我国是一个农业大国,果蔬作为重要的经济作物,农药残留的问题日益显著。随着农药残留的检测技术飞速发展,尤其是随着液相色谱-串联质谱检测技术的成熟应用,农药残留检测技术不断提升,过往的检测存在速度慢、检测单一、灵敏度低等缺点,高通量、高选择性、高灵敏度成为新的发展方向,这就对样品前处理提出了更高标准,以满足检测技术的要求。
目前常用于农药残留前处理技术主要包括:液液萃取法[1-4]、固相萃取法[5-6]、薄层色谱法、QuEChERS[7-10]等样品前处理净化方法,其中QuEChERS方法速度快、通量大、效率高且成本低,能够很好地与液相色谱-串联质谱检测技术进行融合,满足高通量、高选择性、高灵敏度的检测技术要求。QuEChERS方法自提出以来经历了多年的优化和改良,已被国内外学者广泛使用。QuEChERS方法主要利用MgSO4等盐类对样品进行除水,乙二胺-N-丙基硅烷(primary secondary amine, PSA)、C18[11-16]、石墨化碳黑(graphitized carbon black, GCB)、Al2O3等填料进行混合搭配,清除样品中的杂质。QuEChERS方法在使用过程对于极性化和非极性的化合物都有很好的回收率,适用范围广泛,方法操作过程中也可以使用内标法校正使准确度和精密度得到提升,样品前处理分析过程简便、快速、溶剂消耗少且价格低廉。QuEChERS方法在提升样品提取率的同时损失了净化效果,样品的净化效果不如固相萃取法,对于复杂基质的净化不彻底导致样品基质干扰问题严重。随着近年来新型的净化材料的出现,如多壁碳纳米管(multi-walled carbon nanotube,MWCNT)和磁珠材料,提升了复杂样品基质的净化效果,其在农药残留检测领域的应用逐渐引起广泛关注。碳纳米材料表面经过官能化的修饰,在表面键合特殊官能团,大大增加对色素、脂肪酸等干扰物质的选择性。通过表面去活技术,控制了材料对药物的过分吸附力,保证了敏感性农药的回收率。该材料比表面积大,具有典型的层状中空结构特征,增大了其比表面积,增加了材料的负载能力。
本文通过MWCNTs吸附剂与QuEChERS方法的有效融合,建立了MWCNTs分散固相萃取净化技术,结合高效液相色谱串联质谱测定果蔬中50种农药残量的检测方法,以期为食品安全风险监测工作提供有效的技术支持。
1 材料与方法
1.1 试剂与仪器
UPLC-MS/MS-8050超高效液相色谱串联质谱联用系统,日本岛津;离心机,Sigma公司; Milli-Q 高纯水发生器,美国 Millipore公司。
50种农药标准品(纯度均大于98%),德国Dr.E公司;乙腈、甲醇、丙酮、乙酸铵,均为HPLC级,提取包(MgSO4+NaCl)、5种MWCNTs(纯度>95%),天津博纳艾杰尔公司。MWCNTs外径、长度及比表面积见表1。
表1 5种MWCNTs的外径、长度和批号
Table 1 Difference specification of MWCNTs

型号批号外径/nm长度/μmMWNCTs16988496~132.5~20MWNCTs2659258110~1705~9MWNCTs390101950~9065MWNCTs44129887~150.5~10MWNCTs57551339.51.5
1.2 标准溶液配制
称取 10 mg (精确至0.1 mg) 标准品于10 mL的棕色容量瓶中,用甲醇溶解并定容到刻度;对于部分不溶农药,可以选择乙腈、丙酮等试剂辅助溶解。混合标准溶液的配制:移取适量的单标储备溶液,配制混合标准溶液,所有标准溶液于-20 ℃避光保存。
1.3 样品处理
称取10.0 g果蔬样品于50 mL离心管中,加入10.0 mL 1%乙酸乙腈溶液,迅速加入提取包(含有4.0 g MgSO4和1.5 g NaCl),扣上盖子用力摇匀,超声波振荡提取10 min,5 000 r/min离心5 min,取2.0 mL上清液于 IC-NANO(MWCNTs)净化管中进行净化,收集流出液涡旋混匀,取1.0 mL流出液经0.22 μm聚四氟乙烯滤膜过滤后,供UPLC-MS/MS测定。
1.4 色谱工作条件
色谱柱:Shim-pack XR-ODS Ⅲ (100 mm×2.0 mm×2.2 μm);流动相:A为乙腈,B为0.5 mmol/L乙酸铵溶液,梯度洗脱条件:30%A→30%A(0.5 min)→95%A(6.0 min)→30%A(7.0 min)→30%A(8.0 min);流速0.4 mL/min;柱温40 ℃;进样量2 μL。
1.5 质谱工作条件
电喷雾离子源(electrospray ionization,ESI),接口电压4 kV(ESI+)、2.8 kV(ESI-);DL温度250 ℃;加热块温度400 ℃;接口温度300 ℃;雾化气流量3 L/min;加热器流量10 L/min;干燥气流量10 L/min;监测模式多反应监测(multiple reaction monitoring,MRM)正、负离子扫描。50种农药残留质谱条件参数见表2。
