桔青霉素(citrinin,CIT)是一种由青霉属(Penicillium)、曲霉属(Aspergillus)和红曲霉属(Monascus)中的部分菌株产生的聚酮类真菌毒素,其主要存在于储存的谷物中[1]。特别在气候较炎热的国家生产的谷物,CIT的污染是一个严重问题[2]。谷物产品和其他具有药用价值的植物在生长、收获、运输和储存过程中可能会被产生CIT的真菌污染[3-4]。当处理和储存条件没有得到严格控制时,水果也易被CIT污染而造成重大损失。根据调查,工业化国家的作物收获后损失约为25%,其他国家超过50%[5]。而且CIT被证明对动物具有较强的肾毒性以及潜在的遗传毒性、胚胎毒性[6]和致畸性[7]。鉴于其对人类健康及经济发展的危害,开发一种灵敏、快速的CIT检测方法显得非常重要。日本厚生省在2000年版的“日本食品添加剂标准”中率先制定出红曲色素中CIT限量标准为0.2 mg/kg,这一标准是当时能够检出的CIT的最低剂量[8],欧盟规定CIT在红曲霉发酵的大米补充剂中的限量标准为2 mg/kg,中国台湾省制定的《食品中真菌毒素限量标准》规定了食品中CIT的限量,其中红曲色素中CIT的限量为0.2 mg/kg、原料用红曲米的限量为5 mg/kg以下,使用红曲原料制成的食品为2 mg/kg以下[8]。目前在中国大陆地区,还没有建立不同农产品中CIT含量的控制指标。
目前,CIT检测可用的分析方法有薄层色谱法[9]、高效液相色谱法(HPLC)[10]、液相色谱串联质谱法(liquid chromatography-tandem mass spectrometry,LC-MS/MS)[11]、超高效液相色谱和荧光检测法[12]、气相色谱质谱法[13]和酶联免疫分析法(enzyme-linked immunosorbent assay,ELISA)[14]。薄层色谱法虽然操作简单,但是准确性和特异性较差;HPLC等仪器检测方法虽然灵敏度和稳定性较高,但样品前处理操作较为复杂,且需要专业的大型仪器和检测场所,不能满足食品安全快速检测要求。ELISA作为一种灵敏度高、高通量、设备简单、成本低的样品筛查和定量方法,近年来得到了迅速发展和广泛应用。基于辣根过氧化物酶(horse radish peroxidase,HRP)催化底物的比色ELISA法存在灵敏度低、抗基质干扰能力差的局限性。荧光酶联免疫分析法(fluorescein-linked immunosorbent assay,FELISA)与现有的分析平台兼容性良好,被认为是目前有害食源性物质筛选最敏感的方法之一[15-16]。在过去的几十年里,已开发出多种具有高灵敏度的FELISA用于检测目标分子,包括基于碱性磷酸酶的化学发光免疫检测[17]、基于HRP的化学发光免疫检测[18]、基于过氧化氢酶的荧光免疫检测[19]、基于人α凝血酶的荧光免疫检测[20]等,具有更广泛的应用前景。因此,建立一种具有高灵敏度的FELISA方法用于谷物中CIT的定量检测具有较大的应用前景。
胰蛋白酶(trypsin)是一种高特异性的丝氨酸蛋白水解酶,对于含精氨酸(Arg)位点的肽段表现出非常高的水解催化性能[21]。基于trypsin的这个特性,研究人员设计并合成了含氨基保护基的三肽Cbz-Ile-Pro-Arg-OH,再通过酰化反应将Arg的羧基与罗丹明110分子中的2个氨基缩合形成双酰胺底物(Cbz-Ile-Pro-Arg)2-R110,用于trypsin的催化能力测定[22]。在trypsin的催化下,连接在罗丹明110分子两端的酰胺键迅速发生水解断裂,具有微弱荧光的罗丹明110双酰胺底物转变为具有强荧光的罗丹明110分子,荧光信号强度可以得到极强的提高[23]。本研究通过将trypsin和罗丹明110双酰胺底物引入到FELISA中,克服实际样本的基质干扰,以实现检测灵敏度提高的目的。
