食源性生物活性肽被认为是新一代的生物活性调节剂[1],主要来源于动物蛋白和植物蛋白。食源性生物活性肽不仅具有易消化吸收、安全性高的特点,还具有抗氧化、抗疲劳、抗炎、抗菌、调节免疫、降血压、降血脂和抗肿瘤等广泛的生理功能,是极具开发利用潜力的功能因子[2-3]。活性肽的制备方法主要有化学合成法、微生物发酵法和蛋白酶解法等,其中最常使用的是蛋白酶解法,已应用于数百种食源性生物活性肽的制备[4-5]。
银杏(Ginkgo biloba)又名白果,是卫计委认定的药食同源物质,具有敛肺气、定喘嗽等功效。银杏营养成分丰富,干制品中蛋白质、碳水化合物含量分别为13.2%和72.6%[6];清蛋白和球蛋白为银杏蛋白的主要组分,银杏蛋白氨基酸组成合理,为优质植物蛋白[7],具有抗氧化[8]、抗衰老[9]、抗菌[10]、免疫调节[11]和抗肿瘤[12]等作用。目前已有基于控制酶解技术制备银杏肽(Ginkgo biloba peptides, GBP)的研究报道,贾韶千等[13]以总还原力为指标,优化了碱性蛋白酶和胃蛋白酶双酶法制备银杏抗氧化肽的酶解工艺;张焕新等[14]采用Alcalase蛋白酶和超滤法制备得到了具有较好清除![]() 和·OH作用的低相对分子质量的GBP;张灿等[15]利用木瓜蛋白酶水解银杏蛋白,制备得到了具有较好α-葡萄糖苷酶抑制活性的GBP;MA等[16]采用碱性蛋白酶水解银杏蛋白,利用超滤和葡聚糖凝胶层析纯化酶解产物,得到了3种具有降压活性的GBP。上述研究结果表明银杏蛋白源生物活性肽具有良好的开发利用前景。
和·OH作用的低相对分子质量的GBP;张灿等[15]利用木瓜蛋白酶水解银杏蛋白,制备得到了具有较好α-葡萄糖苷酶抑制活性的GBP;MA等[16]采用碱性蛋白酶水解银杏蛋白,利用超滤和葡聚糖凝胶层析纯化酶解产物,得到了3种具有降压活性的GBP。上述研究结果表明银杏蛋白源生物活性肽具有良好的开发利用前景。
酒精性肝损伤是临床上最常见的肝病之一,从单纯性肝脂肪变性可进展为肝纤维化和肝硬化,酒精性肝硬化的死亡率占肝硬化死亡率的46.7%[17]。我国酒精性肝损伤的发病率逐年升高,由2000年的2.27%增加到2015年的8.74%,据估计,发病率将不断升高[18]。酒精性肝损伤的发病机制较为复杂,目前尚未完全阐明,但氧化应激和炎性损伤被普遍认为在该病的发生和进展中具有关键作用[19-20]。摄入生物活性肽,拮抗酒精代谢诱导的氧化应激和炎性损伤,被认为是一种有效的辅助治疗酒精性肝损伤的策略。动物实验表明,玉米肽[21-22]、海洋胶原蛋白肽[23]和核桃低聚肽[24]等生物活性肽对酒精性肝损伤都有较好的保护作用。目前有关GBP对酒精性肝损伤保护作用的研究未见报道。
