青梅(Prunus mume),又称果梅,是药食同源的水果,含有多种有益的活性组分[1]。青梅酒,具有独特的水果和花香,且有益健康[2],是一种非常受欢迎的酒精饮料,主要有发酵型和浸泡型,前者在保留了青梅果香的同时,还贡献了更丰富的营养物质。TIAN等[3]报道,发酵型青梅酒中的氨基酸含量普遍高于浸泡型青梅酒,佐以蔗糖和糯米的发酵型青梅酒,抗氧化性能更好[4]。以不同种属的酵母菌共培养发酵葡萄酒等果酒,是改善其特征风味的有效途径[5-7]。目前,研究人员对青梅酒这类小众果酒的微生物多样性尚缺乏研究,主要是借鉴葡萄酒酿造的相关技术[8]。原料自身携带的微生物是发酵剂的主要来源,青梅果实酸度较高,对微生物群落及代谢影响显著。基于葡萄酒酵母发酵青梅酒不仅乙醇含量低,挥发性酸含量高,且挥发性组分特色不突出。应用葡萄酒共培养发酵的非酿酒酵母,如Torulaspora delbrueckii,发酵果酒,产挥发酸少且对SO2抗性高,其代谢产物中的异戊醇、苯乙醇、乙酯类和萜烯类可赋予果酒独特风味[9-10]。其是否适合青梅发酵尚待探讨,且与Saccharomyces cerevisiae共培对青梅酒发酵的微生物群落及代谢组分的影响尚未见报道。
本文主要研究基于S.cerevisiae单培接种与T.delbruecki顺序共培养接种及多轮补料发酵对青梅酒主要理化参数、代谢组分及微生物群落多样性的影响规律,解析优势微生物种属与主要代谢组分的相关性。旨在揭示酿酒酵母与非酿酒酵母共培养对青梅酒特征风味的贡献规律,奠定过程参数优化的理论基础。
1 材料与方法
1.1 材料与试剂
青梅,四川省大邑县;一级蔗糖,本地副食商店。草酸、柠檬酸、酒石酸、L-苹果酸、琥珀酸、乳酸、乙酸、辛酸甲酯等标准品,Sigma-Aldrich公司(上海);其他化学药品,蔚蓝化学(中国成都)。
1.2 仪器与设备
Trace 1300-TSQ 9000气相色谱-三重四级杆质谱联用仪,配备VF-WAX-MS毛细管色谱柱(30 m×0.25 mm,0.25 μm),美国Thermo Fisher Electron公司;HPLC配备UV/Vis检测器和有机酸柱(Alltech OA-1000,300 mm×6.5 mm×9 μm),美国Agilent公司;TU-1901紫外可见分光光度计,北京普析通用仪器有限公司;CX31显微镜,日本奥林巴斯有限公司。
1.3 实验步骤
原料经挑选、清洗沥水,以m(果)∶m(糖)=5∶1逐层用蔗糖覆盖青梅果(4个50 L发酵罐),糖渍约36 h后,蔗糖溶解,分别加入偏重亚硫酸钠至糖梅汁中SO2的质量浓度为120 mg/L,随后调节(NH4)2HPO4质量浓度为150 mg/L。接种S.cerevisiae(5×106 CFU/mL)到发酵罐中,样品编号为S;接种S.cerevisiae后又接种T.delbrueckii Y7(CCTCC M2019523)的样品编号为S+T,其初始浓度均为5×106 CFU/mL;未接种发酵罐(ZR)为对照。这3个发酵罐同时进行周期性搅拌,另取1个未接种的发酵罐采用静置发酵方式,以更好地模拟自然发酵,编号为ZD。接种发酵的2个发酵罐,发酵至糖的质量浓度降至0.4 g/L以下,取出清汁(第1轮原酒)后,补加与残留青梅果等体积的23°Brix的蔗糖溶液,同样条件发酵结束后,再重复补加蔗糖溶液发酵。第2、3轮接种发酵的原酒标注2F和3F。
1.4 实验方法
1.4.1 理化指标的测定
主要理化指标(残糖、总酸、酒精度、挥发酸)的测定参照GB/T 15038—2006《葡萄酒、果酒通用分析方法》。
1.4.2 色度和色调的测定
根据BIMPILAS等[11]的方法测定青梅酒的色度和色调,用紫外可见分光光度计测定吸光度,其中色度=A420+A520+A620,色调=A420/A520。
1.4.3 抗氧化活性的测定
抗坏血酸含量采用2,6-二氯酚靛酚滴定法[12]测定。多酚含量、DPPH自由基和ABTS阳离子自由基清除活性采用WANG等[13]的方法测定。总抗氧化能力参照总抗氧化能力(total antioxidant capacity,T-AOC)试剂盒说明书(南京建成生物工程研究所)进行分析。
1.4.4 有机酸的测定
HPLC检测程序:按参考文献[14]所述方法,稍作修改。采用Agilent Chemstation软件对色谱数据进行采集和分析,根据柠檬酸、L-苹果酸、琥珀酸、乳酸和乙酸标准品的保留时间来判定样品中的有机酸种类,采用外标法定量,应用HPLC分析建立标准曲线,计算上述有机酸含量。
