惰性碳氢键的选择性单加氧反应是有机合成中具有探索意义的一类化学反应,目前主要采用化学法和酶法催化,化学催化法往往反应条件苛刻,而酶催化法相对温和,且具有很高的区域和对映选择性[1]。来自茶树菇(Agrocybe aegerita)的非特异性过氧化酶(unspecific peroxygenase,Aae UPO,EC 1.11.2.1)仅需H2O2为共底物即可进行复杂的单加氧反应[2],在催化乙苯反应时,(R)-苯乙醇产率可达到99.9%,对映体过量(enantiomeric excess, ee)值大于99.9%[2]。但是,非特异性过氧化酶作为一种血红素依赖型酶,在高浓度H2O2下容易失活[3]。酶催化原位产生H2O2可持续稳定地为AaeUPO提供较低浓度的H2O2,且过程温和高效[4],是一种避免外加高浓度H2O2的有效途径之一。
葡萄糖氧化酶(glucose oxidase,GOx)是原位产生H2O2最常用的酶源。GOx氧化葡萄糖的同时将O2还原为H2O2,仅产生葡萄糖酸内酯一种无毒无害的副产物。此外,与GOx结合的酶级联系统与单次添加H2O2相比,更有助于酶稳定性的提高。在GOx与氯过氧化物酶(chloroperoxidase,CPO)结合的酶级联系统中,总的反应速率由H2O2进入CPO活性口袋的速度决定,催化茴香硫醚的产率最高可达100%[5]。YANG等[6]通过制备复合交联酶聚集体的方式对GOx和辣根过氧化物酶(horseradish peroxidase,HRP)进行共固定,在此级联反应中,没有H2O2积累,说明GOx产生的H2O2被HRP有效利用,同时双酶复合交联酶聚集体(combi-CLEAs)获得了更高的稳定性。
本研究采用交联酶聚集体方法共固定AaeUPO与GOx,通过优化AaeUPO与GOx的酶活力比例、沉淀剂与酶液的体积比、交联剂的体积分数以及牛血清白蛋白(bovine serum albumin, BSA)的质量浓度,以期得到高酶活回收率的combi-CLEAs。在乙苯惰性C—C键上加入氧原子需要约400 kJ/mol的能量,而生物催化法可以高效环保的实现这一过程,因此以乙苯为模型底物,将combi-CLEAs用于催化乙苯生成(R)-苯乙醇,考察反应液pH、反应温度和葡萄糖浓度对乙苯转化率的影响,并以不同底物对combi-CLEAs的催化性能进行验证。该研究制备AaeUPO与GOx的combi-CLEAs,有助于2种酶结合得更紧密,提高催化效率[7-8]。
1 材料与方法
1.1 材料
AaeUPO菌株,实验室构建的基因工程菌P.pastoris/pPIC9k/PaDa-I;GOx(231 U/mg),上海源叶生物科技有限公司;异丙醇、葡聚糖、高碘酸钠、BSA、葡萄糖、ABTS、酵母粉、蛋白胨,天津鼎国生物技术有限责任公司;乙苯、乙酸乙酯、乙腈,凯玛特(天津)化工科技有限公司;H2O2(AR),天津市江天化工技术有限公司;其他试剂均为市售分析纯。