本研究通过甲醛加成法[24]合成了trypsin-CIT,并将其引入到FELISA信号生成系统实现CIT的快速检测。其原理如图1所示,trypsin-CIT可通过抗原-抗体相互作用被固定在酶标孔中,选择trypsin替代常用的HRP作为标记酶,并且选用仅存在极低荧光的罗丹明110双酰胺衍生物作为trypsin的催化底物。通过CIT与trypsin-CIT对其抗体的竞争关系,被固定下来的trypsin催化罗丹明110双酰胺衍生物裂解而触发荧光信号的产生,实现对CIT的快速检测。本研究与传统ELISA的HRP-TMB显色体系进行了对比,显示出较高的分析灵敏度,并将其应用于检测玉米样本,验证了本方法的检测性能和稳定性。

图1 Trypsin催化的罗丹明110双酰胺荧光底物 ELISA方法检测CIT
Fig.1 Trypsin-catalyzed rhodamine 110 bis-amide fluorescent substrate ELISA method for the detection of CIT
1 材料与方法
1.1 材料与仪器
胰蛋白酶、链球菌蛋白G, 美国Sigma-Aldrich公司;(Cbz-Ile-Pro-Arg)2-R110赛默飞世尔科技公司; CIT, Fermentek公司;抗CIT腹水, 南昌大学中德联合研究院;脱脂乳, 广东赛国生物科技有限公司;甲醇(色谱纯、分析纯)及常见试剂(分析纯),西陇科学分有限公司;96孔酶标板, Costar公司。
Waters H CLASS/XEVD TQD, 美国沃特世公司;桔青霉素免疫亲和柱, 北京华安麦克生物技术有限公司;Varioskan LUX多功能酶标仪, 赛默飞世尔公司;超纯水仪, 美国密里博公司。
1.2 trypsin-CIT与HRP-CIT的制备
将5.7 mg trypsin溶解于2 mL浓度为1 mmol/L的HCl(pH 3)中,随后向其中逐滴加入10 mg/mL的CIT甲醇溶液100 μL,混合均匀后加入200 μL体积分数为37%甲醛溶液,37 ℃搅拌反应24 h,即得到trypsin-CIT,同样CIT与HRP反应得到HRP-CIT,反应结束后,置0.01 mol/L PBS中4 ℃透析72 h。
1.3 trypsin催化的荧光底物浓度
罗丹明110双酰胺荧光底物的浓度对于trypsin催化的FELISA信号强度有着较大影响,因此本研究测定了在相同trypsin质量浓度(400 ng/mL)条件下,荧光底物浓度与trypsin浓度的变化规律,获得最佳目标底物浓度。
1.4 trypsin催化的FELISA检测流程
将链球菌蛋白G(proteinG)用PBS(pH 8.6)稀释至25 μg/mL,每孔100 mL,4 ℃过夜包被,去除孔内液体,用含0.05%吐温-20的磷酸缓冲液进行洗板,重复3次,用PBS(pH 7.4)将抗CIT腹水稀释至20 μg/mL,每孔100 μL,37 ℃孵育1 h后洗板,加入300 μL 1%脱脂乳溶液,37 ℃孵育1 h,加入50 μL质量浓度为5.5 μg/mL的trypsin-CIT和50 μL的待测液,37 ℃孵育30 min后洗板,加入浓度为0.312 5 μmol/L的罗丹明双酰胺底物,37 ℃孵育30 min后测定激发波长为498 nm,发射波长为521 nm时的荧光强度。
1.5 trypsin催化的FELISA的精密度与准确性评价
分别通过LC-MS/MS和FELISA的方法对加标的阴性玉米样本进行分析,随后根据GB 5009.222—2016《桔青霉素测定》中的样本提取方法:称取10.0 g加有CIT的玉米样本于锥形瓶中,加入50 mL体积分数70%的甲醇水溶液,在涡旋振荡2 min,5 000 r/min离心,取上清液用快速定性滤纸过滤后待检测。取上清液用0.01 mol/L PBS稀释7倍后,进行FELISA检测;另取1 mL上清液加入49 mL 0.01 mol/L磷酸溶液(pH 7.5)混匀,用微纤维滤纸过滤后取20 mL上述溶液过免疫亲和柱净化,将其以1~2滴/s的流速全部通过亲和柱,再将5 mL 0.01 mol/L磷酸溶液(pH 7.5)也以1~2滴/s的流速通过亲和柱,待液体排干后,用2 mL甲醇以1滴/s的流速进行洗脱,洗脱液收集至样品瓶中用于LC-MS/MS分析。
2 结果与讨论
2.1 trypsin催化的荧光底物浓度
