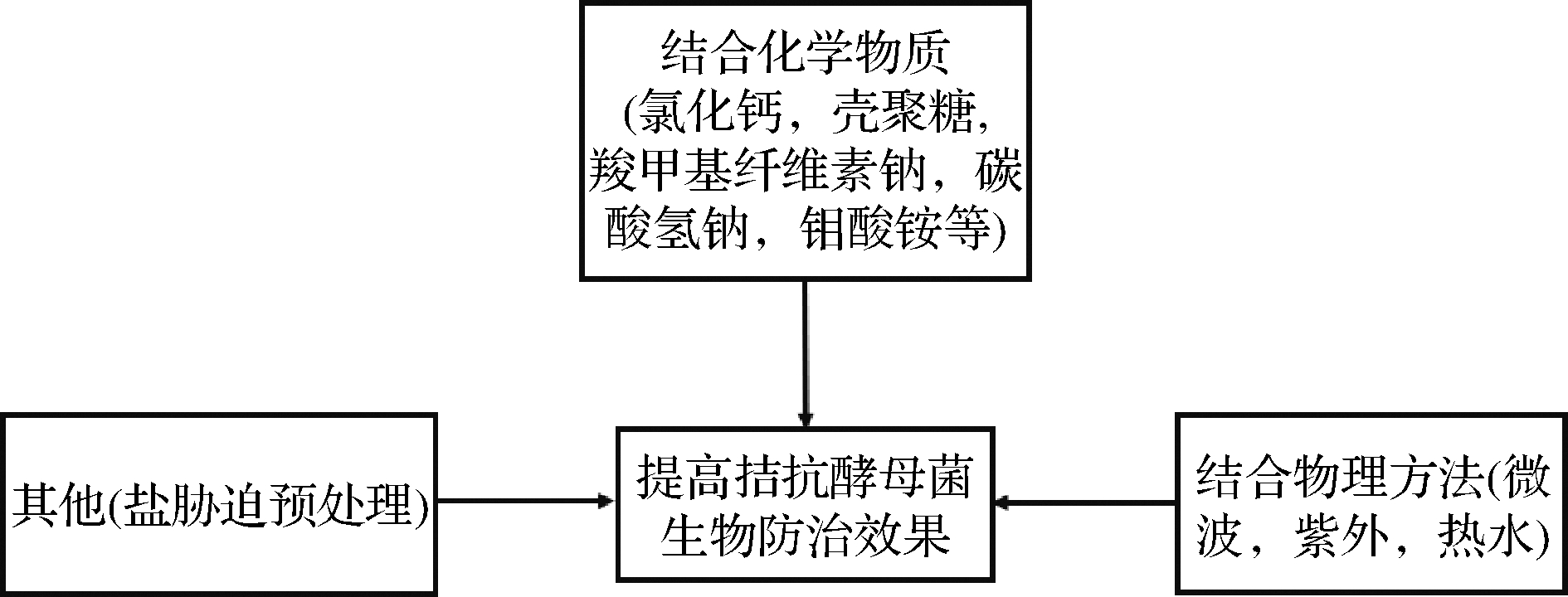
图1 提高拮抗酵母菌防治枣果实采后病害生物防治
效果示意图
Fig.1 Schematic diagram of improving biological control
effect of antagonistic yeast against postharvest diseases of
jujube fruit
枣果实富含氨基酸、蛋白质、膳食纤维、矿物质、抗坏血酸等营养物质,深受广大消费者喜爱[1]。然而,枣果实在采后贮藏期间极易发生微生物潜伏侵染性病害[2],其主要病害包括:由链格孢菌(Alternaria sp.)引起的黑斑病[3],细极链格孢菌(Alternaria alternate sp.)、青霉菌(Penicillium sp.)和芽枝孢菌(Cladosporium tenuissimum)等联合侵染引起的缩果病[4],交链孢霉属(Alternaria Nees)和根菌索菌(Rhizomorpha Roth.ex Fr.)[5]引起的浆胞病等。使用拮抗微生物进行生物防治是有效控制果蔬采后病害的方法之一[6],拮抗微生物防治果蔬采后病害的机制主要包括营养与空间的竞争,分泌抑菌性物质和诱导宿主抗性等[7]。采后应用拮抗微生物能有效地控制病害发生,积极开发有效的拮抗微生物,将拮抗微生物与物理技术或者化学物质结合起来以提高其防治效果是防治枣果实采后病害的研究热点。
本文将分别从拮抗酵母菌和拮抗细菌两方面综述近几年防治枣果实采后病害拮抗微生物的研究进展,包括拮抗微生物的来源、防治效果、增效途径和作用机制等方面。
大多数拮抗酵母菌来源于果实、叶片表面,以及根际土壤[8-9]。美极梅奇酵母(Metschnikowia pulcherrima)[10]、梅奇酵母XY201(Metschnikowia zizyphicola sp.nov.)[11]、隐球酵母(Cryptococcus sp.)[12]以及假丝酵母(Candida metapsilosis)[13]等拮抗酵母菌已成功从枣果实表面分离纯化,且能够有效防治枣果实采后病害;除了从枣果实本身分离筛选,张红印[14]从苹果表面分离得到的罗伦隐球酵母(Cryptococcus laurentii)对控制枣果实采后病害具有良好效果。除了密切相关的果实表面,拮抗酵母菌也可以从其他来源分离得到。如王一非[15]从海水中筛选分离的海洋酵母(Rhodosporidium paludigenum Fell&Tallman),能够有效防治枣果实采后黑斑病。
拮抗酵母菌能够耐受采后低温、紫外线辐射、低氧等极端环境[16-17],而且对于水果采后环境具有独特的适应性而被广泛研究应用于果实采后病害的防治。近10年来,以酵母菌为基础的水果采后病原微生物防治产品的开发、注册、和商业化取得了令人瞩目的进展[18]。不同拮抗酵母菌抑菌谱不同,因此对枣果实采后病害的防治效果也存在差异。拮抗酵母菌能够有效控制枣果实采后病害,但其防治效果相对有限,研究表明结合绿色化学物质或者物理防治技术还能够提高其对枣果实采后病害的防治效果[19]。拮抗酵母菌对于枣果实采后病害防治的增效途径如图1所示。

图1 提高拮抗酵母菌防治枣果实采后病害生物防治
效果示意图
Fig.1 Schematic diagram of improving biological control
effect of antagonistic yeast against postharvest diseases of
jujube fruit
