乳酸菌是一种革兰氏阳性菌,因其安全性和益生效果已被广泛应用于食品工业,其具有抑制致病微生物生长、预防心血管疾病、调节肠道菌群、降低血糖、增强免疫力、改善消化系统症状等多种有益的生理活性功能[1-2]。乳酸菌的抗逆特性在许多生产和应用场景中都具有重要意义,例如耐酸、耐胆盐的性能是乳酸菌开发成为益生菌的重要性能指标,而在发酵食品,如酸奶、泡菜、发酵高酸的果蔬果汁等产品生产过程中,都对菌种耐酸、耐盐等抗逆性能提出了要求。
研究发现绝大多数微生物能够以生物膜形式提高对外界不良环境的抵御能力。微生物分泌到胞外的多糖、核酸等物质使细胞间相互联结的同时为微生物提供了天然屏障,因此生物膜状态的菌株相较浮游态菌株具有更强的耐药、耐热等性能[3-4]。然而现有研究多集中于对有害微生物生物膜的消除中,关注益生菌生物膜的研究较少。部分研究证实通过形成生物膜,乳酸菌提高了对乙醇、乙酸、胆盐等的耐受能力[5-6]。生物膜的形成与发展受外界环境和自身调节机制等多种因素影响,其中群体感应(quorum sensing, QS)系统与生物膜形成间的关系被诸多研究者所关注。在对乳酸菌生物膜的研究中发现,luxS基因参与了其生物膜的形成[7]。luxS基因是AI-2/LuxS型QS系统中的关键基因,在半胱氨酸代谢途径中S-腺苷甲硫氨酸(S-adenosylhomocysteine, SAM)经甲基转移酶作用生成S-腺苷高半胱氨酸(S-adenosylhomocysteine, SAH),S-腺苷高半胱氨酸核苷酶(S-adenosylhomocysteine nucleosidase, Pfs)将SAH水解为S-核糖半胱氨酸(S-ribosylhomocysteine, SRH)和腺嘌呤,SRH经LuxS蛋白催化生成高半胱胺酸和(S)-4,5-二羟基-2,3-戊二酮 [(S)-4,5-dihydroxy-2,3-pentanedione, DPD]。DPD经自身分子环化重排后即为信号分子AI-2[8]。AI-2在环境中被微生物感知进而调控下游基因的表达,然而在乳酸菌等大多数微生物中信号分子AI-2调控的下游途径尚不明确。
综上,已有研究表明乳酸菌AI-2/LuxS型QS系统与环境胁迫间存在相关性,但是具体的调控机制不明确,调控效果也表现出菌株特异性,因此有待进一步研究阐明。此外,现有研究主要集中于通过抑制AI-2/LuxS系统消除有害微生物,通过正向调控AI-2/LuxS提高菌株生理特性的研究较少。基于此,本研究以柠檬酸驯化前后的2株有直接、紧密亲缘关系的植物乳杆菌为研究对象,两者在AI-2活性、生物膜形成能力、柠檬酸耐受性方面具有显著差异。研究从生物膜及QS角度入手,对比出发和子代菌株在不同柠檬酸胁迫下生长、代谢产物、信号分子AI-2及相关基因表达的变化情况,以进一步明确AI-2/LuxS系统与菌株抗逆性的关系。并通过外源物质干扰AI-2/LuxS系统,探究能否通过环境干预方式调控AI-2/LuxS系统进而调控菌株生长及代谢情况,以期为通过QS系统调控菌株抗逆性能以及代谢性能提供参考。
