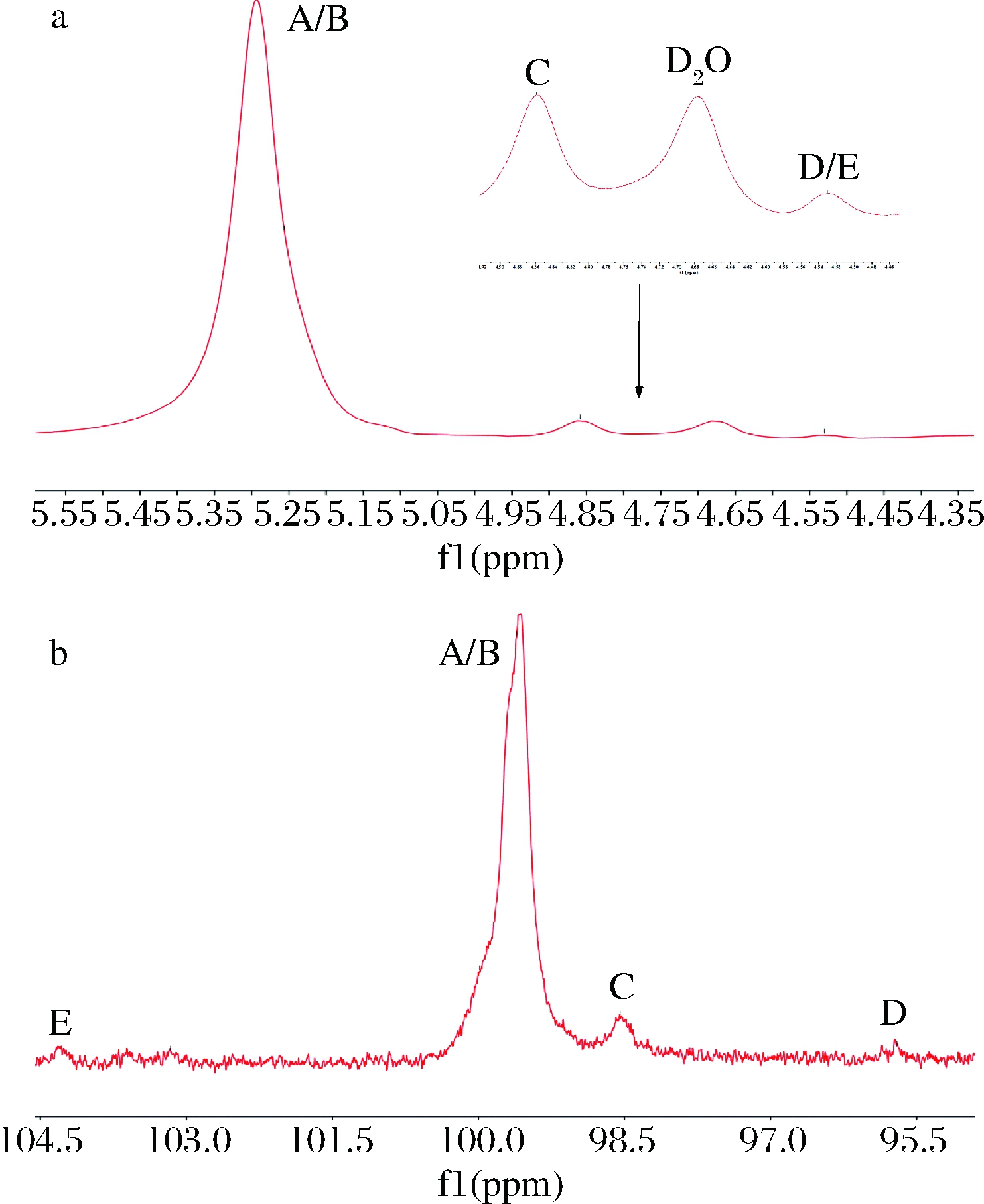
a-1H-NMR;b-13C-NMR
图4 CVPs-III的异头质子区的1H-NMR谱和13C-NMR谱
Fig.4 1H-NMR and 13C-NMR spectra of heterotopic region of CVPs-III
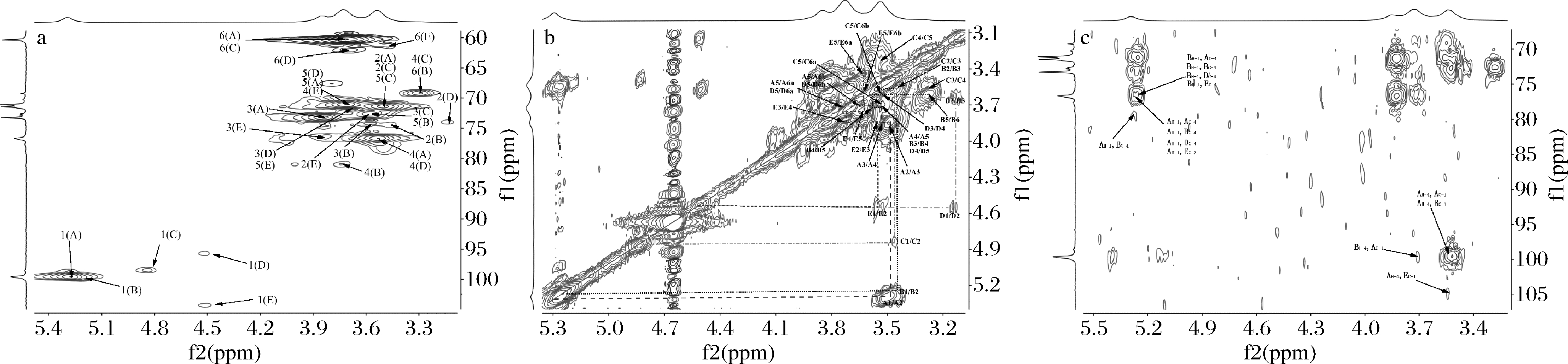
a-HSQC光谱;b-H-H COSY光谱;c-HMBC光谱
图5 CVPs-III的二维核磁光谱
Fig.5 The 2D NMR spectrum of CVPs-III
表1 残基A-G的化学位移
Table 1 The chemical shifts and coupling constant of residue A-G
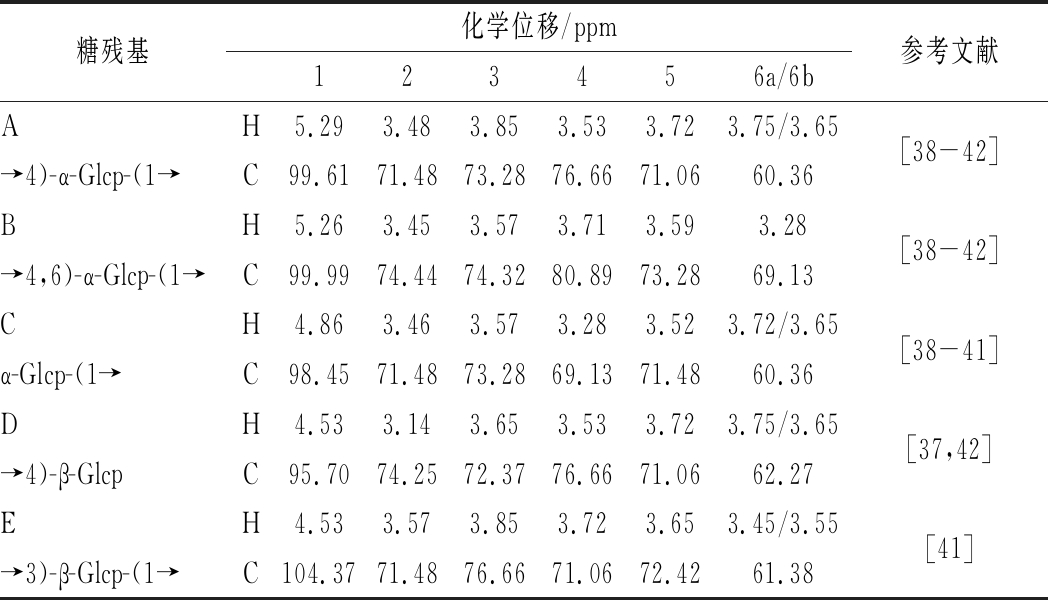
糖残基化学位移/ppm123456a/6b参考文献AH5.293.483.853.533.723.75/3.65[38-42]→4)-α-Glcp-(1→C99.6171.4873.2876.6671.0660.36BH5.263.453.573.713.593.28[38-42]→4,6)-α-Glcp-(1→C99.9974.4474.3280.8973.2869.13CH4.863.463.573.283.523.72/3.65[38-41]α-Glcp-(1→C98.4571.4873.2869.1371.4860.36DH4.533.143.653.533.723.75/3.65[37,42]→4)-β-GlcpC95.7074.2572.3776.6671.0662.27EH4.533.573.853.723.653.45/3.55[41]→3)-β-Glcp-(1→C104.3771.4876.6671.0672.4261.38
2.3 抗氧化活性鉴定
