腈水合酶(nitrile hydratase,NHase)是一种可以将腈类物质通过水合作用转化生成更有利用价值的酰胺类化合物的金属酶[1]。在工业生产中主要用于生产丙烯酰胺和烟酰胺等[2]。其中,烟酰胺是维生素B族的一员,广泛应用于医药、化妆品及食品等行业;丙烯酰胺及其聚合物主要应用于凝结剂、土壤改良剂及石油回收剂等行业。因此,开发高性能的腈水合酶催化剂,实现酰胺类产物的高效合成具有重要的工业应用价值和经济价值。
根据腈水合酶多聚体的分子质量大小,可将其分为低分子质量腈水合酶(low-molecular-mass nitrile hydratase,L-NHase)和高分子质量腈水合酶(high-molecular-mass nitrile hydratase,H-NHase)。L-NHase由B、A、E 3个亚基构成,而H-NHase由B、A、G 3个亚基构成,它们的催化反应性能有较大差异。如在大肠杆菌中表达R.rhodochrous J1来源的L-NHase,重组L-NHase 以烟腈为底物的比酶活力比重组H-NHase(234 U/mg)高42%[3]。热稳定性方面,H-NHase在50 ℃下放置30 min仍然具有完全的活性,在60 ℃下放置1 h则能保存50%的活性,而L-NHase稳定性则稍差。WANG等[4]用含有H-NHase的重组菌全细胞催化烟腈合成烟酰胺,在添加菌体终浓度OD600值为8时,可在105 min合成390 g/L烟酰胺;添加菌体浓度OD600值为160时,烟酰胺产量提高为508 g/L,是目前文献报道的最高值。对底物和产物的耐受性方面,L-NHase用烟腈底物或者烟酰胺产物孵育后会导致酶活力大幅度降低,耐受性远远低于H-NHase。此外,不同的NHase具有不同的底物特异性,NHase的底物主要有芳香型底物(包括杂环型)、脂肪型底物两类。H-NHase对脂肪型底物的亲和性较高,尤其是丙烯腈;而L-NHase对带有芳香环及杂环的腈类物质(例如烟腈)具有较高的活力。可见,不同类型的腈水合酶在催化反应中各有其明显的优势和劣势。目前,主要通过筛选不同菌株来源的腈水合酶或对腈水合酶进行改造以获得具有高稳定性和/或耐受性的腈水合酶,还没有通过协同表达不同类型的腈水合酶以提供更高效的腈水合酶[5]细胞催化剂的报道。
本研究协同表达H-型腈水合酶与L-型腈水合酶,构建兼具对底物和产物耐受性高以及催化活性高的基因工程菌,实现全细胞催化合成烟酰胺及丙烯酰胺。
1 材料与方法
1.1 菌株、质粒与引物
