芽孢通常存在于环境中,包括食品中,它自身独特的结构使其对热、干燥、紫外线和γ辐射等处理以及一些杀菌化学物质具有极强的抵抗力[1-2],很难被杀灭。由于杀菌强度不够,芽孢引起的食物腐败和食物中毒时有发生[3-4]。高压热杀菌(high-pressure thermal sterilization,HPTS)作为一种新型的食品杀菌技术,可以杀灭食品中所有微生物,包括最难杀死的芽孢[5],相比于高温杀菌,HPTS处理过程相对温和,对食品的感官和营养品质的损害较小[6-7]。
近年来,相关研究致力于HPTS与其他因素相结合来进一步提高芽孢的致死性,例如降低介质的pH,TOLA等[8]报道:随着介质pH值的降低,HPTS杀灭地衣杆菌芽孢的数量在增加。ROBERTS等[9]发现pH越低,芽孢的抗性减弱,在400 MPa、45 ℃条件下,当pH值从7.0降到4.0时,多杀灭了1.5个对数的凝结杆菌芽孢。然而在高压热处理过程中,酸度对芽孢失活的影响以及两者结合杀灭芽孢的作用机理,目前尚不明确。HPTS主要影响芽孢内膜的通透性、膜脂的流动性[10]和芽孢蛋白质、核酸的结构[11]。研究表明,HPTS破坏了芽孢内膜对水分子的通透屏障,大量水分子进入芽孢内核而导致内核水合和芽孢死亡[12-13],而不同pH值不仅可以改变蛋白质、核酸等生物大分子的电荷,还可以改变细胞膜的电荷,从而影响微生物的功能和活性[14]。WUYTACK等[15]研究人员发现,低pH值会改变氨基酸侧链的电离状态,从而改变电荷分布和蛋白质构象。SETLOW等[16]研究表明酸对芽孢的杀灭涉及芽孢通透性屏障的破坏。
傅里叶变换红外光谱仪目前广泛应用于综合检测微生物的生理状态[17],具有高分辨率、高灵敏度和高效扫描等优势,利用该仪器对芽孢红外光谱中不同波段进行研究,可以分析出HPTS与酸共同作用下芽孢膜脂相态和蛋白质、核酸等物质的变化情况。
本文将HPTS与酸处理相结合,对枯草杆菌芽孢进行处理,研究HPTS与酸联合作用对枯草杆菌芽孢的杀灭效果并探讨其杀灭机理,为深入研究芽孢杀菌抗性及HPTS在杀灭细菌芽孢上的应用提供理论基础。
1 材料与方法
1.1 材料与试剂
枯草芽孢杆菌(Bacillus subtilis),中国普通微生物菌种保藏管理中心(CGMCC),编号As 1.433;营养琼脂,天津市大茂化学试剂厂;促芽孢生长锰盐营养琼脂培养基,向营养琼脂中加入MnSO4·H2O(使Mn2+质量浓度为 50 mg/L),调pH值,灭菌备用;碘化丙啶(propidium iodide,PI)、TSA-YE培养基,北京索莱宝科技有限公司;盐酸(分析纯)、氢氧化钠(分析纯),天津市天力化学试剂有限公司。
1.2 仪器与设备
UV-9000S型双光束紫外可见分光光度计,上海元析仪器有限公司;Scientz-1LS真空冷冻干燥干燥浓缩仪,宁波新芝生物科技股份有限公司;Spectrum Two型傅里叶变换红外光谱仪,美国PerkinElmer公司;CyFlow Cube 8流式细胞仪,日本SYSMEX(希森美康)株式会社;5L HPP超高压设备,包头科发高压科技有限公司
