慢传输型便秘是最常见的便秘类型之一[1],是由肠道转运时间延长导致的慢性排便困难[2]。药物和手术治疗均会给患者带来不同程度的副作用,同时存在复发的可能性。使用益生菌制剂治疗便秘不易引起不良反应,受到越来越多的关注[3-5]。中国食品科学技术学会发布《益生菌的科学共识(2020年版)》,指出了益生菌的核心特征之一是保证有足够的数量,其功效发挥具有菌株特异性,益生功能需要在数量和菌株水平进行确认。相比使用单一菌株,复合菌株的人群适用性范围更广。
盐酸洛哌丁胺是慢传输型便秘动物模型常用造模药物[6-7],其机制是作用于肠壁阿片受体,阻止胆碱和前列腺素释放,从而抑制肠蠕动,延长肠道内容物通过时间,促进水、电解质等吸收,导致动物肠转运时间延长,粪便数量、质量和含水量减少,出现便秘症状[8]。
本研究通过盐酸洛哌丁胺诱导慢传输型便秘动物模型,研究益生菌组合配方在改善慢传输型便秘方面的作用。
1 材料与方法
1.1 材料和仪器
1.1.1 实验材料与试剂
益生菌组合物配方(鼠李糖乳杆菌LR-168、嗜酸乳杆菌LA-99和动物双歧杆菌BB-115),仙乐健康科技股份有限公司。
盐酸洛哌丁胺胶囊,西安杨森制药有限公司;活性炭粉,生工生物工程(上海)股份有限公司;小鼠胃动素(motilin, MTL)、胃泌素(gastrin, Gas)、P物质(substance P, SP)、内皮素(endothelin 1, ET-1)、生长抑素(somatostatin, SS)、血管活性肠肽(vasoactive intestinal peptide, VIP)、肠道紧密连接蛋白、血清D-乳酸、肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)、干扰素γ(interferon γ, IFN-γ)、白介素2(interleukin 2, IL-2)、白介素4(interleukin 4, IL-4)、白介素6(interleukin6, IL-6)、白介素10(interleukin 10, IL-10)、白介素12(interleukin 12, IL-12)、白介素17(interleukin, IL-17)和抗体(sIgA)ELISA试剂盒,南京森贝伽生物科技有限公司;阿拉伯树胶粉,国药集团化学试剂公司。
墨汁配制方法:称取阿拉伯胶100 g,与800 mL水混合均匀,加热煮沸至溶液透明,再加入活性炭50 g,煮沸3次。待溶液冷却后,用水稀释定容至1 000 mL,4 ℃保存。
1.1.2 仪器设备
PB300-N电子天平,Mettler Toledo;Multi-scan Go多功能酶标仪,美国Thermo公司;CFX384实时PCR系统,BIO-RAD公司。
1.2 实验方法
1.2.1 实验动物设计
6周龄雄性SPF级BABL/c小鼠,北京维通利华公司。实验方案经江南大学伦理委员会批准(JN.No20191115b0500110),并按照欧盟指导方针(2010/63/EU)执行。饲养环境保持在(23±2)℃,相对湿度(50±10)%,12 h/12 h明暗交替,实验期间,小鼠自由进水进食。
1.2.2 模型建立与分组
50只小鼠随机分成空白组、模型组(盐酸洛哌丁胺)、阳性对照组(盐酸洛哌丁胺+动物双歧杆菌BB-12:5×108 CFU)、低剂量组(盐酸洛哌丁胺+益生菌组合物:5×108 CFU)、高剂量组(盐酸洛哌丁胺+益生菌组合物:5×109 CFU),每组10只。