表2 50种农药的化合物名称、保留时间、质荷比、 碰撞能量、扫描方式
Table 2 MS parameters, retention times of the 50 pesticides

化合物名称保留时间母离子定量离子(碰撞能)定性离子(碰撞能)锥孔电压扫描方式杀螟丹0.570237.973.1(35)151.1(27)35ESI+灭蝇胺0.594167.185.1(27)60.1(20)30ESI+吡蚜酮0.636217.9105.1(25)78.1(20)27ESI+多菌灵0.907192.1160.1(22)132.1(28)30ESI+氧乐果0.910214.1183.0(24)155.0(27)32ESI+噻菌灵0.985202.1175.1(40)131.1(32)40ESI+噻虫嗪1.438292.0211.1(21)181.1(33)30ESI+抗蚜威1.508239.272.1(35)182.2(24)35ESI+敌百虫1.556256.9109.1(27)221.1(30)40ESI+三羟克百威1.734238.1163.1(35)181.2(28)35ESI+吡虫啉1.836255.9175.2(26)209.1(22)30ESI+乐果1.937230.0198.9(28)125.1(23)30ESI+啶虫脒1.951222.9126.1(25)56.2(35)40ESI+克百威2.886222.1165.1(25)123.1(30)30ESI+粉唑醇2.961302.1123.0(27)109.1(30)35ESI+氯吡脲3.028247.9129.1(30)93.1(40)40ESI+甲萘威3.063201.9126.7(22)144.6(30)30ESI+抑霉唑3.135199.9107.1(25)168.2(32)35ESI+嘧霉胺3.136200.1107.1(22)168.1(30)35ESI+吡咪唑3.201212.8172.1(25)118.2(30)40ESI+甲维盐3.392886.1158.1(35)126.2(40)45ESI+烯酰吗啉3.595388.2301.1(27)165.1(30)35ESI+多效唑3.601294.170.1(38)125.1(40)45ESI+甲硫威3.701226.1169.1(25)121.1(30)30ESI+咪鲜胺3.723376.1308.1(30)266.1(32)35ESI+腈菌唑3.841289.170.1(32)125.2(35)40ESI+嘧菌酯3.892404.1372.1(25)329.1(27)30ESI+氟环唑3.933330.1121.2(23)141.1(28)35ESI+啶酰菌胺3.995342.8307.1(24)271.1(29)35ESI+戊唑醇4.036308.170.1(25)125.1(30)35ESI+氟硅唑4.076316.1247.1(30)165.1(35)40ESI+腈苯唑4.098336.9125.1(27)70.2(32)35ESI+稻瘟灵4.226291.1231.1(25)189.1(33)40ESI+戊菌唑4.217284.1159.1(32)70.2(40)45ESI+烯唑醇4.321326.170.1(40)159.1.2(38)45ESI+丙环唑4.341341.8159.1(35)205.1(30)40ESI+氯唑磷4.432314.1162.1(24)120.1(35)35ESI+醚菌酯4.499313.9235.1(35)222.1(35)40ESI+啶氧菌酯4.541368.1145.2(27)205.2(22)35ESI+吡唑醚菌酯4.756388.1194.1(28)163.1(33)40ESI+噻嗪酮4.826306.1201.1(29)116.1(24)35ESI+辛硫磷4.860299.177.1(35)129.2(35)40ESI+四螨嗪4.862303.1138.1(27)102.2(28)35ESI+茚虫威4.879527.9150.1(40)218.1(27)45ESI+肟菌酯4.974409.1186.1(23)145.2(28)35ESI+唑虫酰胺5.175384.1197.1(28)154.1(35)40ESI+唑螨酯5.596422.2366.1(25)138.1(30)37ESI+阿维菌素5.695895.4449.3(45)327.4(55)60ESI+螺螨酯5.824411.171.2(27)313.1(32)40ESI+除虫脲5.842309.1289.1(30)156.1(25)35ESI-
2 结果与分析
2.1 色谱与质谱条件的优化