操作方法:准确吸取10.00 mL样品,4 ℃、12 000 r/min离心10 min,取3 mL上清液加载至经甲醇活化的固相萃取(solid phase extraction,SPE)小柱(成都SWELL公司)洗脱,取中间1 mL洗脱液,再用0.22 μm水系滤膜过滤后待HPLC检测。HPLC检测条件:流动相为9.00 mmol/L的H2SO4溶液,恒定流速为0.60 mL/min,柱温箱温度为75 ℃,检测波长为215 nm。
1.4.5 挥发性成分的测定
参考ZHENG等[15]所述方法,稍作修改。准确吸取0.5 mL样品置于15 mL顶空瓶中,加入2 mL纯水,再加入10 μL内标(79 mg/L辛酸甲酯)和1.5 g NaCl后密封,放入60 ℃水浴中预热平衡15 min,然后插入固相微萃取头(50/30 μm DVB/CAR/PDM,美国Supelco公司)萃取吸附40 min,取出插入GC-MS进样口解吸5 min后检测。检测条件与NIU等[16]所述相同,稍作修改。操作条件:进样口温度为270 ℃,升温程序:40 ℃,保持5 min,以4 ℃/min升至100 ℃,然后6 ℃/min升至230 ℃,保持10 min。载气为高纯氦,流速为1 mL/min,不分流模式。质谱离子源传输管的温度分别是300 ℃和250 ℃;电离方式为EI,电子能量70 eV,质量扫描范围35~400 amu。
1.4.6 高通量测序
DNA提取、PCR扩增按照HE等[17]和TANG等[18]所述的方法进行,并稍作修改。按照Fast DNA SPIN提取试剂盒操作说明对第1轮原酒的微生物进行基因组DNA提取,将提取的DNA进行0.8%琼脂糖凝胶电泳进行分子大小判断,取3 μL核酸用紫外分光光度计对DNA进行定量。细菌16S rRNA基因的V3-V4区采用通用引物338F/806R,真菌rRNA基因的ITS1区分别用通用引物ITS5F/ITS1R进行扩增。PCR纯化产物送至上海派森诺生物科技有限公司,使用MiSeq基因测序试剂盒v3进行2×300 bp双端测序。
1.5 数据处理
序列数据分析主要使用QIIME(v1.8.0)和R包(v3.2.0)进行。原始数据采用IBM SPSS Statistics 26.0进行单因素方差分析,以确定样本间的差异,P<0.05表示有统计学意义的显著性差异。采用SIMCA 14.1(瑞典Umetrics公司)软件进行偏最小二乘判别分析(partial least squares discriminant analysis,PLS-DA)。应用Canoco 5.0进行冗余分析(redundancy analysis,RDA)。其他统计分析使用Origin 2018。所有试验重复3次,数据以平均值±相对标准偏差表示。
2 结果与讨论
2.1 主要理化性质及抗氧化活性的差异
如表1所示,主要理化性质、色度和色调等指标随接种方式的不同而变化。与自然发酵相比,其余原酒,无论是单培接种还是顺序共培接种,其残糖含量均较低,酒精度较高。由于酸胁迫对酵母菌的抑制,导致第1轮的S和S+T的酒精度比后两轮低[19]。同时,原酒间的总酸和挥发酸含量具有显著差异,且单培接种的挥发酸低于顺序共培接种的。后两轮总酸和挥发酸以及色度逐轮下降,而色调先上升后下降。在发酵周期方面,ZR和ZD需要1个月左右,而接种强化发酵每轮仅需7~11 d,在充分利用青梅剩余价值的同时,发酵时间明显缩短。
表1 理化指标及抗氧化活性的差异
Table 1 Difference of physicochemical indexes and antioxidant activities