根据《保健食品检验与评价技术规范(2003版)》对通便功能的评价方法,使用盐酸洛哌丁胺造模便秘模型,通过检测首粒黑便排出时间判定造模成功与否。小鼠适应7 d后开始灌胃,从第8—14天空白组、模型组灌胃0.2 mL生理盐水,阳性对照组、低剂量组、高剂量组灌胃相应的用0.2 mL生理盐水重悬的菌粉悬液。从第15—21天,模型组、灌菌组灌胃用0.2 mL生理盐水重悬的盐酸洛哌丁胺悬液(10 mg/kg bw),空白组灌胃0.2 mL生理盐水,1 h后空白组、模型组灌胃0.2 mL生理盐水,灌菌组灌胃相应的用0.2 mL生理盐水重悬的菌粉悬液。第22天处死小鼠取样用作后续实验。
1.2.3 小鼠首粒黑便时间、黑便粒数、黑便质量及小肠推进率的测定
第21天,空白组给予0.2 mL生理盐水溶液、模型组和灌菌组均给予0.2 mL生理盐水溶液重悬的盐酸洛哌丁胺悬液(10 mg/kg bw),1 h后,各组菌灌胃墨汁,从灌胃墨汁开始,记录每一只小鼠排首粒黑便的时间、6 h黑便粒数和黑便质量。
第22天空白组给予0.2 mL生理盐水溶液、模型组和灌菌组均给予0.2 mL生理盐水溶液重悬的盐酸洛哌丁胺悬液(10 mg/kg bw),30 min后,空白组和模型组灌胃墨汁,灌菌组灌胃含各自灌胃内容物的墨汁。30 min后处死小鼠,打开腹腔分离肠系膜,剪取肠管,测定小肠总长度L1,墨汁推进长度L2,按公式(1)计算小肠推进率:
小肠推进率/%=L2/L1×100
(1)
1.2.4 小鼠血清、结肠组织匀浆胃肠调节肽的检测
将收集到的小鼠血液静置2 h,3 000×g离心15 min后获得血清,参照试剂盒说明书进行实验,根据标准曲线计算中血清中胃肠调节肽含量。
用预冷的PBS冲洗结肠组织,去除残留血液,剔除周围脂肪组织,称重后将其剪碎。将剪碎的组织与PBS按照料液比1∶9(g∶mL)在组织破碎仪上进行破碎,最后5 000×g离心10 min,取上清液检测胃肠调节肽浓度。具体操作参照试剂盒说明书。
1.2.5 小鼠血清细胞因子的检测
使用对应ELISA试剂盒小鼠血清中细胞因子含量,具体步骤参考试剂盒说明书。
1.2.6 小鼠结肠组织AQP3和c-kit基因表达水平检测
取超低温冰箱冻存新鲜结肠组织,采用实时荧光定量聚合酶链反应(fluorescence real time quantitative polymerase chain reaction,qRT-PCR)来测定相关蛋白的表达量。严格按说明书用Trizol法提取总RNA,1 μg总RNA经逆转录合成30 μL cDNA,基因引物由生工生物工程(上海)股份有限公司合成。AQP3基因、c-kit基因、actin基因引物序列如表1所示。
表1 AQP3、c-kit和actin引物序列
Table 1 Primer sequences of AQP3, c-kit and actin
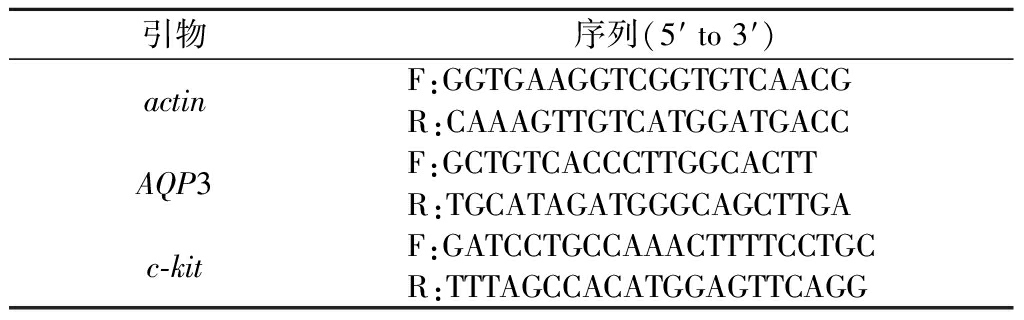
引物序列(5′ to 3′)actinF:GGTGAAGGTCGGTGTCAACGR:CAAAGTTGTCATGGATGACCAQP3F:GCTGTCACCCTTGGCACTTR:TGCATAGATGGGCAGCTTGAc-kitF:GATCCTGCCAAACTTTTCCTGCR:TTTAGCCACATGGAGTTCAGG
根据反应曲线得到各组样品目的基因和内参基因的阈值循环数(cycle threshold,Ct),采用2-△△Ct方法进行数据分析,△Ct=Ct Mean目的基因-Ct Mean内参基因;△△Ct=△Ct实验组-△Ct对照组;实验组和对照组的基因表达差异为2-ΔΔCt。空白对照组的数值为1。
