柠檬酸是三羧酸循环的中间代谢物,广泛存在于自然界中,是一种重要的有机酸[1],天然无害,无色无臭,具有令人愉悦的酸味,在食品工业中作为酸味剂被广泛应用[2]。柠檬酸具有中强酸性,且拥有3个羧基等独特的化学结构特性,广泛用于化学工业,被认为是重要的六碳平台化合物[3]。此外,柠檬酸的应用范围拓展至医药[4]、交联剂[5-7]、土壤修复[8-10]等新领域,需求量逐年上升。
当前,柠檬酸主要通过黑曲霉(Aspergillus niger)发酵获得:先制备黑曲霉孢子,然后接入种子罐培养,种子成熟后再移入发酵罐中发酵,最后提取得到柠檬酸产品[11-13]。在当前的种子培养中,孢子先要经过激活,然后才膨胀发芽,并开始菌丝生长。孢子萌发通常超过8 h,增加了种子培养周期,降低了生产效率[14]。并且,种子培养无法同时匹配孢子萌发和菌丝生长最适条件,导致孢子发芽率低、菌丝活力差。另外,孢子间存在吸附作用,容易聚集成团[15],多个凝聚的孢子只能形成一个菌丝球,降低了成球率;且形成的菌丝球大小不一,部分菌丝球直径较大,中心形成致密区,无法与营养物质和氧气有效接触,导致菌体转化效率降低[16]。因此,如何改进黑曲霉种子培养方法,提高种子培养效率和改善菌丝球形态是柠檬酸生产中亟待解决的重要课题。
本研究提出了孢子预培养策略,即先激活孢子、促进发芽,再利用发芽孢子进行种子培养。孢子萌发是丝状真菌对环境产生应答并开始生长的关键点,但关于工业化黑曲霉孢子萌发的研究报道较少,并且发芽孢子替代休眠孢子接种对于菌丝球形态的影响未见报道。基于此,本研究对孢子萌发的条件进行考察,研究颗粒物添加和振荡分散对发芽孢子、菌丝球形态以及发酵结果的影响;最后,在5 000 L规模进行工艺放大验证,以期有效提升柠檬酸生产效率。
1 材料与方法
1.1 实验材料
1.1.1 菌种
黑曲霉,江苏国信协联能源有限公司。
1.1.2 材料
玉米粉,市售玉米粉碎后过60目筛;α-高温淀粉酶,诺维信(中国)公司。
1.1.3 培养基
固体培养基:马铃薯葡萄糖琼脂培养基(potato dextrose agar,PDA)。
液化液制备:将玉米粉和60 ℃去离子水按照质量比1∶3.2混合,用Ca(OH)2调pH至5.8~6.0,按照25 U/g玉米粉的比例加入α-高温淀粉酶,然后用中试液化系统加热至97 ℃维持2 h得到液化液。70%的液化液经过板框压滤机过滤得到糖液和玉米渣。未过滤的液化液和过滤后的糖液用来配制培养基。
种子培养基:由液化液和硫酸铵配制而成,初始总糖质量浓度为100 g/L,总氮质量浓度为2 g/L。
发酵培养基:由玉米液化液和糖液配制而成,初始总糖质量浓度为140~170 g/L,总氮质量浓度为8.5~10.5 g/L。
1.1.4 仪器与设备
HYW中试液化系统,上海兆光生物工程设计研究院;XA/MY12板框压滤机,浙江金鸟公司;YM75立式高压灭菌锅,上海三申医疗器械有限公司;DHZ-LA振荡摇床,太仓市强乐实验设备有限公司;DFC450光学显微镜、DM1000显微照相机,德国Leica公司;发酵罐(30、500、5 000 L),无锡汇森生物设备有限公司;UDK159凯氏定氮仪,意大利Velp公司;Waters 1525高效液相色谱仪,美国Waters有限公司。