名称S+TSZRZDS+T(2F)S(2F)S+T(3F)S (3F)残糖/%0.40±0.03g0.88±0.01e1.26±0.01d3.71±0.03a0.68±0.01f0.28±0.01h3.01±0.01b1.76±0.05c酒精度/%11.7±0.1d11.4±0.2d11.1±0.2d10.2±0.1e12.8±0.2ab13.1±0.3a12.0±0.1c12.4±0.2c总酸/(g·L-1)62.80±0.45c63.78±0.15b68.80±1.03a60.70±0.28d26.09±0.18e28.68±0.15e12.87±0.02h13.90±0.06g挥发酸/(g·L-1)0.90±0.02c0.85±0.00d1.54±0.02a1.40±0.03b0.86±0.01d0.78±0.01e0.73±0.00f0.66±0.01g色度1.035±0.096a1.039±0.078a0.973±0.000a0.798±0.002b0.424±0.000c0.460±0.001c0.401±0.001c0.443±0.001c色调1.809±0.011f1.845±0.020e2.258±0.000d2.356±0.014c2.461±0.005b2.603±0.013a1.732±0.016e1.791±0.008f抗坏血酸/(g·L-1)5.37±0.15b4.70±0.04c5.42±0.11b5.82±0.14a4.58±0.15c4.72±0.11c3.79±0.08d3.55±0.04e多酚/[mg (GAE)·L-1]101.95±0.74c105.46±0.47b105.19±1.01b117.22±0.56a100.15±0.49d101.32±0.34cd98.53±0.57e100.35±1.05dT-AOC/(U·mL-1)166.46±6.08cd170.83±2.39bc164.14±3.00cd176.38±4.72ab158.54±2.91d180.03±4.24a96.13±4.28f111.05±3.69eABTS/[mg (TE)·L-1]132.69±0.47ab133.43±0.28a128.30±1.83c130.46±3.16bc132.31±0.28ab131.63±1.30ab133.18±0.11ab132.99±0.28abDPPH/[mg (TE)·L-1]167.61±0.58d176.51±2.11b156.67±0.16f161.86±3.47e175.68±0.85bc173.27±1.00c182.35±0.85a180.60±0.70a
注:GAE-没食子酸当量;TE-Trolox当量;不同字母代表显著性差异(P<0.05)(表2同);数据表示为平均值±相对标准偏差(n=3)
所有原酒的总抗氧化力较高,ABTS清除率和多酚含量没有明显差异,DPPH自由基的清除能力高于ABTS的清除能力。然而,抗坏血酸含量相对较低,致使避免芳香化合物氧化能力减弱[20]。
2.2 有机酸的比较分析
如表2所示,样品间有机酸总含量在9.53~58.76 g/L,从而导致总酸显著不同。显然,检出的5种有机酸中柠檬酸是优势有机酸,所占比例在78.36%~89.58%,主要是源于青梅,且含量高于浸泡型青梅酒[3]。除第3轮原酒外,含量次之的是苹果酸,且其含量在样品间存在显著差异,它有助于酒中芳香化合物的释放,提高果酒的鲜味[3]。第3轮,因蔗糖经酵母发酵,琥珀酸成为主要代谢产物之一[21]。此外,后两轮样品中检出了少量乳酸,可能是苹果酸-乳酸发酵[22]所致。除ZD外,乙酸含量与挥发酸含量呈正相关,且3轮的单培接种都高于共培接种。
表2 有机酸含量的差异 单位:g/L
Table 2 Difference in organic acid content
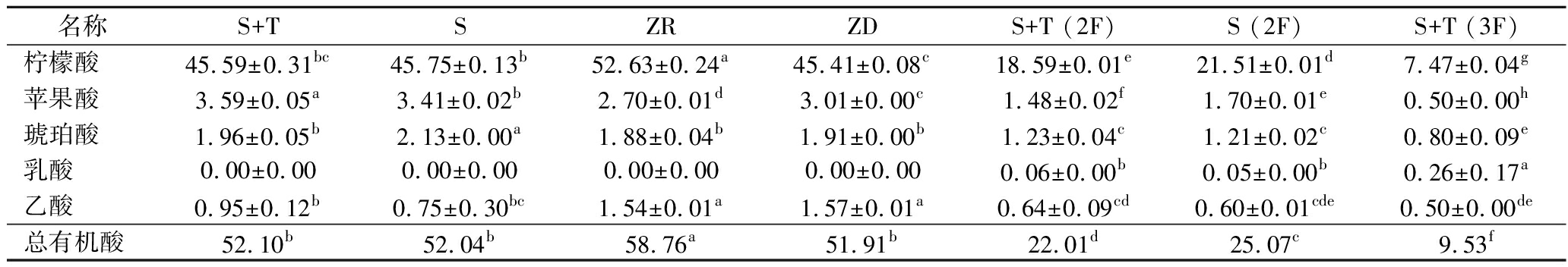
名称S+TSZRZDS+T (2F)S (2F)S+T (3F)S (3F)柠檬酸45.59±0.31bc45.75±0.13b52.63±0.24a45.41±0.08c18.59±0.01e21.51±0.01d7.47±0.04g8.80±0.02f苹果酸3.59±0.05a3.41±0.02b2.70±0.01d3.01±0.00c1.48±0.02f1.70±0.01e0.50±0.00h0.63±0.00g琥珀酸1.96±0.05b2.13±0.00a1.88±0.04b1.91±0.00b1.23±0.04c1.21±0.02c0.80±0.09e0.97±0.01d乳酸0.00±0.000.00±0.000.00±0.000.00±0.000.06±0.00b0.05±0.00b0.26±0.17a0.33±0.01a乙酸0.95±0.12b0.75±0.30bc1.54±0.01a1.57±0.01a0.64±0.09cd0.60±0.01cde0.50±0.00de0.39±0.00e总有机酸52.10b52.04b58.76a51.91b22.01d25.07c9.53f11.12e