根据图2-a可得,在trypsin质量浓度固定于400 ng/mL条件下,随着底物浓度升高,trypsin催化底物产生的荧光信号也逐渐升高。结合荧光信号强度,底物浓度为0.156 μmol/L时具有明显的荧光信号,故选择了0.156 μmol/L为本实验的最终底物浓度。
2.2 trypsin-CIT与trypsin催化性能对比
在0.156 μmol/L底物浓度下,绘制trypsin、trypsin-CIT浓度与荧光强度关系曲线,如图2-b所示,trypsin和trypsin-CIT质量浓度为25 ng/mL时,trypsin-CIT催化产生的荧光强度为35.42,trypsin催化产生的荧光强度为16.68,可推得酶活损失52.91%。

a-trypsin(400 ng/mL)条件下底物浓度与荧光强度关系; b-trypsin、trypsin-CIT浓度与荧光强度关系
图2 底物浓度及不同酶浓度对荧光强度的影响
Fig.2 Effect of substrate concentration, trypsin and trypsin-CIT concentration on fluorescence intensity
2.3 trypsin催化的荧光ELISA的检测条件优化
实验优化了包被腹水浓度、trypsin-CIT浓度、pH、甲醇浓度、NaCl浓度、免疫孵育时间和酶催化时间等影响FELISA的几个关键参数,以获得高灵敏度和强荧光信号。
包被抗体和trypsin-CIT的浓度被认为是影响直接竞争ELISA检测灵敏度的最重要因素。为了提高CIT抗体的生物活性,本实验预先将proteinG包被在酶标板上,定向捕获CIT抗体的Fc片段,从而提高CIT抗体的生物活性。因此本实验设置腹水质量浓度为80、40、20、10 μg/mL和trypsin-CIT质量浓度为22、11、5.5、2.75 μg/mL进行正交实验,结果如表1,通过(F为阴性条件下的荧光值,F0为50 ng/mL加标条件下的荧光值)反映本方法对CIT的检测灵敏度,并基于F与F0的最大差值与B0的最高值原则选择最佳腹水浓度与trypsin-CIT浓度,根据最佳信号显示,包被腹水质量浓度为20 μg/mL、trypsin-CIT质量浓度为5.5 μg/mL是最佳条件。
表1 棋盘滴定法优化包被腹水浓度和trypsin-CIT浓度
Table 1 Optimized coated ascites concentration and trypsin-CIT concentration by a checkboard titration method
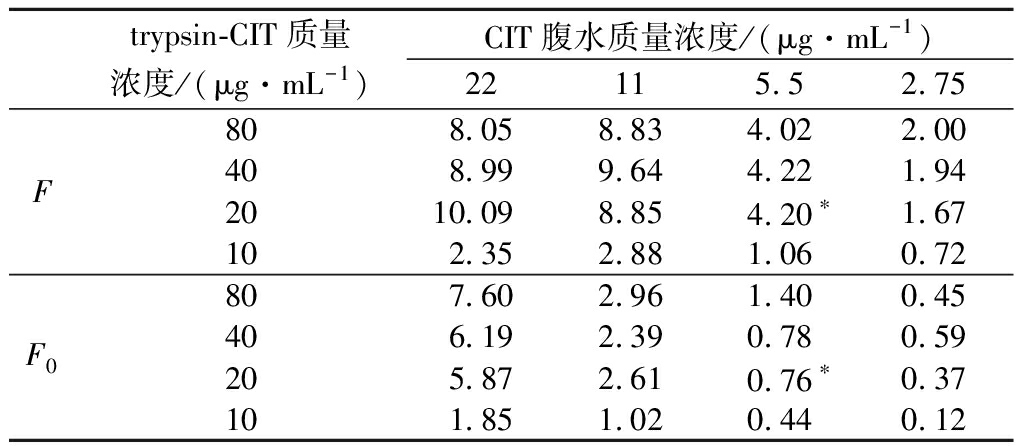
trypsin-CIT质量浓度/(μg·mL-1)CIT腹水质量浓度/(μg·mL-1)22115.52.75F808.05 8.83 4.02 2.00 408.99 9.64 4.22 1.94 2010.09 8.85 4.20∗1.67 102.35 2.88 1.06 0.72F0807.60 2.96 1.40 0.45 406.19 2.39 0.78 0.59 205.87 2.61 0.76∗0.37 101.85 1.02 0.44 0.12
注:*为最佳包被腹水浓度和Trypsin-CIT
pH、甲醇浓度、NaCl浓度能够通过改变抗原抗体结合位点的活性来影响抗原抗体反应,本实验通过B0=(1-F/F0)×100(F为阴性条件下的荧光值,F0为50 ng/mL加标条件下的荧光值)反映加入标准品后的抑制结果,当B0值越大时,表示本方法对于CIT的检测灵敏度越高。如图3-a所示,在pH 6.5时,B0达到最大值,为最佳pH条件。由于CIT分子具有很强的疏水性,为实现对样本中CIT的高回收率提取,需要用含有一定体积分数甲醇的样本提取液,但是待检测溶液中的甲醇体积分数过高时,抗原抗体的反应会受到极大干扰,因此,研究了含不同体积分数甲醇的待检测溶液进行反应的检测结果,如图3-b所示,当甲醇体积分数升高时,B0会逐渐下降,且在甲醇体积分数>10%时B0从34.09%降至15.55%,基于这一结果,本实验选择甲醇体积分数为10%为免疫检测最佳条件。