1.3 实验方法
1.3.1 枯草杆菌芽孢悬浮液的制备
将活化的枯草芽孢杆菌(As 1.433)划线接种到试管斜面促芽孢生长锰盐营养琼脂培养基上,37 ℃条件下培养7 d,用无菌去离子水洗涤离心(4 ℃、9 000 r/min、15 min)芽孢3次,芽孢悬浮液浓度大约调整到1.5×109 CFU/mL,4 ℃下保存[18]。实验前将枯草杆菌芽孢悬浮液浓度调整到1.5×107 CFU/mL左右。
1.3.2 枯草杆菌芽孢悬浮液的HPTS结合酸处理
通过盐酸标准液将芽孢悬浮液pH值调整为1、4、7,各取10 mL芽孢悬浮液于无菌聚乙烯塑料袋中,抽真空封口后置于超高压设备腔体中,处理介质为水,作为压力和温度的传递介质对芽孢悬浮液加压升温。压力为550 MPa,处理温度为 25、65、75 ℃,保压处理20 min,处理时间不包括升压和卸压所需时间。压力水平,时间和温度由计算机控制。
1.3.3 枯草杆菌芽孢平板计数方法
将处理前后的芽孢悬浮液进行梯度稀释后,在平板中吸取1 mL稀释液并倒入15~20 mL TSA-YE培养基混匀,凝固后在 37 ℃下倒置培养24~48 h,计算菌落总数对数值。
1.3.4 枯草杆菌芽孢紫外吸收物质泄漏量的测定
将处理前后的芽孢悬浮液离心(4 ℃、9 000 r/min)15 min,收集上清液,以无菌去离子水为空白参比,使用紫外分光光度计测定260 nm(核酸)和280 nm(蛋白质)处的吸光值。
1.3.5 流式细胞仪检测枯草芽孢杆菌芽孢内膜通透性
取处理前后的芽孢悬浮液,将菌液浓度稀释到106~107 CFU/mL。使用PI染色,在1 mL芽孢悬浮液中加入0.75 μL 20 mmol/L的PI染色液,在室温下暗处孵育15 min。用流式细胞仪检测前向散射光、侧向散射光、荧光通道FL2和FL3。采用488 nm激发光,PI标记细胞在615 nm处发红色荧光(FL3)[19]。数据采集后用FSC Express Version 3.0软件分析。
1.3.6 傅里叶变换红外光谱分析
将处理前后的样品进行冷冻干燥,于玛瑙研钵中磨粉,再与干燥的KBr粉末混合均匀,压片后置于傅里叶红外变换光谱仪上,于4 000~500 cm-1内进行扫描,扫描次数为32次,分辨率为4 cm-1。
1.3.7 统计分析
所有实验至少重复3次。采用Origin 2018软件对实验结果进行分析和作图,以P<0.05为显著差异的标准。
2 结果与分析
2.1 HPTS结合不同pH处理对枯草杆菌芽孢杀灭效果的影响
图1为HPTS结合不同pH处理后枯草杆菌芽孢存活浓度的变化。由图1可知,枯草杆菌芽孢处理前的初始计数约为1×107 CFU/mL。