BMGY培养基:1%酵母粉(质量分数,下同)、2%蛋白胨、1.34%YNB、4×10-5%生物素、1%甘油、100 mmol/L磷酸钾缓冲液(pH 6.0);BMMY培养基:1%酵母粉、2%蛋白胨、1.34%YNB、4×10-5%生物素、1%甲醇、100 mmol/L的磷酸钾缓冲液(pH 6.0)。其中YNB、生物素和甲醇不能高温灭菌,用0.22 μm的水系滤膜除菌,其他培养基在121 ℃下高温灭菌20 min。
1.2 实验方法
1.2.1 AaeUPO的制备
在BMGY培养基中,以1%的接种量将AaeUPO甘油菌进行活化,在30 ℃、220 r/min条件下培养16~18 h,待OD600≥2后,8 000 r/min离心10 min,收集菌体。将菌体加入到BMMY诱导培养基中复溶,在30 ℃、220 r/min条件下进行诱导表达,随后每隔24 h加入1次体积分数1%甲醇,连续诱导5 d。离心分离发酵液(8 000 r/min,10 min),收集上清液,得到粗酶液。经聚丙烯酰胺凝胶电泳测定,AaeUPO为46 kDa的胞外酶(图1),对其酶活性进行测定,AaeUPO的比酶活力为130 U/mg。

M-Marker;1-AaeUPO
图1 AaeUPO粗酶液的SDS-PAGE
Fig.1 SDS-PAGE of AaeUPO
1.2.2 葡聚糖醛的制备
将1.65 g葡聚糖和3.85 g高碘酸钠溶于50 mL蒸馏水中,室温下搅拌反应90 min。倒入透析袋中,透析24 h,中途不断更换超纯水以去除残余的高碘酸钠,冷冻干燥后备用。
1.2.3 Combi-CLEAs的制备
制备过程如图2所示。GOx(30 U/mL,pH 7.0)与AaeUPO(pH 7.0)按比例混合至2 mL,加入一定量的BSA和沉淀剂,25 ℃下磁力搅拌30 min。随后加入一定体积分数的交联剂,10 ℃下交联3 h。8 000 r/min下离心10 min,弃去上清液,所得沉淀经磷酸盐缓冲液(100 mmol/L,pH 7.0)重悬洗涤离心3次,即得combi-CLEAs。
分别考察GOx与AaeUPO酶活力比、沉淀剂与酶液的体积比、交联剂体积分数和BSA质量浓度对combi-CLEAs酶活回收率的影响。
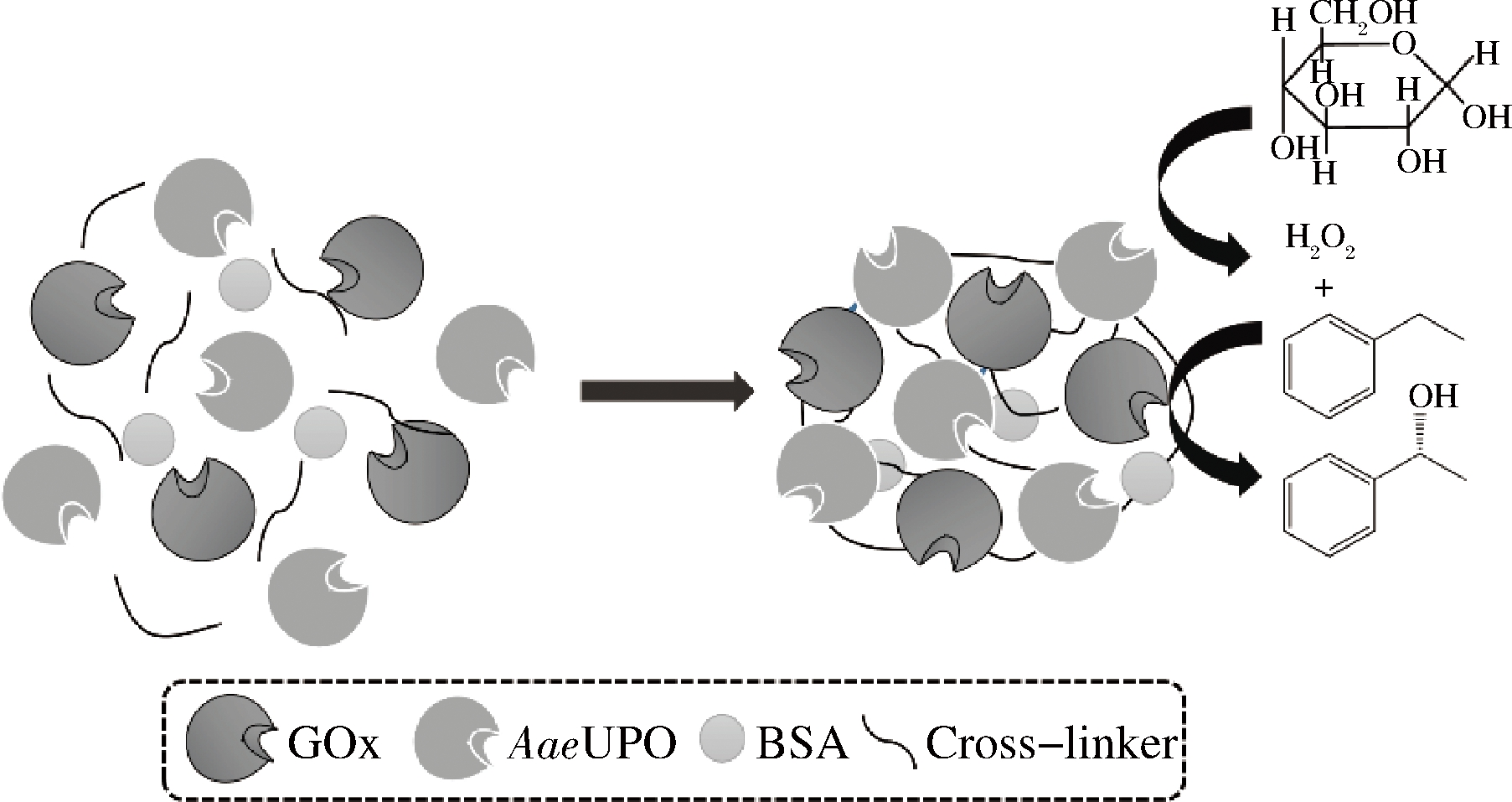
图2 Combi-CLEAs的制备过程
Fig.2 Preparation process of combi-CLEAs
1.2.4 酶活力和蛋白质浓度测定
游离AaeUPO和combi-CLEAs的酶活性测定均采用ABTS显色法[9]。在1 mL反应体系中加入4 mmol/L葡萄糖、0.3 mmol/L ABTS、适量的combi-CLEAs和柠檬酸-柠檬酸钠缓冲液(100 mmol/L,pH 5.0),30 ℃下反应5 min,在波长418 nm下测得反应液的吸光值。活力单位(U)定义:在30 ℃、pH 5.0条件下,在1 min内生成1 μmol ABTS自由基所需要的酶量为1 U。比酶活、相对酶活力和酶活回收率的计算如公式(1)~(3)所示:
比酶活![]()
(1)
相对酶活性![]()
(2)
酶活回收率![]()