以逆转录得到的cDNA为模板,进行qRT-PCR,反应体系共10 μL。反应条件为:95 ℃ 2 min,95 ℃ 30 s,59 ℃ 30 s,72 ℃ 20 s,共38个循环。以actin基因为内参,CFX96 Manager软件分析结果。
1.3 统计分析
应用GraphPad prism8作图,所有的数据采用SPSS 21.0进行显著性分析,应用one-way ANOVA进行组间比较,所有数据均以平均数±标准差表示,P<0.05表示差异显著。
2 结果与分析
2.1 益生菌组合物对小鼠肠道运动和排便情况影响
如图1所示,与空白组相比,模型组小鼠的肠道转运及排便情况发生显著变化(P<0.05),主要包括小肠推进率下降,首粒黑便排出时间延长,6 h黑便排出粒数减少,黑便质量降低。这表明洛哌丁胺造模慢传输型便秘成功。与阳性对照组相比,益生菌组合配方低、高剂量在小肠推进率、首粒时间、黑便粒数和黑便质量方面没有统计学差异。相比模型组,各干预组(阳性对照组、益生菌组合物低、高剂量组)在肠道运动和排便情况方面得到显著改善(P<0.05)。益生菌组合物能够提高肠转运效率,降低排便时间,改善便秘,效果与阳性对照一致。

A-空白组;B-模型组;C-阳性对照组;D-低剂量组;E-高剂量组(以下各图与此相同) a-小肠推进率;b-首粒黑便时间;c-黑便粒数;d-黑便质量
图1 益生菌组合物配方对洛哌丁胺引起的便秘小鼠肠道排泄影响
Fig.1 Effects of probiotics complex on excretion in loperamide-induced constipation mouse model 注:组间不同字母表示差异显著(P<0.05)(下同)
2.2 益生菌组合物对小鼠血清和结肠组织胃肠调节肽的影响
临床研究表明,胃肠调剂肽在便秘和正常状态下的分泌存在差异,为探究益生菌组合物的作用机制,对其表达进行检测。血清胃肠调节肽含量如图2所示。
模型组相比空白组在胃肠调节肽方面差异显著(P<0.05),具体表现为兴奋性胃肠调节肽(SP、MTL、Gas)在造模后含量下降,抑制性胃肠调节肽(VIP、SS、ET-1)造模后含量上升。益生菌组合配方灌胃后,抑制性胃肠调节肽(VIP、SS、ET-1)能够恢复至空白组水平,低、高剂量无显著差异。相比模型组,灌胃益生菌组合物配方低、高剂量组的兴奋性胃肠调节肽(SP、MTL、Gas)含量,表现提升效果,其中血清SP、MTL含量在低剂量组中与空白组无统计学差异, Gas含量在高剂量组中与空白组无显著差异。与阳性对照组相比,低剂量组在促进SP表达水平上显著优于阳性对照,而高剂量组在抑制ET-1的表达水平显著优于阳性对照。

a-SP;b-MTL;c-Gas;d-VIP;e-SS;f-ET-1
图2 益生菌组合物对洛哌丁胺引起的便秘小鼠血清中胃肠调节肽的影响
Fig.2 Effects of probiotics complex on gastrointestinal hormones in serum samples of loperamide-induced constipation
结肠组织胃肠调节肽水平如图3所示,与模型组相比,灌胃益生菌组合物配方后,兴奋性胃肠调节肽(SP、MTL、Gas)含量显著上升,抑制性胃肠调节肽(VIP、SS、ET-1)含量显著下降。在促进MTL和Gas分泌上,阳性对照比益生菌组合物更有优势,而在抑制SS、ET-1分泌中,益生菌组合物则更具优势。

a-SP;b-MTL;c-Gas;d-VIP;e-SS;f-ET-1