2.3 挥发性组分的差异
原酒样品中共检出了69种挥发性成分(>0.1%),包括酯(29)、醇(14)、酸(10)、醛(4)、酚(4)和萜(8)等6类,所有样品都检出的成分有31种,部分挥发性成分含量差异如图1-a所示。
S和S+T的3轮发酵的原酒中第2轮挥发性成分的含量最高,对照样品ZR最低。ZD的总挥发物高于S和S+T的第1轮原酒的,且显著高于ZR的。ZD与ZR的酯类的比例相似,前者的酸类的比例较后者高,萜烯类比例则低。如图1-b所示,酵母强化显著提高了醇类组分的比例,单培接种的酸类组分比例随发酵轮次减少而共培接种的则先减少后增加,酯类的比例是略减,S+T第2轮的萜烯类组分的比例略增。
醇和酯是优势组分,前者主要是苯乙醇和异戊醇,与曾报道的结果[23-24]是一致的,酸类次之。酯类组分中,主要是辛酸、癸酸、琥珀酸、乙酸和苯甲酸等5种酸的乙酯衍生物,这些组分赋予青梅酒果香和花香。S和S+T的原酒中,检出了赋予样品甜味、柑橘味、花香的癸醛。浸泡型青梅酒中检出的赋予杏仁香气的特征组分苯甲醛[25],本研究在除S外的样品中也都检出,HAN等[4]也有类似的报道,可能是源于青梅(核)[26]。4-乙基苯酚、4-乙基愈创木酚、乙基酚等对葡萄酒特征风味贡献显著[27],S.cerevisiae抑制了Brettanomyces/Dekkera生长与代谢所致[28],所有强化的样酒中都未检出。萜烯类含量尽管较低,但阈值小,所以其含量变化显著影响果酒花香和果香等特征风味。

图1 挥发性成分含量(a)及相对含量(b)的差异
Fig.1 Difference of volatile compounds content (a) and relative content (b)
主要香气化合物的气味活度值(odour activity value,OAV)如表3所示。主要香气化合物、有机酸和理化指标的PLS-DA的结果如图2所示。这些原酒彼此距离较远,其72.50%的总方差可被解释。类似HU等[29]报道的结果,酵母接种方式显著影响原酒的代谢组成。在这些香气化合物中,有14种物质的变量投影重要性(variable importance in the projection,VIP)>1,如β-紫罗兰酮、异戊醇、苯乙醇等,这些物质对PLS-DA模型的方差贡献较大[30],且残糖和酒精度的VIP>1。模型的RX2、RY2和Q2的值(0.91、0.979和0.905)表明,PLS-DA模型较准确地揭示发酵所致青梅酒品质的差异。

图2 青梅原酒的PLS-DA
Fig.2 PLS-DA of greengage wines
表3 主要香气化合物气味活度值
Table 3 Odour activity values of major aroma compounds

名称CAS#阈值/(μg·L-1)OAVsS+TSZRZDS+T (2F)S (2F)S+T (3F)S (3F)苯乙酸乙酯101-97-3148ND0.040.040.03NDNDNDND丁酸乙酯105-54-4200.941.220.880.55NDNDNDNDα-松油醇7 785-53-71000.630.520.500.070.290.270.130.24合金欢醇(法尼醇)4 602-84-0---NDNDNDNDNDND癸醛112-31-2101.251.55NDNDNDNDNDND乙酸异戊酯123-92-22000.330.420.090.120.260.28ND0.20苯甲酸甲酯93-58-3301.593.110.190.110.490.94ND0.21乙酸己酯142-92-71 5000.010.02NDNDND0.01NDND己酸乙酯123-66-0144.486.394.265.073.024.281.131.96芳樟醇78-70-61000.370.360.190.300.220.250.060.07乙酸苯乙酯103-45-76500.090.110.040.050.070.090.050.05橙花叔醇40 716-66-3---NDND----苯甲酸乙酯93-89-05700.410.460.40ND0.510.600.740.80异戊醇123-51-340 