自由基作为人体内正常代谢的产物,一般情况下处于动态平衡中,但平衡一旦被打破后,由于其状态极不稳定,会攻击邻近分子(碳水化合物、蛋白质、脂质等)以夺取电子。所以,过多的自由基会导致细胞结构破坏、功能丧失、造成细胞损伤或死亡[43-45]。另外,过多的自由基也是心血管疾病、糖尿病、慢性炎症、神经退行性疾病、风湿病与癌症等慢性疾病和老年病的原因之一[46-48]。因此,采用ABTS阳离子自由基清除能力、DPPH自由基清除能力、·OH清除能力、Fe2+螯合能力及总还原力对川明参多糖的体外抗氧化活性进行考察。
川明参粗多糖及其纯化组分的体外抗氧化活性如图6所示。川明参粗多糖及其纯化组分均具有较好的体外抗氧化活性,其ABTS阳离子自由基清除能力、DPPH自由基清除能力、·OH清除能力、Fe2+螯合能力及总还原力均表现出剂量效应关系。如图所示,CVPs-III的抗氧化活性最高,其次是CVPs、CVPs-II和CVPs-I。利用SPSS软件并通过Probit回归模型所计算得到CVPs-III的ABTS阳离子自由基清除能力、DPPH自由基清除能力、Fe2+螯合能力和·OH清除能力的IC50分别为(1.392±0.006)、(0.596±0.017)、(0.554±0.001 1)、(0.618±0.018)mg/mL。
与文献报道的超声辅助和微波辅助提取的川明参多糖的ABTS阳离子自由基清除能力相比,CVPs-III的ABTS阳离子自由基清除能力较低,但DPPH自由基清除能力与总还原力较高[19-20]。与LIN等[12,27]的研究结果相比,CVPs-III的ABTS阳离子自由基清除能力与川明参非淀粉多糖和川明参叶多糖的ABTS阳离子自由基清除能力较为接近。综上,经分离纯化后得到的CVPs-III具有较好的体外抗氧化活性。
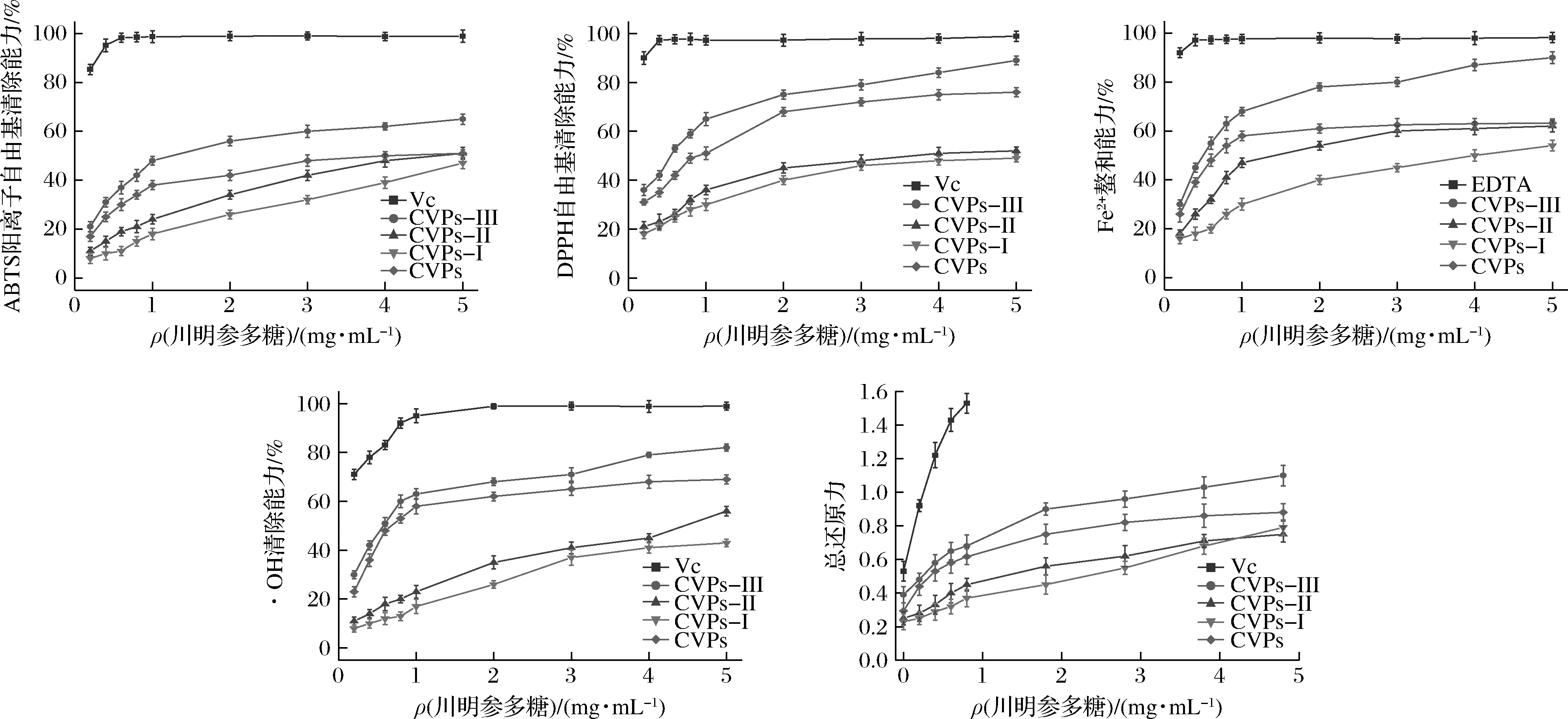
图6 川明参多糖的抗氧化活性
Fig.6 The antioxidant activity of C.violaceum polysaccharides
3 结论
本文通过水提醇沉法提取川明参粗多糖,并对经凝胶柱层析后的主要成分(CVPs-III)的结构和抗氧化活性进行分析。其结果表明,CVPs-III是一种分子质量为176 kDa,以→4)-D-Glcp-(1→4)-α-Glcp-(1→为主链,并含有少量→4)-α-Glcp-(1→3)-β-Glcp-(1→4)-α-Glcp-(1→的葡聚糖。