与化学物质结合可以提高拮抗酵母菌生防效果。无机盐是常见的用于提高拮抗酵母生防效力的化学物质之一,研究表明使用1×108 cells/mL的R.paludigenum接种处理枣果实后其采后黑斑病发病率相对于对照降低了60%左右[15],当与0.3%碳酸氢钠结合处理时将枣果实采后黑斑病的发病率控制在30%以下;离体条件下,1×108 cells/mL的Cryptococcus sp.对交链孢霉抑制率高达54.08%,对根霉抑制率为48.72%,果实实验表明1×108 cells/mL的Cryptococcus sp.能够将冬枣采后的发病率降低约50%,碳酸氢钠以及钼酸铵能够提高Cryptococcus sp.对于枣果实采后病害的生物防治效果[20]。壳聚糖作为天然多糖类物质,具有良好的生物相容性,研究表明0.5%壳聚糖溶液能够将C.metapsilosis对枣果实采后病害的控制效果提高约20%[13]。此外,结合低计量的化学杀菌剂也能达到增效的目的,1×106 cells/mL的M.zizyphicola sp.nov可将由细交链格孢引发的冬枣病害发生率控制在8%以下[11],与低浓度的化学杀菌剂扑海因[10]组合使用能够提高其生防效果。除了上述的几类化学物质,将R.paludigenum结合0.3%羧甲基纤维素钠或尼泊金甲酯接种处理时对冬枣果实病害发病率控制在10%左右[21-22]。
除了通过化学物质提高酵母生防效力,还可以结合物理处理方式。常用的提高拮抗酵母菌防治果蔬采后病害防治效果的物理增效方法包括热水、冷藏、超声波以及紫外等处理[19],研究发现M.pulcherrima结合微波,紫外等物理处理均能够提高其对枣果实采后黑斑病的防治效果,并且不会对枣果实的品质造成影响[23]。
拮抗酵母菌在贮藏、施用过程中会遇到一系列逆境胁迫如低温、果实伤口处高水平活性氧、高渗透压等[24]。很多研究表明温和逆境预培养处理可以提高拮抗酵母菌对逆境的耐受性从而提高其生物防治效力。研究表明经6.6% NaCl预处理后R.paludigenum对于低温以及低水分活度等逆境环境具有更强的耐受性,将R.paludigenum对于枣果实采后病害的控制效果提高了20%左右[25]。除了上述的3种增效途径外,研究报道几种拮抗酵母菌复合使用、在培养基中添加外源物质对拮抗酵母进行诱导培养等均能够有效提高拮抗酵母菌对芒果、柑橘等果实采后病害的防治效果,这为更好的利用拮抗酵母菌防治枣果实采后病害提供新的思路。
全面了解拮抗微生物的作用机制对于有效的将其应用于采后病害的控制具有重要意义。拮抗酵母菌控制果蔬采后病害主要作用机制包括空间和营养的竞争,产生挥发性抑菌物质,诱导果实抗病性[26-28],生物膜的形成[29]等。拮抗酵母菌防治枣果实采后病害的主要机制如图2所示。

图2 拮抗酵母菌防治枣果实采后病害作用机制示意图
Fig.2 Schematic diagram of action mechanism of antagonistic
yeast against postharvest diseases of jujube fruit
不同的拮抗酵母菌的防治机制不尽相同,且同种拮抗酵母菌可能存在多种作用机制[30]。空间和营养的竞争是大多数拮抗酵母菌的生防机制之一,拮抗酵母菌在果实伤口处有效定殖,比病原菌更有效地利用有限的营养和空间资源从而抑制病原菌的生长繁殖。研究表明M.zizyphicola以及R.paludigenum能够在枣果实伤口处迅速定殖,消耗了伤口部位的可用营养物质,通过营养和空间的竞争有效防治枣果实采后病害[31]。