本研究建立急性酒精性肝损伤小鼠模型,基于氨基转移酶、血脂、促炎细胞因子、抗氧化酶和氧化损伤产物水平等生理生化指标,评价GBP对急性酒精性肝损伤小鼠的保护作用,旨在为GBP的开发利用提供理论依据。
1 材料与方法
1.1 材料与仪器
银杏采自江苏省邳州市;雄性昆明小鼠(20±2)g购自济南朋悦实验动物繁育有限公司,生产许可证号SCXY (鲁) 20190003。
碱性蛋白酶(2.25×105 U/g),丹麦诺维信公司;丙氨酸氨基转移酶(alanine aminotransferase, ALT)、天冬氨酸氨基转移酶(aspartate aminotransferase, AST)、甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)、IL-1β、IL-6和TNF-α,肝脏过氧化氢酶(catalase, CAT)、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)、总超氧化物歧化酶(total superoxide dismutase, T-SOD)、丙二醛(malondialdehyde, MDA)和蛋白质羰基(protein carbonyl group, PCG)试剂盒,南京建成生物工程研究所;还原型谷胱甘肽片(国药准字H20050667),重庆药友制药有限责任公司;其他试剂均为国产分析纯。
FA2104 N电子分析天平、723C可见分光光度计,上海精密科学仪器有限公司;BILON92-IID超声波细胞破碎仪,上海比朗仪器有限公司;LGJ-18A冷冻干燥机,北京四环科学仪器厂;TGL-16G型台式离心机,上海安亭科学仪器厂;SHZ-D(Ш)循环水式真空泵,郑州长城科工贸有限公司。
1.2 实验方法
1.2.1 银杏蛋白的制备
银杏去壳后,60 ℃下烘干,粉碎过60目筛,石油醚脱脂,得到脱脂银杏粉。采用超声辅助碱提酸沉法制备银杏蛋白。按液料比20∶1(mL∶g)将脱脂银杏粉与去离子水混合,调节pH至10.0,超声功率200 W,提取温度50 ℃,提取时间40 min。提取结束后,以3 500 r/min离心20 min,收集上清液,调节pH至银杏蛋白的等电点4.4,4 500 r/min离心20 min,将所得沉淀透析(截留相对分子质量3.5 kDa)72 h,浓缩,冷冻干燥,得到银杏蛋白,考马斯亮蓝法测得纯度为84.63%。
1.2.2 银杏肽的制备
