UPLC-MS/MS因分析效率、准确度和灵敏度高等优点被广泛应用于兽药残留的检测[1-4]。但由于动物性食品基质复杂,含蛋白质、脂类、肽类、胺类及等内源性干扰物[5-6]及前处理过程中引入的无机盐、有机酸、塑化剂等外源性杂质[7],会增强或抑制待测物在电喷雾接口处的离子化效率[8-9],对待测物产生一定的干扰,影响检测的选择性和灵敏度,从而导致分析结果的准确度和精密度受到不同程度的影响,这一过程称为基质效应[10-11]。2001年,美国食品和药物管理局指出,在LC-MS/MS的方法开发和验证过程中,需评估基质效应[12]。
本文采用改善的QuEChERS前处理法[27]结合UPLC-MS/MS检测技术,通过离子抑制率(ion suppression,IS)[13-15]对6种不同的动物性食品中24种兽药的基质效应进行系统性地评价;考察同位素内标[16-17]、基质匹配标准曲线[18-19]对基质效应的补偿效果。
1 材料与方法
1.1 仪器、试剂与材料
TSQ Endura三重四极杆串联质谱仪、Ultimate 3000超高效液相色谱仪,美国Thermo-Fisher公司;VORTEX GENIUS3涡旋仪,德国IKA公司;Milli-Q型全自动超纯水机,美国Millipore公司。
乙腈、甲醇、正己烷均为色谱纯,德国Merck公司;甲酸(色谱纯),德国CNW公司;无水硫酸钠(分析纯),重庆川东化工(集团)有限公司;C18 E粉,美国Welch公司。
本实验室所用的标准品纯度除替米考星(tilmicosin,TIL)和诺氟沙星-D5(norfloxacine-D5 hydrate,NOR-D5)外纯度均在92%以上,TIL纯度为80.71%,NOR-D5纯度为80.40%。沙拉沙星(sarafloxacin,SAR)、金刚烷胺(amantadine,AMA)、金刚乙胺(rimantadina,RIM)、环丙沙星-D8(ciprofloxacin-D8,CIP-D8)、恩诺沙星-D5(enrofloxacin-d5,ENR-D5)、NOR-D5,Bepure公司;磺胺间二甲氧嘧啶-D6(sulfadimethoxine-D6,SDT-D6)、磺胺邻二甲氧嘧啶-D3(sulfadoxine,SDX-D3),德国WITEGA公司;金刚烷胺-D15(AMA-D15),德国CNW公司;红霉素-13C2(erythromycin-13C2,ERY-13C2),美国Sigma-Aldrich公司;其余21种标准品均来自德国DR.Ehrenstorfer。
分别称取适量标准品,用甲醇配制成1 mg/mL的标准储备液;准确移取一定量的各标准储备液,用甲醇稀释成1 μg/mL的混合标准工作液,现用现配;同法配制内标工作液。
1.2 样品前处理
称取动物组织5.00 g(精确至0.01 g)于50 mL离心管中,加入2 mL乙腈饱和正己烷;加入10 mL 0.1%(体积分数)甲酸-乙腈,3 g无水硫酸钠,加盖涡旋1 min,超声5 min,8 000 r/min离心5 min;取中间层提取液于含有2 g C18E的净化管中,超声5 min,4 000 r/min离心3 min;准确移取5 mL上清液于氮吹管中,40 ℃氮吹至干,用20%(体积分数)甲醇-水定容至1 mL,涡旋,过0.22 μm有机滤膜,供UPLC-MS/MS测定。
1.3 仪器条件
1.3.1 色谱条件
色谱柱:Thermo-Fisher Hypersil GOLD aQ色谱柱(50 mm×2.1 mm,1.9 μm);柱温:40 ℃;流速:0.4 mL/min;进样体积5 μL;流动相A:0.1%(体积分数)甲酸-水溶液,流动相B:乙腈;梯度洗脱程序:0~0.5 min,90%A;0.5~3 min,90%A~10%A;3~4 min,10%A;4.1~5 min,90%A。
1.3.2 质谱条件
离子化模式:电喷雾离子源(ESI),正离子模式;喷雾电压:3 500 V;鞘气(Arb):45;辅助气(Arb):13;吹扫气(Arb):2;离子传输管温度:350 ℃;蒸发温度:300 ℃;碰撞气压力:氩气2 mTorr;Q1半峰宽:0.7 Da;Q3半峰宽:0.7 Da;扫描模式:多反应监测(multiple reaction monitoring,MRM)。24种兽药质谱参数见表1。