此外,体外抗氧化实验表明,CVPs-III具有较好的抗氧化活性,其次为CVPs、CVPs-II和CVPs-I。其主要组分(CVPs-III)的ABTS阳离子自由基清除能力、DPPH自由基清除能力、Fe2+螯合能力和·OH清除能力的IC50分别为(1.392±0.006)、(0.596±0.017)、(0.554±0.001 1)、(0.618±0.018)mg/mL。综上,川明参多糖具有较好的抗氧化活性,可作为天然抗氧化剂的来源之一。
[1] SHU Z P,YANG Y N,XING N,et al.Structural characterization and immunomodulatory activity of a pectic polysaccharide (CALB-4) from Fructus aurantii[J].International Journal of Biological Macromolecules,2018,116:831-839.
[2] LIU L Q,NIE S P,XIE M Y,et al.Tea polysaccharides inhibits colitis-associated colorectal cancer via interleukin-6/STAT3 pathway[J].Journal of Agricultural and Food Chemistry,2018,66(17):4 384-4 393.
[3] WANG L B,LIU F C,WANG A X,et al.Purification,characterization and bioactivity determination of a novel polysaccharide from pumpkin (Cucurbita moschata) seeds[J].Food Hydrocolloids,2017,66:357-364.
[4] CHEN C,HUANG Q,LI C,et al.Hypoglycemic effects of a Fructus Mori polysaccharide in vitro and in vivo[J].Food & Function,2017,8:2 523-2 535.
[5] GHEDA S F,ELADAWI H,ELDEED N M.Antiviral profile of brown and red seaweed polysaccharides against hepatitis C virus[J].Iranian Journal of Pharmaceutical Research,2016,15(3):483-491.
[6] YUAN Y Q,LI C,ZHENG Q W,et al.Effect of simulated gastrointestinal digestion in vitro on the antioxidant activity,molecular weight and microstructure of polysaccharides from a tropical sea cucumber (Holothuria leucospilota)[J].Food Hydrocolloids,2019,89:735-741.
[7] 国家中医药管理局《中华本草》编委会.中华本草[M].上海:上海科学技术出版社,1999.
Editorial board of Chinese herbal medicine,state administration of traditional Chinese medicine.Chinese MateriaMedica[M].Shanghai:Shanghai Science and Technology Press,1999.
[8] DONG H M,ZHANG Q,LI L,et al.Antioxidant activity and chemical compositions of essential oil and ethanol extract of Chuanminshen violaceum[J].Industrial Crops and Products,2015,76:290-297.
