植物乳杆菌(Lactobacillus plantarum)是一种兼性厌氧细菌,在缺氧条件下能够进行发酵,将糖转化为乳酸[1]。乳酸菌有多种健康益处,包括替代抗生素、降低胆固醇水平、维持肠道菌群平衡和提高免疫力[2-3]。据国际乳业联合协会建议,食品中益生菌活菌数最低为107 CFU/g或107 CFU/mL,以确保益生菌对宿主发挥有益作用[4]。因此,生产上通过高密度培养(high cell density culture,HCDC)来获得足够数量的益生菌,以发挥其促进宿主健康的作用。乳酸菌的生长受培养基组成、pH、温度、接种量和代谢产物的影响较大[5]。由于乳酸菌对营养的要求非常严格,因此需要丰富的培养基来促进生长。在发酵培养基优化方法中,响应曲面法(response surface methodology,RSM)得到了广泛的应用[6]。人工神经网络(artificial neural network,ANN)是一种基于生物神经网络结构和功能的计算模型,在非线性统计数据建模上具有较高的精度和泛化能力[7]。一些研究表明,ANN相较于RSM在发酵条件优化方面具有更好的拟合效果[8]。遗传算法(genetic algorithm,GA)是一种基于达尔文“适者生存”理念的启发式优化算法,采用遗传算子如选择、突变和交叉,以找到最优的问题解决方案[9]。在最近的研究中,ANN-GA混合模型已被证明预测精度高,稳定性好[10]。高密度培养主要通过添加碱性溶液控制培养基pH来降低酸抑制作用。在此基础上,通过分批补料策略来提高菌体产量[11]。本研究通过单因素试验对植物乳杆菌的碳源、氮源和缓冲盐进行了优化,同时基于响应面试验设计,利用ANN-GA混合模型对培养基组成进行了优化。在此基础上,对植物乳杆菌发酵过程进行模拟,建立了发酵动力学方程。在最佳培养基和培养条件下,通过间歇补料方式,进一步提高菌体产量,为植物乳杆菌的推广应用提供参考。
1 材料与方法
1.1 菌种与培养基
植物乳杆菌(Lactobacillus plantarum),分离自江西省萍乡市成品搓菜,由本实验室保藏。MRS培养基(g/L):蛋白胨10,牛肉膏10,酵母膏5,柠檬酸二铵2,葡萄糖10,Tween-80 l mL,磷酸氢二钾2,MgSO4·7H2O 0.58,MnSO4·H2O 0.25,自然pH值。
1.2 仪器与设备
DGG-9140A型电热恒温鼓风干燥箱,上海森信实验仪器有限公司;HWS-150型恒温恒湿箱,上海精宏实验设备有限公司;雷磁PHS-25型数显pH计,上海智城分析仪器制造有限公司;LDZX-50KBS立式压力蒸汽灭菌器,上海申安医疗器械厂;EXL800全自动酶标仪,美国Biotek Instruments有限公司;THZ-82 N台式恒温振荡器,上海跃进医疗器械厂;AR1140电子分析天平,奥豪斯仪器有限公司。
1.3 实验方法
1.3.1 菌种活化与指标测定
L.plantarum菌种保藏于甘油冻存管中,每次实验前将菌种以3%接种量接种于液体MRS培养基中,35 ℃培养24 h,活化2代。
菌体生物量的测定采用烘干法,即取一定量发酵液于8 000 r/min离心5 min去除上清液,用生理盐水洗涤菌体2次,于105 ℃烘干至恒重后称量[12]。然后,将OD600 值转换成菌体干重(dry cell weight,DCW),由实验测定,DCW(g/L)=10.87×OD600-0.096 9。