在免疫反应过程中,一定浓度的NaCl有利于抗原抗体的相互作用,如图3-c所示,随着NaCl浓度的升高,B0也逐渐升高,且当NaCl浓度达到80 mmol/L时,B0达到最高值。为了评价免疫孵育时间和酶催化时间对B0的影响,设置了不同免疫孵育时间(15~75 min)和不同酶催化时间(10~60 min)作为反应条件,30 min是免疫孵育时间最佳条件(图3-d),酶催化时间达到30 min后,B0进入平台期(图3-e),因此最佳酶催化时间为30 min。

a-pH;b-甲醇体积分数;c-NaCl浓度;d-免疫孵育时间;e-酶催化时间
图3 Trypsin催化的FELISA检测条件优化
Fig.3 Optimization of FELISA detection conditions catalyzed by Trypsin
2.4 trypsin催化的FELISA检测性能评价
以CIT浓度为横坐标,B0为横坐标绘制定量曲线(图4-a),随着溶液中的CIT质量浓度从1.56~6 400 ng/mL,B0逐渐增加,且在CIT质量浓度为12.5~400 ng/mL时,B0随CIT浓度增加呈线性增加关系,其定量可用方程y=18.606 lnx-18.713(R2=0.992),检测灵敏度IC20为8.01 ng/mL,IC50为40.16 ng/mL,为了与基于HRP-CIT的传统比色ELISA检测性能对比,设置腹水质量浓度为80、40、20、10 μg/mL,HRP-CIT质量浓度为80、40、20、10 μg/mL进行正交实验同样优化了二者浓度,结果如表2,根据竞争抑制率B0=(1-OD阴性/OD阳性)×100(OD阴性为阴性条件下的吸光度,OD阳性为加标200 ng/mL条件下的吸光度),由最高竞争抑制率所对应的条件得出最佳腹水质量浓度为40 μg/mL,HRP-CIT质量浓度为40 μg/mL,同时基于此条件构建了基于HRP-CIT的传统比色ELISA,并绘制了其定量曲线(图4-b),其定量可用方程y=13.476 lnx-11.975(R2=0.971 2),检测灵敏度IC20为10.73 ng/mL,IC50为110.08 ng/mL,对比可得,本实验构建的FELISA检测灵敏度是传统比色ELISA的1.34倍,IC50是传统比色ELISA的2.741倍。

a-trypsin催化的FELISA检测CIT定量曲线; b-HRP催化的比色ELISA检测CIT定量曲线
图4 Trypsin催化的FELISA与HRP催化的比色 ELISA检测性能比较
Fig.4 Detection efficacy of FELISA catalyzed by trypsin and colorimetric ELISA catalyzed by HRP
表2 棋盘滴定法优化包被腹水浓度和HRP-CIT浓度
Table 2 Optimized coated ascites concentration and HRP-CIT concentration by a checkboard titration method

CIT-HRP浓度/(μg·mL-1)CIT腹水质量浓度/(μg·mL-1)80402010OD阴性801.38 0.83 0.57 0.35401.75 1.11∗0.68 0.43201.98 1.29 0.80 0.50101.72 1.13 0.71 0.46OD阳性800.64 0.42 0.25 0.18400.79 0.48∗0.30 0.21201.04 0.61 0.37 0.26100.87 0.52 0.40 0.27
注:*为最佳包被腹水浓度和HRP-CIT
用本方法进一步检测质量浓度为1 μg/mL的常见真菌毒素CIT、脱氧雪腐镰刀菌烯酮(deoxynivalenone,DON)、玉米赤霉烯酮(zearalenone,ZEN)、黄曲霉毒素B1(aflatoxin B1,AFB1)、黄曲霉毒素B2(aflatoxin B2,AFB2)、黄曲霉毒素G1(aflatoxin AFG1,AFG1)和伏马毒素B1(fumonisin B1,FB1),结果如图5所示,与常见真菌毒素无明显的交叉反应,表明本方法检测CIT具有较好的特异性。