常温常压下pH=1、pH=4和pH=7单独处理后枯草杆菌芽孢的存活浓度分别为6.71、6.77、6.82 lgCFU/mL,与常温常压下pH值单独处理相比,当pH=1时,550 MPa结合25 ℃/65 ℃/75 ℃处理后枯草杆菌芽孢的存活浓度为5.62、2.53、0.57 lgCFU/mL;pH=4时,550 MPa结合25 ℃/65 ℃/75 ℃处理后枯草杆菌芽孢的存活浓度为6.46、3.6、2.52 lgCFU/mL;pH=7时,550 MPa结合25 ℃/65 ℃/75 ℃处理后枯草杆菌芽孢的存活浓度为6.69、3.97、2.59 lgCFU/mL。结果表明在常温常压下pH单独处理均无法造成芽孢失活。当pH=7时,550 MPa结合25 ℃处理对芽孢基本没有杀灭效果,而pH=1和pH=4时,550 MPa结合 25 ℃ 处理对芽孢有一定的杀灭作用。当pH=4和pH=7时,550 MPa结合75 ℃处理后芽孢的杀灭效果没有显著变化,而pH=1时,550 MPa结合75 ℃处理后芽孢存活浓度达到最低,杀灭效果显著增强,芽孢最多被杀灭了6.25 lgCFU/mL,这可能是因为超高压结合低pH促使芽孢变成脱离子H-芽孢。压力和热会增加芽孢对质子的通透性,使得在低pH时芽孢核心部分发生再水化,说明酸性环境有利于HPTS杀灭枯草杆菌芽孢[15]。
2.2 HPTS结合不同pH处理对枯草杆菌芽孢紫外吸收物质泄漏量(OD260和OD280)的影响
260 nm和280 nm 紫外吸收物质的泄漏量常被用来检测细胞膜通透性的变化。图2为HPTS结合不同pH处理对枯草杆菌芽孢紫外吸收物质泄漏量(OD260、OD280)的影响。常温常压下,芽孢紫外吸收物质泄漏量随着pH值降低有所增加。经HPTS结合不同pH处理后,芽孢紫外吸收泄漏量增大,说明HPTS结合不同pH对芽孢的渗透屏障都造成了一定程度的损伤。随着pH值的降低,550 MPa结合25 ℃/65 ℃/75 ℃处理后芽孢紫外吸收物质泄漏量显著增加,表明温度升高到一定程度,酸会进一步破坏芽孢的渗透屏障,使得更多的核酸及蛋白质类紫外吸收物质泄漏出来。
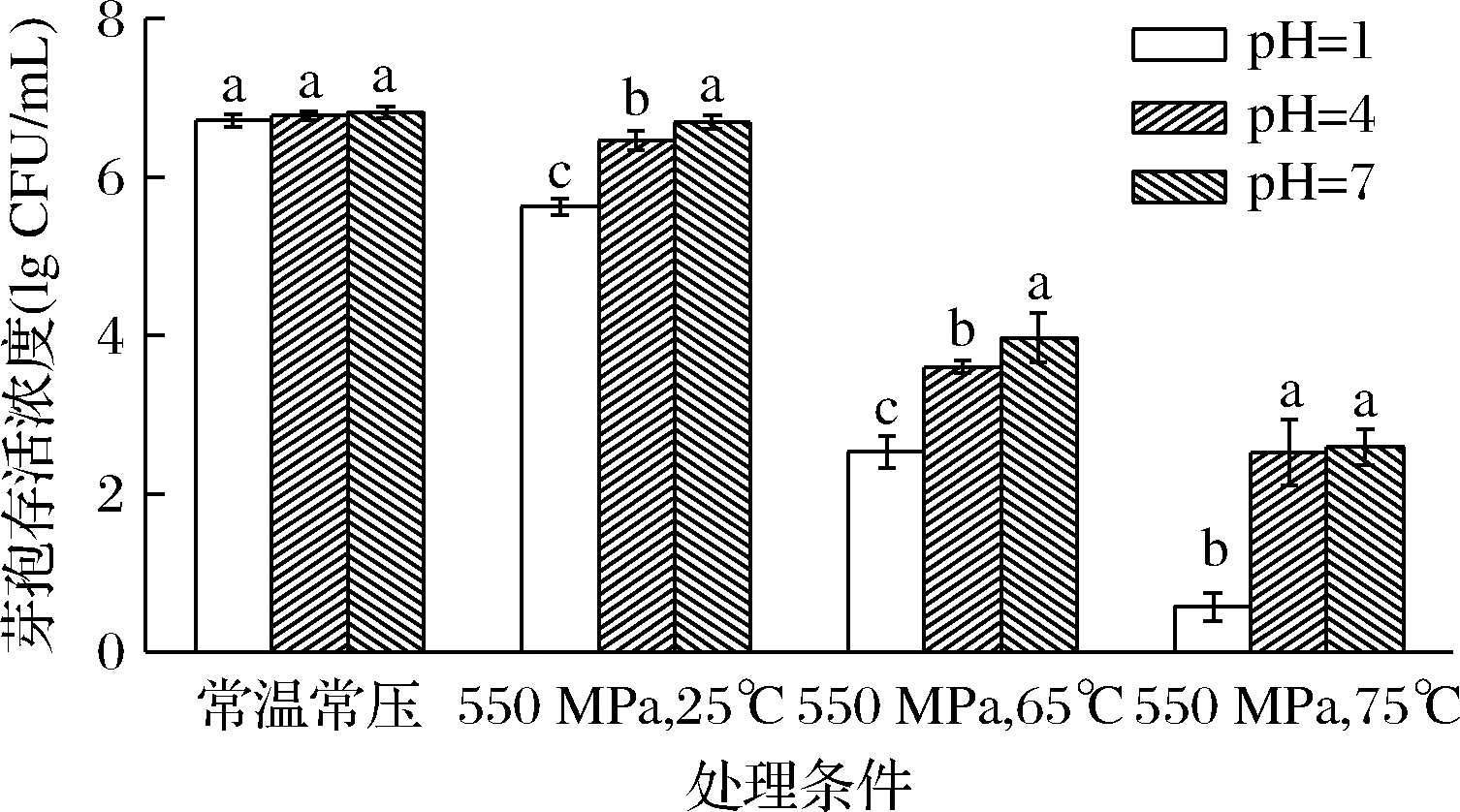
图1 HPTS结合不同pH处理对枯草杆菌芽孢存活浓度的影响
Fig.1 Effect of HPTS combined with different pH treatments on Bacillus subtilis spore survival concentration
注:图中不同小写字母表示组内差异显著(P<0.05)

图2 HPTS结合不同pH处理对枯草杆菌芽孢紫外吸收 物质泄漏量的影响
Fig.2 Effect of HPTS combined with different pH treatments on the leakage of UV absorbing substances from Bacillus subtilis spores
注:不同大写字母表示OD260值组内差异显著;不同小写字母 表示OD280值组内差异显著
2.3 HPTS结合不同pH处理对枯草杆菌芽孢细胞膜损伤的流式细胞仪检测