1 材料与方法
1.1 材料与试剂
1.1.1 菌株
植物乳杆菌D(Lactobacillus plantarum D, LP-D)及柠檬酸驯化后的植物乳杆菌D-840(L.plantarum D-840, LP-D840),实验室保藏菌株。坎氏弧菌(ibrio campbellii) BB-170,北京百欧博伟生物技术有限公司。
1.1.2 培养基
MRS液体培养基参照文献[9]配制;对于AI-2的生物学检测,将葡萄糖等量替换为半乳糖[10]。AB培养基参照文献[11]配制。海水2216E液体培养基,青岛海博生物技术有限公司。
1.1.3 主要仪器
S220型微量pH计,瑞士METTLER-TOLEDO公司;Synergy H1型酶标仪,美国BioTek公司;CFX Connect型荧光定量PCR系统,Bio-Rad公司;DHZ-DA型恒温摇床,太仓市实验设备厂;5425R型高速冷冻离心机,德国Eppendorf公司;SHP-250型恒温培养箱,上海精宏实验设备有限公司;U-1000型分光光度计,翱艺仪器(上海)有限公司;NanoDrop2000超微量分光光度计,美国Thermo Fisher Scientific公司;Waters e2695型高效液相色谱,美国Waters公司。
1.1.4 主要试剂
细菌总RNA提取试剂盒,天根生化科技(北京)有限公司;HiScript® Ⅲ RT SuperMix for qPCR (+gDNA wiper),南京诺唯赞生物科技股份有限公司;PowerUpTM SYBRTM Green预混液、LIE/DEADTM BacLightTM Bacterial iability and Counting Kit,美国Thermo Fisher Scientific公司;DPD,英国Carbosynth公司;SAM,北京索莱宝科技有限公司。
1.2 实验方法
1.2.1 生物膜检测
参考文献[12]的方法并略作修改,将活化3代后的菌液按2%(体积分数)接种至新鲜MRS培养基中,于96孔板中37 ℃静置培养24 h后弃去培养基,使用生理盐水洗涤3~5次后加入200 μL 95%甲醇固定15 min。弃甲醇于室温干燥片刻,加入5 g/L的结晶紫染色20 min后用生理盐水清洗至无色。晾干后加入200 μL 95%乙醇脱色30 min。吸取150 μL脱色液至另一96孔板中并测定570 nm下的吸光度值。
1.2.2 乳酸及苯乳酸的测定
将活化3代后的菌液按2%(体积分数)接种至新鲜MRS培养基中,37 ℃静置培养。发酵液于9 000×g离心10 min后取上清液,使用0.22 μm微孔滤膜过滤。乳酸和苯乳酸的检测均采用Waters e2965高效液相色谱仪,Waters Atlantis T3(4.6 mm×250 mm, 5 μm)色谱柱,柱温30 ℃,检测波长210 nm,进样量10 μL。检测乳酸的流动相为20 mmol/L NaH2PO4(pH=2.7),流速0.7 mL/min[13]。检测苯乳酸的流动相为含有0.05%(体积分数)三氟乙酸的甲醇(A)和含有0.05%(体积分数)三氟乙酸的超纯水(B);梯度洗脱程序:10%~100% A(0~20 min),100% A(20~23 min),100%~10% A(23~25 min),流速1.0 mL/min[14]。