表1 各兽药保留时间及质谱参数
Table 1 Mass spectral parameters of various veterinary drugs
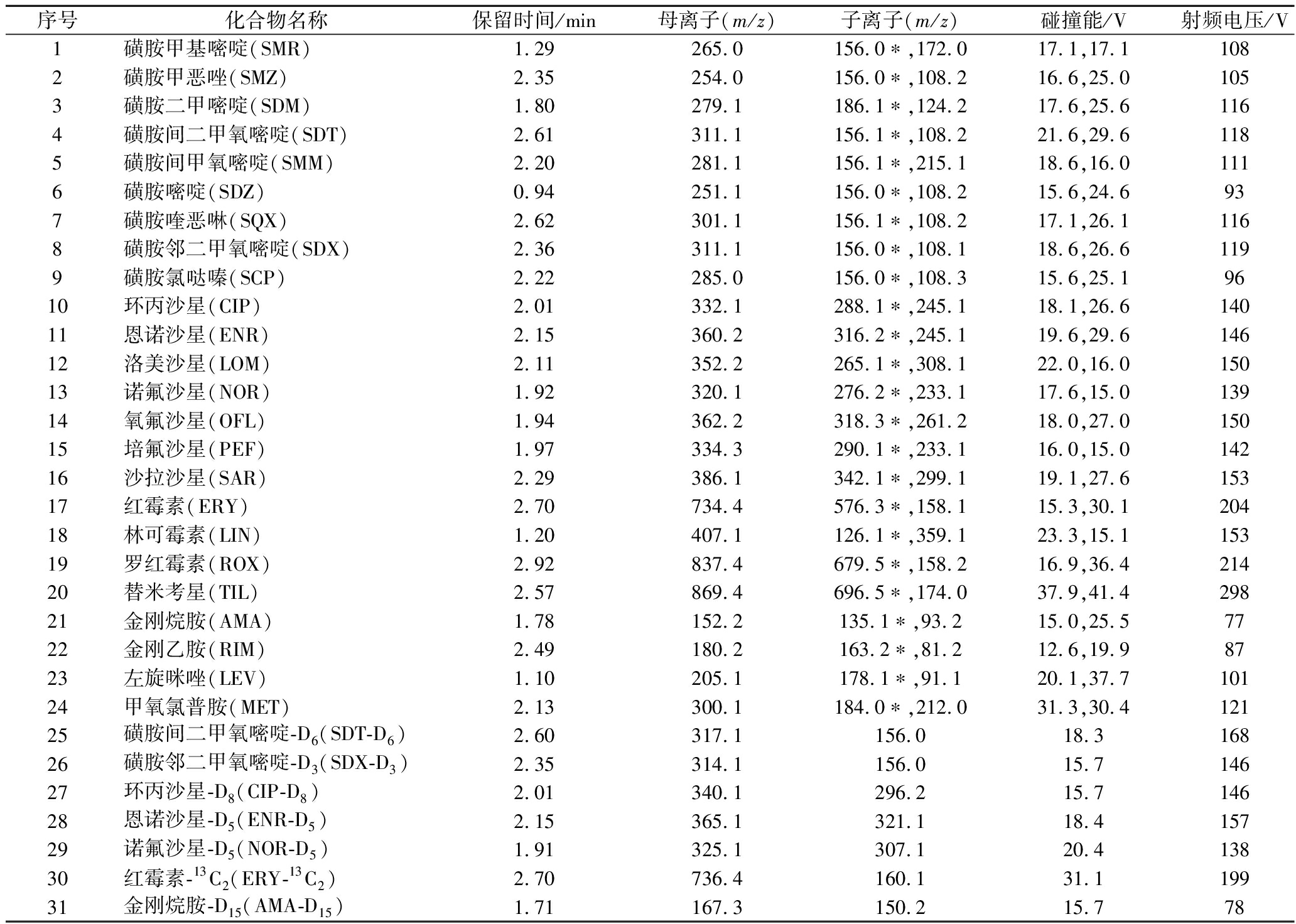
序号化合物名称保留时间/min母离子(m/z)子离子(m/z)碰撞能/V射频电压/V1磺胺甲基嘧啶(SMR)1.29265.0156.0∗,172.017.1,17.11082磺胺甲恶唑(SMZ)2.35254.0156.0∗,108.216.6,25.01053磺胺二甲嘧啶(SDM)1.80279.1186.1∗,124.217.6,25.61164磺胺间二甲氧嘧啶(SDT)2.61311.1156.1∗,108.221.6,29.61185磺胺间甲氧嘧啶(SMM)2.20281.1156.1∗,215.118.6,16.01116磺胺嘧啶(SDZ)0.94251.1156.0∗,108.215.6,24.6937磺胺喹恶啉(SQX)2.62301.1 156.1∗,108.217.1,26.11168磺胺邻二甲氧嘧啶(SDX)2.36311.1156.0∗,108.118.6,26.61199磺胺氯哒嗪(SCP)2.22285.0156.0∗,108.315.6,25.19610环丙沙星(CIP)2.01332.1288.1∗,245.118.1,26.614011恩诺沙星(ENR)2.15360.2316.2∗,245.119.6,29.614612洛美沙星(LOM)2.11352.2265.1∗,308.122.0,16.015013诺氟沙星(NOR)1.92320.1276.2∗,233.117.6,15.013914氧氟沙星(OFL)1.94362.2318.3∗,261.218.0,27.015015培氟沙星(PEF)1.97334.3290.1∗,233.116.0,15.014216沙拉沙星(SAR)2.29386.1342.1∗,299.119.1,27.615317红霉素(ERY)2.70734.4576.3∗,158.115.3,30.120418林可霉素(LIN)1.20407.1126.1∗,359.123.3,15.115319罗红霉素(ROX)2.92837.4679.5∗,158.216.9,36.421420替米考星(TIL)2.57869.4696.5∗,174.037.9,41.429821金刚烷胺(AMA)1.78152.2135.1∗,93.215.0,25.57722金刚乙胺(RIM)2.49180.2163.2∗,81.212.6,19.98723左旋咪唑(LEV)1.10205.1178.1∗,91.120.1,37.710124甲氧氯普胺(MET)2.13300.1184.0∗,212.031.3,30.412125磺胺间二甲氧嘧啶-D6(SDT-D6)2.60317.1156.018.316826磺胺邻二甲氧嘧啶-D3(SDX-D3)2.35314.1156.015.714627环丙沙星-D8(CIP-D8)2.01340.1296.215.714628恩诺沙星-D5(ENR-D5)2.15365.1321.118.415729诺氟沙星-D5(NOR-D5)1.91325.1307.120.413830红霉素-13C2(ERY-13C2)2.70736.4160.131.119931金刚烷胺-D15(AMA-D15)1.71167.3150.215.778
注:*为定量离子
1.4 基质效应评价方法
溶剂标准曲线:准确移取适量混合标准工作液,用20%(体积分数)甲醇-水稀释成5、10、20、50、100、200 ng/mL的混合标准系列溶液。基质匹配标准曲线:分别称取阴性鲫鱼、鸡肉、鸭胗、猪肝、牛肉、猪肉5.00 g(精确至0.01 g)按1.2处理,获得6组不同的空白基质溶液,加入适量混合标准工作液,配制成6组5、10、20、50、100、200 ng/mL的基质匹配混合标准溶液。在1.3的仪器条件下测定,以浓度为横坐标,色谱峰面积为纵坐标绘制标准曲线。离子抑制率计算如公式(1)所示:
(1)