流式细胞仪经常被用来检测细菌细胞膜的损伤。图3分别为HPTS结合不同pH处理芽孢后的流式细胞图,反映芽孢内膜通透性的改变[20]。使用荧光染料PI染色后,在嵌入双链DNA时会发出红色荧光。常温常压下,随着pH值降低,芽孢内膜的荧光分布没有向M2移动,芽孢内膜通透性没有增大,与常温常压下pH值单独处理相比,当pH=1和pH=4时,550 MPa结合25 ℃处理后荧光分布基本没有向M2移动,芽孢损伤程度较小,芽孢内膜通透性变化较小,而pH=7时,550 MPa 结合25 ℃处理荧光分布明显向M2移动,芽孢内膜通透性增大。随着温度上升,HPTS结合不同pH,荧光分布明显向M2移动,说明芽孢内膜通透性增大,这与紫外吸收泄漏量的结果相一致。与HPTS单独作用相比,HPTS结合pH=4和pH=7处理后M2比例基本相差不大,而HPTS结合pH=1处理后M2的比例明显增大,说明HPTS结合pH=1与相同条件下HPTS单独处理对芽孢内膜的破坏作用更强,即可以认为HPTS结合pH=1增强了对芽孢内膜的破坏,提高了对芽孢的杀灭效果。
2.4 HPTS结合酸处理前后枯草杆菌芽孢的傅里叶红外光谱分析
图4为枯草杆菌芽孢在HPTS结合酸处理前后的傅里叶红外光谱图,红外检测的波长范围为4 000~500 cm-1,本实验选取了芽孢杀灭效果最好和内膜通透性破坏最严重的条件即pH=1,550 MPa结合75 ℃ 进行分析,研究HPTS在酸性条件下芽孢内膜相态及化学组分结构的变化。HPTS结合酸处理前后的红外光谱图的峰形、峰高变化细微,从而反映出芽孢处理前后的化学组分基本一致。为了进一步探究芽孢红外光谱中特征波段的变化情况,需要对原红外光谱图进行数据分析处理。
二阶导数处理是红外谱图分析中常用的方法[17],它可以将原红外谱图中细微的差别分辨出来,有利于深入分析原红外谱图。图5-a反映了芽孢内膜脂肪酸相变化情况。波数大约在2 872、2 960 cm-1的谱带分别表示CH3基团对称和反对称伸缩振动;波数约为2 852、2 919 cm-1的谱带分别表示CH2基团对称和反对称伸缩振动[21],与未处理和HPTS处理的枯草杆菌芽孢相比,HPTS结合酸处理后,原2 960、2 919 cm-1处的红外吸收峰发生了移动,红外吸收峰强度变化更加剧烈。以上红外吸收峰反映了芽孢内膜的相变化。图5-a表明,芽孢内膜磷脂的不流动性是由于其磷脂处于凝胶态[22],经过HPTS结合酸处理后,芽孢内膜的磷脂分子疏水尾链混乱度增大,磷脂由凝胶态变为液晶态,从而流动性增加,最终导致芽孢内膜水分子通透屏障的受损。

a-pH=1、25 ℃处理;b-pH=4、25 ℃处理;c-pH=7、25 ℃处理;d-pH=1、550 MPa、25 ℃处理;e-pH=4、550 MPa、25 ℃处理; f-pH=7、550 MPa、25 ℃处理;g-pH=1、550 MPa、65 ℃处理;h-pH=4、550 MPa、65 ℃处理;i-pH=7、550 MPa、65 ℃处理; j-pH=1、550 MPa、75 ℃处理;k-pH=4、550 MPa、75 ℃处理;l-pH=7、550 MPa、75 ℃处理
图3 HPTS结合不同pH处理对枯草芽孢杆菌芽孢内膜通透性的影响