2.1.1 质谱条件的优化
将各农药分别配制成50 μg/L的标准溶液。通过液相色谱直接注入质谱仪,建立正、负两种离子同时扫描模式,得到准确的母离子,首选[M+H]+ 或[M-H]-利用质谱仪自动优化功能,筛选二级碎片离子信息,获得碎片离子及碰撞能量,并将母离子和 2个信号较强的子离子组成监测离子对,以MRM模式进行检测。
2.1.2 流动相条件的优化
实验比较了乙腈-水、甲醇-水、乙腈-0.1%甲酸水溶液、甲醇-乙酸铵水溶液、作为流动相时对化合物分析结果的影响。乙腈-水作为流动相与甲醇-水相比,可提高部分农药的质谱信号,但杀螟丹、抗蚜威、阿维菌素等部分农药的质谱响应信号仍偏低。在乙腈-水中加入甲酸,可增加正离子的电离程度,但负离子的电离受到影响。在乙腈水溶液中加入乙酸铵,绝大部分农药的灵敏度均明显提高,表明乙腈-乙酸铵水溶液更适合作流动相。同时考察了不同浓度(0.5,1.0,5.0,10.0 nmol/L)的乙酸铵对检测结果的影响。随着乙酸铵浓度的增加,大部分农药的响应值降低,形成了离子抑制。且高浓度的乙酸铵溶液会加速质谱的污染,从而增加离子源的清洗频次。因此,最终采用乙腈-0.5 mmol/L乙酸铵水溶液作为流动相响应值最佳。50种农药总离子流色谱图见图1。
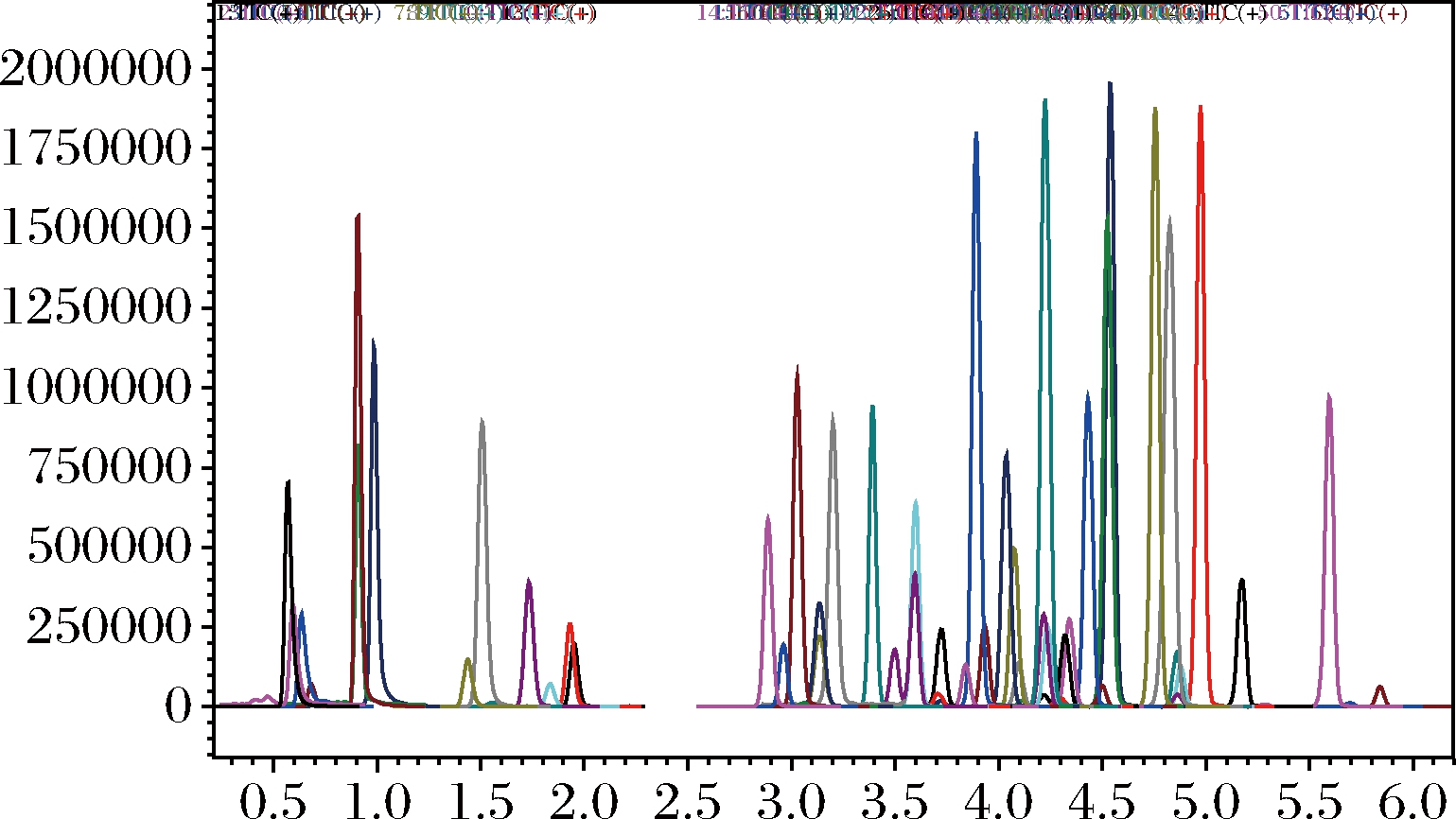
图1 50种农药总离子流色谱图
Fig.1 TIC of 50 pesticides
2.1.3 提取溶剂的选择
农药残留分析中, 常用的提取溶剂包括乙腈、甲醇、二氯甲烷、正己烷、丙酮或有机溶剂添加一定比例的酸或碱等。本实验以回收率为指标,选取回收率相对较差的咪鲜胺、稻瘟灵和肟菌酯3种化合物,分别考察了乙腈、甲醇、二氯甲烷、正己烷、丙酮等有机溶剂,对白菜、苹果、红心火龙果、韭菜空白基质中加入0.010 mg/kg的3种混标,进行提取实验。如图2所示,使用乙腈、甲醇、丙酮作为提取溶剂,目标物回收率普遍高于用二氯甲烷和正己烷;但丙酮作为提取溶剂时,提取液中杂质较多,因此,初步选定甲醇、乙腈作为提取溶剂。

图2 不同基质、不同溶剂的提取回收率统计(n=6)
Fig.2 Effect of different extraction solvent,different sample on recovery (n=6)
2.1.4 混合提取溶剂的选择
不同农药溶解性和极性相差较大,选择的提取溶剂要与样品和农药的性质相符合,目标农药在提取溶剂中要有足够大的可溶性和稳定性。使用单一溶剂提取不能完全兼顾所有待测农药的回收率,为兼顾多种农药同时检测的需要,进一步提高样品提取率,对白菜、苹果、红心火龙果、韭菜空白基质中加入回收率相对较差的咪鲜胺、稻瘟灵和肟菌酯3种化合物混标0.010 mg/kg,进行提取实验,分别考察了甲醇∶乙腈(1∶1、1∶2、2∶1)、1%乙酸甲醇、1%乙酸乙腈混合溶剂的提取效果(图3)。

图3 不同基质、混合溶剂的提取回收率统计(n=6)
Fig.3 Effect of different extraction, different sample solvent on recovery (n=6)
结果表明,使用1%乙酸乙腈作为提取溶剂,农药的回收率普遍偏高,并且杂质干扰少,所以选定1%乙酸乙腈作为提取溶剂。
2.1.5 MWCNTs的种类和使用量
为考察MWCNTs的种类及用量,选用空白样品,添加3种已知浓度有代表性农药目标物,依据前述方法进行样品前处理,并在净化阶段依据正交实验设计方案分别添加不同种类(5种MWCNTs)和用量(3,5,10,15和20 mg)MWCNTs净化,LC-MS/MS检测后计算获得各目标物加标回收率,通过对比回收率来评判净化效果,结果见图4。通常情况下,多农残分析方法中各目标物的平均回收率应满足的范围是60%~120%。由图4可以看出,在相同用量MWCNTs条件下,随着MWCNTs外径尺寸的增大,回收率递增,说明整体净化效果改善;在相同种类 MWCNTs条件下,随着使用量的增加,尽管通过提取液颜色可以直观判断吸附效果增强,但回收率呈明显降低趋势,不能满足检测回收率要求。

图4 不同MWCNTs种类和用量条件下目标物 回收率统计(n=6)
Fig.4 Effect of different amounts of different MWCNTs on recovery(n=6)
由于5种MWCNTs的主要区别在于外径尺寸,外径减小则比表面积增大,吸附力增强,但对基质吸附增强的同时也会对目标物产生吸附。同种MWCNTs使用量越大,吸附效果越强,也会在吸附除杂的同时造成目标物损失。可见,以 MWCNTs作为吸附剂实现净化功能时,并非比表面积越大越好,而是尺寸和用量要合适,控制吸附净化作用以满足实验需要。由图4可知,选用MWCNTs5用量为3和5 mg时目标物回收率均在60%~120%,但实验中发现,用量5 mg较3 mg的除杂效果更好。因此,确定净化剂材料为 MWCNTs5,用量为5 mg。