按液料比15∶1(mL∶g)将银杏蛋白与去离子水配成悬液,沸水浴10 min灭内源性酶;调节pH至8.5,加入4 000 U/g碱性蛋白酶,50 ℃振荡酶解,期间不断补加NaOH溶液,使pH恒定,待水解度至25%时终止反应;酶解后调节pH至7.0,沸水浴5 min灭酶,8 000 r/min离心20 min,将上清液冷冻干燥后得到银杏肽。水解度的测定采用甲醛滴定法[25]。
1.2.3 动物分组与处理
该研究遵循的程序符合本院实验动物伦理委员会所制定的伦理学标准,得到该委员会批准。60只雄性昆明小鼠经适应性饲养后,随机分为6组:正常组、模型组、3组GBP组和阳性对照组。低、中和高剂量GBP组分别灌胃GBP 50、100和200 mg/kg,连续21 d。阳性对照组每天灌胃100 mg/kg还原型谷胱甘肽;正常组和模型组每天灌胃等量去离子水。第22天,除正常组外,其余组小鼠每隔12 h灌胃体积分数50%酒精(12 mL/kg),连续3次[26-28]。末次灌胃12 h后,颈椎脱臼法处死小鼠,无菌摘取肝脏和脾脏,称量小鼠体质量和脏器质量,根据脏器质量与体质量比,计算脏器系数。
1.2.4 血清生理生化指标测定
小鼠眼眶后静脉丛采血,37 ℃水浴1 h,3 500 r/min离心10 min,收集上清液即为血清,按照试剂盒说明书测定ALT、AST、TG、TC、IL-1β、IL-6和TNF-α水平。
1.2.5 肝脏抗氧化酶与氧化损伤产物水平测定
取肝脏,置于玻璃组织匀浆器,加入一定体积的生理盐水,制备质量浓度100 g/L组织匀浆,3 500 r/min离心10 min,收集上清液,按照试剂盒说明书测定CAT、GSH-Px、T-SOD、MDA和PCG水平。
1.2.6 数据处理与统计分析
采用SPSS 24.0软件进行数据统计与分析,结果以平均值±标准差表示,组间多重比较采用Duncan法。
2 结果与分析
2.1 对小鼠脏器系数的影响
肝脏系数和脾脏系数可反映肝脾肿大的程度,而肝脾肿大是酒精性肝损伤临床上常见的症状[29]。由图1可知,模型组小鼠血清肝脏系数和脾脏系数显著高于正常组(P<0.05),提示酒精暴露造成了小鼠肝脾肿大。与模型组相比,GBP组小鼠肝脏系数和脾脏系数显著下降(P<0.05),中剂量GBP组脾脏系数以及高剂量GBP组肝脏系数和脾脏系数恢复至正常组水平(P>0.05),表明GBP缓解了酒精暴露导致的小鼠肝脾肿大。

图1 银杏肽对酒精性肝损伤小鼠脏器系数的影响
