葡萄是全球栽培最广的水果之一。国际葡萄与葡萄酒组织(OIV)最新报告显示,2018年全球葡萄栽培面积约为744万hm2,中国葡萄栽培面积约87.5万hm2,含酿酒葡萄、鲜食葡萄和制干葡萄等。葡萄栽培过程伴随着大量副产物的产生,一个生长周期修剪废弃物量约达1~7.5 t/hm2[1],主要有葡萄叶、卷须、茎等,此类副产物多被丢弃、焚烧或堆肥,这些处理方式既浪费资源,也污染环境。
近年来已有研究者开始关注这类副产物的经济实用性,在法国、西班牙、希腊等部分欧洲国家,葡萄叶被用于制药、化妆品、茶叶和其他食品供应[2],土耳其更是有鲜食或腌渍食用葡萄叶的饮食文化[3],我国亦是有将葡萄叶入药,用以止血、镇痛的中医疗法[4]。故而充分挖掘此类废弃物价值,回收再利用,这对葡萄与葡萄酒产业可持续发展及改善环境十分有意义。本文在阅读近10年国内外相关研究报道的基础上,重点总结了葡萄叶中黄酮醇、黄烷醇、酚酸、芪类物质等多酚化合物组分、常用的提取方法、葡萄叶提取物在体内外的抗氧化活性、抑菌效果等生物活性方面的研究成果,以期为葡萄叶片的开发利用提供参考资料。
1 葡萄叶中多酚类化合物种类
健康葡萄叶中总酚浓度可达27.5~76.0 g GAE/kg DW(其中GAE表示没食子酸当量,DW表示葡萄叶干重),具有抗氧化、抗肿瘤、抑菌及神经保护等多种功效[5]。经超高效液相色谱-电喷雾离子源-三重四级杆质谱联用技术技术[5]、高效液相色谱-紫外二级管阵列-质谱联用技术技术[6]等检测鉴定发现,葡萄叶中酚类化合物主要是黄酮醇类、黄烷醇类、酚酸类及少量的芪类物质。
1.1 黄酮醇及其衍生物
黄酮醇是葡萄叶中含量最为丰富的一类酚类化合物,具有C6-C3-C6结构,叶脉中含量可达0.8~7.7 g/kg DW,叶片(除叶脉)中为3.6~20.6 g/kg DW[7]。遇外界刺激时,参与黄酮类物质合成的相关基因(CHS3、F3`5`H、F3H1、LDOX、LAR1及MybA1等)表达量迅速上调,导致黄酮醇含量增加[8]。
葡萄叶中鉴定出的黄酮醇类多酚主要包括槲皮素、山奈酚、杨梅酮等(表1)。受类黄酮-3-O-葡萄糖基转移酶催化影响,葡萄叶中黄酮醇物质:R3位上羟基易与3-O-葡萄糖苷、3-O-葡萄糖苷酸、3-O-半乳糖苷、3-O-鼠李糖苷及3-O-芸香苷等结合,同时有单体形式、糖苷形式存在。其中以槲皮素-3-O-葡萄糖苷酸含量最高,约占黄酮醇总量的60%~92%,其次是槲皮素-3-O-葡萄糖苷,占黄酮醇总量的2.3%~23.5%[7]。
1.2 黄烷-3-醇及其衍生物
黄烷-3-醇具有与黄酮醇类似的C6-C3-C6母环结构,是黄烷醇类物质的基本单元。葡萄叶片(除叶脉)中黄烷-3-醇含量为0.44~3.39 g/kg DM,叶脉中为0.04~1.55 g/kg DM[7]。根据黄烷-3-醇结构式B环上的取代基不同及C环手性碳原子的存在,可分为儿茶素、表儿茶素和培儿茶素、表培儿茶素(表2)。当C3位羟基被没食子酸酯化后,可形成(表)儿茶素没食子酸酯和(表)培儿茶素没食子酸酯[13]。葡萄叶中以儿茶素含量最丰富,约0.12~4.68 g/kg,而表儿茶素含量相对较低,仅为0.005~0.15 g/kg,可能是表儿茶素多以复杂的聚合体形式存在[14]。SCHOEDL等[15]发现葡萄基叶中的儿茶素浓度显著高于半叶和顶叶,而表儿茶素没食子酸酯和表儿茶素则在顶叶中含量最高,表明葡萄叶位置对黄烷醇物质的含量有较大的影响。
表1 葡萄叶中主要的黄酮醇物质及其结构
Table 1 Structures of favonols in grapevine leaves
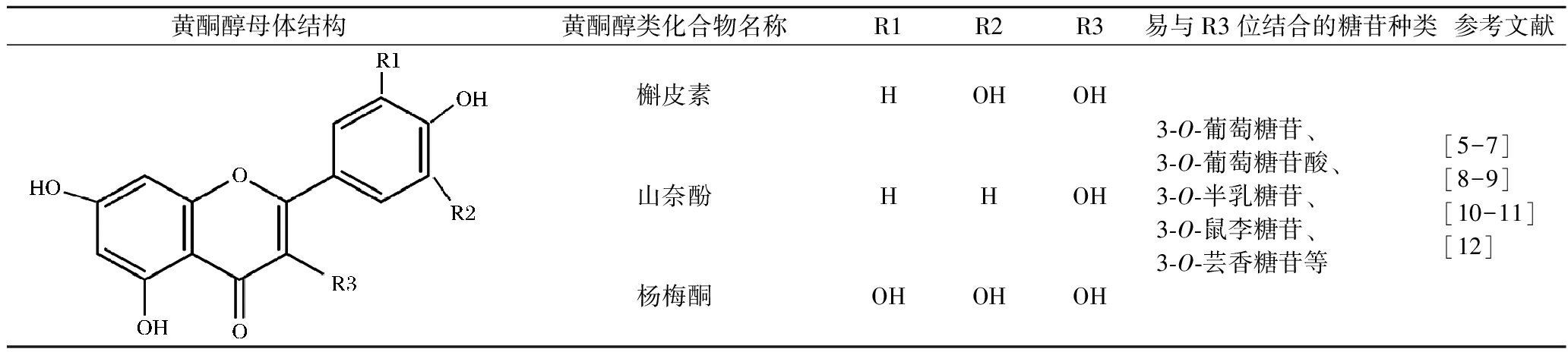
黄酮醇母体结构黄酮醇类化合物名称R1R2R3易与R3位结合的糖苷种类参考文献槲皮素HOHOH山奈酚HHOH杨梅酮OHOHOH3-O-葡萄糖苷、3-O-葡萄糖苷酸、3-O-半乳糖苷、3-O-鼠李糖苷、3-O-芸香糖苷等[5-7][8-9][10-11][12]
表2 葡萄叶中主要的黄烷-3-醇物质及其结构
Table 2 Structures of favan-3-ols in grapevine leaves