1.2.3 被膜态及浮游态菌株的制备
参照文献[15]的方法并稍作修改,将活化后的菌株LP-D840按2%(体积分数)接种至MRS培养基中,其中被膜态菌株于37 ℃静置培养,浮游态菌株于具塞锥形瓶中37 ℃、200 r/min下振荡培养,将培养24 h后的活化3代菌株打散后于6 000×g离心10 min收集菌体并采用磷酸盐缓冲液重悬菌体至OD600=1.0。
1.2.4 被膜态及浮游态菌株抗逆性能测定
参照文献[16]将被膜态和浮游态菌株分别重悬于含有25 g/L柠檬酸、25 g/L苹果酸、5 g/L胆盐的MRS培养基中,37 ℃处理2 h。处理后的菌株于6 000×g离心10 min后采用磷酸盐缓冲液洗涤2次并重悬。使用LIE/DEADTM BacLightTM试剂盒对菌株采用SYTO9/碘化丙啶荧光染料双染后通过流式细胞仪分析细胞存活率。
1.2.5 无菌上清液制备
将活化24 h后的活化3代菌株按2%(体积分数)接种至半乳糖改良的MRS培养基中,37 ℃静置培养,于不同培养时间取样测定其OD600值。另取一份于6000×g离心10 min后取上清液,采用1 mol/L NaOH调节pH至7.0后通过0.22 μm 无菌过滤器过滤,用于后续信号分子AI-2检测。
1.2.6 生物学法检测信号分子AI-2
将坎氏弧菌BB-170接种于海水2216E培养基中活化2代后按1%(体积分数)接种至AB培养基中,过夜培养至OD600为0.7~1.2。按体积比1∶5 000将菌液稀释到新鲜AB培养基中。将样品、阴性对照、介质对照的无菌上清液与稀释后的菌液按体积比1∶10混合,30 ℃、180 r/min振荡孵育。于阴性对照荧光强度最低时测定各样品的荧光强度,并计算其相对荧光强度以代表信号分子AI-2活性[11]。按公式(1)计算:
(1)
式中:RLU,样品相对荧光强度;LUsample,样品荧光强度;LUmedium,介质对照荧光强度。
1.2.7 luxS和pfs基因表达量的测定
将活化24 h后的3代菌株按2%(体积分数)接种至不同柠檬酸浓度的MRS培养基中,37 ℃静置培养,于7 h收集菌体。提取RNA后对RNA进行DNA的去除以及反转录。以反转录得到的cDNA为模板进行荧光定量PCR,反应体系为20 μL(2 μL cDNA,10 μL SYBR,6 μL ddH2O,上下游引物各1 μL)。qPCR反应条件为:95 ℃ 5 min;95 ℃ 5 s,58 ℃ 30 s,72 ℃ 30 s,40个循环。数据分析采用2-ΔΔCT法[17]。
表1 本实验所用引物
Table 1 Primers used in this experiment