Fig.3 Effects of HPTS combined with different pH treatments on the membrane permeability of Bacillus subtilis spores
注:M1代表荧光强度较低的阴性区域;M2代表荧光强度较高的阳性区域
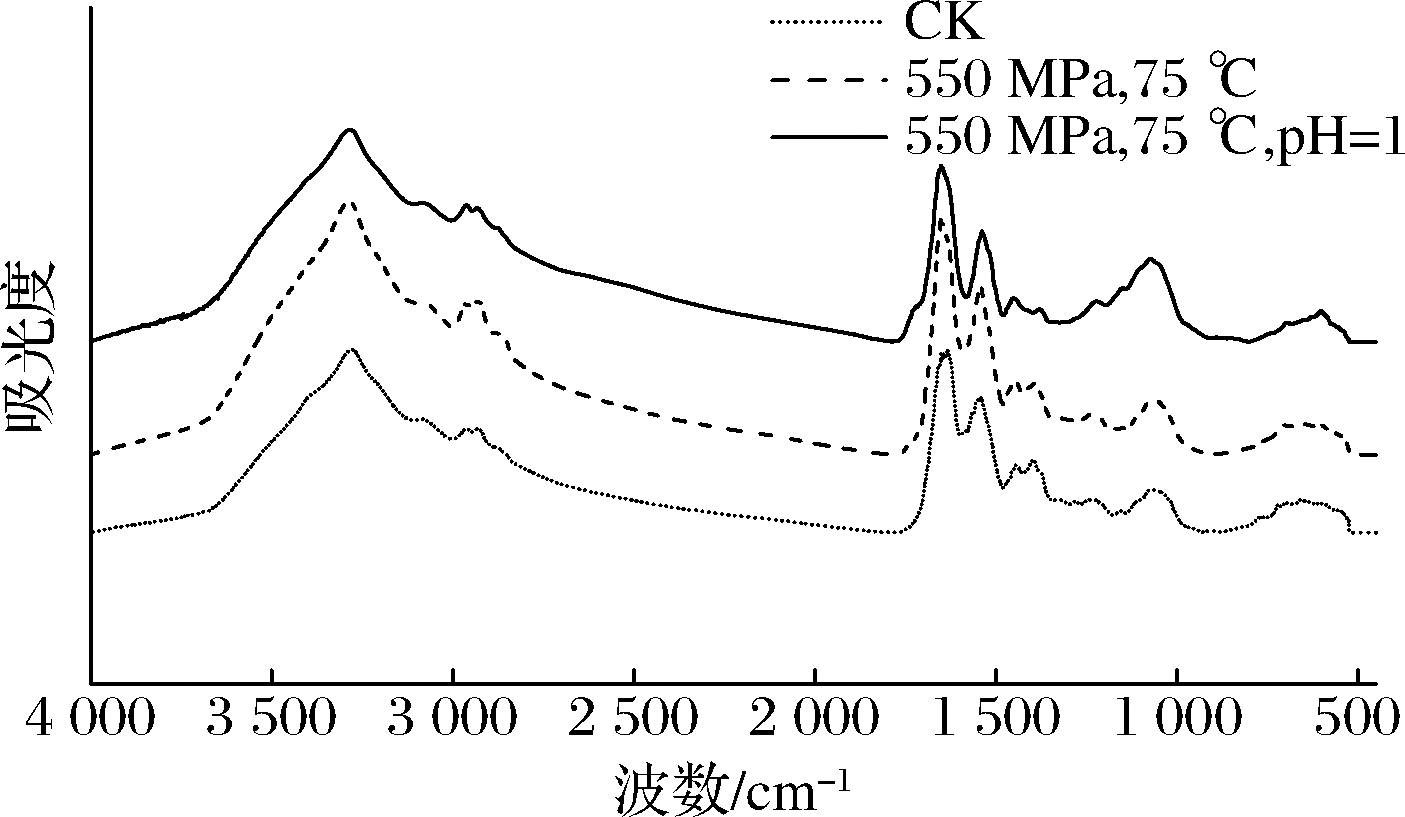
图4 HPTS结合酸处理前后枯草杆菌芽孢的红外光谱图
Fig.4 FT-IR spectra of Bacillus subtilis spores before and after HPTS combined with acid treatment
图5-b反映了芽孢蛋白酰胺Ⅰ带的蛋白质二级结构变化。从图5-b中可以看出,HPTS结合酸处理后,1 674 cm-1处的红外吸收峰发生了明显的变化,从未处理的1 674 cm-1处移动到1 676 cm-1处;1 652 cm-1的红外吸收峰移动到了1 650 cm-1;1 644 cm-1的红外吸收峰移动到了1 646 cm-1处,其中1 674 cm-1的吸收峰代表蛋白质β折叠结构,1 652、1 644 cm-1代表蛋白质的α螺旋结构[23]。由此表明,经过HPTS结合酸处理后,红外吸收峰的位置和峰形均发生了明显的变化,芽孢蛋白质的二级结构遭到破坏,蛋白质发生了变性。原因可能是酸改变了芽孢内膜的电荷,影响其正常生理功能,同时,酸性条件改变了氨基酸分子侧链的电离状态,从而导致蛋白质构象的改变[15],在高压和温度作用下,芽孢蛋白质更容易发生变性。
图5-c反映了芽孢核酸骨架振动变化。波数大约在1 080、1 220 cm-1的谱带分别表示P![]() O基团对称与反对称伸缩振动[17],与未处理和HPTS处理的芽孢相比,HPTS结合酸处理后,以上2处红外吸收峰位置向低波数迁移,吸收峰强度减弱。这是因为HPTS结合酸处理后,进一步加剧了芽孢内核酸物质的变性,核酸分子内的氢键减弱使得峰位向低波数迁移。此外,1 200~900 cm-1波段主要表示C—O—C伸缩振动,可以反映处理前后细胞壁肽聚糖层结构的变化[24]。从图5-c中可以发现,芽孢在HPTS处理后肽聚糖层和细胞壁已经明显改变,结合酸处理后的变化更加强烈(1 015 cm-1)。芽孢皮层肽聚糖支架是其耐压特性的关键结构,在萌发后形成细胞壁[25]。芽孢皮层肽聚糖和细胞壁结构的改变可能使芽孢皮层破裂,从而导致芽孢死亡。
O基团对称与反对称伸缩振动[17],与未处理和HPTS处理的芽孢相比,HPTS结合酸处理后,以上2处红外吸收峰位置向低波数迁移,吸收峰强度减弱。这是因为HPTS结合酸处理后,进一步加剧了芽孢内核酸物质的变性,核酸分子内的氢键减弱使得峰位向低波数迁移。此外,1 200~900 cm-1波段主要表示C—O—C伸缩振动,可以反映处理前后细胞壁肽聚糖层结构的变化[24]。从图5-c中可以发现,芽孢在HPTS处理后肽聚糖层和细胞壁已经明显改变,结合酸处理后的变化更加强烈(1 015 cm-1)。芽孢皮层肽聚糖支架是其耐压特性的关键结构,在萌发后形成细胞壁[25]。芽孢皮层肽聚糖和细胞壁结构的改变可能使芽孢皮层破裂,从而导致芽孢死亡。