(3)
蛋白质浓度采用考马斯亮蓝法测定。
2 结果与分析
2.1 Combi-CLEAs制备条件优化
H2O2是AaeUPO的共底物,较高浓度的H2O2会导致AaeUPO失活,为使中间产物H2O2的产生和消耗达到平衡,需要考察双酶酶活力比对combi-CLEAs酶活力的影响[10-11]。如图3-a所示,当GOx与AaeUPO的酶活力比为6∶1时,酶活回收率最高。其原因是当GOx与AaeUPO酶活力比较低时,GOx催化葡萄糖氧化产生的H2O2较少,导致AaeUPO催化动力不足。而当GOx与AaeUPO酶活力比过高时,GOx为AaeUPO提供的H2O2浓度过高,造成AaeUPO血红素中心的稳定性变差甚至失活。当GOx与AaeUPO的酶活力比为6∶1时,GOx为AaeUPO提供的H2O2处在一个供应和消耗平衡的状态,可使combi-CLEAs持续高效地进行催化。
制备combi-CLEAs的过程中选用了沉淀效果较好的异丙醇,随后考察了异丙醇与酶液的体积比对酶活力回收率的影响。如图3-b所示,当异丙醇与酶液体积比为1∶1时,酶活力回收率较低,这是因为沉淀剂过少时无法破坏酶的水化层,沉淀不完全,部分酶无法交联[12]。当异丙醇与酶液体积比为2∶1时,combi-CLEAs的酶活力回收率达到最大,为63.68%,比酶活力为40.17 U/mg。异丙醇继续增加,过多的异丙醇会争夺酶分子表面的水化层甚至改变它的结构与构象,从而导致酶变性失活。
制备交联酶聚集体时,一般是酶分子表面上赖氨酸的氨基与交联剂的醛基以非共价键的方式结合,但如果交联时涉及到酶活性中心的氨基,则会对酶活性造成较大影响。因此,本实验中选用了分子质量较大的葡聚糖醛(200 kDa)做交联剂,防止交联剂穿透酶的活性位点与催化所必需氨基酸残基反应[13]。如图3-c所示,当葡聚糖醛体积分数过低时,无法使酶充分交联,而体积分数过高,会使酶分子过度交联,柔韧性变差[14],酶活力回收率降低。葡聚糖醛的体积分数为15%时,combi-CLEAs的酶活力回收率最高,为82.55%,此时的比酶活力为59.03 U/mg。为解决酶因赖氨酸残基较少,无法充分交联的问题,在交联过程中加入富含氨基的BSA,BSA可使酶分子被充分交联,减少酶分子的泄露,使combi-CLEAs的机械性能更加稳定[15-16]。如图3-d所示,随着BSA质量浓度的增加,酶活力回收率提高。但当BSA质量浓度过大时,部分交联剂用于BSA之间的交联,使酶分子无法充分交联,所以酶活力回收率降低[17]。当BSA质量浓度为40 μg/mL时,combi-CLEAs的酶活回收率最高,为87.43%,比酶活力为63.12 U/mg。
综上,制备combi-CLEAs时,GOx与AaeUPO酶活力比为6∶1、异丙醇与酶液的体积比为2∶1、200 kDa葡聚糖醛体积分数为15%,且加入40 μg/mL的BSA为保护剂时,combi-CLEAs的酶活力回收率为87.43%,比酶活力为63.12 U/mg。

a-酶活力比;b-异丙醇与酶液体积比;c-200 kDa葡聚糖醛的体积分数;d-BSA的质量浓度