植物具有识别和响应微生物存在的先天免疫系统,将拮抗微生物应用于果实表面可诱导果实产生对入侵病原真菌的系统抗性。拮抗酵母菌诱导枣果实抗病性主要表现为生化防御反应的激活,如R.paludigenum能够诱导枣果实组织内一些防御相关的酶活性如过氧化物酶,多酚氧化酶等并使其活性显著提高,同样的,Cryptococcus sp.也能够显著提高枣果实组织内多酚氧化酶和超氧化物歧化酶活性[20]。研究发现枣果实接种C.laurentii后,果实组织中的β-1,3-葡聚糖酶活性显著升高,编码β-1,3-葡聚糖酶的基因GLU-2表达上调[32]。许多拮抗酵母菌能产生抑菌性物质,有效抑制病原真菌孢子萌发和菌丝生长。M.zizyphicola[31]可以产生一些非抗生素类代谢物质抑制病原菌的生长。
上述内容介绍了近年来枣果实采后病害防治的主要拮抗酵母菌的最新研究进展,并讨论了其作用效果和增效途径,相关内容总结如表1所示。防治枣果实采后的拮抗酵母菌主要来源于枣果实表面,这些拮抗酵母菌主要通过空间和营养的竞争以及诱导枣果实抗病性达到生防的目的。对于拮抗酵母菌防治枣果实采后病害的机制研究不够充分,导致开发经济有效的且能够大规模繁殖的拮抗酵母菌防治枣果实采后病害还存在一些挑战。
表1 防治枣果实采后病害主要的拮抗酵母菌
Table 1 The main antagonistic yeast against postharvest disease of jujube fruit

拮抗酵母菌来源病原物作用机制增效途径参考文献M.pulcherrima枣果实表面A.alternata, P.expansum空间和营养的竞争壳聚糖,微波,紫外,热水[10, 18-19]M.zizyphicola枣果实表面A.alternata, P.expansum空间和营养的竞争,产抑菌性物质扑海因[11, 33]R.paludigenum东海近海海域A.alternata诱导果实抗病性碳酸氢钠,尼泊金乙酯钠[16,20-21]C.laurentii枣果实表面A.alternata, P.expansum诱导果实抗病性碳酸氢钠,钼酸铵[12,22]C.metapsilosi枣果实病健交界A.alternata空间和营养的竞争,提高果实抗病性壳聚糖[13]
土壤是拮抗细菌良好的来源,从枣树根际土壤中分离得到一株枯草芽孢杆菌(Bacillus subtilis)[33]对于冬枣果实采后主要的致腐真菌根索菌、细极链格孢菌、扩展青霉等有显著的拮抗作用;张晓琴[34]从西洋参种植土中分离得到1株解淀粉芽孢杆菌(Bacillus amyloliticus)对枣胶胞炭疽病防治效果明显。果实表面也是拮抗细菌的来源之一,研究表明从冬枣表面分离得到的枯草芽孢杆菌xj063-1以及成团泛菌B501(Pantoea agglomerans)[35]能够有效的防治枣果实采后病害。
研究发现B.subtilis对于冬枣果实采后主要病原菌细交链格孢的抑制率达78.8%,多隔镰孢霉和美澳核果褐腐串珠霉抑制率分别达71.3%和63.8%,能够显著降低枣果实采后发病率[36]。B.subtilis xj063-1对于新疆枣果黑斑病病原菌链格孢菌的抑制率达到86%[33]。B.amyloliticus菌体及其发酵上清液对冬枣果实炭疽病的防治效果分别达到74.9%和75.8%[37],菌株K5-4对于冬枣黑斑病的防治率达到78.50%[38]。结合物理方法或者化学物质提高拮抗细菌对于枣果实采后病害防治效果的相关报道较少,研究表明将B.subtilis的内生孢子与水杨酸结合处理能够显著增强拮抗细菌的防治效果,降低土豆以及桃果实腐烂率[39-41],拮抗细菌与其他方法结合提高生防效果将为其更好地防治枣果实病害提供新思路。