克隆宿主菌株大肠杆菌JM109、表达宿主菌株BL21(DE3)为本实验室保藏菌株。质粒pRSFDuet以及含有腈水合酶编码基因的重组质粒BAE、BAG由本实验室保藏。
1.2 试剂与培养基
LB培养基(g/L):胰蛋白胨10、酵母粉5、氯化钠10。
培养基中根据质粒上携带的抗性基因,选择添加相应的抗生素,抗生素添加量为:卡那霉素终质量浓度为50 μg/mL,氯霉素终质量浓度为34 μg/mL。
本研究所用引物如表1所示。
表1 本研究所用引物
Table 1 Primers used in this study

引物序列(5'→3')DT7-BAE-FTAATAAGGAGATATACCATGGATGGAATCCACGACCTDT7-BAE-RGACTTAAGCATCACCCGTCGGAGTCAGDT7-BAE-S-FACTCCGACGGGTGATGCTTAAGTCGAACAGAAAGTAATCDT7-BAE-S-RCCATCCATGGTATATCTCCTTATTAAAGTTAAACAAAATTATTTCDT7-BAG-FGAGATATACATATGGATGGTATTCATGATACCGGTGDT7-BAG-RGCCTAGGTTAATTAATCAATAATGGCCATACTTTCCATACGDT7-BAG-S-FTATTGATTAATTAACCTAGGCTGCTGCCACDT7-BAG-S-RATACCATCCATATGTATATCTCCTTCTTATACTTAACTAATATACTAAGADT7-BAG-FGAGATATACATATGGATGGTATTCATGATACCGGTGDT7-BAG-2GCAGGAGTCGCTIATTGCTCAGCGGTGGCDT7-GE-S-1CTG AGCAATAAGCGACTCCTGCATTAGGDT7-BAG-S-RATACCATCCATATGTATATCTCCTTCTTATACTTAACTAATATACTAAGA
1.3 仪器与设备
PCR仪、凝胶成像仪、蛋白电泳仪,BIO-RAD公司;UV-1800PC型紫外可见分光光度计,上海美普达有限公司;pH计,梅特勒-托利多仪器(上海)有限公司;生物传感仪,山东省科学院;高效液相色谱仪,日立(HITACHI)公司;Organic Acid色谱柱(250 mm×4.6 mm, 5 μm),Prevail公司。
1.4 重组质粒的构建
1.4.1 BAE-BAG质粒的构建