图3 Combi-CLEAs制备条件的优化
Fig.3 Optimization of preparation conditions of combi-CLEAs
2.2 Combi-CLEAs催化乙苯的羟基化反应
将制备的combi-CLEAs用于催化乙苯生成光学纯(R)-苯乙醇,考察反应液pH、反应温度、葡萄糖浓度对乙苯转化率的影响。
2.2.1 pH和温度对反应的影响
酶的催化效率受多种环境因素影响,其中pH值的变化可改变酶分子活性部位和整个表面的电荷分布,进而导致乙苯转化率的改变[18],而温度的变化则影响酶分子的天然结构,导致其催化活性改变,最终影响乙苯的转化率[19]。由实验结果可知,combi-CLEAs催化乙苯的最适pH为7.0,最适温度为30 ℃。
2.2.2 葡萄糖浓度对反应的影响
GOx以葡萄糖为底物生成H2O2与葡萄糖酸内酯,AaeUPO利用产生的H2O2为共底物催化乙苯的羟基化反应。葡萄糖浓度直接影响GOx生成H2O2的浓度与速率,间接影响了AaeUPO催化乙苯的转化,因此需要对葡萄糖浓度进行优化。
从图4-a可以看出,葡萄糖初始浓度为2 mmol/L,H2O2生成速率较快,此浓度下combi-CLEAs在20 min时对乙苯的转化率为67.0%。随着时间的延长,乙苯的转化率最高可达91.4%,这说明在2 mmol/L葡萄糖浓度下,GOx可以较为迅速地为AaeUPO提供H2O2,但累积的H2O2不足以完全催化乙苯的反应。由图4-b、图4-c可以看出,在葡萄糖初始浓度为4和6 mmol/L时,乙苯最终的转化率均略高于2 mmol/L葡萄糖浓度,说明GOx能为AaeUPO提供较为充足的H2O2。反应20 min时,4 mmol/L葡萄糖浓度下乙苯的转化率要高于6 mmol/L葡萄糖浓度,这可能是由于较高浓度的H2O2对GOx造成了一定程度的产物抑制[20]。由图4-d可以看到,在8 mmol/L的葡萄糖浓度下,在反应20 min时,由于受到明显的产物抑制,GOx无法快速提供足够的H2O2,使得此时combi-CLEAs对乙苯的转化率仅为18.3%。而在3 h后乙苯的转化率为89.2%,较低的乙苯转化率可能是由于产生的H2O2浓度较高,造成了部分combi-CLEAs的不稳定[21]所致。由图4可以得出在6 mmol/L葡萄糖浓度下,可以得到99.9%的乙苯转化率,且(R)-苯乙醇ee值大于99.9%。

a-2 mmol/L葡萄糖;b-4 mmol/L葡萄糖;c-6 mmol/L葡萄糖;d-8 mmol/L葡萄糖
图4 Combi-CLEAs催化乙苯过程中葡萄糖浓度的优化
Fig.4 Optimization of glucose concentration in ethylbenzene catalyzed by combi-CLEAs
2.2.3 Combi-CLEAs重复使用性