与拮抗酵母菌相同,空间和营养的竞争也是拮抗细菌的生防机制之一[42]。研究表明P.agglomerans防治枣果实采后病害主要的作用机制可能是空间和营养的竞争。而很多拮抗细菌如芽孢杆菌,假单胞菌等通过分泌化合物抑制细胞壁合成,破坏或改变细胞膜结构等机制抑制病原菌生长发育从而达到生物防治的目的。研究表明拮抗菌CHY主要产生伊枯草菌素(iturin)和表面活性素(surfactin)两种脂肽类抑菌物质抑制病原菌的生长,从而达到防治枣果实采后病害的目的;B.amyloliticus也能够产生抗生素等抑菌物质[43]。拮抗细菌也能够诱导果实的抗病性,研究表明经过B.amyloliticus处理,枣果实组织内苯丙氨酸解氨酶、过氧化氢酶、多酚氧化酶、氧化物酶4种防御酶的活性显著提高[34]。综上,拮抗细菌主要通过产生抑菌性物质以及诱导枣果实抗病性防治枣果实采后病害。
拮抗细菌具有潜在的抑真菌能力,芽孢杆菌属是常见果蔬采后拮抗细菌包括枯草芽孢杆菌、粉状芽孢杆菌、地衣芽孢杆菌以及解淀粉芽孢杆菌[44]等。以上综述了防治枣果实采后病害的拮抗细菌及其作用机制,总结如表2所示。
表2 防治枣果实采后病害的主要拮抗细菌
Table 2 The main antagonistic bacteria against postharvest disease of jujube fruit

拮抗细菌来源病原菌作用机制文献B.subtilis土壤Rhizomorpha Roth.ex Fr,A.alternata产抑菌性物质[36,38]B.amyloliquefa-ciens土壤A.alternata产生伊枯草菌素和表面活性素,诱导果实抗病性[35,43]P.agglomerans冬枣表面A.alternata, P.expansum空间和营养的竞争[37]
有效控制枣果实采后病害是枣产业可持续发展的重要组成部分,生物防治是有望替代化学杀菌剂的一种绿色环保的防治方法。目前已经成功筛选分离出几种有效防治枣果实采后病害的拮抗微生物,研究表明其作用机制主要包括空间和营养的竞争以及诱导枣果实抗病性,以及产生抑菌性物质等。在枣果实中控制其采后病害的拮抗微生物的研究进展 与柑橘、梨、芒果等果实相比还存在一定差距[19,45],对于拮抗微生物防治枣果实采后病害的机理研究不足,导致目前拮抗微生物的生防效果相对较低,同时要开发商业化可行的拮抗微生物防治枣果实采后病害还具有一定的挑战[46-47]。目前常用的拮抗微生物防治方法是鉴定一种单一的能在受损的果实组织中迅速定殖的拮抗微生物[48],这种方法忽略了拮抗微生物并不是整个微生物群落唯一的参与者,以及忽略了拮抗微生物与宿主微生物之间的相互作用[49-50]。微生物组学将为了解宿主-微生物相互作用对果蔬代谢和抗病性的影响,为探索利用有益的微生物群落,防治枣果实采后病害提供基础,除此之外还需要利用分子生物学手段深入了解微生物拮抗剂防治果蔬采后病害机理,为我们在枣果实及其他果蔬采后病害生物防治以及研究生物防治产品提供新的思路。
[1] RASHWAN A K, KARIM N, SHISHIR M R I, et al.Jujube fruit:A potential nutritious fruit for the development of functional food products[J].Journal of Functional Foods, 2020, 75:104205.