2.1.6 MWCNTs与PSA结构差异对净化效果的比较
MWCNTs吸附剂材料管体由石墨组成具有较好的疏水性能、多孔结构比表面积大、吸附性能强、耐高温、强酸、强碱等化学特性。它可以除去植物源性样品中色素、甾醇、有机酸和酚类杂质,常用于农残检测分析中样品的前处理,结构见图5。

图5 MWCNTs结构图
Fig.5 Structure diagram of MWCNTs
而PSA是与氨基相似的吸附剂,其具有2个氨基,pKa值分别为10.1和10.9左右,比氨基柱具有更强的离子交换能力。同时PSA可与金属离子产生鳌合作用,常用于提取金属离子。它可以去除许多基质类糖,脂肪酸和有机酸等杂质,结构图见图6。对于基质复杂且含有较多天然色素的样品,进行前处理净化时MWCNTs更具有优势。

图6 PSA结构图
Fig.6 Structure diagram of PSA
为比较MWCNTs与PSA对果蔬样品提取液的净化效果,实验选用空白果蔬样品依前述方法提取后分别使用MWCNTs(5 mg)和PSA(25 mg)净化,并进行LC-MS/MS检测。比较二者的总离子流图后发现,使用MWCNTs净化可以获得比PSA更好的净化效果,结果见图7~9。由于MWCNTs市售价格更为低廉,用量也仅为PSA的1/5,因而有效降低了成本。在本实验中,还与以PSA为净化剂的传统QuEChERS方法的定量限进行了比较。结果发现,以MWCNTs作为吸附剂的方法定量限(limit of quantification,LOQ)可低至0.002 g/kg水平,在50种农药目标物中,LOQ多数在0.002~0.010 mg/kg之间;传统QuEChERS方法的最低LOQ为0.010 mg/kg,50种农药目标物中,LOQ多数在0.010~0.050 mg/kg。显然,MWCNTs比PSA有更加优异的表现。这是由于MWCNTs具有纳米级别的中空管状结构、大的比表面积和疏水的表面能强烈吸附某些重金属离子和有机化合物,具有较强的吸附和去除色素的能力,因此目标物的LOQ更低。

图7 空白样品的50种农药总离子流色谱图
Fig.7 Chromatograms of blank sample
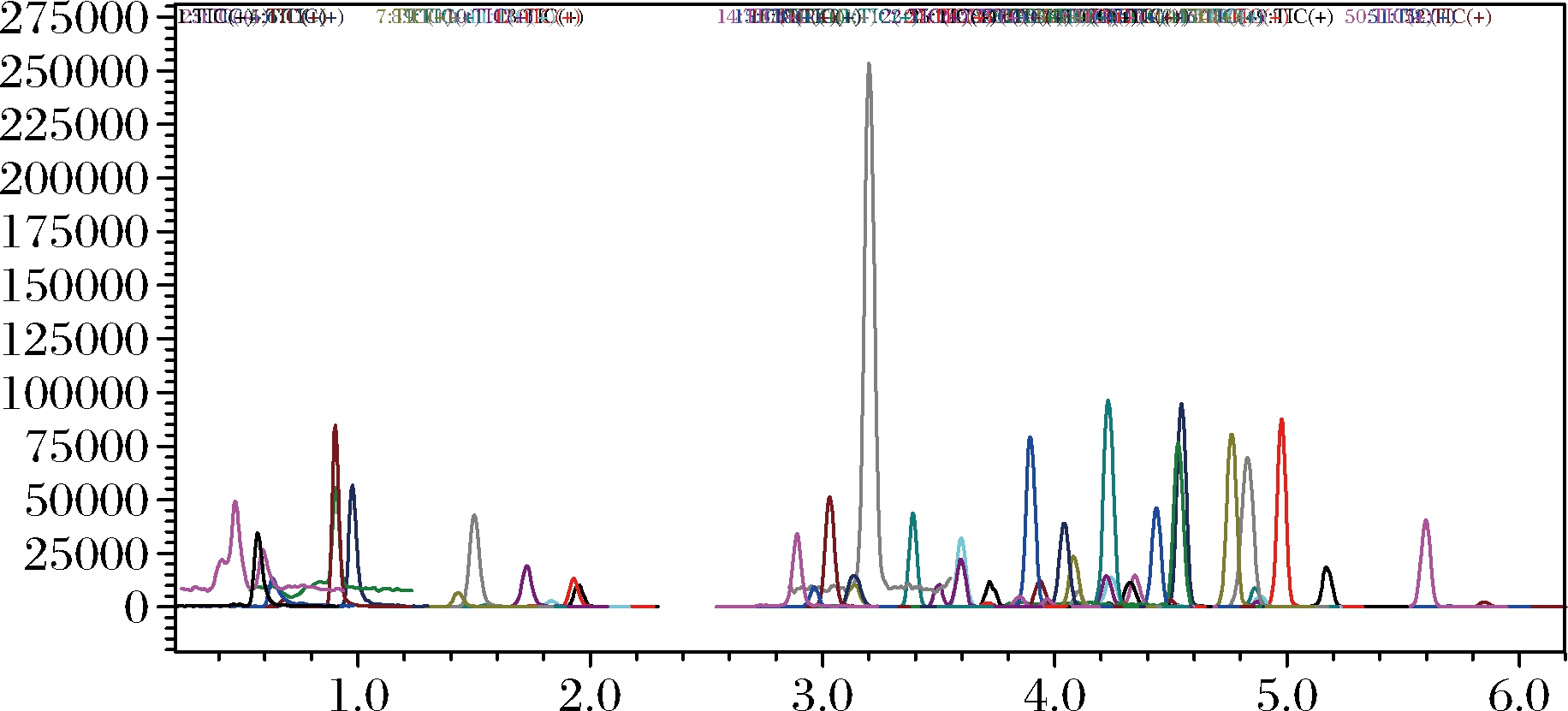
图8 50种农药总离子流色谱图空白加标 0.010 mg/kg使用PSA净化
Fig.8 Chromatograms of sample purified by PSA spiked at 0.010 mg/kg 50 pesticides
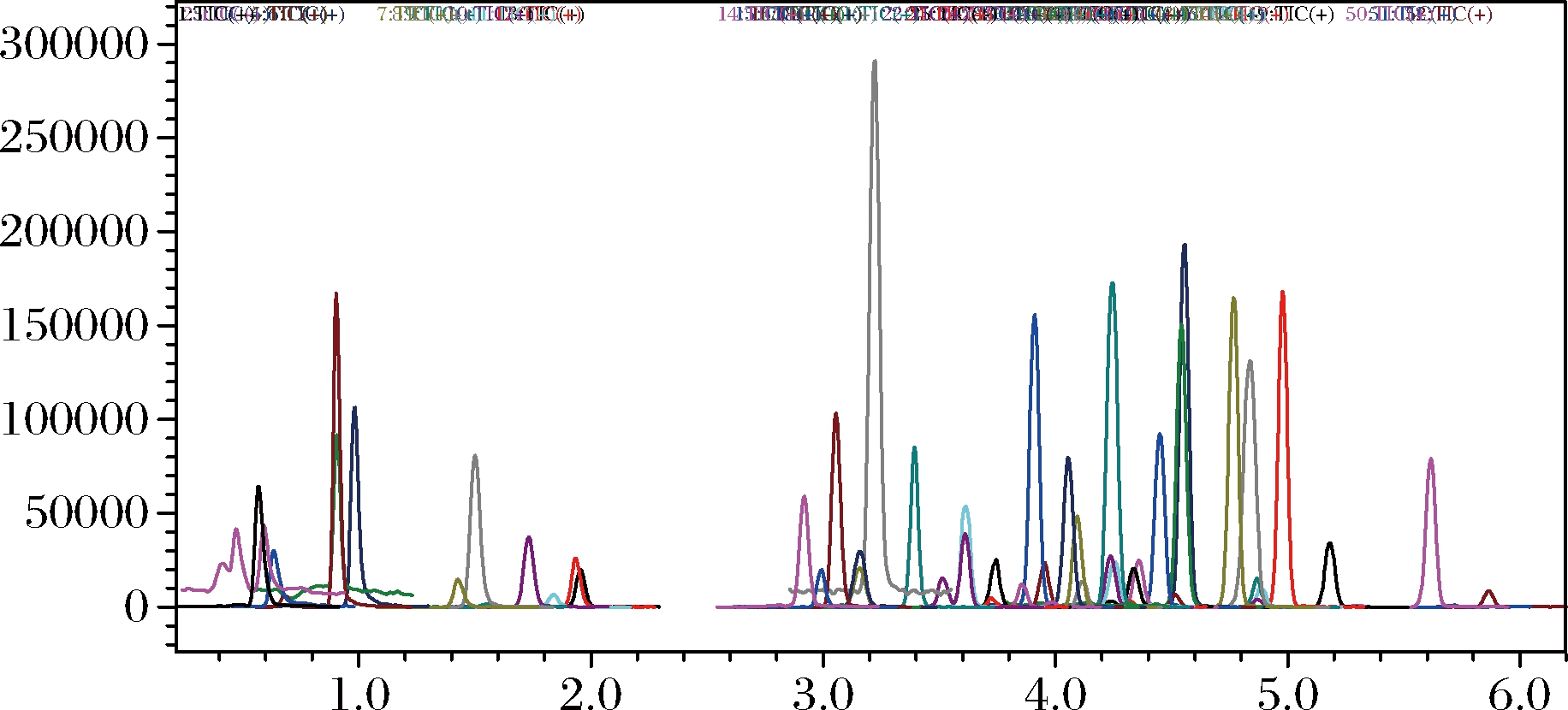
图9 50种农药总离子流色谱图空白加标0.010 mg 使用MWCNTs净化
Fig.9 Chromatograms of sample purified by MWCNTs spiked at 0.010 mg/kg 50 pesticides
2.2 方法验证
2.2.1 标准曲线和检出限(limit of detection,LOD)
选择与被测样品性质相同或相似的空白样品按1.3方法进行前处理,得到空白基质溶液。精确吸取一定量的混合标准溶液,逐级用空白基质溶液稀释成质量浓度为0.000 5、0.001、0.002、0.005、0.01、0.02、0.05、0.1 mg/L的基质匹配标准工作溶液,根据仪器性能和检测需要选择不少于5个浓度点供液相色谱-质谱联用仪测定。以农药定量用子离子的质量色谱图峰面积为纵坐标,相对应的基质匹配标准工作溶液质量浓度为横坐标,绘制基质匹配标准工作曲线。50种化合物的线性方程、相关系数、线性范围和LOD见表3。
表3 50种化合物的线性方程、相关系数、线性范围和检出限
Table 3 Linear equations, correlation coefficients, LODs for 50 pesticides

序号化合物方程r线性范围/(mg·L﹣1)LOD/(mg·kg﹣1)1杀螟丹Y=6.713 69e7X+5 969.490.999 60.000 5~0.100.0012灭蝇胺Y=2.485 9e7X+39 308.30.996 60.000 5~0.100.0203吡蚜酮Y=3.401 67e7X+18 1630.999 60.000 5~0.100.0024多菌灵Y=1.268 1e8X+79 0510.999 50.000 5~0.100.0015氧乐果Y=4.740 39e7X+41 283.10.998 50.000 5~0.100.0026噻菌灵Y=8.357 33e7X-37 262.50.999 10.000 5~0.100.0027噻虫嗪Y=1.478 