实现生物催化剂的重复使用是固定化酶的重要目标之一。Combi-CLEAs操作稳定性如图5-a所示,在重复使用过程中,乙苯转化率逐次降低,重复使用第8次时乙苯转化率降为67.2%。经图5-b可知,造成转化率降低的原因是由于缓冲液对combi-CLEAs的不断冲洗使部分酶脱落,另外重复操作会导致蛋白构象发生变化,使活性部位发生扭曲,导致酶分子自身变性[22]。
2.3 Combi-CLEAs催化其他底物的羟基化反应
Combi-CLEAs通过无载体固定化的方式将GOx与AaeUPO进行紧密结合,使GOx原位稳定的为AaeUPO提供催化所需的H2O2。相较于外加H2O2的方式,combi-CLEAs在催化不同底物进行单加氧反应时更加稳定高效。由表1可以看出,combi-CLEAs对不同底物的转化率均高于AaeUPO-CLEAs,说明其在实际应用中还有很大的空间。

图5 Combi-CLEAs操作稳定性
Fig.5 Operation stability of combi-CLEAs
3 结论
AaeUPO广泛用于单加氧反应中,该类反应均以H2O2为共底物,鉴于GOx可原位制备H2O2,本文构建了GOx与AaeUPO复合交联酶聚集体级联催化系统。论文考察了制备条件对combi-CLEAs的活性的影响,在GOx与AaeUPO的酶活力比为为6∶1、异丙醇与酶液体积比为2∶1、200 kDa的葡聚糖醛体积分数为15%,加入BSA后进行交联,在该条件下获得了结构紧密、机械稳定的combi-CLEAs,其比酶活力为63.12 U/mg。通过设计级联系统为AaeUPO提供H2O2,酶的稳定性大大提高,将combi-CLEAs用于催化乙苯羟基化生成(R)-苯乙醇的反应中,在优化的pH、反应温度和葡萄糖浓度条件下,乙苯的转化率高达99.9%,产物(R)-苯乙醇的ee值>99.9%,重复使用8次后,仍保留67.2%的乙苯转化率。并且将combi-CLEAs应用不同底物的羟基化时,其性能均优于外加H2O2的AaeUPO-CLEAs。由于该固定化方法对酶要求较低,适合于多酶的固定化,且GOx与AaeUPO构成的固定化级联系统稳定好、催化效率高且易于制备,因此在大规模工业生产方面具有很好的应用前景。
表1 AaeUPO-CLEAs与combi-CLEAs用于催化不同底物的羟基化
Table 1 AaeUPO-CLEAs and combi-CLEAs are used to catalyze the hydroxylation of different substrates
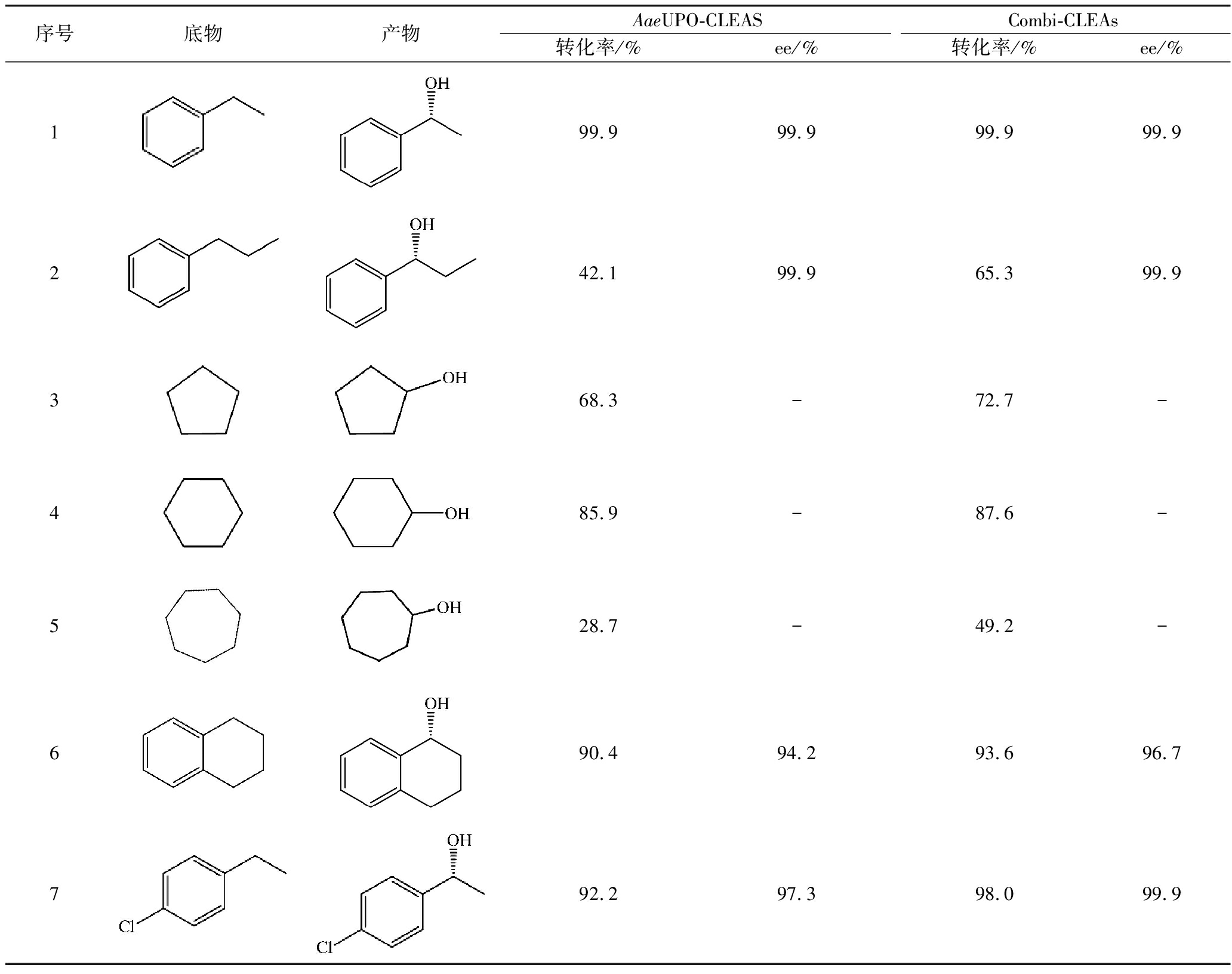
序号底物产物AaeUPO-CLEASCombi-CLEAs转化率/%ee/%转化率/%ee/%199.999.999.999.9242.199.965.399.9368.3-72.7-485.9-87.6-528.7-49.2-690.494.293.696.7792.297.398.099.9
[1] ZHAO L K, WANG C P, GAO X K, et al.Characterization of P450 monooxygenase gene family in the cotton aphid, Aphis gossypii Glover[J].Journal of Asia-Pacific Entomology, 2022, 25(2):101861.