[2] WANG J, SONG L Q, JIAO Q Q, et al.Comparative genome analysis of jujube witches′-broom Phytoplasma, an obligate pathogen that causes jujube witches′-broom disease[J].BMC Genomics, 2018, 19(1):1-12.
[3] HONG L, YANG L F, YANG J, et al.Screening and optimizing fermentation conditions of Actinomycetes spp.antagonistic to Alternaria sp.causing black spot of jujube[J].Southwest China Journal of Agricultural Sciences, 2018, 31(8):1 634-1 637.
[4] 苟建新. 枣缩果病病原形态鉴定及田间药效防治试验[J].中国园艺文摘, 2018,34(3):65-67;73.
GOU J X.Jujube fruit shrink disease pathogen morphological identification and field efficacy prevention orticulture[J].Chinese Horticulture Abstracts.2018,34(3):65-67;73.
[5] LIU Z G, ZHAO Z H, XUE C L, et al.Three main genes in the MAPK cascade involved in the Chinese jujube-phytoplasma interaction[J].Forests, 2019, 10(5):392.
[6] YOU W J, GE C H, JIANG Z C, et al.Screening of a broad-spectrum antagonist-Bacillus siamensis, and its possible mechanisms to control postharvest disease in tropical fruits[J].Biological Control, 2021,157:104584.
[7] LEGEIN M, SMETS W, VANDENHEUVEL D, et al.Modes of action of microbial biocontrol in the phyllosphere[J].Frontiers in Microbiology, 2020, 11:1 619.
[8] DA COSTA W K A, DE SOUZA G T, BRAND O L R, et al.Exploiting antagonistic activity of fruit-derived Lactobacillus to control pathogenic bacteria in fresh cheese and chicken meat[J].Food Research International, 2018, 108:172-182.
O L R, et al.Exploiting antagonistic activity of fruit-derived Lactobacillus to control pathogenic bacteria in fresh cheese and chicken meat[J].Food Research International, 2018, 108:172-182.