1.2 实验方法
1.2.1 黑曲霉孢子制备
将菌种接种至试管斜面,35 ℃培养5~7 d,待孢子成熟后用接种环转接至茄形瓶中,35 ℃培养6~8 d获得成熟的黑曲霉孢子。
1.2.2 孢子预培养
在三角瓶中进行孢子预培养。装液量为50 mL/250 mL。以马铃薯葡萄糖培养基为基础培养基,分别考察碳源(葡萄糖、蔗糖和可溶性淀粉)、氮源(硫酸铵、玉米浆和酵母膏)、Ca2+浓度(0、0.1、1、10、100 mmol/L)、pH(3、4、5、6、7、8)、温度(25、30、35、40 ℃)、转速(0、50、100、200 r/min)、孢子浓度(2.4×108、1.6×108、0.8×108、0.08×108、0.008×108 CFU/mL)和培养时间的影响。
1.2.3 菌丝球形态控制
在预培养培养基中添加2 g/L玉米渣,考察颗粒物对发芽孢子凝聚的影响。将种子培养基经过8层纱布过滤除去玉米渣,考察对菌丝球形态的影响,并统计成球率。聚团的发芽孢子,经过振荡处理,使孢子分散,再转接至种子培养基继续培养,观察菌丝球形态,并统计成球率。摇瓶种子培养条件:装液量为50 mL/250 mL,孢子终浓度为3×105 CFU/mL,培养温度为37 ℃,摇床转速为300 r/min。
1.2.4 菌丝球形态对发酵的影响
将1.2.3部分培养得到不同形态的菌丝球按10%(体积分数)的比例接入发酵培养基进行摇床培养,培养条件同上。
1.2.5 放大验证
对照组中,休眠孢子接入500 L罐进行培养(装液量为400 L,孢子终浓度为3×105 CFU/mL,通风比例0.8 vvm,罐压0.07~0.08 MPa,温度37 ℃,转速300 r/min),种子培养成熟后按照10%(体积分数)比例移入5 000 L罐进行发酵(装液量为4 000 L,通风比例0.5 vvm,罐压0.07~0.08 MPa,温度37 ℃,转速200 r/min),残还原糖质量浓度低于0.5 g/L停止发酵。实验组中先将休眠孢子接入30 L罐内进行预培养,孢子萌发后转至500 L罐进行培养,种子成熟后移入5 000 L罐进行发酵(与对照相同)。
1.2.6 孢子萌发及菌丝球形态分析
孢子计数:样品经过振荡处理,将孢子分散均匀,采用血球计数板计数,有芽管的计为发芽孢子,每个样品重复3次。菌丝球计数:用生理盐水(0.9% NaCl,质量分数)稀释样品,使菌丝球浓度达到102~103 CFU/mL,在显微镜下计数,每个样品重复3次。发芽孢子和菌丝球用装有显微照相机的光学显微镜拍照。利用配套的莱卡应用软件(V4 Leica microsystems)对孢子直径、菌丝长度和菌丝球的直径进行显微测量,每个样品随机选择30个样本,并求平均数。出芽率和成球率按公式(1)(2)计算:
出芽率![]()
(1)
成球率![]()
(2)
1.2.7 检测方法
发酵液采用布氏漏斗过滤,得到菌丝球和滤液。菌丝球用蒸馏水冲洗2遍后105 ℃烘至恒重,称量得到菌丝体干重。残总糖和残还原糖:用斐林滴定法测定,测定残总糖时先将滤液用硫酸完全水解。柠檬酸:滤液先用0.22 μm微孔滤膜再次过滤,然后进行高效液相检测。检测条件:流动相为0.005 moL/L H2SO4,流速为0.6 mL/min,柱温为35 ℃,紫外检测波长为210 nm,进样量为10 μL。总氮:样品先进行消化,然后采用凯氏定氮仪进行测定。