式中:IS,离子抑制率,%;K1,溶剂标曲斜率;K2,基质匹配标曲斜率。IS>0,基质增强[20];IS<0,基质抑制[21];IS绝对值越大,基质效应越强。
2 结果与分析
2.1 不同基质的基质效应评价
为了考察24种兽药受基质影响的程度,本实验选取鲫鱼、鸡肉、鸭胗、猪肝、牛肉、猪肉6种不同的基质,采用1.2方法进行前处理,用离子抑制率表示24种兽药在这6种基质中的基质效应,结果如表2所示。结果表明,同一兽药在不同基质中与不同兽药在同一基质中的基质效应均不同。这可能与基质中含有蛋白质、脂类和色素等物质有关,这些物质的化学结构与待测物的结构有相似之处,与基质产生共提取现象[22-23],共提取程度不同,基质效应也不同。其中鸭胗和猪肝对其影响比较大,可能是内脏富含碳水化合物、蛋白质、脂肪、烟酸、维生素等,比肌肉组织复杂,对待测物的影响更大,故基质效应也越强。
磺胺甲恶唑(sulfamethoxazole,SMZ)、SDT、磺胺嘧啶(sulfadiazine,SDZ)、磺胺喹恶啉(sulfaquinoxaline,SQX)、SDX、NOR、培氟沙星(pefloxacin,PEF)、ERY、罗红霉素(roxithromycin,ROX)、TIL多为中强基质效应,需采用内标法或基质匹配标曲定量;剩余14种兽药多为弱基质效应。根据本实验室内标获得的难易程度,对SDT、SDX、CIP、ENR、NOR、ERY、AMA采用内标法校正基质效应,剩余17种兽药采用基质匹配标准曲线校正基质效应的影响。
表2 24种兽药在6种不同基质中的基质效应(n=3)
Table 2 Matrix effects of 24 veterinary drugs in six food matrices

鲫鱼IS/%鸡肉IS/%鸭胗IS/%猪肝IS/%牛肉IS/%猪肉IS/%磺胺甲基嘧啶(SMR)-10.08±2.55-7.37±1.030.13±0.09-8.15±0.83-31.30±4.091.88±0.64磺胺甲恶唑(SMZ)-35.72±4.39-23.50±2.76-29.73±3.72-37.30±3.62-17.37±2.47-16.51±1.23磺胺二甲嘧啶(SDM)-6.26±0.653.34±0.58-1.05±0.71-22.96±2.33-4.34±0.876.22±0.65磺胺间二甲氧嘧啶(SDT)-31.68±3.59-13.15±1.46-25.84±2.57-26.78±2.87-21.26±3.23-19.19±2.57磺胺间甲氧嘧啶(SMM)-18.14±1.65-6.47±0.98-27.21±2.27-47.74±4.71-4.26±0.66-1.85±0.27磺胺嘧啶(SDZ)-42.05±4.72-37.01±4.07-54.32±4.48-85.63±4.63-45.03±4.27-30.63±3.57磺胺喹恶啉(SQX)-31.23±3.77-10.48±0.93-25.46±2.24-34.99±2.74-28.22±2.59-26.45±2.85磺胺邻二甲氧嘧啶(SDX)-52.17±4.28-40.95±4.58-50.59±4.26-52.31±4.37-45.47±4.76-32.31±3.51磺胺氯哒嗪(SCP)-16.69±2.197.70±0.63-20.37±2.08-39.73±4.097.87±1.0311.00±1.25环丙沙星(CIP)16.21±1.7032.00±2.371.10±1.02-24.57±2.1613.30±1.4811.66±1.44恩诺沙星(ENR)18.13±2.5331.93±3.876.63±0.76-17.36±2.9921.62±3.0715.81±1.78洛美沙星(LOM)4.24±0.878.93±1.09-11.64±1.36-31.96±3.64-1.31±0.47-1.25±0.76诺氟沙星(NOR)31.22±3.2645.44±4.7615.91±1.87-14.39±1.7323.75±2.3817.28±2.18氧氟沙星(OFL)12.50±1.8018.66±2.425.79±0.98-39.76±3.2514.08±1.746.96±1.05培氟沙星(PEF)53.24±4.9570.51