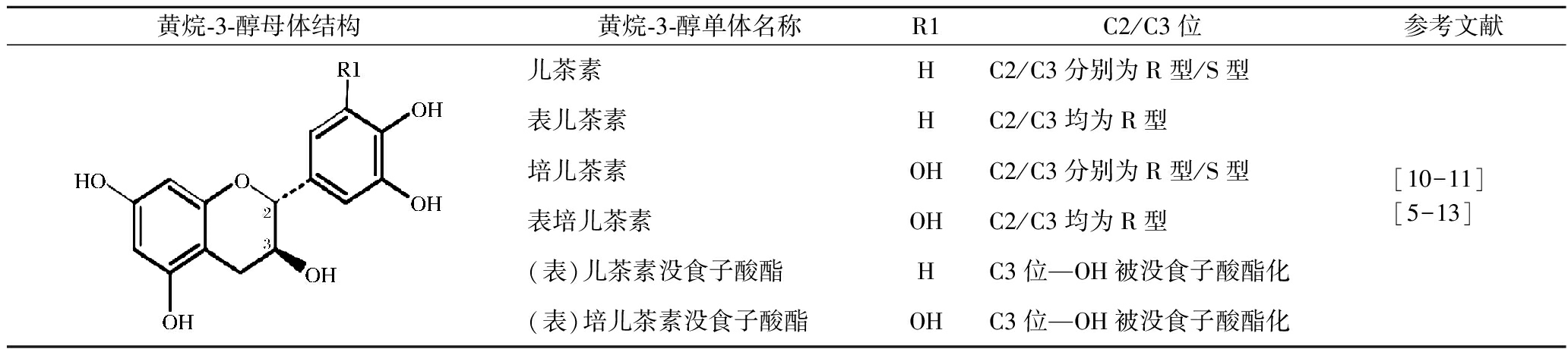
黄烷-3-醇母体结构黄烷-3-醇单体名称R1C2/C3位参考文献 儿茶素HC2/C3分别为R型/S型表儿茶素HC2/C3均为R型培儿茶素OHC2/C3分别为R型/S型表培儿茶素OHC2/C3均为R型(表)儿茶素没食子酸酯HC3位—OH被没食子酸酯化(表)培儿茶素没食子酸酯OHC3位—OH被没食子酸酯化[10-11][5-13]
1.3 酚酸及其衍生物
酚酸是葡萄叶中最丰富的非黄酮类物质,包括有C6-C1结构的羟基苯甲酸和具有C6-C3结构的羟基肉桂酸。葡萄叶中主要的羟基苯甲酸有没食子酸、鞣花酸、原儿茶酸、龙胆酸、丁香酸、香草酸、对羟基苯甲酸等(表3),其中,鞣花酸含量最高,约0.14~0.79 g/kg,龙胆酸(0.000 6~0.008 9 g/kg)含量最低[5]。鉴定出的羟基肉桂酸主要有咖啡酸、咖啡酰酒石酸、对香豆酸、对香豆酰酒石酸、绿原酸、阿魏酸、芥子酸等(表3),以绿原酸浓度最高,为0.002 3~0.66 g/kg(平均0.286 g/kg),其次是咖啡酸(平均浓度约0.043 g/kg)[17]。
KR L等[18]和RYSZARD等[19]发现葡萄叶中咖啡酸有游离态、糖苷结合态、酯化态3种形式,而没食子酸、对香豆酸、阿魏酸等仅存在糖苷形式和酯化形式。低温胁迫可致使糖苷形式的没食子酸、酯化和糖基化的咖啡酸浓度增加,并且可以刺激酯化和糖基化的阿魏酸合成。但长期干旱胁迫会致使葡萄叶中咖啡酸、对香豆酸及阿魏酸浓度降低,同时会造成葡萄叶提取物的抗氧化活性和总还原力下降。
L等[18]和RYSZARD等[19]发现葡萄叶中咖啡酸有游离态、糖苷结合态、酯化态3种形式,而没食子酸、对香豆酸、阿魏酸等仅存在糖苷形式和酯化形式。低温胁迫可致使糖苷形式的没食子酸、酯化和糖基化的咖啡酸浓度增加,并且可以刺激酯化和糖基化的阿魏酸合成。但长期干旱胁迫会致使葡萄叶中咖啡酸、对香豆酸及阿魏酸浓度降低,同时会造成葡萄叶提取物的抗氧化活性和总还原力下降。
1.4 芪类及其衍生物
芪类化合物是一类具有1,2-二苯乙烯骨架的单体及其聚合物的总称,根据C![]() C构型不同,分为顺式和反式2种,其中反式构型的化合物更稳定,分布更广。葡萄叶中的芪类物质主要包括白藜芦醇、云杉新苷、白皮杉醇、葡萄素、蛇葡萄素及紫檀芪和ISOHOPEAPHENOL等[20-22](图1,均以反式构象为例),多分布在叶脉附近[20]。
C构型不同,分为顺式和反式2种,其中反式构型的化合物更稳定,分布更广。葡萄叶中的芪类物质主要包括白藜芦醇、云杉新苷、白皮杉醇、葡萄素、蛇葡萄素及紫檀芪和ISOHOPEAPHENOL等[20-22](图1,均以反式构象为例),多分布在叶脉附近[20]。
芪类化合物是葡萄叶中主要的内源性植物抗毒素,在生物或非生物刺激下迅速合成,并干预植物的应激防御反![]() 等[10]和VRHOVSEK等[24]发现感染霜霉菌的葡萄叶片中芪类化合物种类及含量均明显高于健康叶片,在染病2 d后的F1 21/122(Merzling和Teroldego的杂交品种)葡萄叶中鉴定出了11种芪类及其衍生物,其中反式-白藜芦醇浓度是健康叶片的8倍左右;染病6 d的F1 21/122和Merzling葡萄叶中均检出了12种芪类及其衍生物,F1 21/103中检出11种(反式紫檀芪未检出),3个品系染病叶片中均以Isohopeaphenol含量最高(可达1.31 g/kg FW,FW表示葡萄叶鲜重)。CHOI等[25]发现UV照射能刺激葡萄叶合成大量的反式-白藜芦醇和白皮杉醇,经UV照射后的成熟葡萄叶中反式-白藜芦醇浓度可达18.87 g/kg FW,白皮杉醇浓度达2.31 g/kg FW,约增加了百倍之余。NAIDENOV等[26]用紫外线照射葡萄叶片3 min,3天后在葡萄叶片中鉴定出顺式白藜芦醇、反式白藜芦醇、云杉新苷、ε-葡萄素、δ-葡萄素和紫檀芪6种主要的芪类物质,且这类物质含量极显著高于感染霜霉病的叶片。这可能由于UV处理会增加葡萄叶中与芪类物质合成相关基因(VaSTS1, 3, 4, 5, 6, 10)的表达量[27]。