[9] CHEN C, CAO Z, LI J, et al.A novel endophytic strain of Lactobacillus plantarum CM-3 with antagonistic activity against Botrytis cinerea on strawberry fruit[J].Biological Control, 2020, 148:104306.
[10] 郭东起, 沈永娟.水溶性壳聚糖对美极梅奇酵母菌拮抗效力的影响[J].北方园艺, 2015(24):127-130.
GUO D Q, SHEN Y J.Effect of water-solube chitosan on antagonist Metschnikowia pulcherrima[J].Northern Horticulture, 2015(24):127-130.
[11] 施俊凤, 薛梦林, 张晓宇, 等.梅奇酵母XY201对冬枣采后病原真菌的抑制效果[J].中国生物防治, 2010, 26(3):287-292.
SHI J F, XUE M L, ZHANG X Y, et al.Inhibitory effect of Metschnikowia zizyphicola XY201 on fungal pathogens of postharvest jujube fruit[J] Chinese Journal of Biological Control, 2010, 26(3):287-292.
[12] HE C, ZHANG Z Q, LI B Q, et al.Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit[J].Postharvest Biology and Technology, 2019, 151:134-141.
[13] 李青云. 新疆冬枣贮藏期拮抗酵母菌筛选及鉴定的研究[D].新疆:石河子大学, 2015.
LI Q Y.Study on screening and identification of antagonistic yeast in Xinjiang jujube during storage[D].Xinjiang:Shihezi University, 2015.
[14] 张红印. 罗伦隐球酵母对水果采后病害的生物防治及其防治机理研究[D].杭州:浙江大学, 2004.
ZHANG H Y.The study of biocontrol and mechanism of action on postharevest diseases of fruits by Cryptococcus laurentii[D].Hangzhou:Zhejiang University, 2004.
[15] 王一非. 海洋拮抗酵母Rhodosporidium paludigenum对果实采后病害生物防治的研究[D].杭州:浙江大学, 2008.
WANG Y F.Postharvest biological control of fruits and vegetables by marine yeast Rhodosporidium paludigenum[D].Hangzhou:Zhejiang University, 2008.
[16] FREIMOSER F M, RUEDA-MEJIA M P, TILOCCA B, et al.Biocontrol yeasts:Mechanisms and applications[J].World Journal of Microbiology and Biotechnology, 2019, 35(10):1-19.
[17] NADAI C, FERNANDES LEMOS W J, FAVARON F, et al.Biocontrol activity of Starmerella bacillaris yeast against blue mold disease on apple fruit and its effect on cider fermentation[J].PLoS One, 2018, 13(9):e0204350.
[18] MUKHERJEE A, VERMA J P, GAURAV A K, et al.Yeast a potential bio-agent:Future for plant growth and postharvest disease management for sustainable agriculture[J].Applied Microbiology and Biotechnology, 2020, 104(4):1 497-1 510.
[19] WANG Z S, SUI Y, LI J S, et al.Biological control of postharvest fungal decays in Citrus:A review[J].Critical Reviews in Food Science and Nutrition, 2020.DOI: 10.1080/10408398.2020.1829542.
[20] 班兆军, 李莉, 李喜宏, 等.隐球酵母对长枣果实的生防效果及诱导抗性[J].植物保护, 2009, 35(3):77-81.
BAN Z J, LI L, LI X H, et al.Effects of Cryptococcus on biological control of diseases on post-harvest long jujubes[J].Plant Protection, 2009, 35(3):77-81.
[21] MAHUNU G, ABUBARKARI A, GARTI H.Antagonistic yeasts and alternative chemical combinations as potential biocontrol agents in postharvest application[J].UDS International Journal of Development, 2020, 7(1):273-284.
[22] FU M R, ZHANG X M, JIN T, et al.Inhibitory of grey mold on green pepper and winter jujube by chlorine dioxide (ClO2) fumigation and its mechanisms[J].LWT, 2019, 100:335-340.