±4.8742.03±4.72-16.03±2.0441.21±4.6338.19±3.76沙拉沙星(SAR)-16.50±1.535.21±0.88-21.08±2.88-32.95±3.37-10.40±1.48-2.16±1.03红霉素(ERY)-34.01±3.79-67.69±4.98-65.34±4.88-62.72±4.97-70.46±4.87-68.96±4.75林可霉素(LIN)-13.58±0.69-17.00±0.67-11.26±2.36-6.37±0.38-12.88±0.87-8.80±1.05罗红霉素(ROX)-31.02±3.27-42.31±4.28-53.28±4.06-57.18±4.33-38.23±3.84-39.85±4.28替米考星(TIL)8.97±1.0328.80±2.5722.42±3.5610.99±0.7626.22±2.5630.42±3.59金刚烷胺(AMA)-10.01±1.22-13.86±1.32-15.14±1.75-21.96±2.21-10.82±2.03-9.52±1.64金刚乙胺(RIM)-15.59±1.38-13.90±1.22-15.75±1.52-21.89±1.47-14.53±1.46-12.46±1.53左旋咪唑(LEV)-11.24±1.65-9.01±0.87-6.55±1.52-42.64±4.23-3.63±0.76-0.55±0.27甲氧氯普胺(MET)-11.15±0.87-5.78±0.91-5.42±0.81-13.38±2.05-4.33±1.28-2.83±0.69
2.2 消除或者补偿基质效应
同位素内标与基质匹配标准曲线均能有效地补偿基质效应[22]。基质匹配标准曲线是用空白基质溶液对标准使用液进行稀释,获得系列基质标准工作液,使得样品溶液与标准品在电喷雾接口处具有相同的离子化条件,从而校正基质效应对定量结果的影响[23]。同位素内标是较理想的内标物,它与待分析物的化学性质非常相似,除了可以消除离子化过程对化合物的影响,同时也可以消除样品前处理过程的损耗[24],从而使得同位素内标法成为首选的定量方法。
2.2.1 内标法补偿基质效应
为了考察加入同位素内标后,SDT、SDX、CIP、ENR、NOR、AMA、ERY的基质效应是否减少,本实验绘制了它们在鲫鱼、鸡肉、鸭胗、猪肝、牛肉基质中使用内标前与使用内标后的标准曲线对照图(图1)。在使用内标前,不同的基质标曲与溶剂标曲之所以呈现出不同的夹角,是因为同一化合物受不同基质的影响在仪器上的峰面积响应强度不同。响应强度比溶剂中强的为基质增强,在溶剂标曲上方;响应强度比溶剂中弱的为基质抑制,在溶剂标曲下方;与溶剂标曲的夹角越大,表明基质效应越大,反之亦然。加入内标后,各基质标曲与溶剂标曲的夹角明显减小,说明基质效应得到有效补偿。可能的原因是内标法的标曲绘制是横坐标为待分析物与同位素内标浓度的比值,纵坐标则为两者在仪器上响应强度的比值,待分析物与其同位素内标受基质的影响基本相同,两者相比则抵消了这种影响。

图1 部分兽药在不同基质中的标准曲线和 使用内标后校正的标准曲线
Fig.1 Standard curves of some veterinary drugs in different matrices and calibration curves after using internal standards
注:
图A,C,E,G,I,K,M分别为SDT、SDX、CIP、ENR、NOR、AMA、 ERY在不同基质中的标准曲线;图B,D,F,H,J,L,N分别为SDT、SDX、 CIP、ENR、NOR、AMA、ERY在不同基质中使用内标后校正的标曲; Ci表示溶液中某一兽药浓度,Ci/CIstd表示溶液中某一兽药浓度与该 兽药的同位素内标浓度的比值;Ai/AIstd表示溶液中某一兽药的峰面积 与该兽药的同位素内标峰面积的比值
2.2.2 基质匹配标准曲线补偿基质效应