1.2.8 数据分析
实验中各指标均为3组以上平行,结果表示为平均值±标准差。采用SPSS 21.0软件对实验数据进行统计学分析,2组数据间的比较采用独立样本T检验进行分析;3组以上数据间的比较采用单因素方差分析(One-way ANOVA)进行两两比较分析,显著性水平均设定为0.05。
2 结果与分析
2.1 预培养条件对孢子萌发的影响
图1-a显示,淀粉对黑曲霉孢子萌发几乎没有促进作用,各参数与对照接近;蔗糖能促进孢子萌发,发芽率高于对照;葡萄糖能显著促进孢子萌发(P<0.05),孢子发芽率和芽管长度均最高。孢子从休眠到萌发,需要外源物质激活,相比于蔗糖和淀粉,葡萄糖更有利于吸收利用,从而促进孢子萌发。氮源对孢子萌发具有促进作用,但与无机氮源相比,营养丰富的有机氮源对孢子萌发影响更显著(P<0.05),添加玉米浆时,发芽率和芽管长度均最高(图1-b)。图1-c显示,Ca2+浓度在0~10 mmol/L,发芽率和芽管长度随着Ca2+浓度增加而逐渐升高。Ca2+在细胞膜运输中起到重要作用[17],能帮助输送营养物质,能促进孢子萌发。然而,当Ca2+浓度增加至100 mmol/L时,孢子萌发被抑制,发芽率和芽管长度均降低。因此,后续试验中Ca2+浓度确定为10 mmol/L。图1-d显示,黑曲霉孢子对pH的适应性较宽,在pH 3~8内均可以萌发,最适的pH为5~7。pH过高或过低均不利于芽管生长,pH为5时芽管长度最高,后续实验选择预培养pH为5。图1-e显示,温度为25 ℃时黑曲霉孢子萌发较慢;温度达到40 ℃时,孢子停止萌发,处于休眠状态;预培养温度为35 ℃时,孢子发芽率和芽管长度均最高。图1-f显示,静置培养时,孢子发芽率较低,大部分孢子仍处于休眠状态;而在50~150 r/min摇床振荡培养时,孢子发芽率均较高,且孢子萌发情况相差不大,说明孢子萌发需要供应少量氧气。图1-g显示,孢子浓度>0.8×108 CFU/mL时,发芽率几乎为0;而孢子浓度<0.8×108 CFU/mL时,发芽率和芽管长度均随着孢子浓度降低而升高。结果说明,低浓度孢子有利于萌发,浓度过高会抑制孢子萌发。图1-h显示,随着预培养时间延长,发芽率和芽管长度逐渐增加。培养10 h后,发芽率几乎没有升高,但芽管长度仍继续增加,说明孢子已经完成发芽进入菌丝生长阶段。

a-碳源种类;b-氮源种类;c-Ca2+浓度;d-pH;e-温度;f-摇床转速;g-孢子浓度;h-时间
图1 预培养条件对孢子萌发的影响
Fig.1 Effect of pre-culture conditions on spore germination
2.2 菌丝球形态的控制