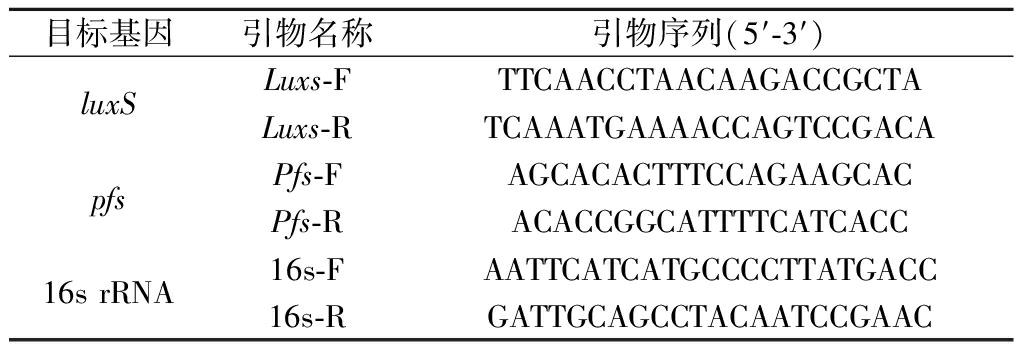
目标基因引物名称引物序列(5′-3′)luxSLuxs-FTTCAACCTAACAAGACCGCTALuxs-RTCAAATGAAAACCAGTCCGACApfsPfs-FAGCACACTTTCCAGAAGCACPfs-RACACCGGCATTTTCATCACC16s rRNA16s-FAATTCATCATGCCCCTTATGACC16s-RGATTGCAGCCTACAATCCGAAC
1.3 数据处理与统计分析
所有实验均进行3次生物学重复,结果用平均值±标准差表示,采用Graphpad Prism 8软件进行统计学分析并绘图,2组间数据比较采用独立样本T检验(unpaired T-test),3组及以上数据采用单因素方差分析(one-way ANOA)的Tukey法进行两两比较,*表示P<0.05,**表示P<0.01,***表示P<0.001,ns表示无显著差异。
2 结果与分析
2.1 柠檬酸驯化前后菌株生理特性比较
LP-D为实验室前期研究发现具有较好的发酵性能的菌株,对高酸的原料也具有较强的耐受性。因此前期以LP-D为出发菌株,通过实验室连续传代驯化,获得了一株能够耐受更高柠檬酸浓度的LP-D840。
出发菌株LP-D和驯化后的LP-D840生理性状如图1所示。
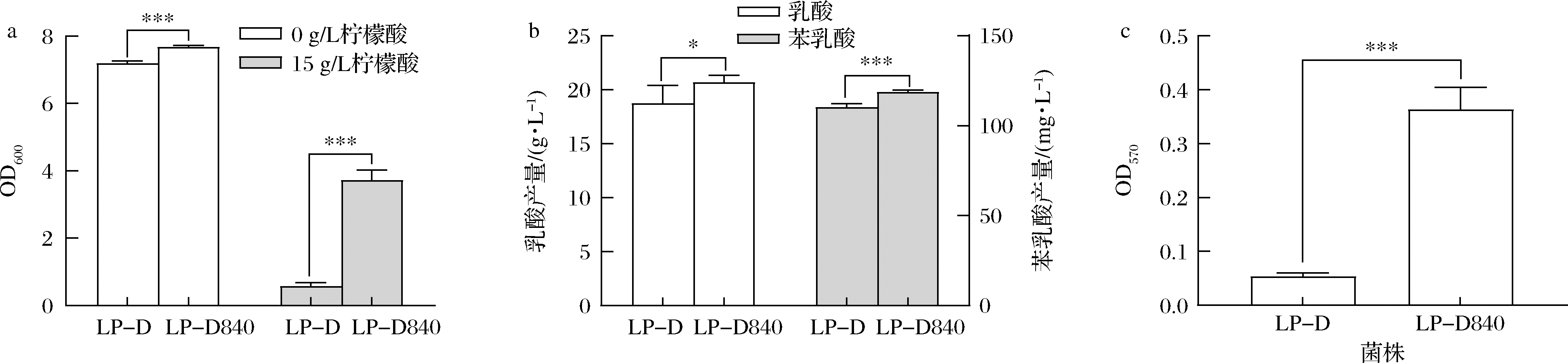
a-菌体密度;b-乳酸及苯乳酸产量;c-生物膜形成能力
图1 柠檬酸驯化前后菌株生理代谢性能比较
Fig.1 Comparison of physiological and metabolic properties of the original and citric acid acclimated strains
LP-D840在含0 g/L和15 g/L柠檬酸的MRS培养基中的菌体密度均高于LP-D,且在柠檬酸浓度为15 g/L时菌体密度较LP-D提高了5.4倍(图1-a)。发酵24 h后LP-D840的乳酸及苯乳酸产量均略高于LP-D(图1-b)。此外观察到出发菌株LP-D几乎不形成生物膜,而经柠檬酸驯化后的菌株LP-D840生物膜形成能力提高了485%(P<0.05)。
生物膜的形成可能是微生物在自然界中抵御各种环境胁迫的一种机制[5,16],结合本研究中发现耐酸性和生物膜形成2种表型在驯化后的菌株LP-D840中同时出现,分析认为LP-D840柠檬酸耐受性的提高可能与其生物膜形成相关。
2.2 被膜态与浮游态菌株抗逆性能对比
为了分析LP-D840显著提高的生物膜合成能力是否与其更高的抗逆性能有关,本研究对比了LP-D840在被膜态和浮游态2种状态下对逆性条件的耐受程度。由图2可知,被膜态菌株在25 g/L柠檬酸、25 g/L苹果酸以及5 g/L胆盐胁迫下的存活率均显著高于浮游态菌株(P<0.05)。

a-流式细胞存活率图;b-存活率柱状图
图2 不同胁迫下LP-D840被膜态与浮游态的存活率