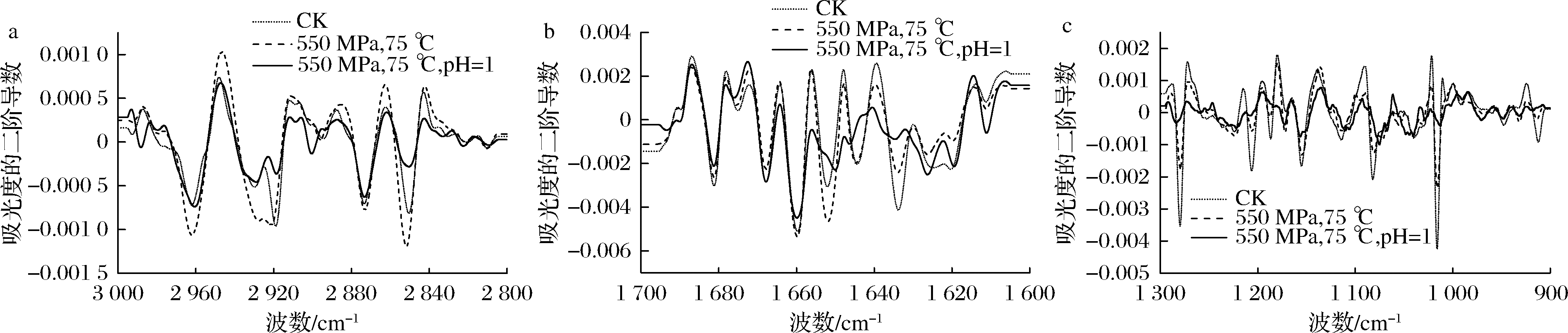
a-3 000~2 800 cm-1波段;b-1 700~1 600 cm-1波段;c-1 300~900 cm-1波段
图5 HPTS结合酸处理前后枯草杆菌芽孢的二阶导数红外光谱图
Fig.5 Second-derivative infrared spectra of Bacillus subtilis spores before and after HPTS combined with acid treatment
3 结论
(1)将芽孢悬浮液的pH值调整为1、4、7,于550 MPa 结合25、65、75 ℃处理20 min,平板计数结果表明,常温常压下pH值单独处理均无法造成芽孢失活。当HPTS结合不同pH处理后,随着pH值降低,芽孢的杀灭效果明显增强,当pH=1时,550 MPa结合75 ℃ 条件下芽孢最多被杀灭了6.25 lgCFU/mL。
(2)对芽孢紫外吸收物质泄漏量和内膜通透性研究发现,常温常压下,芽孢紫外吸收物质泄漏量随着pH值降低有所增加;相比HPTS单独作用,HPTS结合不同pH处理后,芽孢紫外吸收泄漏量大幅增加,pH值越低,芽孢紫外吸收泄漏量越大。常温常压下,随着pH值降低,芽孢内膜通透性没有增大;随着温度上升,HPTS结合不同pH处理后芽孢内膜通透性增大;HPTS结合pH=4和pH=7处理后,阳性区域比例基本相差不大,而HPTS结合pH=1处理后阳性区域的比例明显增大,表明芽孢内膜通透性显著增加,芽孢内膜结构遭到破坏,这与芽孢紫外吸收物质泄漏量的结果相一致。
(3)对不同处理后的芽孢的傅里叶红外光谱分析发现,相比对照组和HPTS单独作用,HPTS结合酸处理后,芽孢内膜磷脂相态发生转变,磷脂由凝胶态变为液晶态,膜脂流动性增加;芽孢蛋白质二级结构变化,导致蛋白质变性;芽孢内核酸物质变性,皮层肽聚糖和细胞壁结构改变。
综上所述,芽孢内膜的极端不通透性和膜脂的不流动性是芽孢难以被杀灭的重要原因,本实验围绕芽孢内膜进行研究,发现单独pH处理无法对芽孢内膜造成损伤,而在HPTS单独作用的基础上,HPTS结合酸使芽孢内膜通透性显著增加,膜脂的流动性增加,推测是因为HPTS促使芽孢快速萌发,并对内膜造成损伤后,低pH进一步破坏芽孢内膜的水分子通透屏障,使得水分子更容易透过芽孢内膜进入芽孢内核,芽孢内核含水量不断增加,芽孢自身的极端抗性就会大大降低,进而可以有效杀灭芽孢,这对于研究芽孢杀菌抗性及HPTS在杀灭细菌芽孢上的应用提供了一定的理论依据。
[1] WELLS-BENNIK M H J, EIJLANDER R T, DEN BESTEN H M W, et al.Bacterial spores in food:Survival, emergence, and outgrowth [J].Annual Review of Food Science and Technology, 2016, 7(1):457-482.