在孢子预培养过程中,孢子容易凝聚成团,形成较大的菌丝球。为控制孢子形成均匀的小菌丝球,考察了颗粒物和振荡分散处理等方式对孢子凝聚和菌丝球形态的影响,结果如图2所示。休眠孢子直接进行种子培养时,颗粒物对菌丝球形态影响显著(图2-A1和图2-A2,P<0.05)。孢子预培养中,添加颗粒物时,形成分散的发芽孢子;而不含颗粒物时,形成聚团的发芽孢子。结果表明,孢子萌发前添加颗粒物,对发芽孢子的凝聚影响明显。同时,无论种子培养基是否含有颗粒物,分散的发芽孢子均能形成大量的小菌丝球(图2-B1和图2-B2);而聚团的发芽孢子均形成少量的大菌丝球(图2-C1和图2-C2)。结果表明,在孢子发芽后,颗粒物对菌丝球形态的影响较小,并且菌丝球形态主要取决于发芽孢子是否凝聚。另外,将聚团发芽孢子分散后再进行种子培养,也能形成大量的小菌丝球(图2-D1和图2-D2)。进一步说明发芽孢子是否聚团对菌丝球形态起决定性作用。因此,孢子萌发前添加颗粒物和孢子聚团后振荡分散可以控制最终菌丝球形态。DRIOUCH等[18-20]认为发酵液添加微粒子可以通过碰撞干扰孢子间的相互作用,阻碍孢子凝集,防止后期形成较大团块。他研究发现添加硅酸盐、滑石粉和氧化铝等颗粒物,黑曲霉形态有较大改观,菌丝球直径减小。本研究通过添加有机物颗粒,补充营养源的同时也控制了菌丝球形态,对菌体的资源化利用不产生影响,实用价值更强。
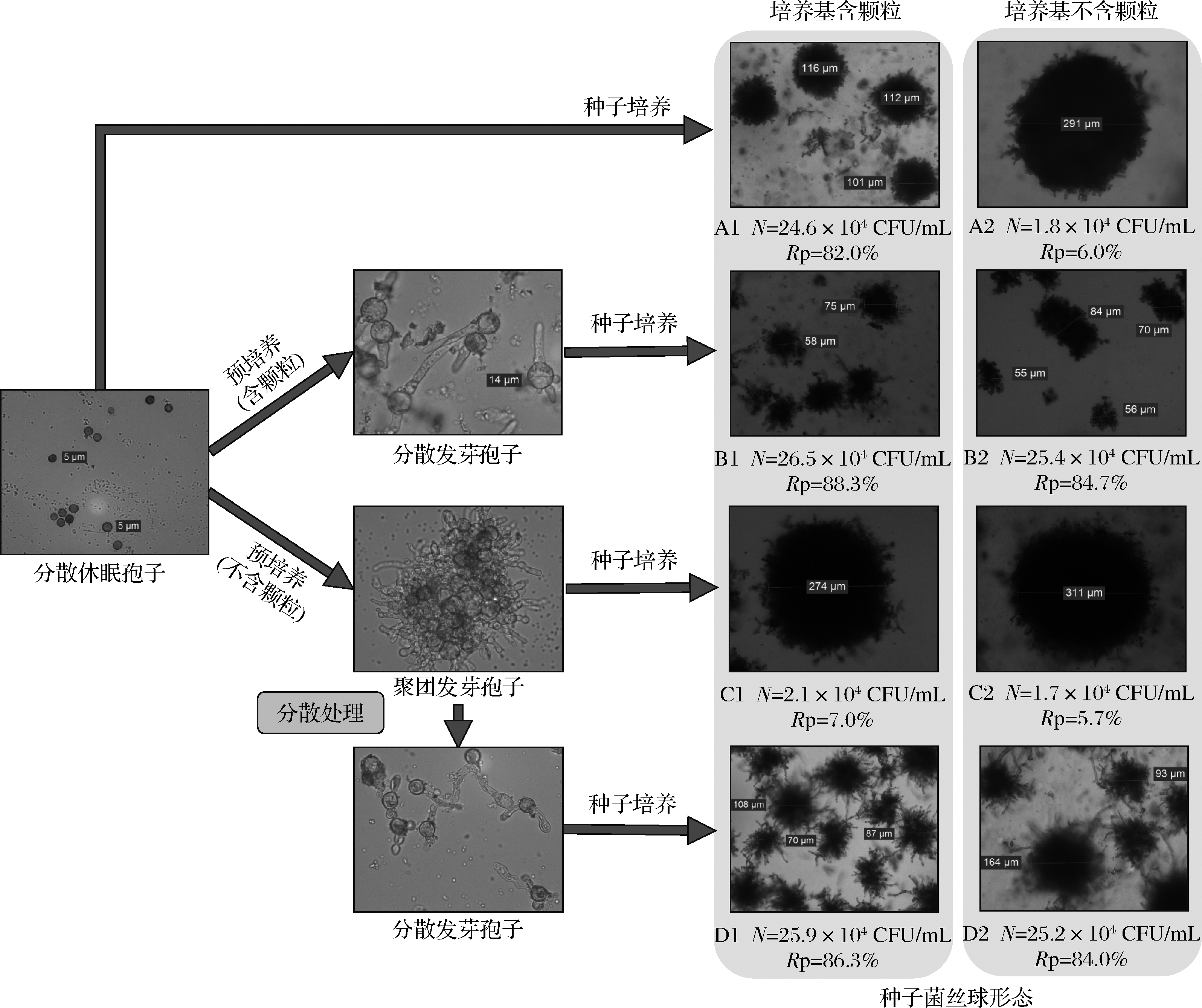
N-菌丝球浓度;Rp-成球率
图2 菌丝球形态的控制
Fig.2 The control of mycelial morphology
2.3 菌丝球形态对柠檬酸发酵的影响
菌丝球形态对柠檬酸发酵的影响如表1所示。无论是采用休眠孢子接种,还是采用发芽孢子接种,或是将聚团的发芽孢子进行分散处理后接种,所有的结果均显示,菌丝球大小对发酵结果具有显著性影响,小菌丝球对应的发酵强度和单位细胞产酸速率均显著高于大菌丝球对应的发酵结果(P<0.05)。
大量文献显示了相同的规律。ZHOU等[21]利用培养基和温度控制戴尔根霉(Rhizopus delemar)菌体形态产富马酸,随着球团直径的减小,干细胞质量和富马酸产量有增加的趋势。WANG等[22]利用菌丝球分散策略分割发酵生产柠檬酸,相比菌丝球发酵,菌丝球分散后对应的柠檬酸产量更高。
表1 菌丝球形态对柠檬酸发酵的影响
Table 1 Effect of mycelium morphology on citric acid fermentation

培养条件菌丝球形态直径/μm发酵强度/[g·(L·h-1)]单位细胞产酸速率/[g·(g·h-1)]A1小菌球108.0±10.6b2.33±0.04d0.109±0.043cA2大菌球291.2±21.6cd1.15±0.02b0.052±0.042abB1小菌球68.3±5.1a2.45±0.03f0.118±0.004eB2小菌球72.5±7.3a2.36±0.03de0.114±0.003cdeC1大菌球270.9±20.7c1.22±0.02c0.056±0.002cC2大菌球315.2±26.4d1.05±0.02a0.05±0.002aD1小菌球86.7±11.3ab2.41±0.04ef0.115±0.003deD2小菌球93.4±13.5ab2.37±0.03de0.112±0.004cd
注:同列不同小写字母代表差异显著性,P<0.05
2.4 放大验证