Fig.2 Surial rates of biofilm and planktonic cells of LP-D840 under different stress
此结果表明驯化子代LP-D840所具有的更强耐受性能与其生物膜的形成密切相关。刘蕾等[16]研究发现被膜态类植物乳杆菌(L.paraplantarum)L-ZS9相较浮游态具有更好的耐热、耐酸、耐胆盐能力,本文结论与之一致。
2.3 发酵过程中QS信号分子AI-2变化
诸多研究表明生物膜的形成与QS系统具有一定的相关性[6,18],对发酵过程中LP-D和LP-D840产信号分子AI-2及其菌体生长情况定量分析,采用Logistic模型拟合生长曲线。如图3所示,LP-D840和LP-D大致在4 h左右进入对数生长期。2株菌的AI-2在4~8 h间迅速积累,LP-D所产AI-2在16 h达到最高后略有下降,而LP-D840所产AI-2在8 h达到最高后略有下降,这一峰值出现时间远早于菌体浓度最大值出现时间(20~24 h)。随发酵进行2株菌的单位菌体AI-2信号合成能力呈下降趋势,发酵后期菌体浓度达到一定值后(12 h),单位菌体AI-2的合成量维持在较低的水平直至发酵结束。
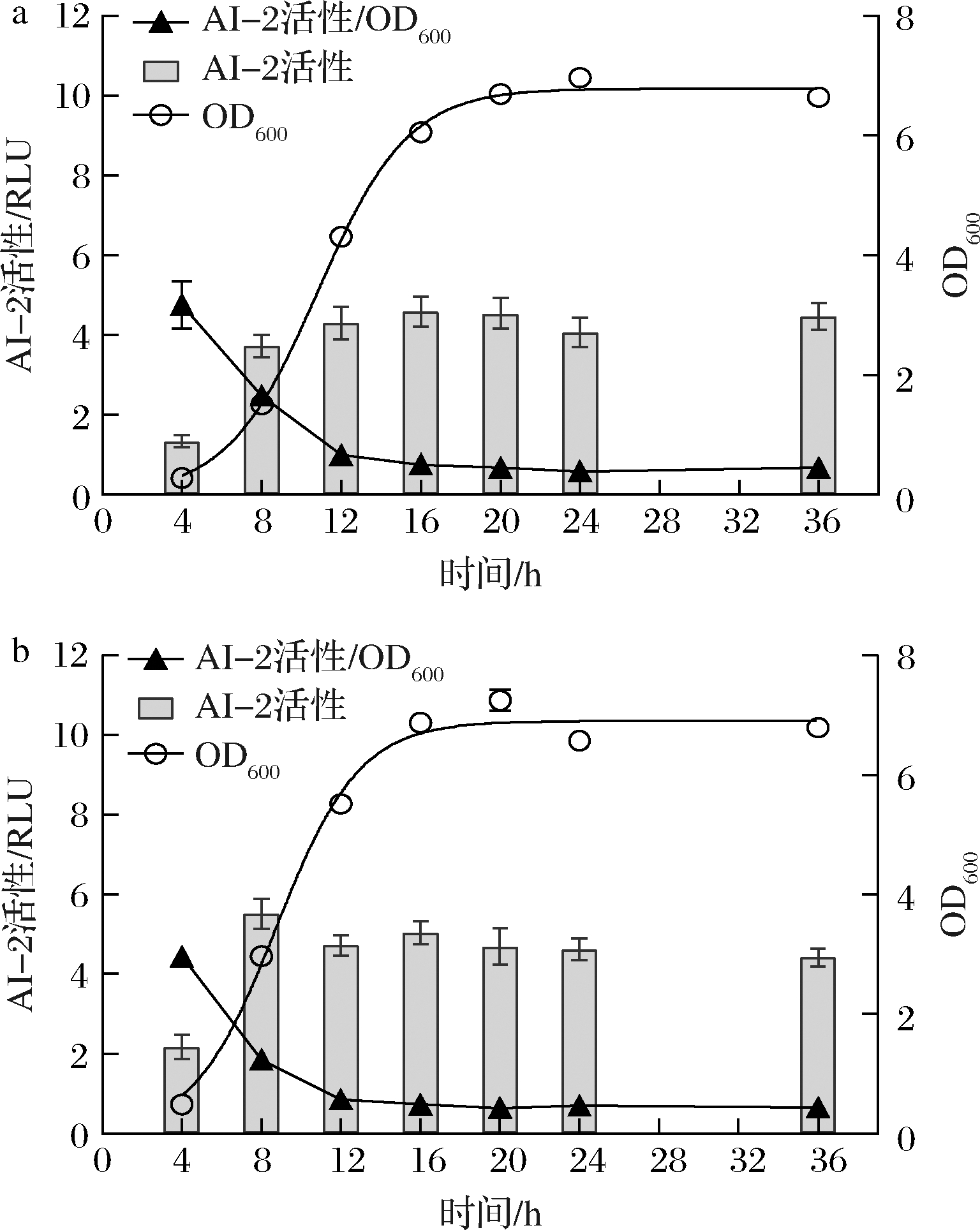
a-LP-D;b-LP-D840
图3 发酵过程中菌体密度与AI-2活性的变化
Fig.3 The changes of cell growth and AI-2 actiity during fermentation
在本研究中,发酵初期细胞上调AI-2/LuxS系统的表达以维持一定环境浓度的AI-2信号,进而发挥细胞交流的作用,且LP-D840能更早地达到较高的AI-2信号分子的积累浓度。因此分析认为AI-2信号的分泌可能是促进菌体生长的一种调控机制。然而MAN等[19]发现植物乳杆菌KLDS1.0391的信号分子AI-2在指数生长后期达到最高值,随后在稳定期迅速下降。有研究认为AI-2可能作为一种代谢产物被菌株自身消耗,从而导致发酵后期AI-2的下降[20],但这一结论还有待进一步探究。
2.4 驯化前后菌株AI-2的QS系统对柠檬酸胁迫响应程度存在差异
2株菌的菌体密度随柠檬酸浓度升高均呈下降趋势(图4-a),但上清液中AI-2活性总体呈上升趋势(图4-b),且在相同柠檬酸浓度下LP-D840上清液中AI-2活性高于LP-D。但LP-D840单位菌体密度下的AI-2强度却显著(P<0.05)低于出发菌株(图4-c)。
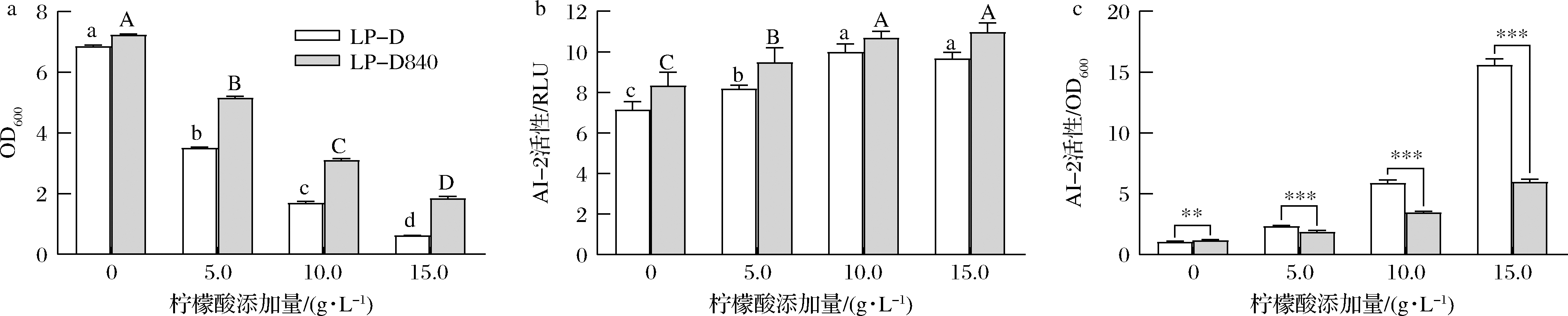
a-菌体密度;b-上清液AI-2活性;c-单位菌体密度下AI-2活性
图4 柠檬酸胁迫对LP-D和LP-D840生长及AI-2活性影响
Fig.4 Effects of citric acid stress on growth and AI-2 actiity of LP-D and LP-D840 注:图中不同小写字母表示LP-D组在P<0.05水平存在显著差异;图中不同大写字母表示LP-D840组在P<0.05水平存在显著差异(下同)