虽然同位素内标法可以有效消除基质效应,但同位素内标价格昂贵,且不易获取。研究表明[25-26],基质匹配标准曲线也可消除基质效应。本实验将其余17种兽药的加标结果(加标量为10 μg/kg)分别用溶剂标曲与空白猪肉基质匹配标曲定量,结果如图2所示。通过空白猪肉基质匹配标曲校正后,17种兽药的回收率均有不同程度地提高。回收率由(17.03±1.21)%~(66.55±2.54)%提升至(71.10±2.68)%~(89.25±3.14)%。这从定量的角度也说明了待分析物与标准品在电喷雾接口处具有相同的离子化条件时,可以有效地校正基质效应。
2.3 方法验证
2.3.1 线性关系、检出限及定量限
本实验选择了对24种兽药基质效应较强的猪肝作为空白基质,加入适量内、外标工作液,配制系列匹配标准溶液,以24种化合物的色谱峰面积为纵坐标,对应的浓度为横坐标,绘制标准曲线。标准曲线、线性范围、相关系数、检出限(limit of detection,LOD)及定量限(limit of quantitation,LOQ),见表3。
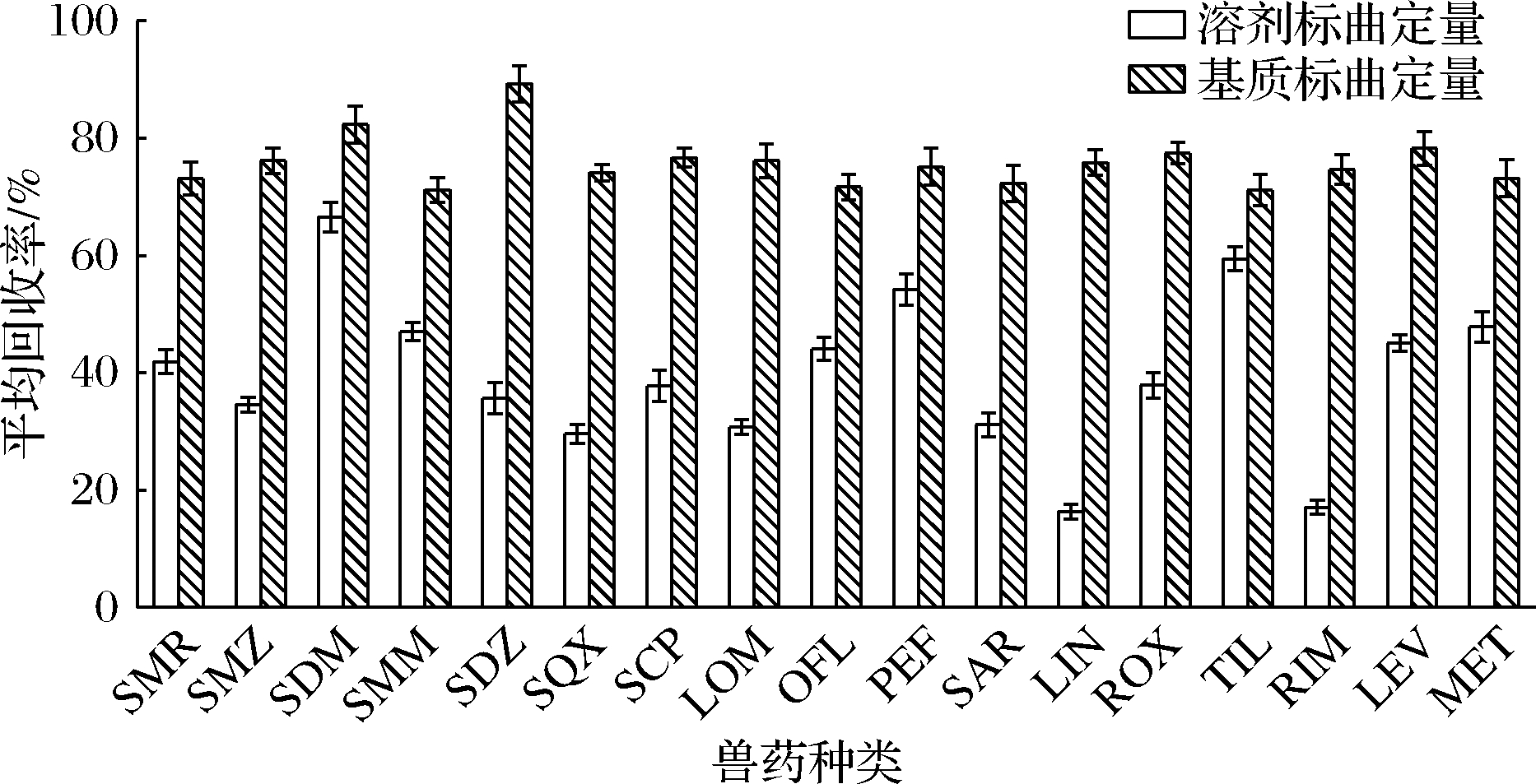
图2 溶剂标准曲线定量结果与空白猪肉基质 匹配标准曲线的比较
Fig.2 Comparison of quantitative results by solvent standard curve and negative pork matrix-matched standard curve
表3 猪肝中24种兽药的线性方程、线性范围、相关系数、检出限及定量限
Table 3 Linear equation, linear range, correlation coefficient, LOD and LOQ for 24 veterinary drugs in pork liver

兽药线性方程线性范围/(ng·mL-1)相关系数RLOD/(μg·kg-1)LOQ/(μg·kg-1)磺胺甲基嘧啶(SMR)Y=8 318X-7 8615.0~5000.997 62.06.0磺胺甲恶唑(SMZ)Y=2 565X-4 1875.0~5000.994 02.06.0磺胺二甲嘧啶(SDM)Y=19 500X-23 3101.0~1000.997 70.752.25磺胺间二甲氧嘧啶(SDT)Y=0.055 04X-0.036 591.0~1000.997 60.51.5磺胺间甲氧嘧啶(SMM)Y=2 209X-2 7655.0~5000.991 12.57.5磺胺嘧啶(SDZ)Y=1 408X+2 04310.0~5000.995 25.015磺胺喹恶啉(SQX)Y=4 250X+1 6525.0~5000.994 63.510.5磺胺邻二甲氧嘧啶(SDX)Y=0.019 34X-0.087 791.0~1000.999 20.752.25磺胺氯哒嗪(SCP)Y=2 586X-4 1275.0~5000.994 52.57.5环丙沙星(CIP)Y=0.018 12X-0.029 475.0~5000.991 12.57.5恩诺沙星(ENR)Y=0.016 38X-0.009 1051.0~1000.999 10.351.05洛美沙星(LOM)Y=10 480X-15 5501.0~1000.994 00.51.5诺氟沙星(NOR)Y=0.028 32X-0.001 29510.0~5000.992 55.015氧氟沙星(OFL)Y=9 879X-12 8001.0~1000.998 50.752.25培氟沙星(PEF)Y=9 023X-10 1202.0~2000.997 01.03.0沙拉沙星(SAR)Y=5 194X-5 8842.0~2000.993 71.54.5红霉素(ERY)Y=0.080 31X+0.384 55.0~5000.990 02.06.0林可霉素(LIN)Y=45 620X-42 7505.0~5000.997 32.57.5罗红霉素(ROX)Y=20 080X-1 3231.0~1000.999 40.51.5替米考星(TIL)Y=4 818X+5 6392.0~2000.998 41.54.5金刚烷胺(AMA)Y=0.016 87X+0.010 585.0~5000.999 72.06.0金刚乙胺(RIM)Y=40 390X-22 8102.0~2000.999 11.253.75左旋咪唑(LEV)Y=20 900X-50 2305.0~5000.995 22.06.0甲氧氯普胺(MET)Y=31 640X-10 7800.5~500.999 50.150.45