27e7X-59.668 70.999 90.000 5~0.100.0028抗蚜威Y=9.445 04e7X-26 887.70.999 80.000 5~0.100.0059敌百虫Y=1.479 27e6X-258.2860.999 50.000 5~0.100.002103-羟基克百威Y=7.760 44e6X+3 348.330.999 30.000 5~0.100.00211吡虫啉Y=5.227 71e6X-1 386.780.999 70.000 5~0.100.00212乐果Y=1.824 46e7X+2 127.490.999 90.000 5~0.100.00213啶虫脒Y=1.993 3e7X+1 026.080.999 90.000 5~0.100.00114克百威Y=3.932 06e7X-5 357.480.999 50.000 5~0.100.00115粉唑醇Y=1.977 65e7X-6 648.390.999 50.000 5~0.100.00216氯吡脲Y=9.591 63e7X-17 498.10.997 80.000 5~0.100.00217甲萘威Y=1.450 61e6X-543.8830.999 70.000 5~0.100.00218抑霉唑Y=4.412 77e7X-13 073.80.999 60.000 5~0.100.00219嘧霉胺Y=2.979 16e7X-7 983.040.999 70.000 5~0.100.00220吡咪唑Y=8.411 73e7X+456 3250.999 20.000 5~0.100.00521甲氨基阿菌素苯甲酸盐Y=1.128 94e8X-46 232.90.999 40.000 5~0.100.00122烯酰吗啉Y=3.487 75e7X-10 619.60.999 60.000 5~0.100.00523多效唑Y=8.106 86e7X-28 690.60.999 70.000 5~0.100.002
续表3
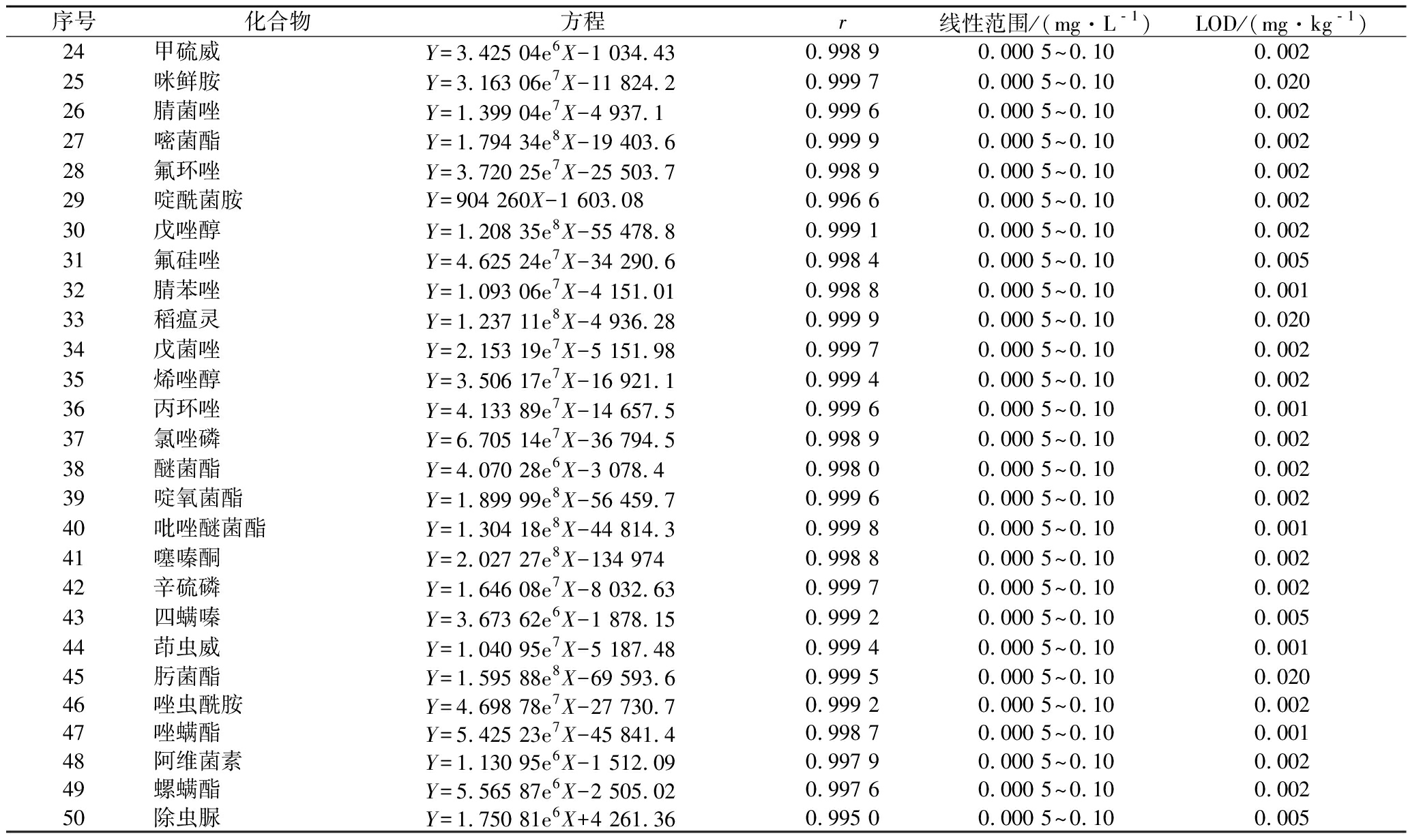
序号化合物方程r线性范围/(mg·L﹣1)LOD/(mg·kg﹣1)24甲硫威Y=3.425 04e6X-1 034.430.998 90.000 5~0.100.00225咪鲜胺Y=3.163 06e7X-11 824.20.999 70.000 5~0.100.02026腈菌唑Y=1.399 04e7X-4 937.10.999 60.000 5~0.100.00227嘧菌酯Y=1.794 34e8X-19 403.60.999 90.000 5~0.100.00228氟环唑Y=3.720 25e7X-25 503.70.998 90.000 5~0.100.00229啶酰菌胺Y=904 260X-1 603.080.996 60.000 5~0.100.00230戊唑醇Y=1.208 35e8X-55 478.80.999 10.000 5~0.100.00231氟硅唑Y=4.625 24e7X-34 290.60.998 40.000 5~0.100.00532腈苯唑Y=1.093 06e7X-4 151.010.998 80.000 5~0.100.00133稻瘟灵Y=1.237 11e8X-4 936.280.999 90.000 5~0.100.02034戊菌唑Y=2.153 19e7X-5 151.980.999 70.000 5~0.100.00235烯唑醇Y=3.506 17e7X-16 921.10.999 40.000 5~0.100.00236丙环唑Y=4.133 89e7X-14 657.50.999 60.000 5~0.100.00137氯唑磷Y=6.705 14e7X-36 794.50.998 90.000 5~0.100.00238醚菌酯Y=4.070 28e6X-3 078.40.998 00.000 5~0.100.00239啶氧菌酯Y=1.899 99e8X-56 459.70.999 60.000 5~0.100.00240吡唑醚菌酯Y=1.304 18e8X-44 814.30.999 80.000 5~0.100.00141噻嗪酮Y=2.027 27e8X-134 9740.998 80.000 5~0.100.00242辛硫磷Y=1.646 08e7X-8 032.630.999 70.000 5~0.100.00243四螨嗪Y=3.673 62e6X-1 878.150.999 20.000 5~0.100.00544茚虫威Y=1.040 95e7X-5 187.480.999 40.000 5~0.100.00145肟菌酯Y=1.595 88e8X-69 593.60.999 50.000 5~0.100.02046唑虫酰胺Y=4.698 78e7X-27 730.70.999 20.000 5~0.100.00247唑螨酯Y=5.425 23e7X-45 841.40.998 70.000 5~0.100.00148阿维菌素Y=1.130 95e6X-1 512.090.997 90.000 5~0.100.00249螺螨酯Y=5.565 87e6X-2 505.020.997 60.000 5~0.100.00250除虫脲Y=1.750 81e6X+4 261.360.995 00.000 5~0.100.005