以BAE质粒为模板,用DT7-BAE-F和DT7-BAE-R引物PCR扩增BAE基因;以pRSFDuet质粒为模板,用DT7-BAE-S-F和DT7-BAE-S-R引物PCR扩增质粒骨架;用In fusion方法组装,构建pRSFDuet-BAE重组质粒。以BAG质粒为模板,用DT7-BAG-F和DT7-BAG-R引物PCR扩增BAG基因;以pRSFDuet-BAE重组质粒为模板,用DT7-BAG-S-F和DT7-BAG-S-R引物PCR扩增质粒骨架;用In fusion方法组装,构建BAE-BAG重组质粒(图1),并经DNA测序验证。
1.4.2 BAG-BAE的质粒的构建
以BAG质粒为模板,用DT7-BAG-F和DT7-BAG-2引物PCR扩增BAE基因;以pRSFDuet-BAE质粒为模板,用DT7-GE-S-1和DT7-BAG-S-R引物PCR扩增质粒骨架;用In fusion方法组装,构建BAG-BAE重组质粒(图1),并经DNA测序验证。

图1 重组菌株质粒结构示意图
Fig.1 Plasmid components of recombinant strains
1.5 重组蛋白诱导表达方法
挑取重组菌株单菌落接种于含有相应抗生素的LB液体培养基,37 ℃、200 r/min条件下振荡培养至OD600值为0.6~0.8。加入终浓度为0.2 mmol/L 异丙基硫代半乳糖苷(isopropyl-beta-D-thiogalactopyranoside,IPTG),在24 ℃、200 r/min继续培养20 h左右诱导目的蛋白表达。
1.6 细胞催化活力的测定方法
400 μL 125 mmol/L烟腈底物中加入100 μL OD600值为1.0的菌体细胞,25 ℃反应10 min,加入500 μL乙腈终止反应。HPLC检测烟酰胺含量。
单位酶活力定义:25 ℃条件下,单位毫升细胞1 min 催化烟腈产生烟酰胺的量(U/mL)。
1.7 细胞耐受性测定方法
将100 μL OD600值为6.0[6]的重组菌细胞分别加入900 μL不同浓度的烟腈、烟酰胺、丙烯腈、丙烯酰胺溶液中,25 ℃下放置30 min,用10 mmol/L 磷酸钾缓冲液(KPB)(pH 7.4)清洗细胞2次,测定细胞残余酶活。相对酶活力计算如公式(1)所示:
相对酶活力![]()
(1)
未经过有机试剂处理的细胞其相对酶活力为100%。
1.8 全细胞催化方法
将重组菌株用去离子水重悬,并将吸光度OD600值调整为8[7]。取50 mL菌液置于烧杯中,边搅拌边反应,并控制反应温度低于30 ℃。以第一次加底物(2 g烟腈/次或者1.5 mL丙烯腈/次)为起点计时,每隔2 min取10 μL反应液加到加入990 μL乙腈中终止反应。用高效液相分析样品中烟酰胺或者丙烯酰胺产物的量,并测定底物是否有剩余,待没有底物剩余时继续加底物。重复前面步骤,直到底物不能继续被消耗。