[23] FIGUEROA J G, BORR S-LINARES I, DEL PINO-GARC
S-LINARES I, DEL PINO-GARC A R, et al.Functional ingredient from avocado peel:Microwave-assisted extraction, characterization and potential applications for the food industry[J].Food Chemistry, 2021, 352:129300.
A R, et al.Functional ingredient from avocado peel:Microwave-assisted extraction, characterization and potential applications for the food industry[J].Food Chemistry, 2021, 352:129300.
[24] KÖHL J, KOLNAAR R, RAVENSBERG W J.Mode of action of microbial biological control agents against plant diseases:Relevance beyond efficacy[J].Frontiers in Plant Science, 2019, 10:845.
[25] WANG Y F, WANG P, XIA J D, et al.Effect of water activity on stress tolerance and biocontrol activity in antagonistic yeast Rhodosporidium paludigenum[J].International Journal of Food Microbiology, 2010, 143(3):103-108.
[26] CONTARINO R, BRIGHINA S, FALLICO B, et al.Volatile organic compounds (VOCs) produced by biocontrol yeasts[J].Food Microbiology, 2019, 82:70-74.
[27] GORE-LLOYD D, SUMANN I, BRACHMANN A O, et al.Snf2 controls pulcherriminic acid biosynthesis and antifungal activity of the biocontrol yeast Metschnikowia pulcherrima[J].Molecular Microbiology, 2019, 112(1):317-332.
[28] CARB A, TORRES R, TEIXID
A, TORRES R, TEIXID N, et al.Predicted ecological niches and environmental resilience of different formulations of the biocontrol yeast Candida sake CPA-1 using the Bioscreen C[J].BioControl, 2018, 63(6):855-866.
N, et al.Predicted ecological niches and environmental resilience of different formulations of the biocontrol yeast Candida sake CPA-1 using the Bioscreen C[J].BioControl, 2018, 63(6):855-866.
[29] PANDIN C, LE COQ D, CANETTE A, et al.Should the biofilm mode of life be taken into consideration for microbial biocontrol agents?[J].Microbial Biotechnology, 2017, 10(4):719-734.
[30] WANG S P, RUAN C Q, YI L H, et al.Biocontrol ability and action mechanism of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit[J].Food Microbiology, 2020, 87:103375.
[31] 薛梦林. 拮抗菌对冬枣采后病害的生物防治[D].杨凌:西北农林科技大学, 2006.
XUE M L.Biological control of postharvest diseases of jujube fruit using antagonists[D].Yangling:Northwest A&F University, 2006.
[32] TIAN S P, YAO H J, DENG X, et al.Characterization and expression of beta-1,3-glucanase genes in jujube fruit induced by the microbial biocontrol agent Cryptococcus laurentii[J].Phytopathology, 2007, 97(3):260-268.
[33] 马荣, 刘晓琳, 孙园园, 等.新疆枣果黑斑病拮抗细菌的筛选及鉴定[J].新疆农业大学学报, 2014, 37(6):460-464.
MA R, LIU X L, SUN Y Y, et al.Screening and identification of the antagonistic bacteria of jujube black spot disease in Xinjiang[J].Journal of Xinjiang Agricultural University, 2014, 37(6):460-464.
[34] 张晓琴. 芽孢杆菌CHY对枣胶胞炭疽病拮抗作用及其拮抗物质的研究[D].西安:西北大学, 2015.
ZAHNG X Q.Study on antagonistic effect of bacillus CHY on jujube colanthracnose and its antagonistic substances[D].Xi′an:Northwestern University, 2015.
[35] 薛梦林, 张力群, 张继澍, 等.拮抗菌B501的鉴定及其对采后冬枣黑斑病的抑制效果[J].中国生物防治, 2008,24(2):122-127.
XUE M L, ZHANG L Q, ZHANG J S, et al. Identification of bacterial strain B501 and its biocontrol activity against black spot on jujube fruits[J].Chinese Journal of Biological Control, 2008,24(2):122-127.
[36] 耿海峰, 张丽珍, 牛伟.冬枣采后病害拮抗菌的筛选和鉴定[J].食品科学, 2010, 31(9):150-155.