图3 益生菌组合物对洛哌丁胺引起的便秘小鼠结肠组织中胃肠调节肽的影响
Fig.3 Effects of probiotics complex on gastrointestinal hormones in colon tissues of loperamide-induced constipation mouse
上述结果表明,益生菌组合物配方通过促进兴奋性胃肠调节肽分泌,同时降低抑制性胃肠调节肽产生来缓解洛哌丁胺诱导的慢传输型便秘,且血清和结肠组织的结果趋势一致。
2.3 益生菌组合物对小鼠血清细胞因子的影响
肠道系统与免疫息息相关,益生菌有调节免疫的能力,通过研究细胞因子水平,能够进一步阐释益生菌缓解便秘的机制。小鼠血清细胞因子水平如表2及表3所示,细胞因子IL-4水平,高剂量组与空白组、阳性对照组之间差异显著;细胞因子IL-6水平,模型组与低、高剂量组之间差异显著,其余各组细胞因子之间基本无差异。结果表明灌胃益生菌组合物基本不影响血清细胞因子水平。
表2 益生菌组合物对洛哌丁胺引起的便秘小鼠细胞因子的影响
Table 2 Effects of probiotics complex on serum cytokines in a loperamide-induced constipation mouse model

组别IL-2/(pg·mL-1)IL-4/(pg·mL-1)IL-6/(pg·mL-1)IL-10/(pg·mL-1)IL-12/(ng·L-1)IL-17/(pg·mL-1)空白组348.44±7.0644.54±2.83a3.35±0.50ab22.89±5.494.45±0.436.65±0.46模型组335.34±11.4840.59±1.80ab2.42±0.62b16.46±1.534.27±0.286.23±0.64阳性对照组343.96±17.9644.57±3.20a3.46±0.72ab19.97±4.834.61±0.395.79±0.26低剂量组345.14±8.8843.52±1.04ab3.99±0.92a18.31±1.244.12±0.326.66±1.09高剂量组343.16±7.1140.08±1.77b4.27±0.22a17.96±1.494.14±0.176.62±0.40
注:同列不同字母表示差异显著(P<0.05)(下同)
表3 益生菌组合物对洛哌丁胺引起的便秘小鼠血清指标的影响
Table 3 Effects of probiotics complex on serum indicators in a loperamide-induced constipation mouse model

组别紧密连接蛋白/(pg·mL-1)D-乳酸/(μg·L-1)TNF-α/(ng·L-1)IFN-γ/(ng·L-1)sIgA/(μg·mL-1)空白组16.76±1.16196.96±8.8072.71±6.39155.10±12.892.02±0.11模型组15.89±0.63190.30±6.2867.69±3.87143.70±7.232.04±0.17阳性对照组16.19±0.99189.38±8.4776.03±7.49159.42±11.181.99±0.16低剂量组17.32±1.44194.46±5.5071.42±4.71151.98±11.052.01±0.12高剂量组15.88±1.02196.72±7.2066.72±4.25149.08±7.002.04±0.11
2.4 益生菌组合物对小鼠结肠组织AQP3和c-kit基因表达量的影响