[2] SETLOW P, JOHNSON E A.Spores and Their Significance [M].United States:Elsevier, 2019.
[3] LUONG T S V, MOIR C, CHANDRY P S, et al.Combined high pressure and heat treatment effectively disintegrates spore membranes and inactivates Alicyclobacillus acidoterrestris spores in acidic fruit juice beverage [J].Innovative Food Science & Emerging Technologies, 2020, 66(1):102523.
[4] JUNEJA V K, GOLDEN C E, MISHRA A, et al.Predictive model for growth of Bacillus cereus during cooling of cooked rice [J].International Journal of Food Microbiology, 2019, 290(2):49-58.
[5] LIANG D, ZHANG L, WANG X, et al.Building of pressure-assisted ultra-high temperature system and its inactivation of bacterial spores [J].Frontiers in Microbiology, 2019, 10:1 275.
[6] EVELYN E, SILVA F V M.Heat assisted HPP for the inactivation of bacteria, moulds and yeasts spores in foods: MLog reductions and mathematical models [J].Trends in Food Science & Technology, 2019, 88(8):143-156.
[7] SEVENICH R, MATHYS A.Continuous versus discontinuous ultra-high-pressure systems for food sterilization with focus on ultra-high-pressure homogenization and high-pressure thermal sterilization:A review [J].Comprehensive Reviews in Food Science and Food Safety, 2018, 17(3):646-662.