本研究中两株菌合成更多的AI-2信号分子响应柠檬酸胁迫,并且这种响应强度和柠檬酸胁迫压力呈正相关。经柠檬酸驯化后的LP-D840,在胁迫下单位菌体AI-2合成能力下降,这可能与该菌株柠檬酸耐受性能提高相关。这与MOSLEHI-JENABIAN等[11]发现经酸适应后的鼠李糖乳杆菌在遭遇酸胁迫时luxS基因表达显著低于野生型菌株相似。
信号分子AI-2合成通路如图5-a所示。对参与AI-2合成的luxS和pfs基因测序发现驯化后的LP-D840较LP-D并未产生非同义突变。RT-qPCR结果如图5-b和图5-c所示,柠檬酸胁迫下2株菌的luxS基因表达下调,而pfs基因表达上调。在15 g/L柠檬酸浓度下,LP-D和LP-D840中的luxS基因表达量分别降低了34.5%、28.8%(P<0.05),而pfs基因表达量分别提高了71.4%、49.3%(P<0.05)。
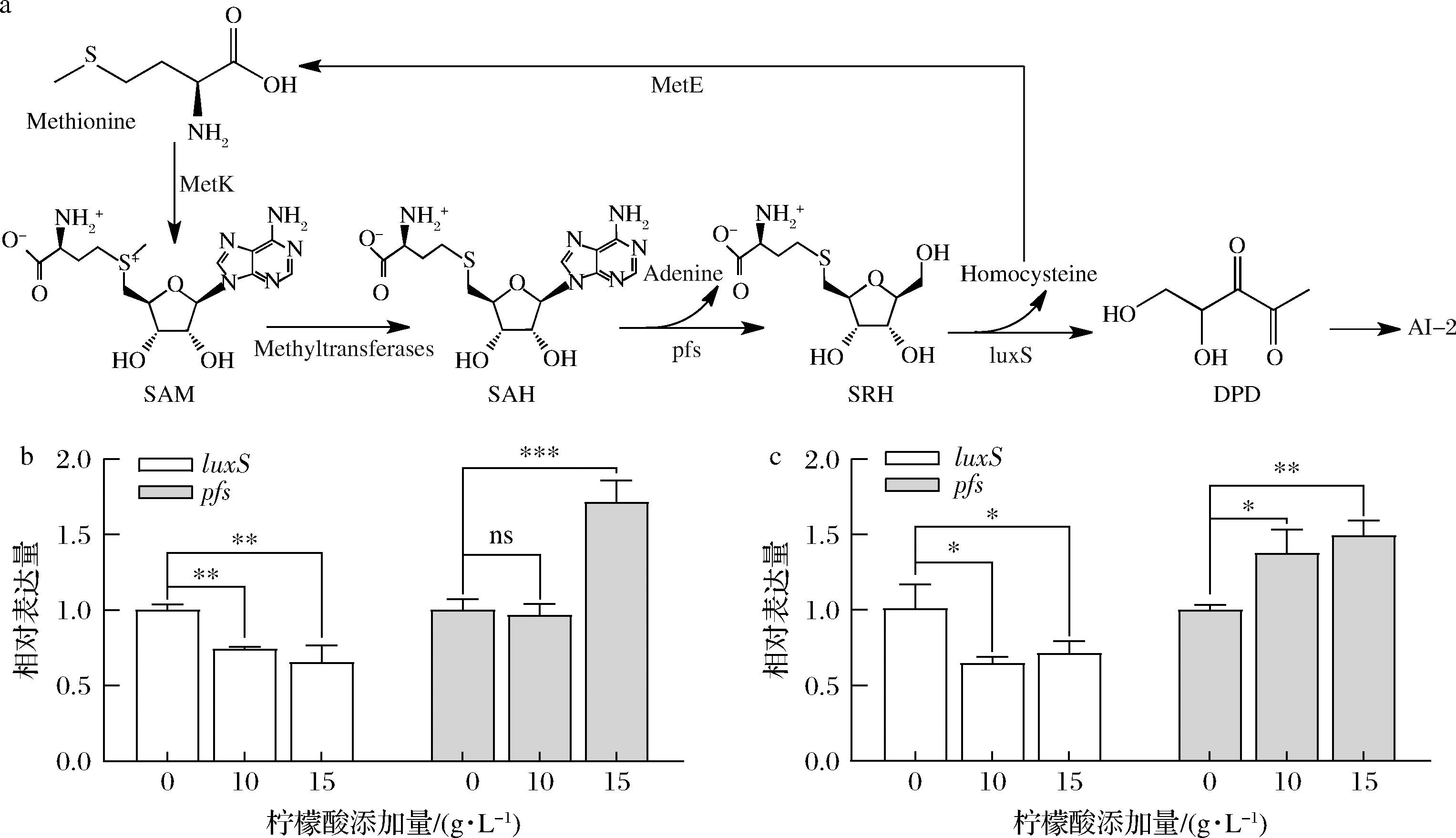
a-信号分子AI-2合成通路;b-LP-D;c-LP-D840
图5 柠檬酸胁迫下LP-D和LP-D840中luxS、pfs基因转录水平
Fig.5 Transcription leel of luxS and pfs genes of LP-D and LP-D840 after exposure to citric acid stress
luxS基因表达量与AI-2产量呈现负相关,这与WANG等[21]对luxS基因的过表达并未提升AI-2产量的结果一致。而pfs基因在柠檬酸胁迫下的表达量与AI-2呈现正相关性,说明在本研究体系中与luxS基因相比,pfs基因对AI-2的合成起更显著的调控作用,并且与菌株柠檬酸耐受能力相关性更强。
2.5 外源添加信号分子前体对菌株生理特性的调控
YANG等[22]研究表明苯乳酸的合成可能受AI-2/LuxS系统调控,因此研究了外源添加信号分子前体对菌株生长、乳酸和苯乳酸合成的影响。图6所示,不同浓度的DPD对驯化前后两株菌生长曲线无显著影响,但能够影响驯化后LP-D840的乳酸和苯乳酸产量。DPD添加量大于0.5 μmol/L时开始显著抑制LP-D840乳酸的合成。0.1 μmol/L的DPD促使LP-D840苯乳酸产量显著提高27.1%。
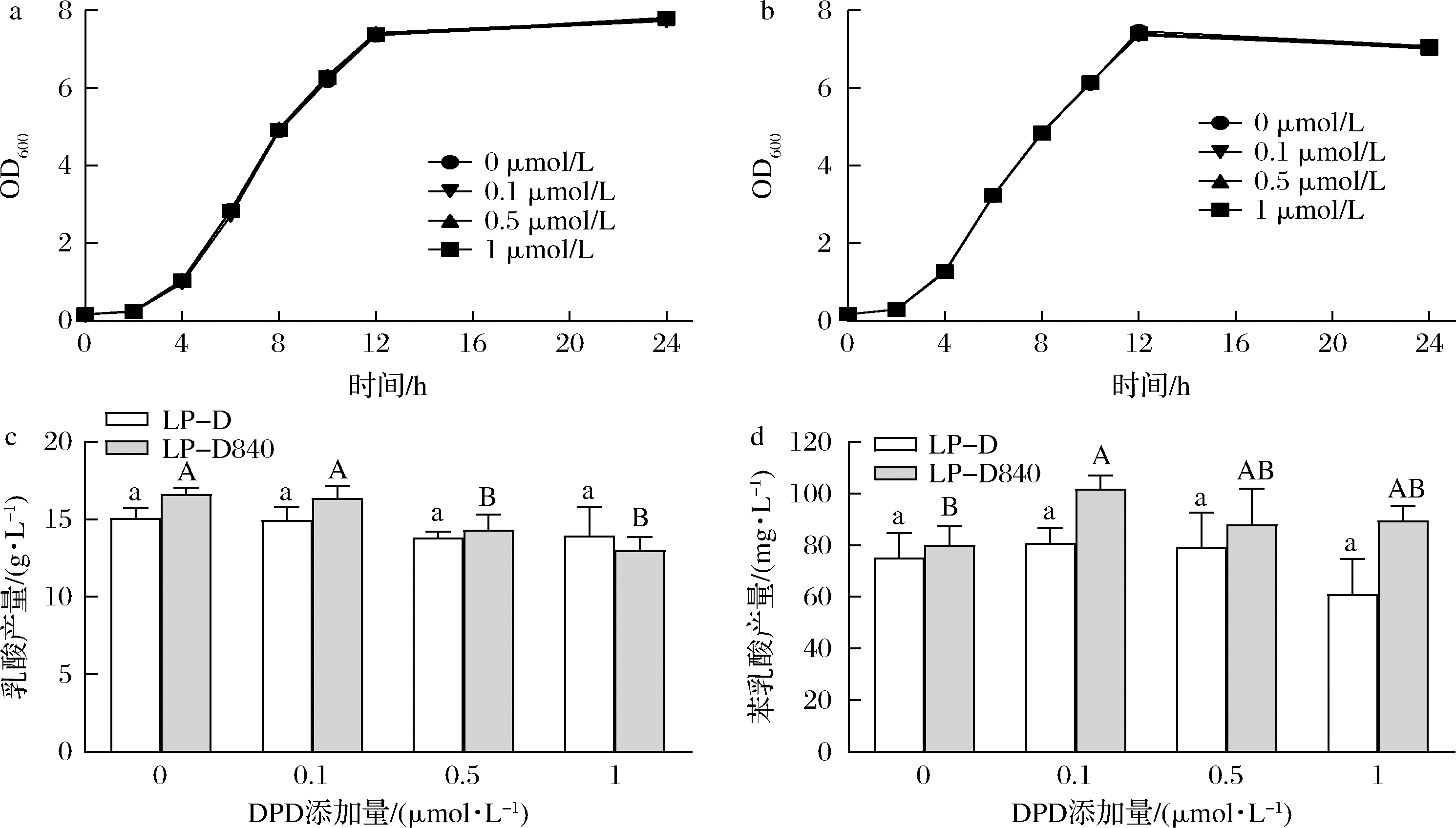
a-LP-D菌体密度;b-LP-D840菌体密度;c-乳酸产量;d-苯乳酸产量
图6 外源添加DPD对LP-D和LP-D840生理代谢的影响
Fig.6 Effects of exogenous DPD on cell growth and metabolism of LP-D and LP-D840