1.9 烟腈、烟酰胺、丙烯腈、丙烯酰胺含量检测方法
采用C18色谱柱及液相色谱,检测烟腈、烟酰胺、丙烯腈、丙烯酰胺的含量。流动相为水和乙腈(体积比2∶1)的混合溶液。检测波长设定220 nm,柱温为40 ℃。流速设定为0.8 mL/min,进样体积10 μL。
2 结果与分析
2.1 协同表达BAG和BAE菌株的构建
将含有BAE、BAG的重组质粒转化E.coli BL21菌株[8],获得5种重组菌株(图1)。重组菌株经诱导表达腈水合酶,全细胞SDS-PAGE如图2所示。BAE和BAG都具有α和β 2个亚基,其β亚基大小略有差别,而α亚基大小相近。BAE菌株表达量明显低于BAG菌株。BAG+BAE双质粒菌株中,同时实现了2种类型腈水合酶的表达,其中BAE的表达水平与BAE单酶菌株相似,而BAG的表达水平低于BAG的单酶菌株。BAG-BAE菌株和BAE-BAG菌株中BAG的表达水平与BAG单酶菌株相仿,而BAE的表达水平较低。
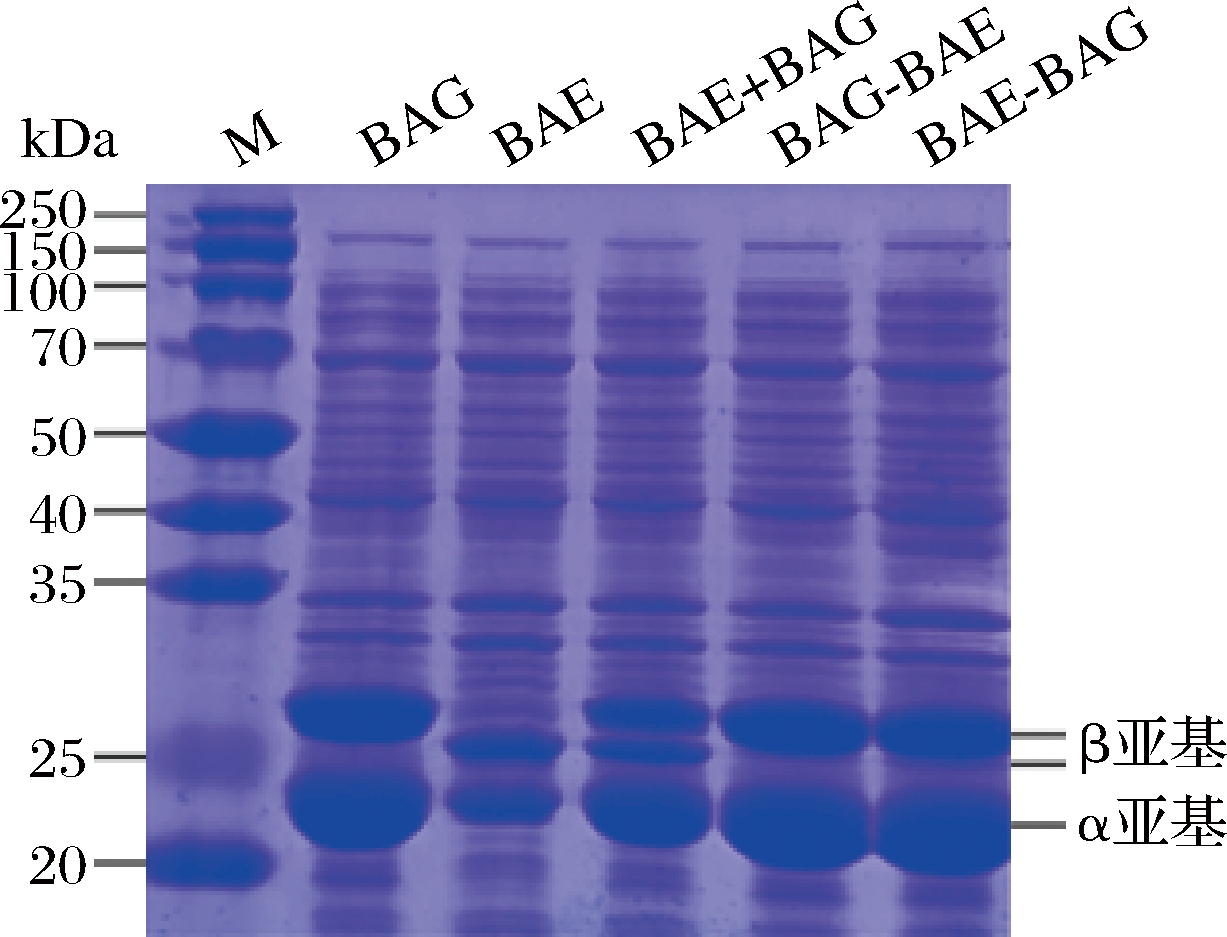
M-标准蛋白marker
图2 重组菌株的SDS PAGE分析
Fig.2 SDS PAGE analysis of the recombinant strain
2.2 重组菌株耐受性分析
由图3可知,BAG菌株对4种化合物的耐受性显著优于BAE菌株。BAG+BAE、BAG-BAE、BAE-BAG菌株中协同表达BAG与BAE,使得其耐受性优于BAE菌株。其中,BAG-BAE、BAE-BAG菌株在一个质粒上同时表达了BAE和BAG两种酶,它们对4种化合物的耐受性显著优于将BAE和BAG两种酶分别放在2个质粒上表达的BAG+BAE菌株,达到了与BAG菌株相仿的水平。因此,本研究协同表达BAG与BAE两种腈水合酶,提高全细胞催化反应速率的同时,仍保留了重组菌株对底物和产物较高的耐受性。

a-烟腈;b-烟酰胺;c-丙烯腈;d-丙烯酰胺
图3 菌株对有机溶剂耐受性
Fig.3 Resistance of the strains to organic solvents
2.3 全细胞催化合成烟酰胺
利用重组菌株细胞催化烟腈底物合成烟酰胺[9]的反应过程如图4-a所示。反应前期BAG+BAE与BAE的反应速率基本相同;但是在反应后期,BAE由于对产物耐受性较差,反应速率明显降低,且反应会较早停止,而BAE+BAG比BAE的耐受性更好,而且后期反应速率也很高,最终产物烟酰胺[10]的合成量高于BAE。BAG的反应速率较慢,但是耐受性最强,可以持续反应较长时间,产物量高。该结果表明通过协同表达BAG与BAE,实现了提高反应速率的目的。