2.2.2 方法的回收率和精密度
在蔬菜、水果样品基质中加入不同用量的混合标准溶液,进行样品前处理,得到信噪比为10∶1的每种化合物的样品最低浓度,此添加量就是该种化合物的方法LOQ。分别取白菜、苹果空白样品,添加低、中、高3个不同添加量水平的50种化合物混合标准溶液,进行前处理,测定目标化合物。每个水平进行6次实验,50种化合物的平均回收率为66.82%~105.91%,相对标准偏差为3.07%~9.96%。以白菜、苹果样品为代表,50种化合物的添加量、回收率、相对标准偏差见表4。
表4 50种化合物的添加量、回收率、相对标准偏差(n=6)
Table 4 Recoveries,relative standard deviations of 50 pesticides spiked in 4 kinds of samples(n=6)

序号化合物基质添加量/(mg·kg-1)回收率/%LOQ/(mg·kg-1)RSD/%序号化合物基质添加量/(mg·kg-1)回收率/%LOQ/(mg·kg-1)RSD/%1杀冥丹白菜苹果火龙果韭菜0.001,0.002,0.010797874710.0023.35.57.68.92灭蝇胺白菜苹果火龙果韭菜0.002,0.005,0.010767772710.0054.98.69.311.23吡蚜酮白菜苹果火龙果韭菜0.002,0.005,0.010929185810.0053.47.28.69.74多菌灵白菜苹果火龙果韭菜0.001,0.002,0.010939689820.0023.33.45.78.45氧乐果白菜苹果火龙果韭菜0.002,0.005,0.010908883820.0054.55.77.99.36噻菌灵白菜苹果火龙果韭菜0.002,0.005,0.010969892910.0053.15.16.98.27噻虫嗪白菜苹果火龙果韭菜0.002,0.005,0.010959483860.0057.16.98.47.28抗蚜威白菜苹果火龙果韭菜0.005,0.010,0.020859182790.017.98.76.78.19敌百虫白菜苹果火龙果韭菜0.002,0.005,0.010848379810.0058.17.66.79.4103-羟基克百威白菜苹果火龙果韭菜0.002,0.005,0.010909288810.0054.87.25.36.1
续表4

序号化合物基质添加量/(mg·kg-1)回收率/%LOQ/(mg·kg-1)RSD/%序号化合物基质添加量/(mg·kg-1)回收率/%LOQ/(mg·kg-1)RSD/%11吡虫啉白菜苹果火龙果韭菜0.002,0.005,0.010969889820.0055.17.29.18.412乐果白菜苹果火龙果韭菜0.002,0.005,0.01096102911040.0058.17.25.39.613啶虫脒白菜苹果火龙果韭菜0.001,0.002,0.010979795920.0023.23.15.76.914克百威白菜苹果火龙果韭菜0.001,0.002,0.010989692850.0025.14.26.78.115粉唑醇白菜苹果火龙果韭菜0.002,0.005,0.01010210595930.0057.35.56.79.816氯吡脲白菜苹果火龙果韭菜0.020,0.050,0.100879588910.056.57.95.19.417甲萘威白菜苹果火龙果韭菜0.002,0.005,0.010827984770.0055.14.73.46.918抑霉唑白菜苹果火龙果韭菜0.002,0.005,0.010858881760.0056.25.84.37.119嘧霉胺白菜苹果火龙果韭菜0.002,0.005,0.010817983740.0054.55.76.18.920吡咪唑白菜苹果火龙果韭菜0.005,0.010,0.020827985730.016.95.27.46.721甲氨基阿菌素苯甲酸盐白菜苹果火龙果韭菜0.001,0.002,0.010798289830.0028.19.47.65.822烯酰吗啉白菜苹果火龙果韭菜0.005,0.010,0.020879186810.016.58.15.79.223多效唑白菜苹果火龙果韭菜0.002,0.005,0.010768