在优化的条件下进行放大验证,结果如图3所示。种子培养时,对照组总糖8 h后开始消耗(图3-a);酸度和菌丝球直径也相应从8 h开始增加(图3-b和3-c),原工艺存在约8 h的延滞期。而实验组菌体快速进入生长阶段,几乎没有延滞期(图3-d),节省了孢子萌发时间,周期缩短12 h以上,提高了种子培养效率。虽然实验组需要10 h用于孢子预培养,但是预培养具有孢子浓度高、体积小、氧气和营养消耗少等特点,可以实现大批量集中培养。同时,比较发酵过程曲线显示,实验组糖消耗速度和产酸速度较快(图3-e和3-f);菌丝球直径偏小(图3-g),产酸速率较快(图3-h),优势较明显。

a-种子总糖;b-种子酸度;c-种子菌球直径;d-种子菌体生长速率;e-发酵总糖;f-发酵酸度;g-发酵菌球直径;h-发酵产酸速率
图3 孢子预培养工艺放大验证
Fig.3 Scale-up verification of spore pre-culture process
发酵结果如表2所示。实验组提高了孢子分散性,菌丝球浓度和成球率增加非常显著(P<0.01),分别增加了0.4×104 CFU/mL和18.3%;菌丝球平均直径下降了28 μm,变化显著(P<0.05)。数量更多的小菌丝球更有利于营养物质吸收和转化,最大产酸速率提高了7.5%,发酵强度提高显著(P<0.05),实验组发酵周期缩短2 h。最终柠檬酸浓度和糖酸转化率分别增加了2 g/L和1.2%。
表2 工艺放大验证结果
Table 2 Results of process amplification verification
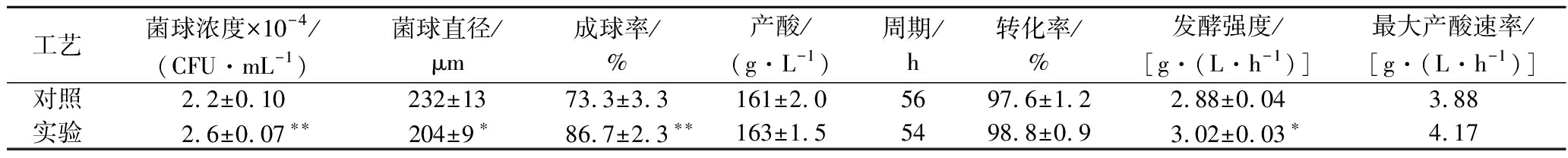
工艺菌球浓度×10-4/(CFU·mL-1)菌球直径/μm成球率/%产酸/(g·L-1)周期/h转化率/%发酵强度/[g·(L·h-1)]最大产酸速率/[g·(L·h-1)]对照2.2±0.10232±1373.3±3.3161±2.05697.6±1.22.88±0.043.88实验2.6±0.07∗∗204±9∗86.7±2.3∗∗163±1.55498.8±0.93.02±0.03∗4.17
注:**表示差异极显著,P<0.01;*表示差异显著,P<0.05
3 结论
本研究针对现有柠檬酸生产种子延滞期长,发酵水平波动大的问题,提出孢子预培养和菌丝形态控制策略。研究获得了孢子最佳萌发条件,并发现孢子萌发前添加颗粒物和孢子聚团后振荡分散可以控制最终菌丝球形态,发芽孢子是否凝聚对最终菌丝球形态起决定性作用。另外,小菌丝球导致发酵产酸速率显著提高。在中试规模工艺放大中,种子培养周期缩短12 h以上;成球率增加了18.3%,最大产酸速率提高了7.5%,发酵强度增加0.14 g/(L·h),发酵效率显著提升。本研究对丝状真菌孢子萌发、形态控制和生产效率的提升具有重要参考意义。
[1] KARAFFA L,KUBICEK C P.Aspergillus niger citric acid accumulation:Do we understand this well working black box?[J].Applied Microbiology and Biotechnology,2003,61(3):189-196.
[2] SOCCOL C R,VANDENBERGHE L P,RODRIGUES C,et al.New perspectives for citric acid production and application[J].Food Technology and Biotechnology,2006,44(2):141-149.