2.3.2 方法的准确度和精密度
取猪肝阴性样品,对24种兽药作3水平加标实验,结果见表4。24种兽药在猪肝中的回收率为71.6%~109.8%(n=6),相对标准偏差(relative standard deviation,RSD)≤10.6%,该方法符合兽残检测要求。
表4 猪肝中24种兽药回收率和精密度(n=6)
Table 4 Recoveries and RSDs of 24 veterinary drugs in pork liver(n=6)

兽药加标水平/(μg·kg-1)回收率/%RSD/%兽药加标水平/(μg·kg-1)回收率/%RSD/%693.4 1.2 15 102.7 8.2 磺胺甲基嘧啶(SMR)1295.6 2.1 诺氟沙星(NOR)30101.2 3.5 6088.4 3.3 15094.0 4.1 6 90.7 7.3 395.6 4.9 磺胺甲恶唑(SMZ)1287.1 7.1 氧氟沙星(OFL)696.7 7.1 6091.3 1.7 3093.3 7.2 3 90.5 3.4 3 94.8 3.5 磺胺二甲嘧啶(SDM)693.5 7.2 培氟沙星(PEF)695.4 10.6 3094.4 4.9 3089.8 5.3 2 103.7 1.0 587.4 9.0 磺胺间二甲氧嘧啶(SDT)4107.9 1.4 沙拉沙星(SAR)1080.9 9.0 2091.7 5.3 5077.4 3.1 896.4 1.9 691.3 2.5 磺胺间甲氧嘧啶(SMM)1686.6 8.8 红霉素(ERY)12100.5 9.5 8084.8 2.7 60109.5 4.8 15 88.0 3.3 8 73.6 6.8 磺胺嘧啶(SDZ)3094.7 8.7 林可霉素(LIN)1671.6 5.9 15093.5 7.8 8076.2 4.5 10 90.3 8.5 2 77.3 4.3 磺胺喹恶啉(SQX)2086.0 3.7 罗红霉素(ROX)474.9 8.8 10076.4 6.6 2072.6 4.4 3 102.4 1.1 5 79.0 6.8 磺胺邻二甲氧嘧啶(SDX)6100.2 2.1 替米考星(TIL)1077.4 8.1 3094.4 1.3 5074.3 6.7
续表4

兽药加标水平/(μg·kg-1)回收率/%RSD/%兽药加标水平/(μg·kg-1)回收率/%RSD/%8 88.4 9.0 6109.2 6.7 磺胺氯哒嗪(SCP)1684.6 9.9 金刚烷胺(AMA)12109.8 1.0 8080.2 3.3 60104.4 1.8 8 106.5 1.9 4 75.2 6.1 环丙沙星(CIP)16105.2 1.9 金刚乙胺(RIM)872.5 2.4 8095.5 8.1 4071.7 1.1 1 107.3 7.3 9 93.3 4.8 恩诺沙星(ENR)2104.5 5.8 左旋咪唑(LEV)1889.3 8.5 1099.8 3.5 9090.4 4.1 192.9 4.5 0.5 92.7 8.8 洛美沙星(LOM)294.3 4.1 甲氧氯普胺(MET)191.3 4.4 1091.2 4.5 586.5 2.3
3 结论
本研究以离子抑制率为衡量指标,系统性地评价了采用QuEChERS-UPLC-MS/MS检测技术,6种不同的动物性食品基质对24种兽药的基质效应。结果表明,SMZ、SDT、SDZ、SQX、SDX、NOR、PEF、ERY、ROX、TIL 10种兽药存在着较强的基质效应,剩余14种兽药为弱基质效应。采用同位素内标及基质匹配标准曲线能有效地补偿基质效应,本研究为动物性食品中多兽残快速准确检测提供了一定的参考依据。
[1] SCHNEIDER M J, LEHOTAY S J, LIGHTFIELD A R.A multiclass multiresidue LC-MS/MS method for analysis of veterinary drugs in bovine kidney[J].Progress in Energy & Combustion Science,2012:515-519.