[2] HOFRICHTER M, ULLRICH R.Oxidations catalyzed by fungal peroxygenases[J].Current Opinion in Chemical Biology, 2014, 19:116-125.
[3] AYALA M, BATISTA C V, VAZQUEZ-DUHALT R.Heme destruction, the main molecular event during the peroxide-mediated inactivation of chloroperoxidase from Caldariomyces fumago[J].JBIC Journal of Biological Inorganic Chemistry, 2011, 16(1):63-68.
[4] PESIC M, WILLOT S J P, FERN NDEZ-FUEYO E, et al.Multienzymatic in situ hydrogen peroxide generation cascade for peroxygenase-catalysed oxyfunctionalisation reactions[J].Zeitschrift Fur Naturforschung.C, Journal of Biosciences, 2019, 74(3-4):101-104.
NDEZ-FUEYO E, et al.Multienzymatic in situ hydrogen peroxide generation cascade for peroxygenase-catalysed oxyfunctionalisation reactions[J].Zeitschrift Fur Naturforschung.C, Journal of Biosciences, 2019, 74(3-4):101-104.
[5] PEREIRA P C, ARENDS I W C E, SHELDON R A.Optimizing the chloroperoxidase-glucose oxidase system:The effect of glucose oxidase on activity and enantioselectivity[J].Process Biochemistry, 2015, 50(5):746-751.
[6] YANG K L. Combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase for catalyzing cascade chemical reactions[J]. Enzyme and Microbial Technology, 2017,100:52-59.