[3] YIN X,LI J H,SHIN H D,et al.Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms:Advances and prospects[J].Biotechnology Advances,2015,33(6):830-841.
[4] CHEN H,LI B Y,FENG B,et al.Tetracycline hydrochloride loaded citric acid functionalized chitosan hydrogel for wound healing[J].RSC Advances,2019,9(34):19 523-19 530.
[5] GONZ LEZ SELIGRA P,MEDINA JARAMILLO C,FAM
LEZ SELIGRA P,MEDINA JARAMILLO C,FAM L,et al.Data of thermal degradation and dynamic mechanical properties of starch-glycerol based films with citric acid as crosslinking agent[J].Data in Brief,2016,7:1 331-1 334.
L,et al.Data of thermal degradation and dynamic mechanical properties of starch-glycerol based films with citric acid as crosslinking agent[J].Data in Brief,2016,7:1 331-1 334.
[6] SHAO H,SUN H,YANG B,et al.Facile and green preparation of hemicellulose-based film with elevated hydrophobicity via cross-linking with citric acid[J].RSC Advances,2019,9(5):2 395-2 401.
[7] WILPISZEWSKA K,ANTOSIK A K,ZDANOWICZ M.The effect of citric acid on physicochemical properties of hydrophilic carboxymethyl starch-based films[J].Journal of Polymers and the Environment,2019,27(6):1 379-1 387.
[8] CHEN Y X,LIN Q,LUO Y M,et al.The role of citric acid on the phytoremediation of heavy metal contaminated soil[J].Chemosphere,2003,50(6):807-811.