等[10]和VRHOVSEK等[24]发现感染霜霉菌的葡萄叶片中芪类化合物种类及含量均明显高于健康叶片,在染病2 d后的F1 21/122(Merzling和Teroldego的杂交品种)葡萄叶中鉴定出了11种芪类及其衍生物,其中反式-白藜芦醇浓度是健康叶片的8倍左右;染病6 d的F1 21/122和Merzling葡萄叶中均检出了12种芪类及其衍生物,F1 21/103中检出11种(反式紫檀芪未检出),3个品系染病叶片中均以Isohopeaphenol含量最高(可达1.31 g/kg FW,FW表示葡萄叶鲜重)。CHOI等[25]发现UV照射能刺激葡萄叶合成大量的反式-白藜芦醇和白皮杉醇,经UV照射后的成熟葡萄叶中反式-白藜芦醇浓度可达18.87 g/kg FW,白皮杉醇浓度达2.31 g/kg FW,约增加了百倍之余。NAIDENOV等[26]用紫外线照射葡萄叶片3 min,3天后在葡萄叶片中鉴定出顺式白藜芦醇、反式白藜芦醇、云杉新苷、ε-葡萄素、δ-葡萄素和紫檀芪6种主要的芪类物质,且这类物质含量极显著高于感染霜霉病的叶片。这可能由于UV处理会增加葡萄叶中与芪类物质合成相关基因(VaSTS1, 3, 4, 5, 6, 10)的表达量[27]。
表3 葡萄叶中主要的酚酸物质及其结构
Table 3 Structures of phenolic acids in grapevine leaves
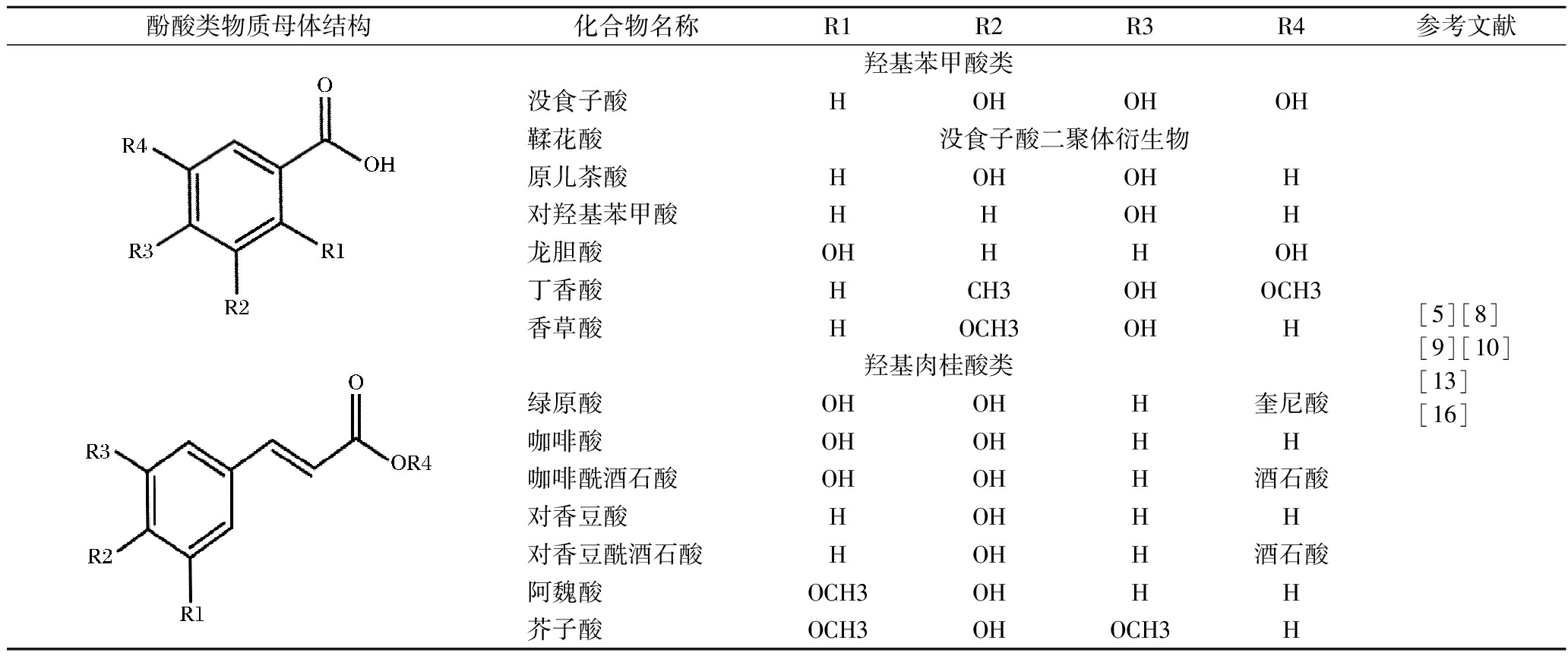
酚酸类物质母体结构化合物名称R1R2R3R4参考文献羟基苯甲酸类没食子酸HOHOHOH鞣花酸没食子酸二聚体衍生物原儿茶酸HOHOHH对羟基苯甲酸HHOHH龙胆酸OHHHOH丁香酸HCH3OHOCH3香草酸HOCH3OHH[5][8][9][10][13][16]羟基肉桂酸类绿原酸OHOHH奎尼酸咖啡酸OHOHHH咖啡酰酒石酸OHOHH酒石酸对香豆酸HOHHH对香豆酰酒石酸HOHH酒石酸阿魏酸OCH3OHHH芥子酸OCH3OHOCH3H
2 葡萄叶中多酚类物质的提取
目前常用于葡萄叶片酚类化合物提取的方法主要有传统溶剂提取法、微波辅助提取法、超声波辅助提取法和超临界CO2萃取法等。
溶剂提取法是根据各化学组分在溶剂中的溶解性差异,选用对活性成分溶解度大、对不需要溶出成分溶解度小的溶剂,从而将有效成分从植物组织内溶解出来。该方法操作简单,成本低廉,使用普遍,但提取得率较低。罗英花等[29]研究发现影响美洲葡萄叶总酚提取率的工艺因素依次为:乙醇浓度>浸提时间>浸提温度>料液比,当乙醇体积分数50%、浸提时间60 min、浸提温度70 ℃、料液比1∶15时,总酚提取率最高(1.72%)。而张纵圆等[30]研究表明对葡萄叶黄酮提取率影响最大的是乙醇体积分数,其次为提取温度。当提取条件为乙醇体积分数45%、提取温度60 ℃、固液比为1∶40、回流提取2 h时,葡萄叶黄酮提取量最高,达5.33 g/kg。王周利等[31]认为不同的提取溶剂亦会对葡萄叶总酚和白藜芦醇提取效果造成显著影响,50%甲醇溶液提取所得总酚浓度最大(34.9 g/kg),β-环糊精溶液提取所得次之(16.7 g/kg),而水提物中多酚浓度最低,仅12.1 g/kg。但β-环糊精的疏水性空腔可以容纳白藜芦醇分子,并通过氢键的形成维持复合物的稳定,所以β-环糊精溶液提取葡萄叶中白藜芦醇效果明显强于水和甲醇溶液,当提取条件为β-环糊精溶液质量浓度为28 g/L,提取温度为50 ℃,处理时间68 min时,白藜芦醇提取量最大,约0.15 g/kg。