0000.030.030.020.020.050.050.050.02苯乙醇1960-12-810 0000.070.080.06ND0.120.140.12ND苯甲醇100-51-620 000NDND0.02ND0.010.020.03ND乙酸苄酯140-11-4270NDNDND0.06NDND0.050.07月桂酸乙酯106-33-216 000NDND0.010.020.010.01NDNDL-乳酸乙酯97-64-314 000<0.01<0.01<0.01<0.01<0.01<0.01<0.01<0.01棕榈酸乙酯628-97-715 0000.040.050.190.110.040.040.030.024-乙基愈创木酚2 785-89-950NDND0.320.65NDNDNDND癸酸乙酯110-38-32000.470.371.242.131.100.800.420.60乙酸乙酯141-78-617 000NDND0.020.02ND0.010.010.01辛酸乙酯106-32-12500.740.670.801.361.000.790.340.49琥珀酸乙酯123-25-1200 000<0.01<0.01<0.01<0.01<0.01<0.01<0.01<0.01肉桂酸乙酯4 192-77-21.1021.3330.2541.8541.8122.7045.4215.0324.22β-紫罗兰酮79-77-6901.681.532.81 1.67 2.29 2.34 1.19 1.23 γ-癸内酯706-14-9881.291.74 1.34 1.260.881.410.450.51香茅醇40 607-48-51000.1000.120000.100
注:ND表示为没有或未检出。
2.4 不同青梅酒风味轮廓的差异
以挥发性成分浓度除以相应阈值的OAVs评估其对风味的贡献,通常OAV>1的贡献度显著[31]。OAV>1的共有9种成分,包括γ-癸内酯、癸醛、β-紫罗兰酮、苯甲酸甲酯、丁酸乙酯、辛酸乙酯、癸酸乙酯、肉桂酸乙酯和己酸乙酯,构建其风味轮廓如图3所示。这些成分除丁酸乙酯和癸醛外,在所有原酒中都有检出。肉桂酸乙酯阈值非常低,因而OAV在各样品中最高,赋予了原酒梅果香、肉桂香和花香,且自然发酵的梅果香更浓。强化发酵的原酒因接种方式及轮次不同导致OAV不同,其特征风味有差异,主要是花香、蜜香、菠萝果香、脂肪味和果香的芳香强度不同。
2.5 微生物多样性分析
接种方式对主要发酵过程微生物α-多样性的影响结果如表4所示。强化发酵提高了细菌丰富度和多样性。但在S+T的后期,真菌丰富度小于自然发酵样品,S+T和S在中期,真菌多样性显著低于自然发酵样品。S在后期真菌多样性显著高于S+T,但仍低于ZR。

图3 青梅原酒的风味轮廓图
Fig.3 Flavor profile of greengage wines
表4 不同样品α-多样性差异
Table 4 Difference of α-diversity in different samples
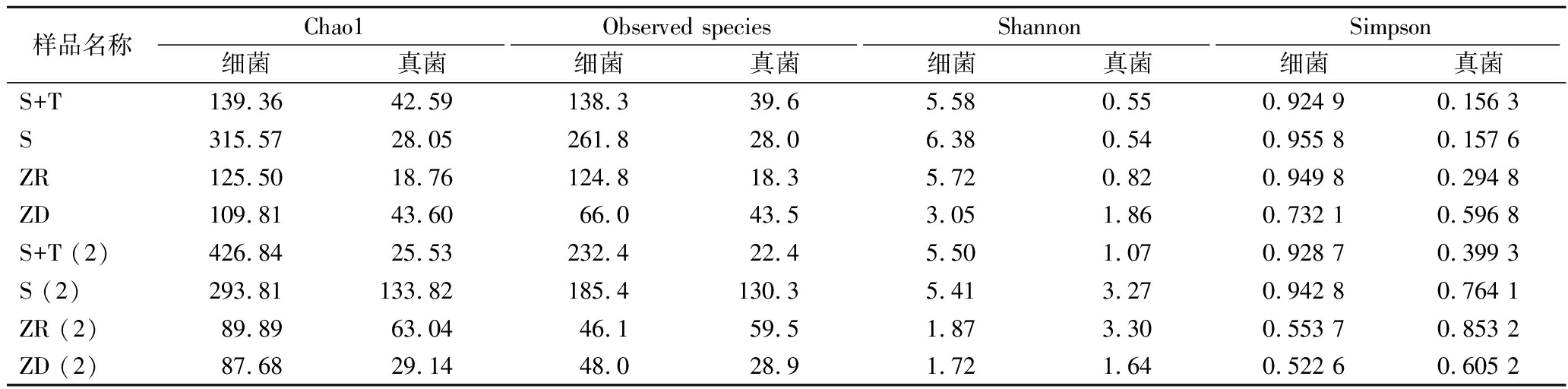
样品名称Chao1Observed speciesShannonSimpson细菌真菌细菌真菌细菌真菌细菌真菌S+T139.3642.59138.339.65.580.550.924 90.156 3S315.5728.05261.828.06.380.540.955 80.157 6ZR125.5018.76124.818.35.720.820.949 80.294 8ZD109.8143.6066.043.53.051.860.732 10.596 8S+T (2)426.8425.53232.422.45.501.070.928 70.399 3S (2)293.81133.82185.4130.35.413.270.942 80.764 1ZR (2)89.8963.0446.159.51.873.300.553 70.853 2ZD (2)87.6829.1448.028.91.721.640.522 60.605 2
注:(2)表示一轮后期(下同)