GENG H F, ZHANG L Z, NIU W.Screening and identification of antagonistic bacteria against post-harvest diseases of jujube fruits[J].Food Science, 2010, 31(9):150-155.
[37] 李嘉维, 徐兰依, 王冬霜, 等.枣炭疽病菌拮抗芽孢杆菌的筛选鉴定及其抑菌物质分析[J].园艺学报, 2019, 46(12):2 406-2 414.
LI J W, XU L Y, WANG D S, et al.Screening and identification of Bacillus antagonist against anthracnose of jujube and analysis of its antifungal substances[J].Acta Horticulturae Sinica, 2019, 46(12):2 406-2 414.
[38] 宋聪, 黄亚丽, 谢晨星, 等.冬枣黑斑病菌拮抗菌株的筛选鉴定及其对该病的防治作用[J].河南农业科学, 2016, 45(7):71-75.
SONG C, HUANG Y L, XIE C X, et al.Screening and control effect determination of antagonistic bacteria against black spot disease of jujube[J].Journal of Henan Agricultural Sciences, 2016, 45(7):71-75.
[39] LASTOCHKINA O, BAYMIEV A, SHAYAHMETOVA A, et al.Effects of endophytic Bacillus subtilis and salicylic acid on postharvest diseases (Phytophthora infestans, Fusarium oxysporum) development in stored potato tubers[J].Plants, 2020, 9(1):76.
[40] YOU W J, GE C H, JIANG Z C, et al.Screening of a broad-spectrum antagonist-Bacillus siamensis, and its possible mechanisms to control postharvest disease in tropical fruits[J].Biological Control, 2021, 157:104584.
[41] LASTOCHKINA O, GARSHINA D, IVANOV S, et al.Seed priming with endophytic Bacillus subtilis modulates physiological responses of two different Triticum aestivum L. cultivars under drought stress[J].Plants-Basel, 2020, 9(12):1 810.
[42] LIU M, ZHAO X, LI X, et al.Antagonistic effects of Delia antiqua (Diptera:Anthomyiidae)-associated bacteria against four phytopathogens[J].Journal of Economic Entomology, 2021, 114(2):597-610.
[43] WANG X, XIAO C, JI C, et al.Isolation and characterization of endophytic bacteria for controlling root rot disease of Chinese jujube[J].Journal of Applied Microbiology, 2021, 130(3):926-936.
[44] HUANG X Q, REN J, LI P H, et al.Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables[J].Journal of the Science of Food and Agriculture, 2021, 101(5):1 744-1 757.
[45] SUN C, FU D, LU H P, et al.Autoclaved yeast enhances the resistance against Penicillium expansum in postharvest pear fruit and its possible mechanisms of action[J].Biological Control, 2018, 119:51-58.
[46] KUSSTATSCHER P, CERNAVA T, ABDELFATTAH A, et al.Microbiome approaches provide the key to biologically control postharvest pathogens and storability of fruits and vegetables[J].FEMS Microbiology Ecology, 2020, 96(7).DOI:10.1093/femsec/fiaa119.
[47] BÖSCH Y, BRITT E, PERREN S, et al.Dynamics of the apple fruit microbiome after harvest and implications for fruit quality[J].Microorganisms,2021, 9(2): 272.
[48] WISNIEWSKI M, DROBY S, ABDELFATTAH A, et al.Spatial and compositional diversity in the microbiota of harvested fruits:What can it tell us about biological control of postharvest diseases[J].Postharvest Pathology:Springer, 2021.DOI:10.1007/978-3-030-56530-5_4.
[49] IQRAR I, SHINWARI Z K, EL-SAYED A S A F, et al.Exploration of microbiome of medicinally important plants as biocontrol agents against Phytophthora parasitica[J].Archives of Microbiology, 2021,203(5):2 475-2 489.
[50] WISNIEWSKI M, DROBY S.The postharvest microbiome:The other half of sustainability[J].Biological Control, 2019, 137:104025.