为解决传统溶剂提取法提取得率低的缺点,常采用超声波提取、微波提取、酶解提取等辅助提取技术。孙磊磊等[32]发现当提取条件为乙醇体积分数为40%,固液比为1∶26,提取温度56 ℃,提取时间为59 min,超声功率280 W时,白藜芦醇提取量最大,为16.26 g/kg,比有机溶剂高14%。程玉[33]研究表明乙醇体积分数为80%,提取温度80 ℃,固液比1∶50,超声处理10 min,浸提4 h时,葡萄叶中总黄酮提取量为35.03 g/kg DM。库尔班江·巴拉提等[34]研究发现与传统的索氏提取法相比,超声波辅助提取法具有提取效率高,提取时间短,当料液比为1∶30,提取温度为50 ℃,超声时间为45 min,超声功率150 W时,葡萄叶乙酸乙酯提取液的抑菌效果最好,对金黄色葡萄球菌的抑菌圈平均直径可达1.81 cm。孙磊磊[35]采用微波辅助法提取葡萄叶中的白藜芦醇,其最佳工艺条件为:料液比1∶92,微波时间76 s,微波功率640 W,在此条件下提取白藜芦醇为13.52 g/kg。相较于超声波(16.26 g/kg)和酶提取法(16.45 g/kg),微波辅助提取获得的白藜芦醇量最低,但其提取时间最短。
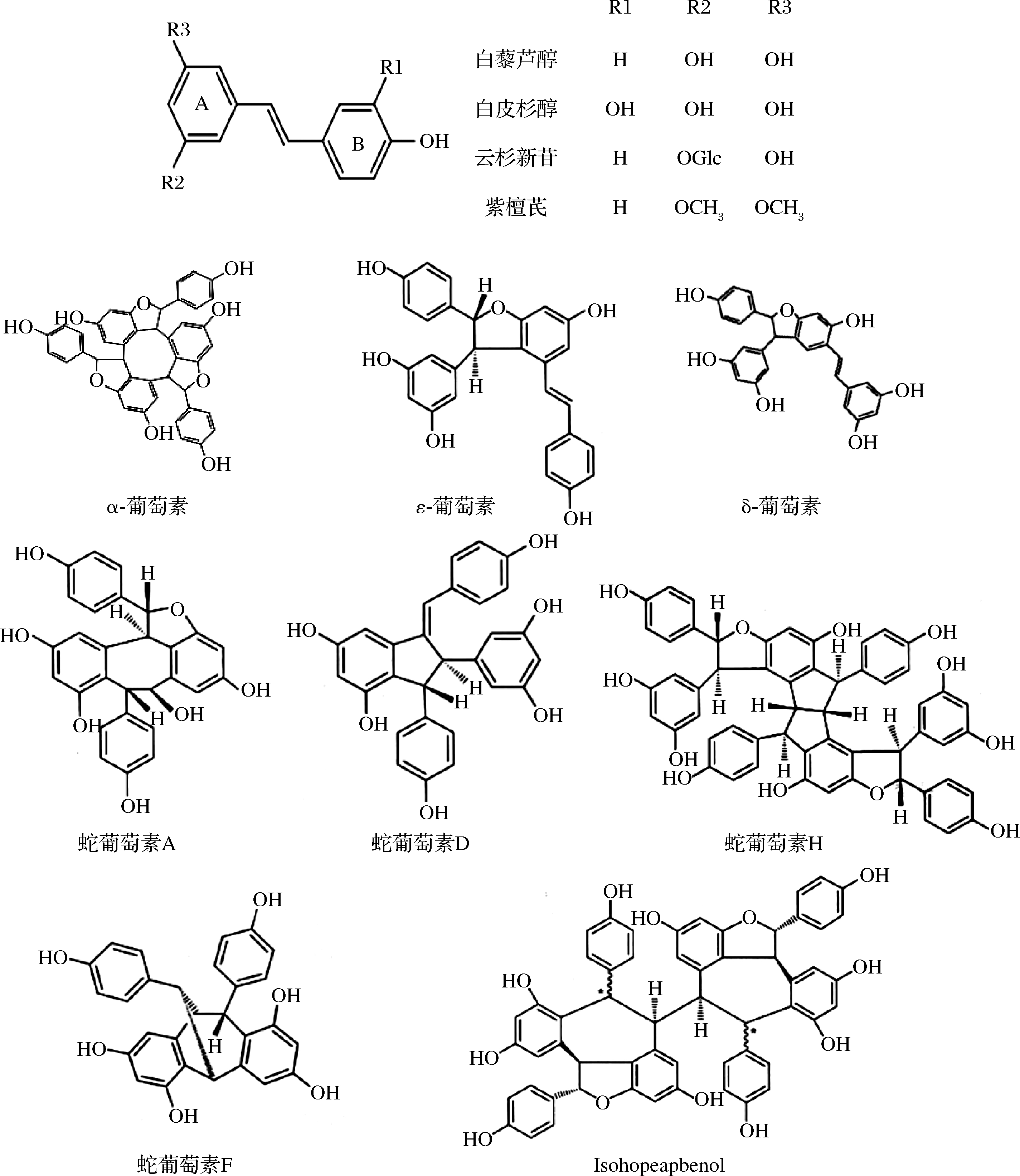
图1 葡萄叶中主要的芪类物质及其结构[28]
Fig.1 Structures of stilbenes in grapevine leaves
超临界CO2萃取是一种新型的萃取分离技术。超临界流体具有高密度、高扩散系数等特殊的物理性质,通过微调CO2流体在超临界状态下的压力和温度,即可改变其对目标物质的溶解性,从而达到分离的目的。超临界CO2萃取技术操作简便快速,提取得率和纯度亦高于传统溶剂提取法[36]。OGANESYANTS等[37]利用超临界CO2萃取技术提取分析了葡萄叶中总酚和白藜芦醇等活性物质,发现超临界CO2萃取液中生育酚浓度(0.046 g/L)极显著高于溶剂提取液(微量),白藜芦醇浓度(0.442 g/L)约为溶剂提取液(0.22 g/L)的2倍。由于超临界CO2萃取方法对于极性大的物质提取率较低,OGANESYANTS等[37]采用高度浓缩泉水萃取方法从超临界CO2萃取后的残留原料中提取出了丰富的多酚物质,总酚浓度可达16.37 g trolox/L,约为超临界CO2萃取液的4倍,传统溶剂提取液的30倍,白藜芦醇浓度(0.444 g/L)与超临界CO2萃取液相近。
3 葡萄叶酚类物质的生物活性
3.1 抗氧化活性
植物多酚结构中的酚羟基可与环境中的分子氧结合,被氧化成醌,同时对超氧阴离子自由基、羟自由基、金属离子等十分敏感,可快速捕捉,故而表现出很强的抗氧化活性[38]。
黑皮诺葡萄叶具有很高的抗氧化能力(24.02 mol TE/kg FW),可与红甘蓝媲美(25.91 mol TE/kg FW),高于草莓(18.46 mol TE/kg FW)和树莓(18.25 mol TE/kg FW)[39]。葡萄叶抗氧化活性与总酚含量和类黄酮含量呈显著正相关(相关性可达90.3%和84.9%),BA
![]() 等[40]研究发现葡萄叶所含总酚(16.37 g GAE/kg)和总黄酮浓度(9.32 g CE/kg)显著高于柑橘、桑葚、大豆、荨麻、樱桃及食用甜菜等植物叶片,表现出很高的抗氧化活性,约为桑葚叶片和大豆叶片抗氧化活性的30倍左右(DPPH法)。
等[40]研究发现葡萄叶所含总酚(16.37 g GAE/kg)和总黄酮浓度(9.32 g CE/kg)显著高于柑橘、桑葚、大豆、荨麻、樱桃及食用甜菜等植物叶片,表现出很高的抗氧化活性,约为桑葚叶片和大豆叶片抗氧化活性的30倍左右(DPPH法)。
DANI等[11]发现大鼠脑脂质和蛋白质氧化程度与摄入的葡萄叶提取物中多酚类化合物水平呈显著负相关。研究表明,葡萄叶提取物能有效清除NO-自由基、超氧阴离子自由基、羟自由基等[41],增加细胞中过氧化氢酶、超氧化物歧化酶和谷胱甘肽过氧化物酶活性,抑制丙二醛合成[42],亦可抑制天门冬氨酸转氨酶和谷氨酰转移酶2种酶浓度增加,减轻细胞中脂肪和蛋白氧化[43]。
3.2 抑菌活性
葡萄叶提取物中含有丰富的白皮杉醇、反式-白藜芦醇、反式-ε-葡萄素、(+)-蛇葡萄素A、(+)-蛇葡萄素F、2-γ-葡萄素和Amuresin G 等多酚化合物[22],可有效抑制革兰氏阳性菌(G+)、革兰氏阴性菌(G-)及真菌类微生物。