Fig.1 Effects of G.biloba peptides on organ index in acute alcoholic liver injury mice *P<0.05,表示与模型组比较;#P<0.05,表示与正常组比较; (n=10)(下同)
2.2 对小鼠血清氨基转移酶水平的影响
酒精既有脂溶性又有水溶性,极易造成细胞膜损伤,可改变细胞膜的结构,使细胞膜介电常数、流动性、通透性等物理性质发生变化,造成细胞膜脂质过氧化[30-31]。ALT和AST分别存在于生物体肝细胞的胞浆和线粒体中,生理状态下血清中ALT和AST水平极低;当肝细胞膜受损后,ALT和AST渗漏至血清,导致血清ALT和AST水平急剧升高,ALT和AST水平是监测肝功能是否受损的敏感标志物。
图2为GBP对急性酒精性肝损伤小鼠血清ALT和AST水平的影响。模型组小鼠血清ALT和AST水平分别是正常组的3.8和4.7倍,2种氨基转移酶水平显著升高(P<0.05),表明酒精暴露造成了小鼠肝细胞受损,致使血清ALT和AST水平升高。

图2 银杏肽对酒精性肝损伤小鼠血清ALT和 AST水平的影响
Fig.2 Effects of G.biloba peptides on serum ALT and AST levels in acute alcoholic liver injury mice
GBP组小鼠血清ALT和AST活性显著低于模型组(P<0.05),且呈剂量依赖性,高剂量GBP组小鼠血清AST恢复至正常水平(P>0.05),提示GBP可缓解酒精暴露导致的小鼠肝细胞损伤。阳性对照还原型谷胱甘肽也能显著降低小鼠血清中这2种氨基转移酶的水平(P<0.05)。
2.3 对小鼠血清血脂水平的影响
脂质代谢紊乱是酒精性肝损伤的常见症状,酒精代谢过程中可诱导脂肪变性,导致脂肪沉积在肝细胞中,同时酒精可影响血脂转运,造成血清TG和TC水平异常[32]。图3为GBP对急性酒精性肝损伤小鼠血清TG和TC水平的影响。模型组小鼠血清TG和TC水平分别是正常组的2.2和2.5倍,显著高于正常组(P<0.05),提示酒精暴露可导致脂质代谢紊乱,这与前人的研究结果一致[33-34]。给药GBP后,小鼠血清TG和TC水平显著低于模型组,表明GBP干预缓解了急性酒精暴露诱导的脂质代谢紊乱。
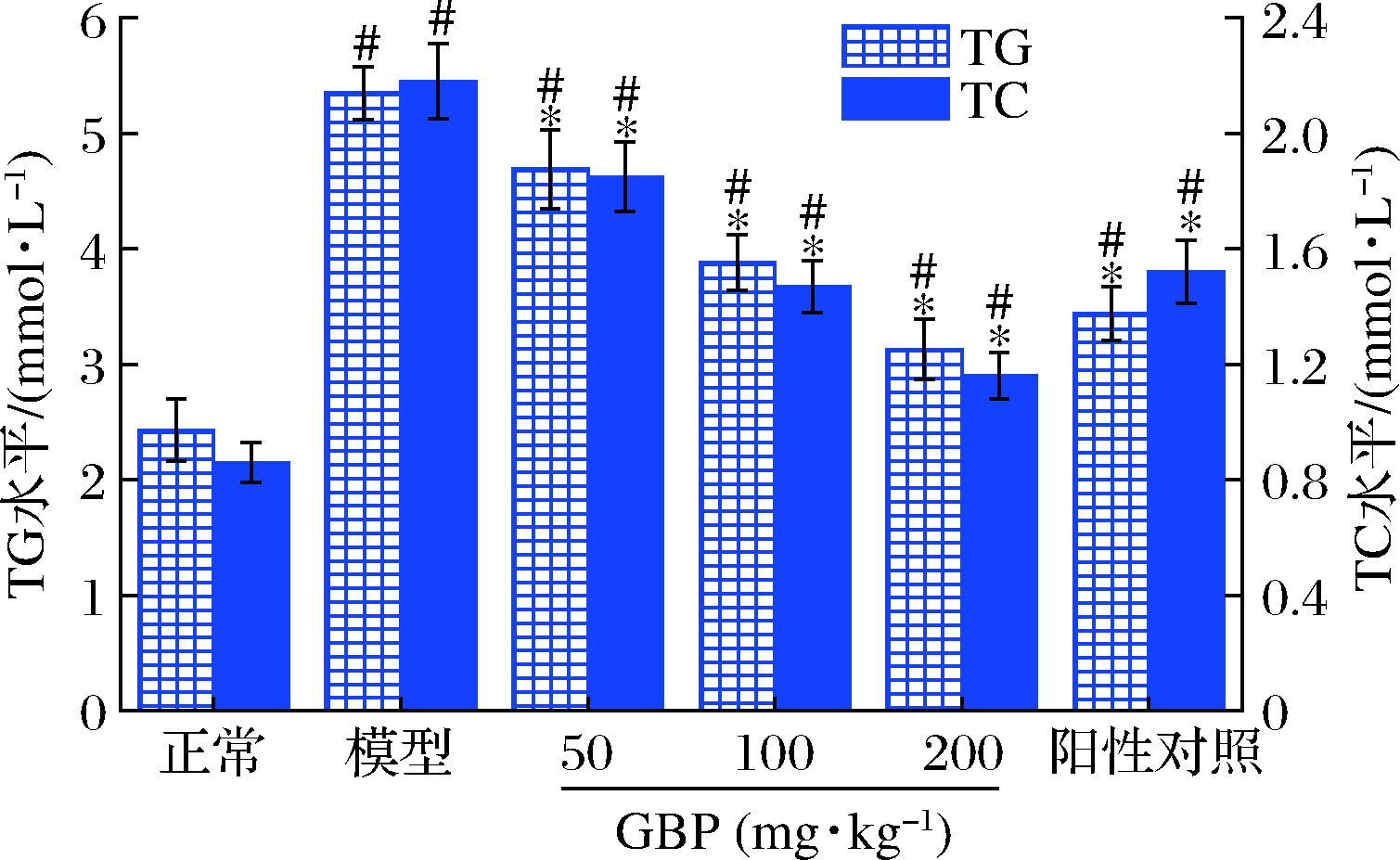
图3 银杏肽对酒精性肝损伤小鼠血清TG和TC水平的影响
Fig.3 Effects of G.biloba peptides on serum TG and TC levels in acute alcoholic liver injury mice
2.4 对小鼠血清炎性细胞因子水平的影响