379850.0057.27.66.25.124甲硫威白菜苹果火龙果韭菜0.002,0.005,0.010838982770.0057.66.58.35.125咪鲜胺白菜苹果火龙果韭菜0.020,0.050,0.100757876710.056.27.15.28.326腈菌唑白菜苹果火龙果韭菜0.002,0.005,0.010818389810.0057.15.24.38.927嘧菌酯白菜苹果火龙果韭菜0.002,0.005,0.010929589810.0056.17.25.19.228氟环唑白菜苹果火龙果韭菜0.002,0.005,0.010757479810.0058.15.89.28.729啶酰菌胺白菜苹果火龙果韭菜0.002,0.005,0.010949587810.0055.23.76.79.130戊唑醇白菜苹果火龙果韭菜0.002,0.005,0.010989987820.0057.85.86.410.231氟硅唑白菜苹果火龙果韭菜0.005,0.010,0.020817683770.013.84.56.37.532腈苯唑白菜苹果火龙果韭菜0.001,0.002,0.010878481760.0027.56.89.28.533稻瘟灵白菜苹果火龙果韭菜0.020,0.050,0.100788285720.055.14.87.19.234戊菌唑白菜苹果火龙果韭菜0.002,0.005,0.010868475720.0053.85.36.87.935烯唑醇白菜苹果火龙果韭菜0.002,0.005,0.010748175760.0055.74.86.98.136丙环唑白菜苹果火龙果韭菜0.001,0.002,0.010869082780.0025.48.16.57.237氯唑磷白菜苹果火龙果韭菜0.002,0.005,0.010959892910.0059.67.48.36.738醚菌酯白菜苹果火龙果韭菜0.002,0.005,0.010818489800.0055.26.67.25.139啶氧菌酯白菜苹果火龙果韭菜0.002,0.005,0.010857983810.0057.86.25.87.140吡唑醚菌酯白菜苹果火龙果韭菜0.001,0.002,0.010979690910.0024.66.75.19.241噻嗪酮白菜苹果火龙果韭菜0.002,0.005,0.010979591930.0058.99.98.37.842辛硫磷白菜苹果火龙果韭菜0.002,0.005,0.010859088910.0056.88.75.19.2
续表4
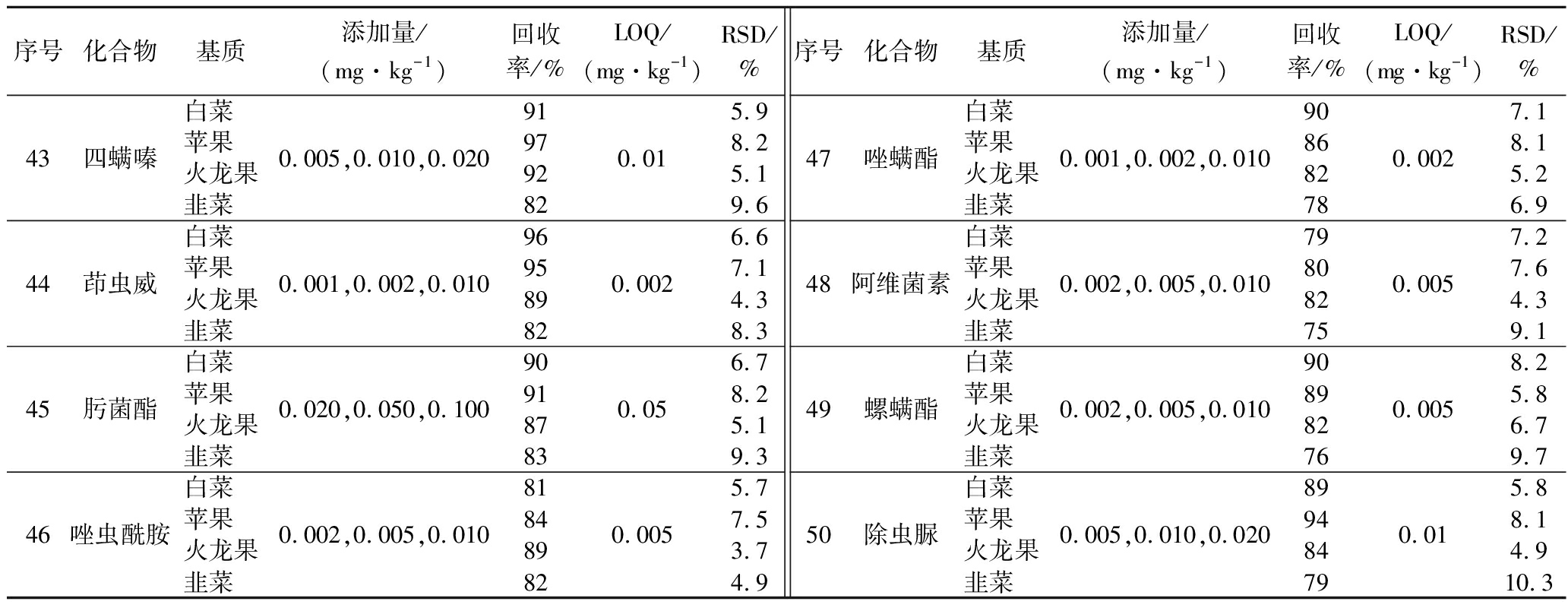
序号化合物基质添加量/(mg·kg-1)回收率/%LOQ/(mg·kg-1)RSD/%序号化合物基质添加量/(mg·kg-1)回收率/%LOQ/(mg·kg-1)RSD/%43四螨嗪白菜苹果火龙果韭菜0.005,0.010,0.020919792820.015.98.25.19.644茚虫威白菜苹果火龙果韭菜0.001,0.002,0.010969589820.0026.67.14.38.345肟菌酯白菜苹果火龙果韭菜0.020,0.050,0.100909187830.056.78.25.19.346唑虫酰胺白菜苹果火龙果韭菜0.002,0.005,0.010818489820.0055.77.53.74.947唑螨酯白菜苹果火龙果韭菜0.001,0.002,0.010908682780.0027.18.15.26.948阿维菌素白菜苹果火龙果韭菜0.002,0.005,0.010798082750.0057.27.64.39.149螺螨酯白菜苹果火龙果韭菜0.002,0.005,0.010908982760.0058.25.86.79.750除虫脲白菜苹果火龙果韭菜0.005,0.010,0.020899484790.015.88.14.910.3
3 结论
本实验建立了一套可同时测定蔬菜水果中50种高风险农药残留量的检测分析方法,采用MWCNTs结合QuEChERS的农药残留样品前处理方法,解决了多种农药残留QuEChERS样品前处理部分化合物回收率低及净化效果差的问题。利用UPLC-MS/MS技术进行快速定性、定量分析检测,解决了常规仪器分析方法灵敏度低、分离效果差、选择性单一、抗干扰能力弱等技术难题。本方法能够满足各种不同的检测需求,该方法快速、简便、灵敏、准确、适用的样品基质广泛,尤其适合应对食品安全突发事件应急处置工作,可快速地确定农药残留的检测项目、发现风险点,进行有针对性的检测,为实现对市场的有效监管提供技术支撑。
[1] 王慧君, 薛亚薇, 康健, 等.SPE和QuEChERS前处理方法结合LC-Q-TOF/MS检测苹果和番茄中282种农药残留对比研究[J].分析试验室, 2015, 34(4):383-387.