[9] ZHANG Q,TIAN J R,WANG C,et al.Discrimination ofChuanminshen violaceum Sheh et Shen from different regions based on fatty acid profiles of roots and leaves[J].Food Quality and Safety.DOI:10.1093/fqsafe/fyaa010.
[10] 杨婷,杨晓阳,陈文静,等.柱前衍生HPLC测定川明参中18种氨基酸含量[J].中国中药杂志,2020,45(6):1 316-1 322.
YANG T,YANG X Y,CHEN W J,et al.Assay of 18 amino acids in Chuanmingshen violaceum roots by pre-column derivative HPLC[J].China Journal of Chinese Materia Medica,2020,45(6):1 316-1 322.
[11] 赵兴洪.川明参多糖的提取工艺及其免疫活性研究[D].雅安:四川农业大学,2015.
ZHAO X H.Study of Chuanmingshen violaceum polysaccharides extraction process and its immune activity[D].Ya'an:Sichuan Agricultural University,2015.
[12] 赵兴洪,殷中琼,贾仁勇,等.川明参多糖及其硫酸化物对免疫低下小鼠的影响[J].中国免疫学杂志,2015,31(1):52-55;60.
ZHAO X H,YIN Z Q,JIA R Y,et al.Effect of Chuanmingshen violaceum polysaccharides and its sulfated derivatives on immunosuppression induced by cyclophosphamide in mice[J].Chinese Journal of Immunology,2015,31(1):52-55;60.
[13] SONG X,YIN Z Q,LI L,et al.Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against duck enteritis virus in vitro[J].Antiviral Research,2013,98(2):344-351.
[14] DONG H M,LIN S,ZHANG Q,et al.Effect of extraction methods on the properties and antioxidant activities of Chuanminshen violaceum polysaccharides[J].International Journal of Biological Macromolecules,2016,93:179-185.
[15] DONG H M,ZHANG Q,LI Y,et al.Extraction,characterization and antioxidant activities of polysaccharides of Chuanminshen violaceum[J].International Journal of Biological Macromolecules,2016,86:224-232.
[16] LIN S,LI H Y,YUAN Q,et al.Structural characterization,antioxidant activity,and immunomodulatory activity of non-starch polysaccharides from Chuanminshen violaceum collected from different regions[J].International Journal of Biological Macromolecules,2020,143:902-912.
[17] 赵兴洪,殷中琼,贾仁勇,等.川明参多糖及其硫酸化物对小鼠脾淋巴细胞增殖的影响[J].中国免疫学杂志,2014,30(2):213-215;221.
ZHAO X H,YIN Z Q,JIA R Y,et al.Effect of Chuanmingshen violaceum polysaccharides and its solfated derivatives on proliferation of mice spleen lymphocytes in vitro[J].Chinese Journal of Immunology,2014,30(2):213-215;221.
[18] ZHAO X H,ZHANG Y T,SONG X,et al.Effect of Chuanminshen violaceum polysaccharides and its sulfated derivatives on immunosuppression induced by cyclophosphamide in mice[J].International Journal of Clinical and Experimental Medicine,2015,8(1):558-568.
[19] FENG H B,FAN J,BO H Q,et al.Selenylation modification can enhance immune-enhancing activity of Chuanminshen violaceum polysaccharide[J].Carbohydrate Polymers,2016,153:302-311.
[20] FENG H B,FAN J,QIU H,et al.Chuanminshen violaceum polysaccharides improve the immune responses of foot-and-mouth disease vaccine in mice[J].International Journal of Biological Macromolecules,2015,78:405-416.
[21] FAN J,FENG H B,YU Y,et al.Antioxidant activities of the polysaccharides of Chuanminshen violaceum[J].Carbohydrate Polymers,2017,157:629-636.
[22] WANG L,CHEN C,ZHANG B,et al.Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its α-glucosidase inhibitory activity[J].Food & Function,2018,9(7):3 974-3 985.
[23] DUAN G L,YU X B.Isolation,purification,characterization,and antioxidant activity of low-molecular-weight polysaccharides from Sparassis latifolia[J].International Journal of Biological Macromolecules,2019,137:1 112-1 120.
[24] JI Y H,LIAO A M,HUANG J H,et al.Physicochemical and antioxidant potential of polysaccharides sequentially extracted from Amana edulis[J].International Journal of Biological Macromolecules,2019,131:453-460.
[25] CHEN Y S,HUANG J Q,HU J,et al.Comparative study on the phytochemical profiles and cellular antioxidant activity of phenolics extracted from barley malts processed under different roasting temperatures[J].Food & Function,2019,10(4):2 176-2 185.