YIM等[22]发现葡萄叶提取物中反式-ε-葡萄素抑菌活性最强,其对变异链球菌和血液链球菌 2种口腔病原菌的最低抑制浓度(minimum inhibitory concentration,MIC)分别为0.025 g/L和0.012 5 g/L。MOLDOVAN等[12]的研究也表明葡萄叶提取物对引发口腔疾病的微生物,如牙龈卟啉单胞菌ATCC 33277、粪肠球菌ATCC 29212、变异链球菌ATCC 25175、金黄色葡萄球菌 ATCC 25923和大肠杆菌ATCC 25922等均表现出明显的抑制作用。此外,实验发现葡萄叶提取物对蜡样芽孢杆菌、枯草芽孢杆菌ATCC 6633等G+,空肠弯曲杆菌、婴儿沙门氏菌、铜绿假单胞菌ATCC9027、克雷伯氏肺炎菌ATCC 10031、嗜水气单胞菌ATCC 19570等G-、黑曲霉菌ATCC 9142、白色念珠菌ATCC 10231等真菌均有明显的抑制作![]() 等[14]研究认为葡萄叶提取物对各微生物的抑制效果依次表现为蜡样芽孢杆菌>空肠弯曲杆菌>金黄色葡萄球菌>大肠杆菌>婴儿沙门氏菌。SHARIF-RAD等[44]研究发现葡萄叶甲醇提取物对真菌的抑制作用要强于G+和G-,当葡萄叶提取物浓度达0.5 g/L时,对黑曲霉菌ATCC 9142和白色念珠菌ATCC 10231的抑菌圈分别为95.6 mm和100.7 mm,极显著高于其他菌。CEYHAN等[17]研究发现葡萄叶乙醇提取液对微生物的MIC范围在0.006 3~0.2 g/L,甲醇提取液MIC在0.05~0.4 g/L,而水提液MIC>0.4 g/L,综合考虑认为葡萄叶的乙醇提取物抑菌效果最好,可作为天然抑菌剂用于食品保鲜和人体健康。
等[14]研究认为葡萄叶提取物对各微生物的抑制效果依次表现为蜡样芽孢杆菌>空肠弯曲杆菌>金黄色葡萄球菌>大肠杆菌>婴儿沙门氏菌。SHARIF-RAD等[44]研究发现葡萄叶甲醇提取物对真菌的抑制作用要强于G+和G-,当葡萄叶提取物浓度达0.5 g/L时,对黑曲霉菌ATCC 9142和白色念珠菌ATCC 10231的抑菌圈分别为95.6 mm和100.7 mm,极显著高于其他菌。CEYHAN等[17]研究发现葡萄叶乙醇提取液对微生物的MIC范围在0.006 3~0.2 g/L,甲醇提取液MIC在0.05~0.4 g/L,而水提液MIC>0.4 g/L,综合考虑认为葡萄叶的乙醇提取物抑菌效果最好,可作为天然抑菌剂用于食品保鲜和人体健康。
3.3 抗肿瘤活性
葡萄叶提取物可上调促凋亡基因BAX表达,降低抗凋亡基因Bcl-2表达,从而抑制HepG2和MCF-7细胞增殖,但对人体正常的HUVEC细胞无毒害作用[45]。且![]() 等[41]研究亦表明,葡萄叶提取物可抑制HeLa、MCF7 和 HT-29肿瘤细胞的增殖,对人体正常细胞MRC-5无毒害作用,且霞多丽葡萄叶提取物抗肿瘤细胞效果最好,其次为美乐,这可能与其所含熊果酸浓度较高有关。
等[41]研究亦表明,葡萄叶提取物可抑制HeLa、MCF7 和 HT-29肿瘤细胞的增殖,对人体正常细胞MRC-5无毒害作用,且霞多丽葡萄叶提取物抗肿瘤细胞效果最好,其次为美乐,这可能与其所含熊果酸浓度较高有关。
3.4 抗炎活性
红葡萄叶含有丰富的花青素和黄酮类多酚。SANGIOVANNI 等[46]在2种体外胃和肠道炎症模型中研究了红葡萄叶水提物的抗炎活性,发现红葡萄叶提取物可通过干扰肿瘤坏死因子诱导的核因子转录和核转录,从而抑制白细胞介素-8分泌和表达。当葡萄叶提取物经胃肠消化后,花青素含量急剧下降(-71%),导致对白细胞介素-8分泌的抑制作用减弱;而槲皮素糖苷含量相对稳定,故而可认为槲皮素糖苷物质在肠胃水平上的抗炎作用比花青素显著。葡萄叶甲醇提取物可缓解卡拉胶和组胺诱导的大鼠足跖水肿,当提取液质量浓度为0.4 g/kg时,对卡拉胶和组胺诱导的大鼠足跖水肿抑制率分别为52.63%和54.67%,其抗炎效果与双氯芬酸钠接近[47]。
此外,葡萄叶提取物还具有神经保护、镇痛、舒缓支气管扩张、抗糖尿病等功效[48]。
4 展望
葡萄叶富含白藜芦醇等芪类化合物及其衍生物、酚酸及其衍生物、黄酮类化合物及其衍生物,有很大的开发利用潜质。是故继续研究葡萄叶中活性成分的分离纯化技术,以利于工业生产可作为未来研究方向之一。葡萄叶多酚提取物的抗氧化、抑菌、抗肿瘤等功效已被部分学者证明,但多数为体外研究,对于其体内作用效果及机理研究甚少。这一方向也可作为未来的研究热点。葡萄叶在食品、化妆品和医药等领域的转化应用是本领域研究的最终目的。