还原糖含量采用3,5-二硝基水杨酸(3,5-dinitrosalicylic acid,DNS)法测定[13]。简要地,取1 mL稀释发酵液,加入2 mL DNS试剂于100 ℃下水浴5 min。冷却后定容至15 mL,于540 nm测定吸光度。
乳酸含量采用紫外分光光度法测定[14]。用离心机将发酵液从菌体中分离出来,上清液用去离子水稀释10倍。取50 μL稀释后的上清液加入到2 mL的2 g/L氯化铁溶液中,在390 nm下测定吸光度。
1.3.2 碳源、氮源和缓冲盐优化
以植物乳杆菌为研究对象,在MRS培养基的基础上,对不同种类的碳源、氮源和缓冲盐进行了优化,用20 g/L的不同碳源和25 g/L的不同氮源分别替代MRS培养基中已有的碳源或氮源,而所有其他成分保持在固定水平。通过测定生物量确定不同物质对植物乳杆菌生长促进作用。
1.3.3 培养基优化试验设计
利用RSM中的中心组合设计(central composite design,CCD)进行试验,对植物乳杆菌培养基的碳源、氮源、缓冲盐进行优化,以菌体生物量为响应值,确定培养基中主要成分的最优配比。其中碳源含量(X1)、氮源含量(X2)、柠檬酸二铵含量(X3)为自变量,试验因素及水平设计如表1所示。
表1 CCD参数水平设计
Table 1 Levels of CCD parameter design
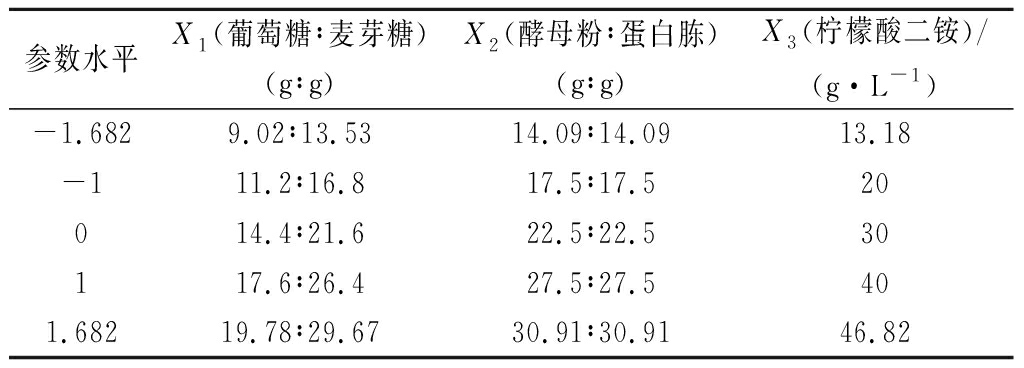
参数水平X1(葡萄糖∶麦芽糖)(g∶g)X2(酵母粉∶蛋白胨)(g∶g)X3(柠檬酸二铵)/(g·L-1)-1.6829.02∶13.5314.09∶14.0913.18-111.2∶16.817.5∶17.520014.4∶21.622.5∶22.530117.6∶26.427.5∶27.5401.68219.78∶29.6730.91∶30.9146.82
1.3.4 ANN模型
ANN是一种受大脑和神经系统研究启发的数学算法。它可以连接输入和输出参数,通过迭代从示例中学习,而不需要关于过程变量之间关系的先验知识[15]。隐藏层神经元的传递函数为tansig,输出层神经元的传递函数为purelin,采用trainbr方法进行训练,当均方误差(mean square error,MSE)达到1×10-3时,训练停止[16]。通过试错法,比较不同神经网络结构预测实际值的能力,确定隐藏层神经元的数量。在此过程中,采用MSE作为质量参数,在不同结构中选择最优结构。本实验选择碳源(X1)、氮源(X2)和缓冲盐(X3)作为模型输入,采用BP神经网络算法,以菌体生物量作为模型输出,建立单隐藏层和双隐藏层神经网络结构。通过比较单隐藏层和双隐藏层结构的MSE,确定最优神经网络结构。MSE的计算如公式(1)所示:
(1)
式中:N为样本数量;Yp,i为预测值;Ya,i为实际值。
1.3.5 GA
GA已经证明了它能够以最优性能优化各种线性和非线性问题,该算法基于自然进化原理,利用交叉和变异来保持最优个体的生存[17]。ANN-GA混合算法模型将训练好的ANN网络结构作为遗传算法的适应度函数,以菌体干重为响应变量,通过寻找全局最优解,获得最优培养基组合。遗传算法参数设定如下:变量类型为实数型,种群大小50,最大迭代数500,交叉概率0.8,变异概率0.1。