[8] TOLA Y B, RAMASWAMY H S.Combined effects of high pressure, moderate heat and pH on the inactivation kinetics of Bacillus licheniformis spores in carrot juice [J].Food Research International, 2014, 62:50-58.
[9] ROBERTS C M, HOOVER D G.Sensitivity of Bacillus coagulans spores to combinations of high hydrostatic pressure, heat, acidity and nisin [J].Journal of Applied Bacteriology, 1996, 81(4):363-368.
[10] INOKUCHI T, ARAI N.Relationship between water permeation and flip-flop motion in a bilayer membrane [J].Physical Chemistry Chemical Physics, 2018, 20(44):28 155-28 161.
[11] CLÉRY-BARRAUD C, GAUBERT A, MASSON P, et al.Combined effects of high hydrostatic pressure and temperature for inactivation of Bacillus anthracis spores[J].Applied and Environmental Microbiology, 2004, 70(1):635-637.
[12] SEVENICH R, REINEKE K, HECHT P, et al.Impact of different water activities (Aw) adjusted by solutes on high pressure high temperature inactivation of Bacillus amyloliquefaciens spores [J].Frontiers in Microbiology, 2015, 6:689.
[13] ALDRETE-TAPIA J A, TORRES J A.Enhancing the inactivation of bacterial spores during pressure-assisted thermal processing [J].Food Engineering Reviews, 2021, 13(3):431-441.
[14] PORÉBSKA I, SOKO OWSKA B, SKA,PSKA S, et al.Treatment with high hydrostatic pressure and supercritical carbon dioxide to control Alicyclobacillus acidoterrestris spores in apple juice [J].Food Control, 2017, 73:24-30.
OWSKA B, SKA,PSKA S, et al.Treatment with high hydrostatic pressure and supercritical carbon dioxide to control Alicyclobacillus acidoterrestris spores in apple juice [J].Food Control, 2017, 73:24-30.
[15] WUYTACK E Y, MICHIELS C W.A study on the effects of high pressure and heat on Bacillus subtilis spores at low pH [J].International Journal of Food Microbiology, 2001, 64(3):333-341.
[16] SETLOW B, LOSHON C A, GENEST P C, et al.Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol [J].Journal of Applied Microbiology, 2002, 92(2):362-375.
[17] AL-QADIRI H M, AL-ALAMI N, AL-HOLY M, et al.Using Fourier transform infrared (FT-IR) absorbance spectroscopy and multivariate analysis to study the effect of chlorine-induced bacterial injury in water [J].Journal of Agricultural and Food Chemistry, 2008, 56(19):8 992-8 997.
[18] 章中, 孙静, 张津瑜,等.高压热杀菌处理对枯草杆菌芽孢皮层裂解酶活力的影响 [J].食品工业科技, 2018, 39(15):90-95.
ZHANG Z, SUN J, ZHANG J Y, et al.Effects of high pressure thermal sterilization on the activity of cortex-lytic enzyme extracted from Bacillus subtilis spores [J].Science and Technology of Food Industry, 2018, 39(15):90-95.
[19] AMOR K B, BREEUWER P, VERBAARSCHOT P, et al.Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead bifidobacterium cells during bile salt stress [J].Applied and Environmental Microbiology, 2002, 68(11):5 209-5 216.
[20] MOUSSA M, PERRIER-CORNET J M, GERVAIS P.Damage in Escherichia coli cells treated with a combination of high hydrostatic pressure and subzero temperature [J].Applied and Environmental Microbiology, 2007, 73(20):6 508-6 518.
[21] YU C X, IRUDAYARAJ J.Spectroscopic characterization of microorganisms by Fourier transform infrared microspectroscopy [J].Biopolymers, 2005, 77(6):368-377.
[22] 张良. 高静压与温度协同杀灭芽孢的效果与机制研究 [D].北京:中国农业大学, 2015.
ZHANG L.Research on effectiveness and mechanism of spore inactivation by high hydrostatic pressure combined with heat [D].Beijing:China Agricultural University, 2015.
[23] KILIMANN K.High pressure inactivation of bacteria:Mathematical and microbiological aspects [D].Munich:Technischen Universität München, 2005.
[24] PAUL C, FILIPPIDOU S, JAMIL I, et al.Bacterial spores, from ecology to biotechnology [J].Advances in Applied Microbiology, 2019, 106:79-111.
[25] POPHAM D L.Specialized peptidoglycan of the bacterial endospore:The inner wall of the lockbox [J].Cellular and Molecular Life Sciences, 2002, 59(3):426-433.