[1] S NCHEZ A, YSUNZA F, BELTR
NCHEZ A, YSUNZA F, BELTR N-GARC
N-GARC A M J, et al.Biodegradation of viticulture wastes by pleurotus:A source of microbial and human food and its potential use in animal feeding[J].Journal of Agricultural and Food Chemistry, 2002, 50(9):2 537-2 542.
A M J, et al.Biodegradation of viticulture wastes by pleurotus:A source of microbial and human food and its potential use in animal feeding[J].Journal of Agricultural and Food Chemistry, 2002, 50(9):2 537-2 542.
[2] DRESCH R R, DRESCH M K, GUERREIRO A F, et al.Phenolic compounds from the leaves of Vitis labrusca and Vitis vinifera L.as a source of waste byproducts:Development and validation of LC method and antichemotactic activity[J].Food Analytical Methods, 2014, 7(3):527-539.
[3] AL JUHAIMI F, USLU N, ÖZCAN M M, et al.Effect of fermentation on antioxidant activity and phenolic compounds of the leaves of five grape varieties[J].Journal of Food Processing and Preservation, 2019, 43(7):e13979.
[4] 中医研究院中药研究所. 全国中草药汇编.下册[M].北京:人民卫生出版社, 1978.
Institute of Chinese Materia Medica and Academy of Chinese Medicine.National Compila- tion of Chinese Herbal Medicine.Vol.2 [M].Beijing: People′s Medical Publishing House, 1978.
[5] ![]() , ZAGORAC D
, ZAGORAC D ![]() et al., et al.Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia[J].Journal of Food Composition and Analysis, 2017, 62:76-83.
et al., et al.Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia[J].Journal of Food Composition and Analysis, 2017, 62:76-83.
[6] KEDRINA-OKUTAN O, NOVELLO V, HOFFMANN T, et al.Constitutive polyphenols in blades and veins of grapevine (Vitis vinifera L.) Healthy Leaves[J].Journal of Agricultural and Food Chemistry, 2018, 66(42):10 977-10 990.