自然发酵及强化的中、后期的群落组成的差异如图4所示。强化方式显著影响真菌群落结构,尤其在发酵中期,这是研究微生物组成及代谢组分关系的关键时期[32]。S.cerevisiae强化后,在发酵中期成为优势菌,在S和S+T中的丰度>91.50%,Pichia的丰度显著降低(图4-b)。自然发酵的ZR和ZD则在发酵后期才检出S.cerevisiae。这些结果证实了接种酵母菌的主要作用之一是缩短发酵时间。KONG等[33]报道了在葡萄酒发酵中Saccharomyces和Pichia具有良好的互作关系,在青梅酒的发酵中后期情况亦是如此。然而Saccharomyces和Torulaspora系偏生关系,S+T的Torulaspora的丰度<0.01%。在ZR和ZD后期发酵醪中检出Dekkera的丰度较高,这也是挥发性乙基酚的产生原因[28]。对于细菌属,在S、S+T和ZR中Sphingomonas是优势菌,可能与发酵过程常搅拌、溶氧较高有关,所以其细菌菌群组成非常类似(图4-a)。而ZD中Gluconobacter和Acetobacter是优势菌,直到发酵后期才与ZR的细菌群落趋于相同。

a-细菌;b-真菌
图4 属水平上细菌和真菌的微生物组成
Fig.4 Microbial compositions of bacteria and fungi at genus level
进一步对1轮原酒中后期微生物群落进行了主坐标分析(principal co-ordinates analysis,PCoA)分析和层次聚类分析(hierarchical cluster analysis,HCA),结果如图5所示。细菌方面,S+T和S同对照样品ZR在发酵中期因细菌群落相似(图5-a)而聚为一簇(图5-c),ZD则单独聚为一簇;发酵后期,接种强化的样品细菌群落组成与发酵中期相比略有差异,ZR和ZD细菌群落组成趋于一致但因变化较大而远离接种强化的样品。真菌方面,S+T和S发酵前期因真菌群落组成重叠性较大(图5-b)而聚为一簇(图5-d),且远离ZR和ZD;而在发酵后期,与细菌不同,接种强化的样品因菌株不同和顺序接种而逐渐远离。

a-一轮细菌PCoA;b-一轮真菌PCoA;c-一轮细菌HCA;d-一轮真菌HCA
图5 属水平上细菌和真菌的主坐标分析和层次聚类分析
Fig.5 PCoA and HCA of bacteria and fungi at genus level
第1轮原酒的主要挥发性成分与微生物组成的RDA结果如图6所示。

a-发酵中期;b-发酵后期
图6 微生物与香气化合物的相关性分析
Fig.6 Correlation analysis between microbes and aroma compounds
在发酵中期(图6-a),接种的S.cerevisiae的丰度与己酸乙酯、苯甲酸甲酯、乙酸异戊酯、异戊醇、橙花叔醇、法尼醇、癸醛等的含量呈显著正相关,T.delbrueckii的丰度与这些组分也呈正相关,但由于Saccharomyces和Torulaspora系偏生关系而相关性较弱。Pichia的丰度与肉桂酸、棕榈酸、乙酸、乳酸等4种酸的乙酯及γ-癸内酯等的含量呈正相关,对这些香气成分贡献度较大。而在发酵后期(图6-b),Dekkera的丰度与4-乙基愈创木酚的含量呈正相关,且在发酵中期与接种酵母呈强烈竞争关系的Pichia在发酵后期则与Saccharomyces表现出良好的协同关系。
3 结论
3种接种方式对青梅酒的理化特性和代谢组分的影响规律的研究结果表明,强化发酵提高了酒精度及自由基清除能力,降低了总酸和挥发酸的含量,且第2轮的糖酸平衡较好。青梅因酸度高,首轮发酵抑制了酵母菌,其乙醇浓度略低于后两轮的。菌株不同及顺序接种影响乙醇、代谢组分的含量,接种强化提高了醇类组分的比例,减少了酸类组分的比例,单培的后两轮乙醇含量更高而共培的第2轮的萜烯类比例略增。强化导致4-乙基苯酚、4-乙基愈创木酚和乙基酚等特征组分含量显著降低,突出了萜烯类组分对风味组分的贡献度,花香和果香更浓郁。对1轮原酒微生物多样性研究结果表明,强化显著改变青梅酒的微生物群落结构。S.cerevisiae和T.delbrueckii的贡献类似,主要是提高了己酸乙酯、苯甲酸甲酯、乙酸异戊酯、异戊醇、橙花叔醇、法尼醇、癸醛等的含量。研究结果为解决原料酸度高影响产品品质,及基于酿酒酵母和非酿酒酵母共培养的发酵果酒过程参数优化奠定了理论基础。
[1] 刘功德,苏艳兰,黄富宇,等.青梅的功能价值及加工研究进展[J].农业研究与应用,2018,31(4):1-8.