1.3.6 发酵动力学
微生物发酵动力学模型主要有结构化和非结构化模型,与结构化模型相比,非结构化模型更易于对间歇发酵进行预测和监测[18]。根据优化后的培养基,建立植物乳杆菌的菌体生长、底物消耗和产物生成模型,每隔2 h测定其培养过程中的生物量、乳酸和残糖量,计算出动力学模型的参数,预测其发酵过程变化。
对于菌体生长动力学,Logistic方程是一个与底物无关的模型,可以很好地描述微生物生长过程[19]。菌体生长Logistic方程的表达如公式(2)所示:
(2)
式中:X,菌体生物量,g/L;X0,菌体初始生物量,g/L;Xm,菌体最大生物量,g/L;μm,最大比生长速率,g/(L·h);t,时间,h。
植物乳杆菌产物生成模型符合“S”型曲线,使用Logistic方程预测产物生成模型,方程的表达如公式(3)所示:
(3)
式中:P,菌体乳酸含量,g/L;P0,菌体初始乳酸含量,g/L;Pm,菌体最大乳酸含量,g/L;μm1,最大比生成速率,g/(L·h);t,时间,h。
植物乳杆菌底物消耗动力学使用Luedeking-Piret模型,该模型既考虑了菌体自身维持生长的底物消耗,也考虑了底物向菌体生物量和代谢产物的转化[5]。鉴于菌体用于维持自身的底物消耗远低于菌体生物量和产物生成量的底物消耗,故对Luedeking-Piret模型进行简化,方程的表达如公式(4)所示:
(4)
式中:S,残糖量,g/L;S0,初始残糖量,g/L;YX/S,菌体得率系数,g/g;YP/S,产物得率系数,g/g;t,时间,h。
1.3.7 培养条件和分批补料优化
以植物乳杆菌菌体干重为指标,通过对温度、初始pH和接种量进行优化,绘制其发酵动力学曲线。在此基础上进行补料优化,采用间歇补料方式,研究中和剂、补料浓度和补料次数对菌体密度的影响。
1.3.8 数据处理
每组实验均进行3次平行测定,响应面设计采用Design-Expert软件进行设计与分析,ANN和GA分别采用R语言中的NeuralNetTools和GA开源软件包[20-21],使用Origin进行数据分析和图表处理。
2 结果与分析
2.1 碳源和氮源优化
不同种类的碳源和氮源对植物乳杆菌生长的影响如图1所示。图1-a显示,麦芽糖对菌体生长的促进作用最为显著,生物量最高可达6.38 g/L,葡萄糖和麦芽糖(质量比2∶3)次之,菌体量可达6.28 g/L。可以发现,相较于葡萄糖,麦芽糖对植物乳杆菌生长促进效果更好,可能是因菌体快速利用葡萄糖产酸,降低培养基的pH,抑制其自身的生长。考虑到单独使用麦芽糖的成本较高,因此选择葡萄糖和麦芽糖作为最佳碳源。如图1-b所示,有机氮源显著优于无机氮源,而当选择酵母粉和蛋白胨(质量比1∶1)为氮源时,菌体最大产量为6.7 g/L。一些研究表明,酵母粉和低成本的蛋白胨作为氮源时,对乳酸菌生长有显著影响[22]。相较于使用单一氮源,酵母粉复合大分子肽的蛋白胨对植物乳杆菌的生长有显著促进作用。不同浓度的复合碳源和复合氮源对菌体的生长有着重要的影响。复合碳源质量浓度为30 g/L时,菌体最大产量为6.96 g/L(图1-c)。复合氮源质量浓度为25 g/L时,菌体最大产量为7.27 g/L(图1-d)。