[7] KEDRINA-OKUTAN O, NOVELLO V, HOFFMANN T, et al., et al.Polyphenolic diversity in Vitis sp.leaves[J].Scientia Horticulturae, 2019, 256:108569.
[8] GUTHA L R, CASASSA L F, HARBERTSON J F, et al.Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves[J].BMC Plant Biology, 2010, 10(1):187.
![]() U, et al.Phenolic profiles of leaves, grapes and wine of grapevine variety vranac(Vitis vinifera L.) from Montenegro [J].Foods, 2020, 9(2):138.
U, et al.Phenolic profiles of leaves, grapes and wine of grapevine variety vranac(Vitis vinifera L.) from Montenegro [J].Foods, 2020, 9(2):138.
[10] ![]() M, RADOVANOVI B,
M, RADOVANOVI B, ![]() A M, et al.Phenolic compounds and bioactivity of healthy and infected grapevine leaf extracts from red varieties merlot and vranac (Vitis vinifera L.)[J].Plant Foods for Human Nutrition, 2015, 70(3):317-323.
A M, et al.Phenolic compounds and bioactivity of healthy and infected grapevine leaf extracts from red varieties merlot and vranac (Vitis vinifera L.)[J].Plant Foods for Human Nutrition, 2015, 70(3):317-323.
[11] DANI C, OLIBONI L S, AGOSTINI F, et al.Phenolic content of grapevine leaves (Vitis labrusca var.bordo) and its neuroprotective effect against peroxide damage[J].Toxicology in Vitro, 2010, 24(1):148-153.
[12] MOLDOVAN M L, CARPA R, FIZE AN I, et al.Phytochemical profile and biological activities of tendrils and leaves extracts from a variety of Vitis vinifera L[J].Antioxidants, 2020, 9(5):373.
AN I, et al.Phytochemical profile and biological activities of tendrils and leaves extracts from a variety of Vitis vinifera L[J].Antioxidants, 2020, 9(5):373.
[13] 张欣珂, 赵旭, 成池芳,等.葡萄酒中的酚类物质Ⅰ:种类,结构及其检测方法研究进展[J].食品科学, 2019, 40(15):255-268.
ZHANG X K, ZHAO X, CHENG C F, et al.Phenolics in wines I:A review of categories, structures and detection methods[J].Food Science, 2019, 40(15):255-268
[14] ![]() et al.Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L.varieties[J].International Journal of Food Properties, 2013,16(1):45-60.
et al.Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L.varieties[J].International Journal of Food Properties, 2013,16(1):45-60.
[15] SCHOEDL K, SCHUHMACHER R, FORNECK A.Studying the polyphenols of grapevine leaves according to age and insertion level under controlled conditions[J].Scientia Horticulturae, 2012, 141:37-41.
[16] JRIDI M, ABDELHEDI O, KECHAOU H, et al.Vine (Vitis vinifera L.) leaves as a functional ingredient in pistachio calisson formulations[J].Food Bioscience, 2019, 31:100436.
[17] CEYHAN N, KESKIN D, ZORLU Z, et al.In vitro antimicrobial activities of different extracts of grapevine leaves (Vitis vinifera L.) from West Anatolia against some pathogenic microorganisms[J].Journal of Pure and Applied Microbiology, 2012, 6(3):1 303-1 308.
[18] KR L, A, AMAROWICZ R, WEIDNER S.Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress[J].Acta Physiologiae Plantarum, 2014, 36(6):1 491-1 499.
L, A, AMAROWICZ R, WEIDNER S.Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress[J].Acta Physiologiae Plantarum, 2014, 36(6):1 491-1 499.
[19] RYSZARD, AMAROWICZ, STANISLAW, et al.Influence of low-temperature stress on the changes in the composition of grapevine leaf phenolic compounds and their antioxidant properties [J].Functional Plant Science and Biotechnology, 2010, 4(Special Issue 1):90-96.
[20] BECKER L, CARRÉ V, POUTARAUD A, et al.MALDI mass spectrometry imaging for the simultaneous location of resveratrol, pterostilbene and viniferins on grapevine leaves[J].Molecules, 2014, 19(7):10 587-10 600.
[21] CHITARRINI G, ZULINI L, MASUERO D, et al.Lipid, phenol and carotenoid changes in ‘Bianca’ grapevine leaves after mechanical wounding:A case study[J].Protoplasma, 2017, 254(6):2 095-2 106.
[22] YIM N H, HA D T, TRUNG T N, et al.The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens[J].Bioorganic & Medicinal Chemistry Letters, 2010, 20(3):1 165-1 168.
[23] ADRIAN M, JEANDET P, BESSIS R, et al.Induction of phytoalexin (resveratrol) synthesis in grapevine leaves treated with aluminum chloride (AlCl3)[J].Journal of Agricultural and Food Chemistry, 1996, 44(8):1 979-1 981.
[24] VRHOVSEK U, MALACARNE G, MASUERO D, et al.Profiling and accurate quantificat-ion of trans-resveratrol, trans-piceid, trans-pterostilbene and 11 viniferins induced by Plasmopara viticola in partially resistant grapevine leaves[J].Australian Journal of Grape and Wine Research, 2012, 18(1):11-19.
[25] CHOI S J.The identification of stilbene compounds and the change of their contents in UV-irradiated grapevine leaves[J].Korean Journal of Horticultural Science and Technology, 2011, 29(4):374-381.
[26] NAIDENOV A P, LATOUCHE G, CEROVIC Z, et al.Quantification of stilbene in grapevine leaves by direct fluorometry and high performance liquid chromatography:Spatial localisation and time course of synthesis[J].Journal International des Sciences de la Vigne et du Vin, 2010(special issue Macrowine): 27-32.
[27] KISELEV K V, ALEYNOVA O A, GRIGORCHUK V P, et al.Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr[J].Planta, 2017,245(1):151-159.
[28] 张东明. 酚酸化学(天然产物化学丛书)[M].北京:化学工业出版社, 2009.
ZHANG D M.Phenolic Acidification (Natural Product Chemistry Series)[M].Beijing:Chemical Industry Press, 2009.
[29] 罗英花, 王晓微, 臧延青,等.美洲品系葡萄叶多酚的提取工艺优化及抗氧化活性研究[J].黑龙江八一农垦大学学报, 2016, 28(4):55-60.