LIU G D,SU Y L,HUANG F Y,et al.Function value and processing research progress of Prunus mume[J].Agricultural Research and Application,2018,31(4):1-8.
[2] ADACHI M,SUZUKI Y,MIZUTA T,et al.The "Prunus mume Sieb.et Zucc" (Ume) is a rich natural source of novel anti-cancer substance[J].International Journal of Food Properties,2007,10(2):375-384.
[3] TIAN T T,SUN J Y,WU D H,et al.Objective measures of greengage wine quality:From taste-active compound and aroma-active compound to sensory profiles[J].Food Chemistry,2021,340:128179.
[4] HAN X Y,PENG Q,YANG H Y,et al.Influence of different carbohydrate sources on physicochemical properties and metabolites of fermented greengage (Prunus mume) wines[J].LWT-Food Science and Technology,2020,121:108 929.
[5] SADOUDI M,TOURDOT-MARÉCHAL R,ROUSSEAUX S,et al.Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts[J].Food Microbiology,2012,32(2):243-253.
[6] TOFALO R,PATRIGNANI F,LANCIOTTI R,et al.Aroma profile of montepulciano d’Abruzzo wine fermented by single and co-culture starters of autochthonous Saccharomyces and non-Saccharomyces yeasts[J].Frontiers in Microbiology,2016,7:610.
[7] LIU J,LIU M,YE P,et al.Characterization of major properties and aroma profile of kiwi wine co-cultured by Saccharomyces yeast (S.cerevisiae,S.bayanus,S.uvarum) and T.delbrueckii[J].European Food Research and Technology,2020,246(4):807-820.
[8] 丁莹, 李亚辉,蒲青,等.我国果酒行业发展现状及前景分析[J].酿酒科技,2019(4):104-107.
DING Y,LI Y H,PU Q,et al.Analysis of the present status and development prospects of fruit wine industry in china[J].Liquor-Making Science & Technology,2019(4):104-107.
[9] CHEN D,YAP Z Y,LIU S Q,et al.Evaluation of the performance of Torulaspora delbrueckii,Williopsis saturnus,and Kluyveromyces lactis in lychee wine fermentation[J].International Journal of Food Microbiology,2015,206:45-50.
[10] BENITO S.The impact of Torulaspora delbrueckii yeast in winemaking[J].Applied Microbiology and Biotechnology,2018,102(7):3 081-3 094.
[11] BIMPILAS A,PANAGOPOULOU M,TSIMOGIANNIS D,et al.Anthocyanin copigmentation and color of wine:The effect of naturally obtained hydroxycinnamic acids as cofactors[J].Food Chemistry,2016,197(Pt A):39-46.
[12] SINGLETON,V.L.,ROSSI,et al.Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents[J].American Journal of Enology and Viticulture,1965,16(3):144-158.
[13] WANG X,XIE K L,ZHUANG H N,et al.Volatile flavor compounds,total polyphenolic contents and antioxidant activities of a China gingko wine[J].Food Chemistry,2015,182:41-46.
[14] ZHANG L Q,HUANG J,ZHOU R Q,et al.Evaluating the feasibility of fermentation starter inoculated with Bacillus amyloliquefaciens for improving acetoin and tetramethylpyrazine in Baoning bran vinegar[J].International Journal of Food Microbiology,2017,255:42-50.
[15] ZHENG J,LIANG R,WU D C,et al.Discrimination of different kinds of Luzhou-flavor raw liquors based on their volatile features[J].Food Research International,2014,56:77-84.
[16] NIU M C,HUANG J,JIN Y,et al.Volatiles and antioxidant activity of fermented goji (Lycium Chinese) wine:Effect of different oak matrix (barrel,shavings and chips)[J].International Journal of Food Properties,2017,20(sup2):2 057-2 069.