库普弗细胞在酒精性肝损伤的进展中扮演着重要的角色,酒精暴露会刺激库普弗细胞通过MyD88介导的Toll样受体4(toll like receptor 4, TLR4)通路产生IL-1β、IL-6和TNF-α等促炎细胞因子,而这些促炎细胞因子会导致脂肪变性,甚至造成肝细胞炎性损伤和凋亡,加剧急性酒精性肝损伤的进展[35-36]。
图4为GBP对急性酒精性肝损伤小鼠血清IL-1β、IL-6和TNF-α水平的影响。由图4可知,模型组小鼠血清IL-1β、IL-6和TNF-α水平分别是正常组的1.9、2.3和1.9倍,显著高于正常组(P<0.05),提示酒精暴露可诱导小鼠肝细胞发生炎性损伤。与模型组相比,GBP组小鼠血清中IL-1β、IL-6和TNF-α水平显著降低(P<0.05),且呈剂量依赖性;高剂量GBP组小鼠血清TNF-α可恢复至正常水平(P>0.05),提示GBP通过抗炎途径发挥其对急性酒精性肝损伤小鼠的保护作用。与模型组相比,还原型谷胱甘肽也能显著降低小鼠血清中IL-1β、IL-6和TNF-α水平(P<0.05)。
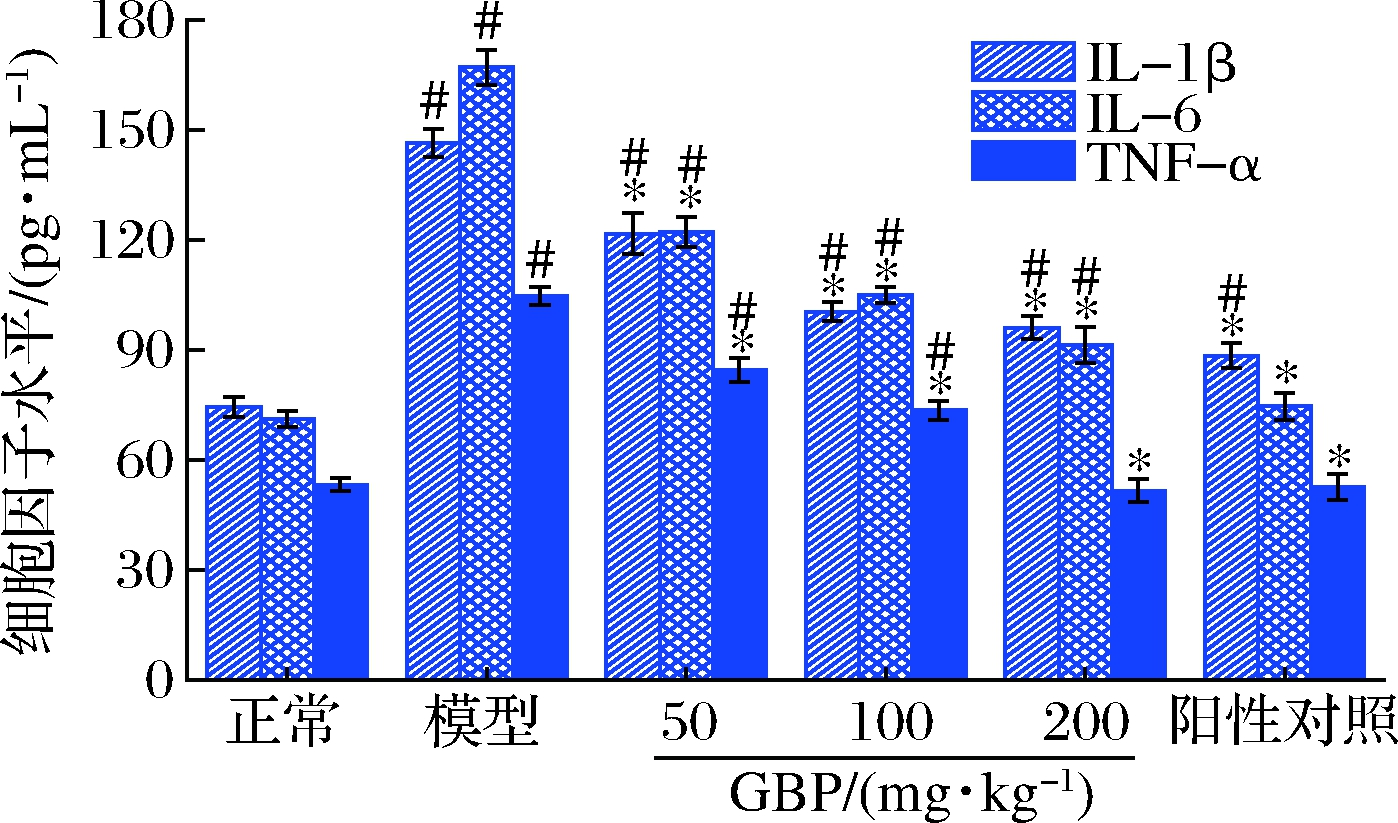
图4 银杏肽对酒精性肝损伤小鼠血清IL-1β、IL-6和 TNF-α水平的影响
Fig.4 Effects of G.biloba peptides on serum IL-1β, IL-6 and TNF-α levels in acute alcoholic liver injury mice
2.5 对小鼠肝脏抗氧化酶活性的影响
肝脏是机体内酒精生物转化的主要场所,也是酒精毒性作用的主要靶器官。酒精代谢过程中会产生过量的活性氧(reactive oxygen species, ROS),造成机体氧化与抗氧化防御系统的失衡,从而导致氧化应激,这被认为是急性酒精性肝损伤发生与进展的重要机制之一[37]。CAT、GSH-Px和T-SOD是机体抗氧化酶促防御系统的主要组分,过量的ROS可作用于CAT、GSH-Px和T-SOD等抗氧化酶,使其结构和构象发生改变,导致抗氧化酶活性降低[38]。
GBP对急性酒精性肝损伤小鼠肝脏CAT、GSH-Px和T-SOD活性的影响如图5所示。模型组小鼠肝脏CAT、GSH-Px和T-SOD活性分别比正常组降低了46.4%、32.8%和28.5%,3种抗氧化酶活性均显著低于正常组(P<0.05),提示酒精暴露致使小鼠肝脏处于氧化应激状态。给药GBP后,小鼠肝脏CAT、GSH-Px和T-SOD活性显著高于模型组(P<0.05),高剂量GBP组小鼠GSH-Px和T-SOD活性恢复至正常水平(P>0.05),表明GBP通过提高抗氧化酶活性,发挥对急性酒精性肝损伤小鼠的保护作用。