LUO Y H,WANG X W, ZANG Y Q, et al.Study on the extraction process optimization of polyphenols from american strain of grape leaves and its antioxidant activity[J].Journal of Heilongjiang Bayi Agricultural University, 2016, 28(4):55-60.
[30] 张纵圆, 彭秧, 索金玲, 等.葡萄叶中总黄酮的提取工艺优化及体外抗氧化作用研究[J].中成药, 2009, 31(2):292-294.
ZHANG Z Y, PENG Y, SUO J L, et al.Optimization of extraction process of total flavonoids from grape leaves and study on antioxidant activity in vitro[J].Chinese Traditional Patent Medicine,2009, 31(2):292-294.
[31] 王周利, 袁亚宏, 刘宇璇, 等.响应面法优化β-环糊精提取葡萄叶白藜芦醇工艺[J].食品科学, 2016, 37(22):13-19.
WANG Z L, YUAN Y H, LIU Y X, et al.Response surface optimization of extraction of resveratrol from grapevine leaves using β-cyclodextrin[J].Food Science, 2016, 37(22):13-19.
[32] 孙磊磊, 康健.响应面试验优化超声波法提取葡萄叶白藜芦醇工艺[J].食品工业, 2015,36(5):16-20.
SUN L L, KANG J.Optimization of extraction process of resveratrol from grape leaves by ultrasonic method of response surface experiments[J].The Food Industry,2015,36 (5):16-20.
[33] 程玉. 葡萄叶总黄酮的萃取及其在葡萄酒酿造中应用的初步研究[D].昆明:云南大学, 2015.
CHENG Y.A Preliminary study on extraction of total flayonoids from grape leaves and its application in wine brewing[D].Kunming:Yunnan University,2015.
[34] 库尔班江·巴拉提, 赛力曼·玉山江.葡萄叶乙酸乙酯提取物的提取及其体外抑菌活性研究[J].伊犁师范学院学报, 2016, 10(3):46-56.
KORBANJHON BRAD, SALIMA YUSANJAN.Extraction of ethyl acetate extract from grape leaves and its antimicrobial activity[J].Journal of Yili Normal University(Natural Science Edition), 2016, 10(3):46-56.
[35] 孙磊磊. 新疆赤霞珠葡萄叶白藜芦醇的提取纯化及其应用研究[D].乌鲁木齐:新疆大学, 2015.
SUN L L.Study on Extraction and purification and application of resveratrol from grape leaf of cabernet sauvignon in Xingjiang[D].Urumqi:Xinjiang University,2015.
[36] 邓巧玉, 江姗, 陈誉丹, 等.超临界二氧化碳萃取技术在中药领域的应用进展[J].中国药业, 2020, 29(17):1-5.
DENG Q Y, JIANG S, CHEN Y D, et al.Application of supercritical fluid extraction with CO2 in traditional chinese medicine[J].China Pharmaceuticals,2020, 29(17):1-5.
[37] OGANESYANTS L, PANASYUK A, KUZMINA H, et al.Study of features of the biochemical composition of red vine leaves of autochthonous varieties in Russia[J].BIO Web of Conferences, 2015, 5:02018.
[38] 韩文凤, 郭红英, 贾娟,等.果蔬多酚及其抗氧化性研究进展[J].保鲜与加工, 2019, 19(4):191-194.
HAN W F, GUO H Y, JIA J, et al.Research progress on polyphenols and antioxygenic property of fruits and vegetables[J].Storage and Process,2019,19 (4):191-194.
[39] MAIA M, ANT NIO E.N.FERREIRA, LAUREANO G, et al.Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds[J].Food and Function, 2019, 10(7):3 822-3 827.
NIO E.N.FERREIRA, LAUREANO G, et al.Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds[J].Food and Function, 2019, 10(7):3 822-3 827.
[40] BA
![]() .Phenolic compounds content, antioxidant and antidiabetic potentials of seven edible leaves[J].The Journal of Food, 2018, 43(5):876-885.
.Phenolic compounds content, antioxidant and antidiabetic potentials of seven edible leaves[J].The Journal of Food, 2018, 43(5):876-885.
[41] ![]() D,
D, ![]() S, et al.Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties[J].Food Chemistry, 2019, 286:686-695.
S, et al.Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties[J].Food Chemistry, 2019, 286:686-695.
[42] SAADAOUI N, WESLATI A, BARKAOUI T, et al.Gastroprotective effect of leaf extract of two varieties grapevine (Vitis vinifera L.) native wild and cultivar grown in North of Tunisia against the oxidative stress induced by ethanol in rats[J].Biomarkers, 2020, 25(1):48-61.
[43] SCHAFFER T K, WOHLENBERG M F, MEDEIROS N, et al.Evaluation of antioxidant activity of grapevine leaves extracts (Vitis labrusca) in liver of Wistar rats[J].Anais Da Academia Brasileira De Ciências, 2016, 88(1):187-196.
[44] SHARIFI-RAD J , MIRI A , SHARIFI-RAD M, et al.Antifungal and antibacterial properties of grapevine(Vitis vinifera L.) leaves methanolic extract from iran in vitro study[J].American-Eurasian Journal of Agricultural and Environmental Sciences, 2014, 14 (11):1 312-1 316.
[45] FERHI S, SANTANIELLO S, ZERIZER S, et al.Total phenols from grape leaves counteract cell pro -liferation and modulate apoptosis-related gene expression in MCF-7 and HepG2 human cancer cell lines[J].Molecules, 2019, 24(3):612-626.
[46] SANGIOVANNI E, LORENZO C D, COLOMBO E, et al.The effect of in vitro gastrointestinal diges -tion on the anti inflammatory activity of Vitis vinifera L.leaves[J].Food and Function, 2015, 6(8):2 453-2 463.
[47] SINGH J, SINGH A K, SINGH A.Analgesic and anti-inflammatory activity of methanolic extract of Vitis vinifera leaves[J].Pharmacologyonline, 2009, 3:496-504.
[48] AHN S Y, KIM S A, YUN H K.Inhibition of Botrytis cinerea and accumulation of stilbene compounds by light-emitting diodes of grapevine leaves and differential expression of defense-related genes[J].European Journal of Plant Pathology, 2015, 143(4):753-765.