由图4-b所示,反应前期BAE-BAG反应速率很快;在反应后期,BAE-BAG由于对产物耐受性较好,反应速率也较高,但随时间延长,底物不断被消耗,产物不断积累,反应速率减弱,最终产量显著高于BAE+BAG双质粒菌株。该结果表明将BAE和BAG构建在同一质粒上,实现了提高反应速率和产量的目的。

a-BAE、BAG、BAG+BAE菌株;b-BAE-BAG、BAG-BAE菌株
图4 全细胞催化合成烟酰胺
Fig.4 Whole-cell catalytic synthesis of nicotinamide
郭军玲等[11]报道了用含有BAG的重组大肠杆菌细胞催化烟腈合成烟酰胺,在添加菌体终浓度OD600值为8时,可在105 min合成390 g/L烟酰胺;添加菌体浓度OD600值为160时,烟酰胺产量提高为508 g/L,是目前文献报道的最高值。本文将BAG与BAE协同表达,菌株BAE-BAG在反应70 min就可以产生400 g/L烟酰胺,反应速度显著高于文献报道;同时,最终烟酰胺产物的产量可以提高为516 g/L;表明成功实现了协同提高催化速率和产量的目标。
2.4 全细胞催化合成丙烯酰胺
利用重组菌株细胞催化丙烯腈底物合成丙烯酰胺[12]的反应过程如图5所示。BAE催化丙烯腈合成丙烯酰胺的速率高于BAG菌株。协同表达BAE与BAG使得催化速率接近BAE菌株。其中BAE-BAG与BAG-BAE菌株在产物达到较高浓度后,仍能继续反应,最终产物浓度比BAE菌株提高了19.1%。该结果表明协同表达BAE、BAG同样协同提高了催化丙烯腈合成丙烯酰胺[13]的催化速率和产量。
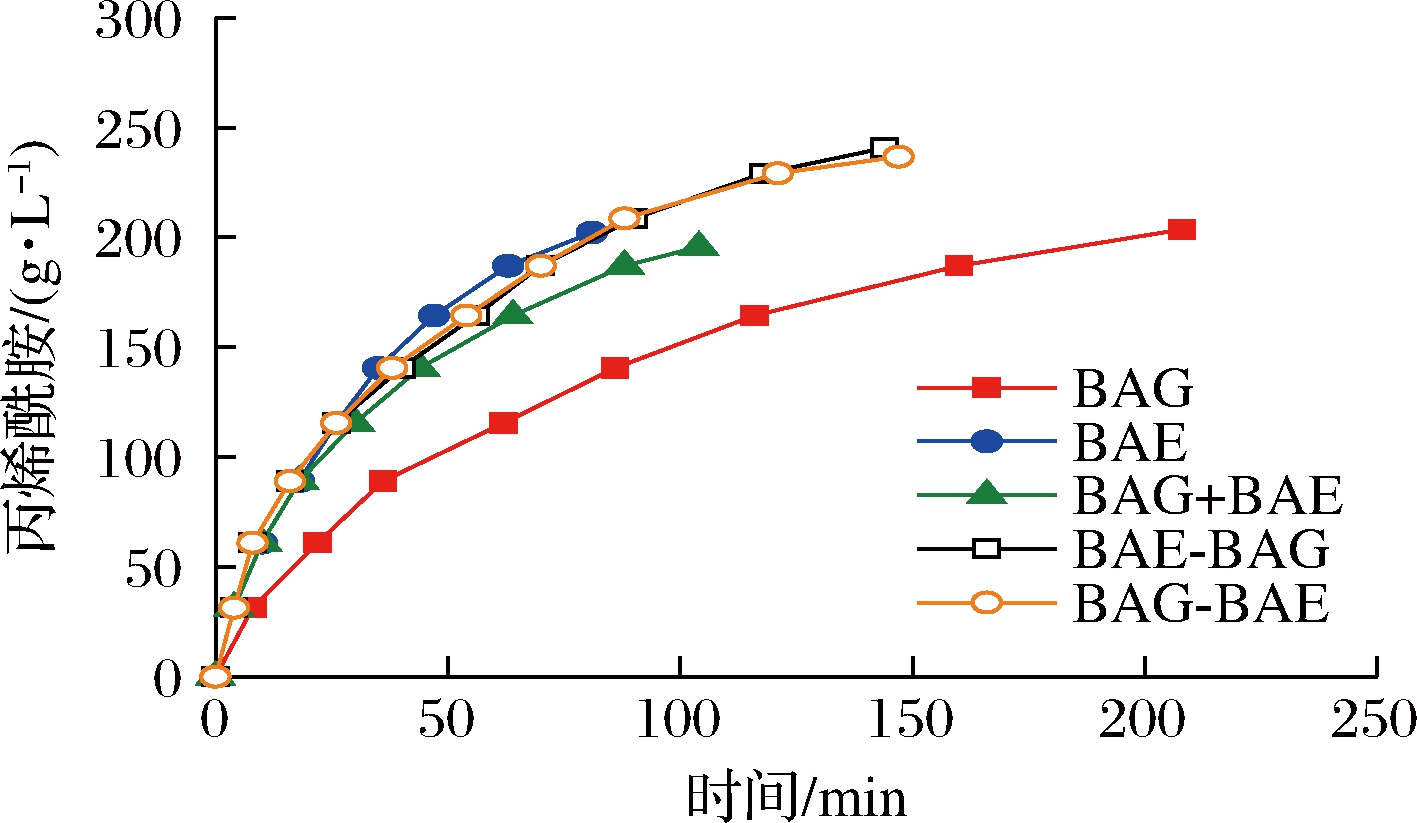
图5 全细胞催化合成丙烯酰胺
Fig.5 Whole-cell catalytic synthesis of acrylamide
3 结论
本文协同表达H-Nhase与L-Nhase型腈水合酶,成功构建了兼备这两种腈水合酶优势的BAE-BAG菌株。该菌株对底物、产物耐受性较强并具有较高催化反应速率。最终烟酰胺产量可达到516 g/L,是目前报道的最高产量;丙烯酰胺产量比原始BAE或BAG菌株提高19.1%以上,有利于工业化应用。
[1] 娄文勇, 宗敏华, 李宁, 等.微生物腈水合酶的研究进展[J].分子催化, 2001, 15(5):394-399.