[26] ZHANG H,CAI X T,TIAN Q H,et al.Microwave-assisted degradation of polysaccharide from Polygonatum sibiricum and antioxidant activity[J].Journal of Food Science,2019,84(4):754-761.
[27] LIN S,LI H Y,WANG Z Y,et al.Analysis of methanolic extracts and crude polysaccharides from the leaves of Chuanminshen violaceum and their antioxidant activities[J].Antioxidants (Basel,Switzerland),2019,8(8):E266.
[28] TANG H L,CHEN C,WANG S K,et al.Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L[J].International Journal of Biological Macromolecules,2015,77:235-242.
[29] CHENG H R,FENG S L,JIA X J,et al.Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum[J].Carbohydrate Polymers,2013,92(1):63-68.
[30] SHEN S A,XU Z,FENG S L,et al.Structural elucidation and antiaging activity of polysaccharide from Paris Polyphylla leaves[J].International Journal of Biological Macromolecules,2018,107:1 613-1 619.
[31] LUO Q L,TANG Z H,ZHANG X F,et al.Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale[J].International Journal of Biological Macromolecules,2016,89:219-227.
[32] XU Y Q,CAI F,YU Z Y,et al.Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity[J].Food Chemistry,2016,194:650-658.
[33] MENG M,CHENG D,HAN L R,et al.Isolation,purification,structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola frondosa fruiting body[J].Carbohydrate Polymers,2017,157:1 134-1 143.
[34] LIU Y,HUANG G L,LV M J.Extraction,characterization and antioxidant activities of mannan from yeast cell wall[J].International Journal of Biological Macromolecules,2018,118:952-956.
[35] REN Y,BAI Y P,ZHANG Z D,et al.The preparation and structure analysis methods of natural polysaccharides of plants and fungi:A review of recent development[J].Molecules (Basel,Switzerland),2019,24(17):E3122.
[36] CAI Z N,LI W,MEHMOOD S,et al.Structural characterization,in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta[J].Carbohydrate Polymers,2018,195:29-38.
[37] ZHAO D,DAI W J,TAO H,et al.Polysaccharide isolated from Auricularia auricular-judae (Bull.) prevents dextran sulfate sodium-induced colitis in mice through modulating the composition of the gut microbiota[J].Journal of Food Science,2020,85(9):2 943-2 951.
[38] ZHANG Z,GUO L,YAN A P,et al.Fractionation,structure and conformation characterization of polysaccharides from Anoectochilus roxburghii[J].Carbohydrate Polymers,2020,231:115688.
[39] SHI X D,LI O Y,YIN J Y,et al.Structure identification of α-glucans from Dictyophora echinovolvata by methylation and 1D/2D NMR spectroscopy[J].Food Chemistry,2019,271:338-344.
[40] WANG J Q,NIE S P,CUI S W,et al.Structural characterization and immunostimulatory activity of a glucan from natural Cordyceps sinensis[J].Food Hydrocolloids,2017,67:139-147.
[41] SONG G L,DU Q Z.Structure characterization and antitumor activity of an α β-glucan polysaccharide from Auricularia polytricha[J].Food Research International,2012,45(1):381-387.
[42] ZHANG A Q,DENG J Y,LIU X Q,et al.Structure and conformation of α-glucan extracted from Agaricus blazei Murill by high-speed shearing homogenization[J].International Journal of Biological Macromolecules,2018,113:558-564.
[43] FINKEL T,HOLBROOK N J.Oxidants,oxidative stress and the biology of ageing[J].Nature,2000,408(6 809):239-247.
[44] GUO Q P,LI F N,DUAN Y H,et al.Oxidative stress,nutritional antioxidants and beyond[J].Science China Life Sciences,2020,63(6):866-874.
[45] SANTOS C X C,NABEEBACCUS A A,SHAH A M,et al.Endoplasmic Reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature:Potential role in hypertension[J].Antioxidants & Redox Signaling,2014,20(1):121-134.
[46] KIM G H,KIM J E,RHIE S J,et al.The role of oxidative stress in neurodegenerative diseases[J].Experimental Neurobiology,2015,24(4):325-340.
[47] ZAMORA R,HIDALGO F J.The triple defensive barrier of phenolic compounds against the lipid oxidation-induced damage in food products[J].Trends in Food Science and Technology,2016,54:165-174.
[48] ZHANG H,TSAO R.Dietary polyphenols,oxidative stress and antioxidant and anti-inflammatory effects[J].Current Opinion in Food Science,2016,8:33-42.