[2] FRENICH A G, AGUILERA-LUIZ M D,VIDAL J L M, et al.Comparison of several extraction techniques for multiclass analysis of veterinary drugs in eggs using ultra-high pressure liquid chromatography-tandem mass spectrometry[J].Analytica Chimica Acta,2010, 661(2):150-160.
[3] 郭海霞,肖桂英,张禧庆,等.QuEChERS-超高效液相色谱-串联质谱法同时检测猪肉中121种兽药[J].色谱, 2015, 33(12):1 242-1 250.
GUO H X, XIAO G Y, ZHANG X Q, et al.Simultaneous determination of 121 veterinary drugs in pork by QuEChERS and ultra performance liquid chromatography-tandem mass spectrometry[J].Chinese Journal of Chromatography, 2015, 33(12):1 242-1 250.
[4] KINSELLA B, WHELAN M, CANTWELL H, et al.A dual validation approach to detect anthelmintic residues in bovine liver over an extended concentration range[J].Talanta, 2011,83(1):14-24.
[5] 冯晓杰, 杜丽英, 冯章英, 等.LC-MS/MS法测定人血药浓度的基质效应研究进展[J].中国新药杂志,2015, 24(13):1 488-1 492.
FENG X J, DU L Y, FENG Z Y, et al.Research progress of matrix effect in determining blood concentration by LC-MS/MS[J]. Chinese Journal of New Drugs, 2015, 24(13):1 488-1 492.
[6] 苏萌, 艾连峰.液相色谱-串联质谱基质效应及其消除方法[J].食品安全质量检测学报,2014, 5(2):511-515.
SU M, AI L F.Matrix effects and elimination methods of liquid chromatography-tandem mass spectrometry[J].Journal of Food Safety and Quality,2014, 5(2):511-515.
[7] JANUSCH F, KALTHOFF L, HAMSCHER G, et al.Evaluation and subsequent minimization of matrix effects caused by phospholipids in LC-MS analysis of biological samples[J].Bioanalysis, 2013, 5(17):2 101-2 114.
[8] LIU D H, WU Y L, WANG B, et al.Analysis of five types of veterinary drug residues in meat products by dispersive solid phase extraction-UPLC-MS/MS[J].Modern Food Science and Technology, 2016, 32(10):290-296.
[9] SUI T, FU J, LI X Y, et al.Simultaneous determination of veterinary drug residues in aquatic products by UPLC-MS/MS[J].Food Science, 2011.DOI:10.3724/SP.J.1011.2011.00211.
[10] HOFF R B, RÜBENSAM G, JANK L, et al.Analytical quality assurance in veterinary drug residue analysis methods:Matrix effects determination and monitoring for sulfonamides analysis[J].Talanta, 2015, 132:443-450.
[11] ZHAO L M, LUCAS D, LONG D, et al.Multi-class multi-residue analysis of veterinary drugs in meat using enhanced matrix removal lipid cleanup and liquid chromatography-tandem mass spectrometry[J].Journal of Chromatography A, 2018,1 549:14-24.
[12] ZHANG N, FAN S, XUE Y, et al.Rapid detection of 8 veterinary drug residues in chicken by QuEChERS-ultra performance liquid chromatography tandem mass spectrometry[J].Journal of Hygiene Research, 2017, 46(1):89-93.
[13] MALLET C R, LU Z, MAZZEO J R.A study of ion suppression effects in electrospray ionization from mobile phase additives and solid-phase extracts[J].Rapid Communications in Mass Spectrometry, 2010, 18(1):49-58.
[14] ANTIGNAC J P, WASCH K D, MONTEAU F, et al.The ion suppression phenomenon in liquid chromatography-mass spectrometry and its consequences in the field of residue analysis[J].Analytica Chimica Acta, 2005, 529(1-2):129-136.