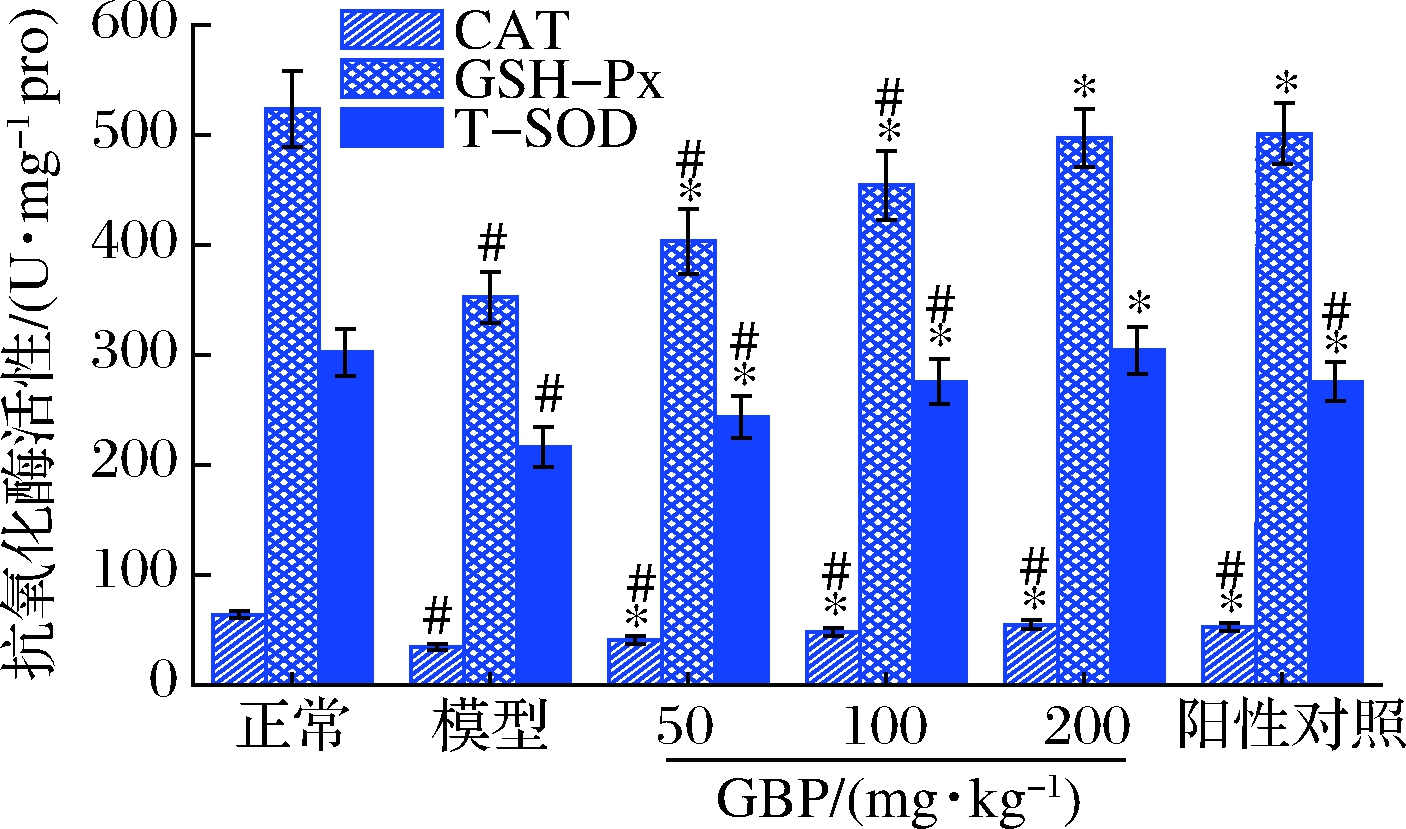
图5 银杏肽对酒精性肝损伤小鼠肝脏CAT、GSH-Px和 T-SOD活性的影响
Fig.5 Effects of G.biloba peptides on liver CAT, GSH-Px and T-SOD activities in acute alcoholic liver injury mice
2.6 对小鼠肝脏氧化损伤产物水平的影响
酒精代谢过程中产生的过量ROS可作用于机体内的蛋白质和脂质等生物分子,造成蛋白质和脂质的氧化损伤。MDA是脂质过氧化的终产物,PCG是蛋白质氧化损伤的产物,MDA和PCG水平是评估脂质和蛋白质氧化损伤的敏感标志物[39-40]。GBP对急性酒精性肝损伤小鼠肝脏MDA和PCG水平的影响如图6所示。模型组小鼠肝脏MDA和PCG水平显著高于正常组(P<0.05),表明酒精摄入造成脂质和蛋白质氧化损伤。灌胃给药GBP后,MDA和PCG水平以剂量依赖的方式显著降低(P<0.05),提示GBP可缓解酒精暴露诱导的肝组织氧化损伤。
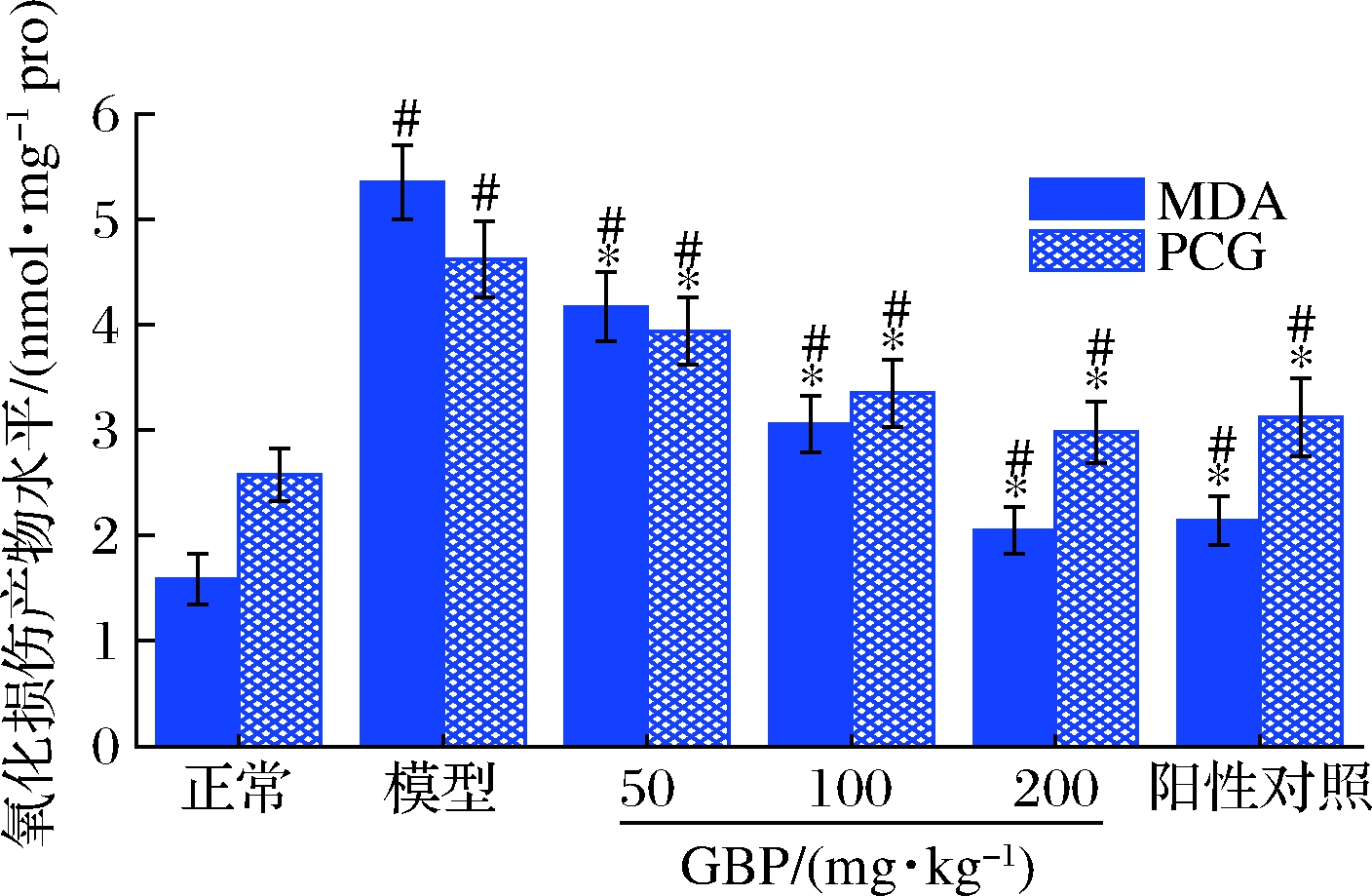
图6 银杏肽对酒精性肝损伤小鼠肝脏MDA和 PCG水平的影响
Fig.6 Effects of G.biloba peptides on liver MDA and PCG levels in acute alcoholic liver injury mice
3 结论
GBP对急性酒精性肝损伤小鼠具有较好保护作用,能缓解酒精性肝损伤小鼠的肝脾肿大,降低小鼠血清ALT和AST活性,抑制血清TG和TC水平,下调血清IL-1β、IL-6和TNF-α水平,提高肝脏CAT、GSH-Px和T-SOD活性,降低肝脏MDA和PCG水平。结果显示,GBP通过抗氧化和抗炎途径发挥其对急性酒精性肝损伤小鼠的保护作用,作用机制与提高机体抗氧化酶活性和抑制促炎细胞因子表达有关。
[1] S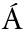 NCHEZ A,V
NCHEZ A,V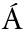 ZQUEZ A.Bioactive peptides:A review[J].Food Quality and Safety,2017,1(1):29-46.