为了评价该方法检测实际样本的可行性,将不同质量浓度的CIT添加至玉米样本中,加标量分别为3.5、1.75、0.88、0.22 mg/kg,结果如表3,FELISA批内回收率分别为97.05%、98.97%、110.58%、90.94%,批间回收率分别为90.10%、109.11%、101.63%、93.46%,且批内、批间测定的标准偏差值均小于15%。检测结果与液相色谱串联质谱法作为参考方法进行对比,表4结果显示trypsin催化的FELISA检测结果与LC-MS/MS结果具有良好的一致性。以上结果表明,trypsin催化的FELISA具有良好的回收率和准确性,可对玉米样本中的CIT进行定量检测。

图5 Trypsin催化的FELISA特异性分析
Fig.5 FELISA specific analysis catalyzed by trypsin
表3 Trypsin催化的FELISA检测不同浓度CIT的 重现性与精密度
Table 3 Reproducibility and precision of FELSA catalyzed by Trypsin in detecting CIT at different concentrations
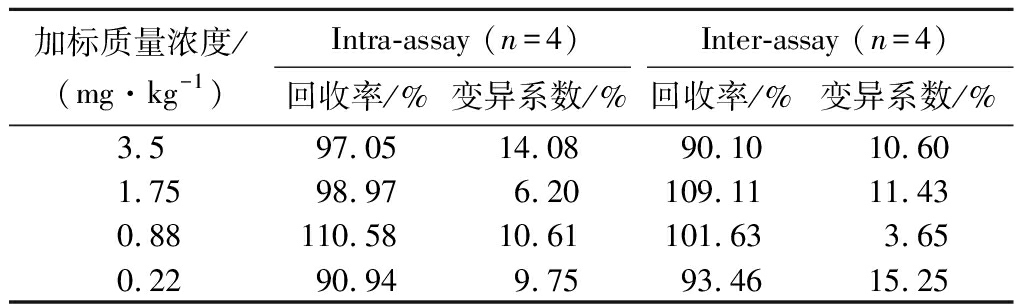
加标质量浓度/(mg·kg-1)Intra-assay (n=4)Inter-assay (n=4)回收率/%变异系数/%回收率/%变异系数/%3.597.0514.0890.1010.601.7598.976.20109.1111.430.88110.5810.61101.633.650.2290.949.7593.4615.25
表4 加标玉米对Trypsin催化的FELISA进行准确度评估
Table 4 Accuracy assessment of Trypsin-catalyzed FELISA by spiked corn
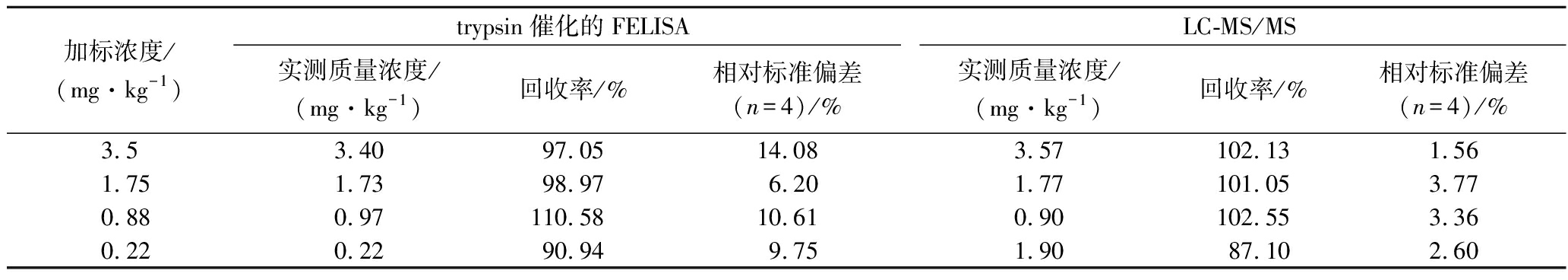
加标浓度/(mg·kg-1)trypsin催化的FELISALC-MS/MS实测质量浓度/(mg·kg-1)回收率/%相对标准偏差(n=4)/%实测质量浓度/(mg·kg-1)回收率/%相对标准偏差(n=4)/%3.53.4097.0514.083.57102.131.561.751.7398.976.201.77101.053.770.880.97110.5810.610.90102.553.360.220.2290.949.751.9087.102.60
3 结论
本研究基于trypsin催化罗丹明110双酰胺荧光底物的特性,结合CIT和CIT标记的trypsin与CIT抗体的相互竞争作用关系,建立了一种FELISA方法用于检测玉米中的CIT。在最佳条件下:抗CIT腹水质量浓度20 μg/mL、trypsin-CIT质量浓度为5.5 μg/mL、pH 6.5、甲醇体积分数10%、NaCl浓度80 mmol/L、免疫反应时间30 min、酶催化时间30 min,该方法检测CIT的最低灵敏度可达8.01 ng/mL,IC50可达40 ng/mL,是HRP催化的比色ELISA检测灵敏度的1.34倍,IC50的2.741倍,此外,该方法具有良好的特异性、重现性和精密度。如果使用CIT的单克隆抗体,检测灵敏度还可以更高,为更快速、灵敏定量检测CIT提供了新的选择。
[1] ZHANG H Y, AHIMA J, YANG Q Y, et al.A review on citrinin:Its occurrence, risk implications, analytical techniques, biosynthesis, physiochemical properties and control[J].Food Research International, 2021, 141:110075.