LOU W Y, ZONG M H, LI N, et al.Advances in study on microbial nitrile hydratase[J].Journal of Molecular Catalysis, 2001, 15(5):394-399.
[2] 王丽燕. 重组腈水合酶NHaseK的功能表达及分子改造[D].杭州:浙江大学, 2016.
WANG L Y.Functional expression of recombinant NHaseK and its molecular modification[D].Hangzhou:Zhejiang University, 2016.
[3] 郭法谋. 功能表达来源于Klebsiella oxytoca KCTC 1686的腈水合酶和酰胺酶及其应用[D].杭州:浙江大学, 2016.
GUO F M.Functional expression of nitrile hydratase and amidase from Klebsiella oxytoca KCTC 1686 and their application[D].Hangzhou:Zhejiang University, 2016.
[4] WANG Z, LIU Z M, CUI W J, et al.Establishment of bioprocess for synthesis of nicotinamide by recombinant Escherichia coli expressing high-molecular-mass nitrile hydratase[J].Applied Biochemistry and Biotechnology, 2017, 182(4):1 458-1 466.
[5] ASANO Y, TANI Y, YAMADA H.A new enzyme “nitrile hydratase” which degrades acetonitrile in combination with amidase[J].Agricultural and Biological Chemistry, 1980, 44(9):2 251-2 252.
[6] CHENG Z Y, XIA Y Y, ZHOU Z M.Recent advances and promises in nitrile hydratase:From mechanism to industrial applications[J].Frontiers in Bioengineering and Biotechnology, 2020, 8:352.
[7] 张晓欢, 崔文璟, 周哲敏.高分子量腈水合酶在大肠杆菌中的表达策略及重组菌的细胞催化[J].微生物学通报, 2016, 43(10):2 121-2 128.
ZHANG X H, CUI W J, ZHOU Z M.Strategy of high molecular mass nitrile hydratase expression in Escherichia coli and the whole-cell catalysis by the recombinant strains[J].Microbiology, 2016, 43(10):2 121-2 128.
[8] LAN Y, ZHANG X H, LIU Z M, et al.Overexpression and characterization of two types of nitrile hydratases from Rhodococcus rhodochrous J1[J].PLoS One, 2017, 12(6):e0179833.
[9] 裴晓林. 腈水合酶基因资源开发及其重组表达体系在制备烟酰胺中的应用[D].杭州:浙江大学, 2013.
PEI X L.Discovery of nitrile hydratase genes and their recombinant expression for the production of nicotinamide[D].Hangzhou:Zhejiang University, 2013.
[10] 尹灵富. 生物催化技术生产烟酰胺的研究[D].杭州:浙江工业大学, 2004.
YIN L F.Production of nicotinamide by biological catalyst[D].Hangzhou:Zhejiang University of Technology, 2004.
[11] 郭军玲, 王哲, 刘中美, 等.高分子量腈水合酶工程菌生产烟酰胺的工艺建立[J].食品与发酵工业, 2018, 44(2):8-14.
GUO J L, WANG Z, LIU Z M, et al.Establishment of the process of nicotinamide produced by Escherichia coli expressing high molecular mass nitrile hydratase[J].Food and Fermentation Industries, 2018, 44(2):8-14.
[12] KOBAYSHI M, NAGASAWA T, YAMADA H.Enzymatic synthesis of acrylamide:A success story not yet over[J].Trends in Biotechnology, 1992, 10(11):402-408.
[13] 张赛兰, 李婷, 程中一, 等.新型耐热腈水合酶的异源表达及其催化工艺研究[J].食品与发酵工业, 2020, 46(14):108-113.
ZHANG S L, LI T, CHENG Z Y, et al.Heterologous expression of a novel thermostable nitrile hydratase and its catalytic process[J].Food and Fermentation Industries, 2020, 46(14):108-113.