ZQUEZ A.Bioactive peptides:A review[J].Food Quality and Safety,2017,1(1):29-46.
[2] LORENZO J M,MUNEKATA P E S,G MEZ B,et al.Bioactive peptides as natural antioxidants in food products-A review[J].Trends in Food Science & Technology,2018,79:136-147.
MEZ B,et al.Bioactive peptides as natural antioxidants in food products-A review[J].Trends in Food Science & Technology,2018,79:136-147.
[3] CICERO A F G,FOGACCI F,COLLETTI A.Potential role of bioactive peptides in prevention and treatment of chronic diseases:A narrative review[J].British Journal of Pharmacology,2017,174(11):1 378-1 394.
[4] KORHONEN H,PIHLANTO A.Bioactive peptides:Production and functionality[J].International Dairy Journal,2006,16(9):945-960.
[5] MAZORRA-MANZANO M A,RAMíREZ-SUAREZ J C,YADA R Y.Plant proteases for bioactive peptides release:A review[J].Critical Reviews in Food Science and Nutrition,2018,58(13): 2 147-2 163.
[6] 杨月欣. 中国食物成分表:第一册[M].第6版.北京:北京大学医学出版社,2018.
YANG Y X.China Food Composition:Book 1[M].6th Edition.Beijing:Peking University Medical Press,2018.
[7] 黄文. 白果活性蛋白的分离、纯化、结构及其生物活性研究[D].武汉:华中农业大学,2002.
HUANG W.Studies on separation purification and structure of Ginkgo seed protein and its biologic activities[D].Wuhan:Huazhong Agricultural University,2002.
[8] HUANG W,DENG Q C,XIE B J,et al.Purification and characterization of an antioxidant protein from Ginkgo biloba seeds[J].Food Research International,2010,43(1):86-94.
[9] 邓乾春, 汪兰,吴佳,等.一种白果清蛋白的抗衰老活性研究[J].中国药理学通报,2006,22(3):352-357.
DENG Q C,WANG L,WU J,et al.Studies on anti-aging activity of Ginkgo Albumin Protein (GAP)[J].Chinese Pharmacological Bulletin,2006,22(3):352-357.
[10] GAO N N,WADHWANI P,MÜHLH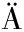 USER P,et al.An antifungal protein from Ginkgo biloba binds actin and can trigger cell death[J].Protoplasma,2016,253(4):1 159-1 174.
USER P,et al.An antifungal protein from Ginkgo biloba binds actin and can trigger cell death[J].Protoplasma,2016,253(4):1 159-1 174.
[11] 邓乾春, 段会轲,谢笔钧,等.白果清蛋白对免疫功能低下小鼠的调节作用[J].食品科学,2006,27(6):195-199.
DENG Q C,DUAN H K,XIE B J,et al.Regulating function of Ginkgo Albumin Protein (GAP) on immunosuppressive model in mice[J].Food Science,2006,27(6):195-199.
[12] 邓乾春, 黄文,谢笔钧.白果清蛋白抑制肿瘤活性及其机制的初步研究[J].营养学报,2006,28(3):259-262.
DENG Q C,HUANG W,XIE B J.The preliminary studies on anti-tumor activity of ginkgo albumin and its mechanism[J].Acta Nutrimenta Sinica,2006,28(3):259-262.
[13] 贾韶千, 吴彩娥,范龚健,等.双酶法制备银杏抗氧化肽工艺研究[J].食品科学,2011,32(21):201-206.
JIA S Q,WU C E,FAN G J,et al.Preparation of antioxidant peptides derived from Ginkgo biloba kernel by dual-enzymatic method[J].Food Science,2011,32(21):201-206.
[14] 张焕新, 臧大存,刘靖,等.银杏肽的抗氧化性研究[J].食品研究与开发,2008,29(12):27-29.
ZHANG H X,ZANG D C,LIU J,et al.Antioxidant activity of Ginkgo peptides[J].Food Research and Development,2008,29(12):27-29.
[15] 张灿, 吴彩娥,范龚健,等.酶解银杏蛋白制备α-葡萄糖苷酶抑制肽的研究[J].食品与机械,2016,32(11):137-141.
ZHANG C,WU C E,FAN G J,et al.Preparation of α-glucosidase inhibitory peptides derived from Ginkgo biloba by enzymatic method[J].Food & Machinery,2016,32(11):137-141.