水通道蛋白AQP3在肠道水转运过程中起到重要作用,Cajal间质细胞(intestinal cells of Cajal,ICC)参与慢传输便秘的形成机制,而c-kit是其特异性标志物,通过研究AQP3和c-kit的基因表达水平可以探索益生菌缓解便秘的机制。如图4所示,与模型组相比,灌菌组显著提高AQP3和c-kit基因表达水平(P<0.05),且结果与空白组及阳性对照组无统计学差异。益生菌组合物低、高剂量无显著差异。结果表明益生菌组合物能够通过恢复AQP3和c-kit基因的转录水平来改善便秘。

图4 益生菌组合物对AQP3和c-kit基因表达量的影响
Fig.4 Effects of probiotics complex on expression of AQP3 and c-kit in mouse colon
3 结论与讨论
慢传输型便秘是功能性便秘的一种,主要症状包括排便困难、排便次数减少、排便时间延长或排便不尽。肠内容物的移动主要通过蠕动进行[9],通过测定小肠推进率可以了解到肠道蠕动情况,小肠推进率越高越有利于粪便的排出[10]。本研究通过盐酸洛哌丁胺造模便秘小鼠,益生菌组合物干预后能够显著提高小肠推进率,首粒黑便时间,黑便粒数和黑便质量,并且低剂量组和高剂量组之间无统计学差异。
研究表明胃肠调节肽SP、MTL、Gas、VIP、SS和ET-1在肠胃运动调节中起重要作用,其表达水平与便秘症状密切相关。其中,SP、MTL和Gas是兴奋性神经递质,VIP、SS和ET-1是抑制性神经递质[11-13]。提高SP、MTL和Gas水平能够加速肠道蠕动,提高转运率,改善便秘症状,而VIP、SS和ET-1是减慢肠转运时间的重要因素,降低其分泌,可以缓解慢传输型便秘[14]。本研究中益生菌干预后能够显著提高兴奋性神经递质的分泌,同时降低抑制性神经递质的表达。
细胞因子是由多种组织细胞(主要为免疫细胞)所合成和分泌的小分子多肽或糖蛋白。细胞因子能介导细胞间的相互作用,具有多种生物学功能,如调节细胞生长、分化成熟、功能维持、调节免疫应答、参与炎症反应、创伤愈合和肿瘤消长等[15]。细胞因子的含量异常会导致各种并发症状的出现[16]。本研究中通过检测小鼠血清中的细胞因子水平,发现各组小鼠细胞因子水平基本未发生变化。
ICC相当于胃肠道的起搏细胞,它能产生一种促进肠道蠕动的慢波。而c-kit受体在ICC发育过程中具有十分重要的作用,所以可以通过检测肠道中c-kit蛋白的表达量来反映肠道中Cajal细胞的数量。已有研究发现可通过提高胃肠道ICC的c-kit基因表达,从而促进结肠的蠕动,缓解便秘症状[17]。水通道蛋白是一种细胞膜上的蛋白质,可控制水在细胞的进出。水通道蛋白有许多的亚家族,其中AQP3基因在小鼠结肠中高表达[18]。研究表明,腹泻和便秘患者水通道蛋白的表达水平均存在异常[19]。本研究中盐酸洛哌丁胺造模会导致c-kit和AQP3的转录水平显著低于空白组,而灌胃益生菌组合物后能恢复其转录水平,结果表明益生菌组合物可能通过提高c-kit和AQP3转录水平缓解便秘,与前人研究相符[20]。
综上所述,表明益生菌组合物能够通过促进SP、MTL和Gas的分泌,抑制VIP、SS和ET-1的表达,调控AQP3和c-kit的基因转录,从而改善慢传输型便秘。
[1] TILLOU J, POYLIN V.Functional disorders:Slow-transit constipation[J].Clinics in Colon and Rectal Surgery, 2017, 30(1):76-86.