[17] HE G Q,DONG Y,HUANG J,et al.Alteration of microbial community for improving flavor character of Daqu by inoculation with Bacillus velezensis and Bacillus subtilis[J].LWT-Food Science and Technology,2019,111:1-8.
[18] TANG Q X,HE G Q,HUANG J,et al.Characterizing relationship of microbial diversity and metabolite in Sichuan Xiaoqu[J].Frontiers in Microbiology,2019,10:696.
[19] TIAN T T,WU D H,NG C T,et al.A multiple-step strategy for screening Saccharomyces cerevisiae strains with improved acid tolerance and aroma profiles[J].Applied Microbiology and Biotechnology,2020,104(7):3 097-3 107.
[20] COJOCARU G A,ANTOCE A O.Influence of glutathione and ascorbic acid treatments during vinification of feteasca regala variety and their antioxidant effect on volatile profile[J].Biosensors,2019,9(4):140.
[21] BERENGUER M,VEGARA S,BARRAJ N E,et al.Physicochemical characterization of pomegranate wines fermented with three different Saccharomyces cerevisiae yeast strains[J].Food Chemistry,2016,190:848-855.
N E,et al.Physicochemical characterization of pomegranate wines fermented with three different Saccharomyces cerevisiae yeast strains[J].Food Chemistry,2016,190:848-855.
[22] BALMASEDA A,ROZ S N,LEAL M
S N,LEAL M  ,et al.Impact of changes in wine composition produced by non-Saccharomyces on malolactic fermentation[J].International Journal of Food Microbiology,2021,337:108954.
,et al.Impact of changes in wine composition produced by non-Saccharomyces on malolactic fermentation[J].International Journal of Food Microbiology,2021,337:108954.
[23] 熊勤梅, 肖胜舰,黄钧等.发酵型青梅酒的微滤:膜污染分析与品质评价[J].食品与发酵工业,2020,46(15):77-83.
XIONG Q M,XIAO S J,HUANG J,et al.Microfiltration of greengage wine:Membrane fouling analysis and quality evaluation[J].Food and Fermentation Industries,2020,46(15):77-83.
[24] TIAN T T,YANG H,YANG F,et al.Optimization of fermentation conditions and comparison of flavor compounds for three fermented greengage wines[J].LWT - Food Science and Technology,2018,89:542-550.
[25] 杨红亚, 吴少华,王兴红,等.气质联用分析青梅发酵酒和浸泡酒的香气成分[J].酿酒科技,2005(9):80-83.
YANG H Y,WU S H,WANG X H,et al.Analysis of flavoring compositions in green plum fruit fermenting wine and in green plum fruit steeping wine by GC-MS[J].Liquor-Making Science & Technology,2005(9):80-83.
[26] ZHENG X H,ZHANG M,FANG Z X,et al.Effects of low frequency ultrasonic treatment on the maturation of steeped greengage wine[J].Food Chemistry,2014,162:264-269.
[27] KHEIR J,SALAMEH D,STREHAIANO P,et al.Impact of volatile phenols and their precursors on wine quality and control measures of Brettanomyces/Dekkera yeasts[J].European Food Research and Technology,2013,237(5):655-671.
[28] CHATONNET P,DUBOURDIE D,BOIDRON,et al.The origin of ethylphenols in wines[J].Journal of the Science of Food and Agriculture,1992,60(2):165-178.
[29] HU B R,CAO Y,ZHU J Y,et al.Analysis of metabolites in chardonnay dry white wine with various inactive yeasts by 1H NMR spectroscopy combined with pattern recognition analysis[J].AMB Express,2019,9(1):140.
[30] R OS-REINA R,SEGURA-BORREGO M P,GARC
OS-REINA R,SEGURA-BORREGO M P,GARC A-GONZ
A-GONZ LEZ D L,et al.A comparative study of the volatile profile of wine vinegars with protected designation of origin by headspace stir bar sorptive extraction[J].Food Research International,2019,123:298-310.
LEZ D L,et al.A comparative study of the volatile profile of wine vinegars with protected designation of origin by headspace stir bar sorptive extraction[J].Food Research International,2019,123:298-310.
[31] GROSCH W.Evaluation of the key odorants of foods by dilution experiments,aroma models and omission[J].Chemical Senses,2001,26(5):533-545.
[32] BÖHMER M,SMOL’AK D,ŽENI OV
OV K,et al.Comparison of microbial diversity during two different wine fermentation processes[J].FEMS Microbiology Letters,2020,367(18):fnaa150.
K,et al.Comparison of microbial diversity during two different wine fermentation processes[J].FEMS Microbiology Letters,2020,367(18):fnaa150.
[33] KONG C L,LI A H,SU J,et al.Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S.cerevisiae[J].LWT-Food Science and Technology,2019,109:83-92.