[16] MA F F,WANG H,WEI C K,et al.Three novel ACE inhibitory peptides isolated from Ginkgo biloba seeds:Purification,inhibitory kinetic and mechanism[J].Frontiers in Pharmacology,2019,9:1 579.
[17] MASARONE M,ROSATO V,DALLIO M,et al.Epidemiology and natural history of alcoholic liver disease[J].Reviews on Recent Clinical Trials,2016,11(3):167-174.
[18] WANG W J,XIAO P,XU H Q,et al.Growing burden of alcoholic liver disease in China:A review[J].World Journal of Gastroenterology,2019,25(12):1 445-1 456.
[19] WANG H J,GAO B,ZAKHARI S,et al.Inflammation in alcoholic liver disease[J].Annual Review of Nutrition,2012,32(1):343-368.
[20] ALBANO E.Oxidative mechanisms in the pathogenesis of alcoholic liver disease[J].Molecular Aspects of Medicine,2008,29(1):9-16.
[21] MA Z L,HOU T,SHI W,et al.Inhibition of hepatocyte apoptosis:An important mechanism of corn peptides attenuating liver injury induced by ethanol[J].International Journal of Molecular Sciences,2015,16(9):22 062-22 080.
[22] WU Y H,PAN X C,ZHANG S X,et al.Protective effect of corn peptides against alcoholic liver injury in men with chronic alcohol consumption:A randomized double-blind placebo-controlled study[J].Lipids in Health and Disease,2014,13:192.
[23] LIN B,ZHANG F,YU Y C,et al.Marine collagen peptides protect against early alcoholic liver injury in rats[J].The British Journal of Nutrition,2012,107(8):1 160-1 166.
[24] 刘睿, 珠娜,刘欣然,等.核桃低聚肽对急性酒精性肝损伤大鼠保护作用[J].中国公共卫生,2020,36(2):192-195.
LIU R,ZHU N,LIU X R,et al.Protective effect of walnut oligopeptides on acute alcohol-induced liver injury in rats[J].Chinese Journal of Public Health,2020,36(2):192-195.
[25] WEI C K,THAKUR K,LIU D H,et al.Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products[J].Food Chemistry,2018,263:186-193.
[26] SONG X L,LIU Z H,ZHANG J J,et al.Antioxidative and hepatoprotective effects of enzymatic and acidic-hydrolysis of Pleurotus geesteranus mycelium polysaccharides on alcoholic liver diseases[J].Carbohydrate Polymers,2018,201:75-86.
[27] WANG M C,ZHU P L,JIANG C X,et al.Preliminary characterization,antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis[J].Food and Chemical Toxicology,2012,50(9):2 964-2 970.
[28] ZHENG Y,CUI J,CHEN A H,et al.Optimization of ultrasonic-microwave assisted extraction and hepatoprotective activities of polysaccharides from Trametes orientalis[J].Molecules,2019,24(1):147.
[29] ZHOU C Y,LAI Y L,HUANG P,et al.Naringin attenuates alcoholic liver injury by reducing lipid accumulation and oxidative stress[J].Life Sciences,2019,216:305-312.
[30] ZAKHARI S.Overview:How is alcohol metabolized by the body?[J].Alcohol Research & Health,2006,29(4):245-254.
[31] CEDERBAUM A I.Alcohol metabolism[J].Clinics in Liver Disease,2012,16(4):667-685.
[32] TESCHKE R.Alcoholic liver disease:Alcohol metabolism,cascade of molecular mechanisms,cellular targets,and clinical aspects[J].Biomedicines,2018,6(4):106.
[33] OSNA N A,DONOHUE T M,KHARBANDA K K.Alcoholic liver disease:Pathogenesis and current management[J].Alcohol Research,2017,38(2):147-161.
[34] LAI Y L,ZHOU C Y,HUANG P,et al.Polydatin alleviated alcoholic liver injury in zebrafish larvae through ameliorating lipid metabolism and oxidative stress[J].Journal of Pharmacological Sciences,2018,138(1):46-53.
[35] AN L,WANG X D,CEDERBAUM A I.Cytokines in alcoholic liver disease[J].Archives of Toxicology,2012,86(9):1 337-1 348.
[36] GAO B.Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease[J].Journal of Gastroenterology and Hepatology,2012,27:89-93.
[37] ![]() H,MICHALAK A.Oxidative stress as a crucial factor in liver diseases[J].World Journal of Gastroenterology,2014,20(25):8 082-8 091.
H,MICHALAK A.Oxidative stress as a crucial factor in liver diseases[J].World Journal of Gastroenterology,2014,20(25):8 082-8 091.
[38] LI S,TAN H Y,WANG N,et al.The role of oxidative stress and antioxidants in liver diseases[J].International Journal of Molecular Sciences,2015,16(11):26 087-26 124.
[39] DALLE-DONNE I,ROSSI R,GIUSTARINI D,et al.Protein carbonyl groups as biomarkers of oxidative stress[J].Clinica Chimica Acta,2003,329(1-2):23-38.
[40] TUMA D J.Role of malondialdehyde-acetaldehyde adducts in liver injury[J].Free Radical Biology and Medicine,2002,32(4):303-308.