WANG H J, XUE Y W, KANG J, et al.Comparison of SPE and QuEChERS combined with LC-Q-TOF/MS for determination of 282 pesticide residues in apple and tomato[J].Chinese Journal of Analysis Laboratory, 2015, 34(4):383-387.
[2] 李凌云, 许晓敏, 林桓, 等.超高效液相色谱-串联质谱法快速检测蔬菜中248种农药残留[J].色谱, 2016, 34(9):835-849.
LI L Y, XU X M, LIN H, et al.Rapid detection of 248 pesticide residues in vegetables by ultra high performance liquid chromatographytandem mass spectrometry[J].Chinese Journal of Chromatography, 2016, 34(9):835-849.
[3] 曹静, 庞国芳, 王明林, 等.液相色谱-电喷雾串联质谱法测定生姜中的215种农药残留[J].色谱, 2010, 28(6):579-589.
CAO J, PANG G F, WANG M L, et al.Determination of 215 pesticide residues in ginger using liquid chromatography coupled with electrospray ionization tandem mass spectrometry[J].Chinese Journal of Chromatography, 2010, 28(6):579-589.
[4] 李岩, 郑锋, 王明林, 等.液相色谱-串联质谱法快速筛查测定浓缩果蔬汁中的156种农药残留[J].色谱, 2009, 27(2):127-137.
LI Y, ZHENG F, WANG M L, et al.Rapid screening and confirmation of 156 pesticide residues in concentrated fruit and vegetable juices using liquid chromatography-tandem mass spectrometry[J].Chinese Journal of Chromatography, 2009, 27(2):127-137.
[5] 徐娟, 陈捷, 王岚, 等.QuEChERS提取与超高效液相色谱-电喷雾电离串联质谱联用法检测果蔬中的230种农药残留[J].分析测试学报, 2013, 32(3):293-301.
XU J, CHEN J, WANG L, et al.Large-scale analysis of pesticides in fruits and vegetables by liquid chromatography-electrospray tandem mass spectrometry with QuEChERS as cleanup step[J].Journal of Instrumental Analysis, 2013, 32(3):293-301.
[6] 刘宝峰, 刘罡一, 马又娥, 等.高效液相色谱-串联质谱法检测蔬菜水果中65种农药残留方法研究[J].科技通报, 2010, 26(1):93-99.
LIU B F, LIU G Y, MA Y E, et al.Study on determination of multi-residues for 65 pesticides in vegetables and fruits using liquid chromatography coupled with mass spectrometry[J].Bulletin of Science and Technology, 2010, 26(1):93-99.
[7] 闫震, 聂继云, 徐国锋, 等.超高效液相色谱-串联质谱法对比4种净化方式对不同色素含量基质中19种农药残留检测的影响[J].分析测试学报, 2014, 33(9):1 000-1 009.
YAN Z, NIE J Y, XU G F, et al.Effects of four kinds of purification methods for determination of 19 pesticide residues in substrates of different pigments analyzed by ultra performance liquid chromatography-tandem mass spectrometry[J].Journal of Instrumental Analysis, 2014, 33(9):1 000-1 009.
[8] 曹慧, 朱岩, 李祖光, 等.磁性多壁碳纳米管净化技术快速测定甘蓝中10种农药残留[J].分析试验室, 2015, 34(12):1 480-1 484.
CAO H, ZHU Y, LI Z G, et al.Simultaneous determination of ten pesticide residues in ball cabbage by ultra performance liquid chromatography-tandem mass spectrometry and magnetic multiwalled carbon nanotubes cleaning[J].Chinese Journal of Analysis Laboratory, 2015, 34(12):1 480-1 484.
[9] 帖金鑫, 余斐, 廖付, 等.多壁碳纳米管在农药残留检测中的应用进展[J].农产品加工, 2017(8):61-65.
TIE J X, YU F, LIAO F, et al.Progress in applications of multi-walled carbon nanotubes in pesticide residue detection[J].Farm Products Processing, 2017(8):61-65.
[10] 张爱芝, 王全林, 曹丽丽, 等.QuEChERS-超高效液相色谱-串联质谱法测定蔬菜中250种农药残留[J].色谱, 2016, 34(2):158-164.
ZHANG A Z, WANG Q L, CAO L L, et al.Determination of 250 pesticide residues in vegetables using QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry[J].Chinese Journal of Chromatography, 2016, 34(2):158-164.
[11] 刘炎, 欧阳迪庆, 叶玉凤, 等.QuEChERS结合超高效液相色谱-串联质谱法同时测定番茄中噻虫嗪、噻虫胺、螺虫乙酯及其代谢物残留[J].分析测试学报, 2017, 36(12):1 431-1 438.
LIU Y, OUYANG D Q, YE Y F, et al.Analysis of thiamethoxam, clothianidin and spirotetramat and their metabolites residues in tomato using QuEChERS method with ultra performance liquid chromatography-mass spectrometry[J].Journal of Instrumental Analysis, 2017, 36(12):1 431-1 438.
[12] 董亚蕾, 刘文婧, 曹进, 等.QuEChERS-超高效液相色谱-串联质谱法测定坚果中38种农药残留[J].分析化学, 2017, 45(9):1 397-1 404.
DONG Y L, LIU W J, CAO J, et al.Determination of 38 kinds of pesticide residues in nuts by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry[J].Chinese Journal of Analytical Chemistry, 2017, 45(9):1 397-1 404.
[13] 陈跃, 王金花, 卢晓宇, 等.超高效液相色谱-串联质谱法快速测定农产品中残留的种衣剂农药[J].色谱, 2008, 26(6):720-725.
CHEN Y, WANG J H, LU X Y, et al.Determination of pesticide residues from seed coating reagent in agricultural products using ultra performance liquid chromatography-tandem mass spectrometry[J].Chinese Journal of Chromatography, 2008, 26(6):720-725.
[14] 苏明伟, 郑言波, 杨海, 等.果蔬农药残留检测的预处理和检测条件优化研究[J].食品科学, 2006, 27(5):199-201.
SU M W, ZHENG Y B, YANG H, et al.Study on opitimization of sample preparation and determination for pesticide residues in fruits and vegetables[J].Food Science, 2006, 27(5):199-201.
[15] 朱俊杰, 陆利霞, 熊晓辉.食品中农残检测及预处理新技术进展[J].食品研究与开发, 2008, 29(2):129-132.
ZHU J J, LU L X, XIONG X H.New technologies of pre-disposal treatment and detection of pesticide residues in food[J].Food Research and Development, 2008, 29(2):129-132.
[16] 彭晓俊, 温绮靖, 庞晋山, 等.改性多壁碳纳米管固相萃取测定农产品中苯氧羧酸类除草剂残留[J].分析测试学报, 2012, 31(11):1 373-1 378.
PENG X J, WEN Q J, PANG J S, et al.Determination of phenoxyacid herbicides in agricultural products by solid phase extraction using modified multi-walled carbon nanotubes as adsorbent[J].Journal of Instrumental Analysis, 2012, 31(11):1 373-1 378.