[2] BHARUCHA A E, WALD A.Chronic constipation[J].Mayo Clinic Proceedings, 2019, 94(11):2 340-2 357.
[3] DIMIDI E, CHRISTODOULIDES S, SCOTT S M, et al.Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation[J].Advances in Nutrition, 2017, 8(3):484-494.
[4] GE X L, ZHAO W, DING C, et al.Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility[J].Scientific Reports, 2017, 7:441.
[5] DIMIDI E, SCOTT S M, WHELAN K.Probiotics and constipation:Mechanisms of action, evidence for effectiveness and utilisation by patients and healthcare professionals[J].The Proceedings of the Nutrition Society, 2020, 79(1):147-157.
[6] ZHANG X Y, YANG H B, ZHENG J P, et al.Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice[J].Carbohydrate Polymers, 2021, 253:117218.
[7] EOR J Y, TAN P L, LIM S M, et al.Laxative effect of probiotic chocolate on loperamide-induced constipation in rats[J].Food Research International, 2019, 116:1 173-1 182.
[8] MORI T, SHIBASAKI Y, MATSUMOTO K, et al.Mechanisms that underlie μ-opioid receptor agonist-induced constipation:Differential involvement of μ-opioid receptor sites and responsible regions[J].The Journal of Pharmacology and Experimental Therapeutics, 2013, 347(1):91-99.
[9] VAN DER WERF M J, VENEMA K.Bifidobacteria:Genetic modification and the study of their role in the colon[J].Journal of Agricultural and Food Chemistry, 2001, 49(1):378-383.
[10] WANG L L, HU L J, XU Q, et al.Bifidobacteria exert species-specific effects on constipation in BALB/c mice[J].Food and Function, 2017, 8(10):3 587-3 600.
[11] LIANG Y X, WEN P, WANG Y, et al.The constipation-relieving property of d-tagatose by modulating the composition of gut microbiota[J].International Journal of Molecular Sciences, 2019, 20(22):5721.
[12] ZHAI X C, LIN D H, ZHAO Y, et al.Bacterial cellulose relieves diphenoxylate-induced constipation in rats[J].Journal of Agricultural and Food Chemistry, 2018, 66(16):4 106-4 117.
[13] LI T, YAN Q J, WEN Y P, et al.Synbiotic yogurt containing konjac mannan oligosaccharides and Bifidobacterium animalis ssp. lactis BB12 alleviates constipation in mice by modulating the stem cell factor (SCF)/c-Kit pathway and gut microbiota[J].Journal of Dairy Science, 2021, 104(5):5 239-5 255.
[14] WANG L L, HU L J, XU Q, et al.Bifidobacterium adolescentis exerts strain-specific effects on constipation induced by loperamide in BALB/c mice[J].International Journal of Molecular Sciences, 2017, 18(2):318.
[15] OPAL S M, DEPALO V A.Anti-inflammatory cytokines[J].Chest, 2000, 117(4):1 162-1 172.
[16] MAHAPATRO M, ERKERT L, BECKER C.Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut[J].Cells, 2021, 10(1):111.
[17] ZHANG X, ZHENG F J, ZHANG Z.Therapeutic effect of Cistanche deserticola on defecation in senile constipation rat model through stem cell factor/C-kit signaling pathway[J].World Journal of Gastroenterology, 2021, 27(32):5 392-5 403.
[18] IKARASHI N, KON R, SUGIYAMA K.Aquaporins in the colon as a new therapeutic target in diarrhea and constipation[J].International Journal of Molecular Sciences, 2016, 17(7):1172.
[19] KON R, TSUBOTA Y, MINAMI M, et al.CPT-11-induced delayed diarrhea develops via reduced aquaporin-3 expression in the colon[J].International Journal of Molecular Sciences, 2018, 19(1):170.
[20] YIN J Q, LIANG Y C, WANG D L, et al.Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation[J].International Journal of Molecular Medicine, 2018, 41(2):649-658.