[2] MÜLLER L, CARIS-VEYRAT C, LOWE G, et al.Lycopene and its antioxidant role in the prevention of cardiovascular diseases-A critical review[J].Critical Reviews in Food Science and Nutrition, 2016, 56(11):1 868-1 879.
[3] LI Y, ZHOU Y-C, YANG M-H, et al.Natural occurrence of citrinin in widely consumed traditional Chinese food red yeast rice, medicinal plants and their related products[J].Food Chemistry, 2012, 132(2):1 040-1 045.
[4] KABAK B, DOBSON A D W.Mycotoxins in spices and herbs-An update[J].Critical Reviews in Food Science and Nutrition, 2017, 57(1):18-34.
[5] JANISIEWICZ W J.Biological control of postharvest diseases:Hurdles, successes and prospects[J].Acta Horticulturae, 2013(1001):273-283.
[6] MEERPOEL C, VIDAL A, HUYBRECHTS B, et al.Comprehensive toxicokinetic analysis reveals major inter species differences in absorption, distribution and elimination of citrinin in pigs and broiler chickens[J].Food and Chemical Toxicology, 2020, 141:111365.
[7] DEGEN G H, ALI N, GUNDERT-REMY U.Preliminary data on citrinin kinetics in humans and their use to estimate citrinin exposure based on biomarkers[J].Toxicology Letters, 2018, 282:43-48.
[8] LIAO C D, CHEN Y C, LIN H Y, et al.Incidence of citrinin in red yeast rice and various commercial Monascus products in Taiwan from 2009 to 2012[J].Food Control, 2014, 38:178-183.
[9] TOUHAMI N, SOUKUP S T, SCHMIDT-HEYDT M, et al.Citrinin as an accessory establishment factor of P.expansum for the colonization of apples[J].International Journal of Food Microbiology, 2018, 266:224-233.
[10] ATAPATTU S N, POOLE C F.Recent advances in analytical methods for the determination of citrinin in food matrices[J].Journal of Chromatography A, 2020, 1627:461399.