[9] GAO Y Z,REN L L,LING W T,et al.Effects of low-molecular-weight organic acids on sorption-desorption of phenanthrene in soils[J].Soil Science Society of America Journal,2010,74(1):51-59.
[10] WAN J Z,MENG D,LONG T,et al.Simultaneous removal of lindane,lead and cadmium from soils by rhamnolipids combined with citric acid[J].PLoS One,2015,10(6):e0129978.
[11] 王宝石,陈坚,孙福新,等.发酵法生产柠檬酸的研究进展[J].食品与发酵工业,2016,42(9):251-256.
WANG B S,CHEN J,SUN F X,et al.Advances in production of citric acid through microbial fermentation[J].Food and Fermentation Industries,2016,42(9):251-256.
[12] ANWAR S,ALI S,SARDAR A.Citric acid fermentation of hydrolysed raw starch by Aspergillus niger IIB-A6 in stationary culture[J].Sindh University Research Journal(Science Series),2009,4(1):1-8.
[13] BARRINGTON S,KIM J S,WANG L,et al.Optimization of citric acid production by Aspergillus niger NRRL 567 grown in a column bioreactor[J].Korean Journal of Chemical Engineering,2009,26(2):422-427.
[14] 王宝石,陈坚,孙福新,等.基于超声波诱导孢子萌发与糖化酶活力表征移种策略高效合成柠檬酸[J].食品与发酵工业,2016,42(12):1-6.
WANG B S,CHEN J,SUN F X,et al.High efficient production of citric acid integrated strategies of the ultrasonic pretreated the spores and glucoamylase representing seed-transferring[J].Food and Fermentation Industries,2016,42(12):1-6.
[15] 卢俊文,张良慧,张建国.静电作用和疏水作用对黑曲霉孢子凝聚的影响[J].工业微生物,2017,47(6):31-37.
LU J W,ZHANG L H,ZHANG J G.Combination effects of Zeta potential and hydrophobicity on Aspergillus niger spore aggregation[J].Industrial Microbiology,2017,47(6):31-37.
[16] LU F,PING K K,WEN L,et al.Enhancing gluconic acid production by controlling the morphology of Aspergillus niger in submerged fermentation[J].Process Biochemistry,2015,50(9):1 342-1 348.
[17] 马新,史娟,李杨,等.苜蓿假盘菌子囊孢子萌发特性及子实体超微结构观察[J].微生物学通报,2020,47(11):3 564-3 576.
MA X,SHI J,LI Y,et al.Ascospore germination characteristics and the ultrastructure observation of fruit body of Pseudopeziza medicaginis[J].Microbiology China,2020,47(11):3 564-3 576.
[18] DRIOUCH H,SOMMER B,WITTMANN C.Morphology engineering of Aspergillus niger for improved enzyme production[J].Biotechnology and Bioengineering,2010,105(6):1 058-1 068.
[19] DRIOUCH H,ROTH A,DERSCH P,et al.Filamentous fungi in good shape:Microparticles for tailor-made fungal morphology and enhanced enzyme production[J].Bioengineered Bugs,2011,2(2):100-104.
[20] DRIOUCH H,H NSCH R,WUCHERPFENNIG T,et al.Improved enzyme production by bio-pellets of Aspergillus niger:Targeted morphology engineering using titanate microparticles[J].Biotechnology and Bioengineering,2012,109(2):462-471.
NSCH R,WUCHERPFENNIG T,et al.Improved enzyme production by bio-pellets of Aspergillus niger:Targeted morphology engineering using titanate microparticles[J].Biotechnology and Bioengineering,2012,109(2):462-471.
[21] ZHOU Z X,DU G C,HUA Z Z,et al.Optimization of fumaric acid production by Rhizopus delemar based on the morphology formation[J].Bioresource Technology,2011,102(20):9 345-9 349.
[22] WANG B S,CHEN J,LI H,et al.Efficient production of citric acid in segmented fermentation using Aspergillus niger based on recycling of pellets-dispersion strategy[J].RSC Advances,2016,6(107):105 003-105 009.