上游物质SAM能够抑制LP-D及LP-D840菌体生长。如图7所示,在SAM添加量为1 g/L时,LP-D和LP-D840的菌体密度分别显著降低了12.8%和7.1%。在SAM的干扰下乳酸的产量变化幅度较小,苯乳酸产量总体呈现上升趋势,在SAM添加量为1 g/L时,LP-D和LP-D840苯乳酸产量分别显著提高了12.2%和25.5%。
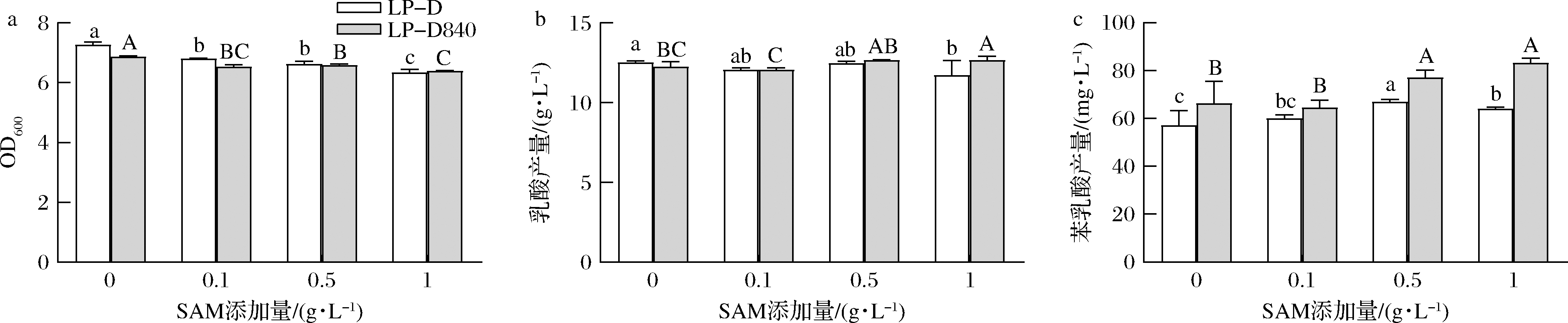
a-菌体密度;b-乳酸产量;c-苯乳酸产量
图7 外源添加SAM对LP-D和LP-D840生理代谢影响
Fig.7 Effects of exogenous SAM on cell growth and metabolism of LP-D and LP-D840
DPD的添加对菌株生长曲线并无影响,这可能因为luxS基因与菌株生长不相关,这与JIA等[23]对植物乳杆菌luxS基因敲除的实验结果相符。由于菌株之间存在AI-2代谢途径速率的差异,导致其对不同前体的响应幅度存在差异。外源添加AI-2前体物质能够干扰菌株QS系统,进而提高代谢产物苯乳酸的积累,这一方法相较于基因工程改造菌株以及驯化育种在安全性和时间周期上有一定的优势。
3 结论
本研究证实了生物膜的形成能够显著提升菌株的抗逆性能(P<0.05),明确了AI-2/LuxS系统参与了菌株对柠檬酸胁迫的抵御。结合基因转录及外源添加DPD、SAM结果,本研究的两株植物乳杆菌参与AI-2合成的上游pfs基因、SAM对菌株生理代谢的调控,其作用效果优于luxS基因和DPD,因此pfs基因可能是调节AI-2的关键靶点。本文的结果拓展了AI-2/LuxS QS系统对环境胁迫响应的研究,并为人工调控AI-2/LuxS QS系统,进而改善菌株发酵性能提供了新的方向和研究依据。
[1] MARTEAU P, LE NEÉ B, QUINQUIS L, et al.Consumption of a fermented milk product containing Bifidobacterium lactis CNCM I-2494 in women complaining of minor digestie symptoms:Rapid response which is independent of dietary fibre intake or physical actiity[J].Nutrients, 2019, 11(1):92.
[2] WANG J C, BAI X Y, PENG C T, et al.Fermented milk containing Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis 9 alleiated constipation symptoms through regulation of intestinal microbiota, inflammation, and metabolic pathways[J].Journal of Dairy Science, 2020, 103(12):11 025-11 038.