[11] JI X F, XU J F, WANG X F, et al.Citrinin determination in red fermented rice products by optimized extraction method coupled to liquid chromatography tandem mass spectrometry (LC-MS/MS)[J].Journal of Food Science, 2015, 80(6):T1438-T1444.
[12] HUERTAS-PÉREZ J F, ARROYO-MANZANARES N, GARC A-CAMPA
A-CAMPA A A M, et al.High-throughput determination of citrinin in rice by ultra-high-performance liquid chromatography and fluorescence detection (UHPLC-FL)[J].Food Additives & Contaminants.Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2015, 32(8):1 352-1 357.
A A M, et al.High-throughput determination of citrinin in rice by ultra-high-performance liquid chromatography and fluorescence detection (UHPLC-FL)[J].Food Additives & Contaminants.Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2015, 32(8):1 352-1 357.
[13] HE S S, LIU X, WANG Y L, et al.Metabolomics analysis based on UHPLC-Q-TOF-MS/MS reveals effects of genistein on reducing mycotoxin citrinin production by Monascus aurantiacus Li AS3.4384[J].LWT, 2020, 130:109613.
[14] SINGH G, VELASQUEZ L, HUET A C, et al.Development of a sensitive polyclonal antibody-based competitive indirect ELISA for determination of citrinin in grain-based foods[J].Food Additives & Contaminants.Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2019, 36(10):1 567-1 573.
[15] WU Y Q, ZENG L F, XIONG Y, et al.Fluorescence ELISA based on glucose oxidase-mediated fluorescence quenching of quantum dots for highly sensitive detection of Hepatitis B[J].Talanta, 2018, 181:258-264.
[16] LIANG Y, HUANG X L, YU R J, et al.Fluorescence ELISA for sensitive detection of ochratoxin A based on glucose oxidase-mediated fluorescence quenching of CdTe QDs[J].Analytica Chimica Acta, 2016, 936:195-201.
[17] HERMAN D S, RANJITKAR P, YAMAGUCHI D, et al.Endogenous alkaline phosphatase interference in cardiac troponin I and other sensitive chemiluminescence immunoassays that use alkaline phosphatase activity for signal amplification[J].Clinical Biochemistry, 2016, 49(15):1 118-1 121.
[18] ZHOU Y, ZHOU T, ZHOU R, et al.Chemiluminescence immunoassay for the rapid and sensitive detection of antibody against porcine parvovirus by using horseradish peroxidase/detection antibody-coated gold nanoparticles as nanoprobes[J].Luminescence, 2014, 29(4):338-343.
[19] HUANG X L, ZHAN S N, XU H Y, et al.Ultrasensitive fluorescence immunoassay for detection of ochratoxin A using catalase-mediated fluorescence quenching of CdTe QDs[J].Nanoscale, 2016, 8(17):9 390-9 397.
[20] WU Y D, GUO W S, PENG W P, et al.Enhanced fluorescence ELISA based on HAT triggering fluorescence “turn-on” with enzyme-antibody dual labeled AuNP probes for ultrasensitive detection of AFP and HBsAg[J].ACS Applied Materials & Interfaces, 2017, 9(11):9 369-9 377.
[21] HUANG S, LI H M, LIU Y, et al.Investigations of conformational structure and enzymatic activity of trypsin after its binding interaction with graphene oxide[J].Journal of Hazardous Materials, 2020, 392:122285.
[22] MAYORAL J G, ALARC N F J, MART
N F J, MART NEZ T F, et al.An improved end-point fluorimetric procedure for the determination of low amounts of trypsin activity in biological samples using rhodamine-110-based substrates[J].Applied Biochemistry and Biotechnology, 2010, 160(1):1-8.
NEZ T F, et al.An improved end-point fluorimetric procedure for the determination of low amounts of trypsin activity in biological samples using rhodamine-110-based substrates[J].Applied Biochemistry and Biotechnology, 2010, 160(1):1-8.
[23] WANG F A, LU C H, WILLNER I.From cascaded catalytic nucleic acids to enzyme-DNA nanostructures:Controlling reactivity, sensing, logic operations, and assembly of complex structures[J].Chemical Reviews, 2014, 114(5):2 881-2 941.
[24] ATAR N, EREN T J, YOLA M L.A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice[J].Food Chemistry, 2015, 184:7-11.