[7] ABD RAHMAN N H, JAAFAR N R, ABDUL MURAD A M, et al.Novel cross-linked enzyme aggregates of levanase from Bacillus lehensis G1 for short-chain fructooligosaccharides synthesis:Developmental, physicochemical, kinetic and thermodynamic properties[J].International Journal of Biological Macromolecules, 2020, 159:577-589.
[8] LUCENA G N, SANTOS C C D, PINTO G C, et al.Synthesis and characterization of magnetic cross-linked enzyme aggregate and its evaluation of the alternating magnetic field (AMF) effects in the catalytic activity[J].Journal of Magnetism and Magnetic Materials, 2020, 516:167326.
[9] 张鹏. 以ABTS为底物测定漆酶活力的方法[J].印染助剂, 2007, 24(1):43-45.
ZHANG P.Test method for the laccase activity with ABTS as the substrate[J].Textile Auxiliaries, 2007, 24(1):43-45.
[10] ÖZACAR M, MEHDE A A, MEHDI W A, et al.The novel multi cross-linked enzyme aggregates of protease, lipase, and catalase production from the sunflower seeds, characterization and application[J].Colloids and Surfaces B:Biointerfaces, 2019, 173:58-68.
[11] ARAYA E, URRUTIA P, ROMERO O, et al.Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose[J].Food Chemistry, 2019, 288:102-107.
[12] ZHANG D Q, MU T H, SUN H N, et al.Comparative study of potato protein concentrates extracted using ammonium sulfate and isoelectric precipitation[J].International Journal of Food Properties, 2017, 20(9):2 113-2 127.
[13] MATEO C, PALOMO J M, VAN LANGEN L M, et al.A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates[J].Biotechnology and Bioengineering, 2004, 86(3):273-276.
[14] JUNG D H, JUNG J H, SEO D H, et al.One-pot bioconversion of sucrose to trehalose using enzymatic sequential reactions in combined cross-linked enzyme aggregates[J].Bioresource Technology, 2013, 130:801-804.
[15] CUI J D, LIU R L, LI L B.A facile technique to prepare cross-linked enzyme aggregates of bovine pancreatic lipase using bovine serum albumin as an additive[J].Korean Journal of Chemical Engineering, 2016, 33(2):610-615.
[16] GUAUQUE TORRES M P, FORESTI M L, FERREIRA M L.CLEAs of Candida Antarctica lipase B (CALB) with a bovine serum albumin (BSA) cofeeder core:Study of their catalytic activity[J].Biochemical Engineering Journal, 2014, 90:36-43.
[17] HILL A, KARBOUNE S, MATEO C.Investigating and optimizing the immobilization of levansucrase for increased transfructosylation activity and thermal stability[J].Process Biochemistry, 2017, 61:63-72.
[18] 陈海龙, 田耀旗, 李丹, 等.脂肪酶交联聚集体的制备及其催化合成月桂酸淀粉酯的研究[J].食品与发酵工业, 2017, 43(2):21-25.
CHEN H L, TIAN Y Q, LI D, et al.Preparation of cross linked lipase aggregates for synthesis of starch laurate[J].Food and Fermentation Industries, 2017, 43(2):21-25.
[19] 陈宁, 延文星, 路福平, 等.磷脂酶D交联聚集体的制备及其酶学性能研究[J].食品与发酵工业, 2022, 48(5):1-7.
CHEN N, YAN W X, LU F P, et al.Preparation and characterization of cross-linked enzyme aggregates of phospholipase D[J].Food and Fermentation Industries, 2022, 48(5):1-7.
[20] NGUYEN L T, YANG K L.Combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase for catalyzing cascade chemical reactions[J].Enzyme and Microbial Technology, 2017, 100:52-59.
[21] KARICH A, SCHEIBNER K, ULLRICH R, et al.Exploring the catalase activity of unspecific peroxygenases and the mechanism of peroxide-dependent heme destruction[J].Journal of Molecular Catalysis B:Enzymatic, 2016, 134:238-246.
[22] 姜艳军, 王旗, 王温琴,等.交联酶聚集体与仿生硅化技术结合制备固定化脂肪酶[J].催化学报, 2012, 33(5):857-862.
JIANG Y J, WANG Q, WANG W Q, et al.Preparation of immobilized lipase through combination of cross-linked enzyme aggregates and biomimetic silicification[J].Chinese Journal of Catalysis, 2012, 33(5):857-862.