[3] 陆文伟, 林炳谕, 尹一婷, 等.双歧杆菌生物膜成膜规律及特性研究[J].中国食品学报, 2017, 17(12):42-49.
LU W W, LIN B Y, YIN Y T, et al.The biofilm formation of Bifidobacterium and its characteristics[J].Journal of Chinese Institute of Food Science and Technology, 2017, 17(12):42-49.
[4] 吴旭光, 王红, 徐一凡, 等.猪源肠外致病性大肠埃希氏菌的生物膜形成能力及耐药性[J].微生物学通报, 2020, 47(1):162-171.
WU X G, WANG H, XU Y F, et al.Biofilm-forming capability and drug resistance of extraintestinal pathogenic Escherichia coli isolated from diseased swine[J].Microbiology China, 2020, 47(1):162-171.
[5] KUBOTA H, SENDA S, NOMURA N, et al.Biofilm formation by lactic acid bacteria and resistance to enironmental stress[J].Journal of Bioscience and Bioengineering, 2008, 106(4):381-386.
[6] LIU L, WU R Y, ZHANG J L, et al.Oerexpression of luxS promotes stress resistance and biofilm formation of Lactobacillus paraplantarum L-ZS9 by regulating the expression of multiple genes[J].Frontiers in Microbiology, 2018, 9:2628.
[7] LEBEER S, CLAES I J J, ERHOEEN T L A, et al.Impact of luxS and suppressor mutations on the gastrointestinal transit of Lactobacillus rhamnosus GG[J].Applied and Enironmental Microbiology, 2008, 74(15):4 711-4 718.
[8] CHEN X, SCHAUDER S, POTIER N, et al.Structural identification of a bacterial quorum-sensing signal containing boron[J].Nature, 2002, 415(6871):545-549.
[9] DE MAN J C, ROGOSA M, SHARPE M E.A medium for the cultiation of lactobacilli[J].Journal of Applied Bacteriology, 1960, 23(1):130-135.
[10] DEKEERSMAECKER S C J, ANDERLEYDEN J.Constraints on detection of autoinducer-2 (AI-2) signalling molecules using ibrio hareyi as a reporter[J].Microbiology (Reading, England), 2003, 149(Pt 8):1 953-1 956.
[11] MOSLEHI-JENABIAN S, GORI K, JESPERSEN L.AI-2 signalling is induced by acidic shock in probiotic strains of Lactobacillus spp.[J].International Journal of Food Microbiology, 2009, 135(3):295-302.
[12] SUN L L, ZHANG Y, GUO X J, et al.Characterization and transcriptomic basis of biofilm formation by Lactobacillus plantarum J26 isolated from traditional fermented dairy products[J].LWT, 2020, 125:109333.
[13] WU L H, LU Z M, ZHANG X J, et al.Metagenomics reeals flaour metabolic network of cereal inegar microbiota[J].Food Microbiology, 2017, 62:23-31.
[14] LI X F, JIANG B, PAN B L, et al.Purification and partial characterization of Lactobacillus species SK007 lactate dehydrogenase (LDH) catalyzing phenylpyruic acid (PPA) conersion into phenyllactic acid (PLA)[J].Journal of Agricultural and Food Chemistry, 2008, 56(7):2 392-2 399.
[15] LIU L, GUO S Y, CHEN X, et al.Metabolic profiles of Lactobacillus paraplantarum in biofilm and planktonic states and inestigation of its intestinal modulation and immunoregulation in dogs[J].Food & Function, 2021, 12(12):5 317-5 332.
[16] 刘蕾, 武瑞赟, 李军, 等.基于AI-2介导的类植物乳杆菌生物被膜态与浮游态抗胁迫能力比较与分析[J].食品科学, 2017, 38(22):41-47.
LIU L, WU R Y, LI J, et al.Stress resistance of biofilm and planktonic cells of Lactobacillus paraplantarum L-ZS9 regulated by autoinducer 2[J].Food Science, 2017, 38(22):41-47.
[17] LIAK K J, SCHMITTGEN T D.Analysis of relatie gene expression data using real-time quantitatie PCR and the 2-ΔΔCT method[J].Methods, 2001, 25(4):402-408.
[18] XIONG Q, LIU D, ZHANG H H, et al.Quorum sensing signal autoinducer-2 promotes root colonization of Bacillus elezensis SQR9 by affecting biofilm formation and motility[J].Applied Microbiology and Biotechnology, 2020, 104(16):7 177-7 185.
[19] MAN L L, MENG X C, ZHAO R H, et al.The role of plNC8HK-plnD genes in bacteriocin production in Lactobacillus plantarum KLDS1.0391[J].International Dairy Journal, 2014, 34(2):267-274.
[20] WINZER K, HARDIE K R, BURGESS N, et al.LuxS:its role in central metabolism and the in itro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone[J].Microbiology, 2002, 148(4):909-922.
[21] WANG Y, YI L, ZHANG Z C, et al.Oerexpression of luxS cannot increase autoinducer-2 production, only affect the growth and biofilm formation in Streptococcus suis[J].The Scientific World Journal, 2013, 2013:924276.
[22] YANG X Y, LI J P, SHI G C, et al.Improing 3-phenyllactic acid production of Lactobacillus plantarum AB-1 by enhancing its quorum-sensing capacity[J].Journal of Food Science and Technology, 2019, 56(5):2 605-2 610.
[23] JIA F F, ZHENG H Q, SUN S R, et al.Role of luxS in stress tolerance and adhesion ability in Lactobacillus plantarum KLDS1.0391[J].BioMed Research International, 2018, 2018:4506829.