[15] ISMAIEL O A, HALQUIST M S, ELMAMLY M Y, et al.Monitoring phospholipids for assessment of ion enhancement and ion suppression in ESI and APCI LC/MS/MS for chlorpheniramine in human plasma and the importance of multiple source matrix effect evaluations[J].Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences,2008, 875(2):333-343.
[16] 王大鹏, 张娴, 颜昌宙.高效液相色谱串联质谱法测定污水污泥中4种磺胺类药物及其乙酰化代谢物[J].环境化学, 2018, 37(10):2 143-2 151.
WANG D P, ZHANG X, YAN C Z.Determination of four sulfonamides and their corresponding acetyl metabolites in wastewater and sludge by high performance liquid chromatography tandem mass spectrometry[J].Nvironmental Chemistry, 2018, 37(10):2 143-2 151.
[17] 唐吉旺, 袁列江, 肖泳, 等.固相萃取-高效液相色谱-质谱联用法同时测定食品中9种人工合成甜味剂[J].色谱, 2019, 37(6):619-625.
TANG J W, YUAN L J, XIAO Y, et al.Simultaneous determination of nine artificial sweeteners in foodby solid phase extraction coupled with high performanceliquid chromatography tandem mass spectrometry[J].Chinese Journal of Chromatography, 2019, 37(6):619-625.
[18] 邱增枝, 郑增忍, 赵思俊, 等.UPLC-MS/MS法测定猪尿中β2-受体激动剂的基质效应研究[J].动物医学进展, 2013, 34(9):66-70.
QIU Z Z, ZHENG Z R, ZHAO S J,et al.Matrix effects in the residue detection of β2-agonists in pig urine by UPLC-MS/MS[J].Progress in Veterinary Medicine, 2013, 34(9):66-70.
[19] 周悦榕, 李丹妮, 吴剑平, 等.超高效液相色谱-电喷雾电离-串联质谱法测定猪粪便中6种抗生素残留的基质效应研究[J].分析测试学报, 2017, 36(8):1 010-1 017.
ZHOU Y R, LI D N, WU J P.Study on matrix effects in analysis of six antibiotics in swine manure by ultra performance liquid chromatography-electrospray ionization-tandem mass spectrometry[J].Journal of Instrumental Analysis, 2017, 36(8):1 010-1 017.
[20] XU R N, FAN L, RIESER M J, et al.Recent advances in high-throughput quantitative bioanalysis by LC-MS/MS[J].Journal of Pharmaceutical & Biomedical Analysis, 2007, 44(2):342-355.
[21] HERN NDEZ F, SANCHO J V, POZO O J.Critical review of the application of liquid chromatography/mass spectrometry to the determination of pesticide residues in biological samples[J].Analytical and Bioanalytical Chemistry, 2005, 382(4):934-946.
NDEZ F, SANCHO J V, POZO O J.Critical review of the application of liquid chromatography/mass spectrometry to the determination of pesticide residues in biological samples[J].Analytical and Bioanalytical Chemistry, 2005, 382(4):934-946.
[22] 王立琦, 贺利民, 曾振灵, 等.液相色谱-串联质谱检测兽药残留中的基质效应研究进展[J].质谱学报, 2011, 32(6):321-332.
WANG L Q, HE L M, ZENG Z L, et al.Progress in matrix effect of veterinary drug residues analysis by high-performance liquid chromatography tandem mass spectrometry[J]. Journal of Chinese Mass Spectrometry Society, 2011, 32(6):321-332.
[23] TAYLOR P J.Matrix effects:The achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry[J].Clinical Biochemistry, 2005, 38(4):328-334.
[24] SEAN O, ILETT K F.Evaluation of a deuterium-labeled internal standard for the measurement of sirolimus by high-throughput HPLC electrospray ionization tandem mass spectrometry[J].Clinical Chemistry, 2008, 54(8):1 386-1 389.
[25] 张凯, 秦宇, 卞华, 等.QuEChERS-超高效液相色谱-串联质谱法检测羊肉中8种抗真菌药[J].色谱, 2018, 36(10):999-1 004.
ZHANG K, QIN Y, BIAN H, et al.Determination of eight antifungal drugs in mutton by QuEChERS coupled with ultra-performance liquid chromatography-tandem mass spectrometry[J] Chinese Journal of Chromatography, 2018, 36(10):999-1 004.
[26] 李丽春, 刘书贵, 尹怡, 等.QuECHRS结合UPLC-MS/MS法测定水产品中酰胺醇类抗生素残留及基质效应[J].中国渔业质量与标准,2018,8(6):32-39.
LI L C, LIU S G, YIN Y, et al. The amphenicols residues analysis in aquatic products and their matrix effects by QuECHERS method and UPLC-MS/MS[J].Chinese Fishery Quality and Standards, 2018, 8(6):32-39.
[27] 李红丽. QuEChERS-UPLC-MS/MS同时测定动物性食品中24种残留兽药方法及基质效应的研究[D].重庆:西南大学, 2020.
LI H L.Study on simultaneous determination of 24 veterinary drug residues contained in animal derived foods and the matrix effect with QuEChERS-UPLC-MS/MS[D].Chongqing:Southwest University, 2020.