a-碳源种类优化;b-氮源种类优化;c-复合碳源浓度优化;d-复合氮源浓度优化
图1 不同碳源和氮源对植物乳杆菌生物量的影响
Fig.1 Effect of different carbon sources and nitrogen source on the biomass of L.plantarum
2.2 缓冲盐优化
乳酸菌在生长过程中代谢碳水化合物生成乳酸,降低培养基pH,抑制菌体生长[23]。在培养基中添加缓冲盐可以在一定程度上平衡pH,降低对菌体的抑制作用。本研究对不同浓度的缓冲盐体系进行了研究,如表2所示,菌体量最初随着缓冲盐浓度的增加而增加,但随后随着缓冲盐浓度的增加而减少。可以看到,当柠檬酸二铵质量浓度为25 g/L时,培养基缓冲效果最好,菌体生物量可达6.46 g/L。
表2 不同缓冲盐体系对菌体生物量的影响
Table 2 Effects of different buffer systems on bacterial biomass
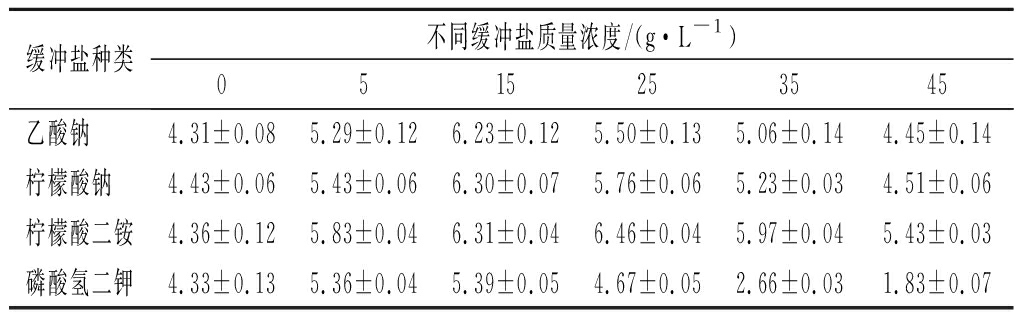
缓冲盐种类不同缓冲盐质量浓度/(g·L-1)0515253545乙酸钠 4.31±0.085.29±0.126.23±0.125.50±0.135.06±0.144.45±0.14柠檬酸钠 4.43±0.065.43±0.066.30±0.075.76±0.065.23±0.034.51±0.06柠檬酸二铵4.36±0.125.83±0.046.31±0.046.46±0.045.97±0.045.43±0.03磷酸氢二钾4.33±0.135.36±0.045.39±0.054.67±0.052.66±0.031.83±0.07
2.3 ANN模型建立
本研究以CCD试验设计数据作为训练样本,采用BP神经网络建立ANN模型(表3)。对于构建神经网络模型,首先需要确定最优网络结构。如图2-a和图2-b所示,单隐藏层神经元数量为9时,MSE最小为0.009 1。双隐藏层神经元数量为7和9时,MSE最小为0.024 2。如图2-c所示,单隐藏层模型相较于双隐藏层模型对生物量拟合效果更好,MSE分别为0.009 4 和0.161 7。因此,本研究选用单隐藏层网络结构模型进行预测,结构模型如图3-a所示。人工神经网络在优化发酵条件方面取得了很大的成功,YANG等[15]利用ANN建立协同发酵法以提高真菌蒽醌产量和抗氧化活性,蒽醌产量提高至2.11%。
2.4 GA优化
将神经网络模型作为GA的适应度函数,采用GA求解器进行过程优化,从而得到问题最优解[24]。如图3-b所示,经过369次迭代,确定了植物乳杆菌生物量最佳输入条件,分别是复合碳源36.64 g/L、复合氮源47.83 g/L和柠檬酸二铵33.27 g/L,输出值为11.02 g/L。同时,在最优条件下的生物量实验值为10.86 g/L,误差值<2%。实验值与预测值吻合较好,说明所生成的ANN-GA模型具有良好的预测和优化能力。
表3 BP神经网络训练样本试验设计
Table 3 Training experimental design for BP neural network
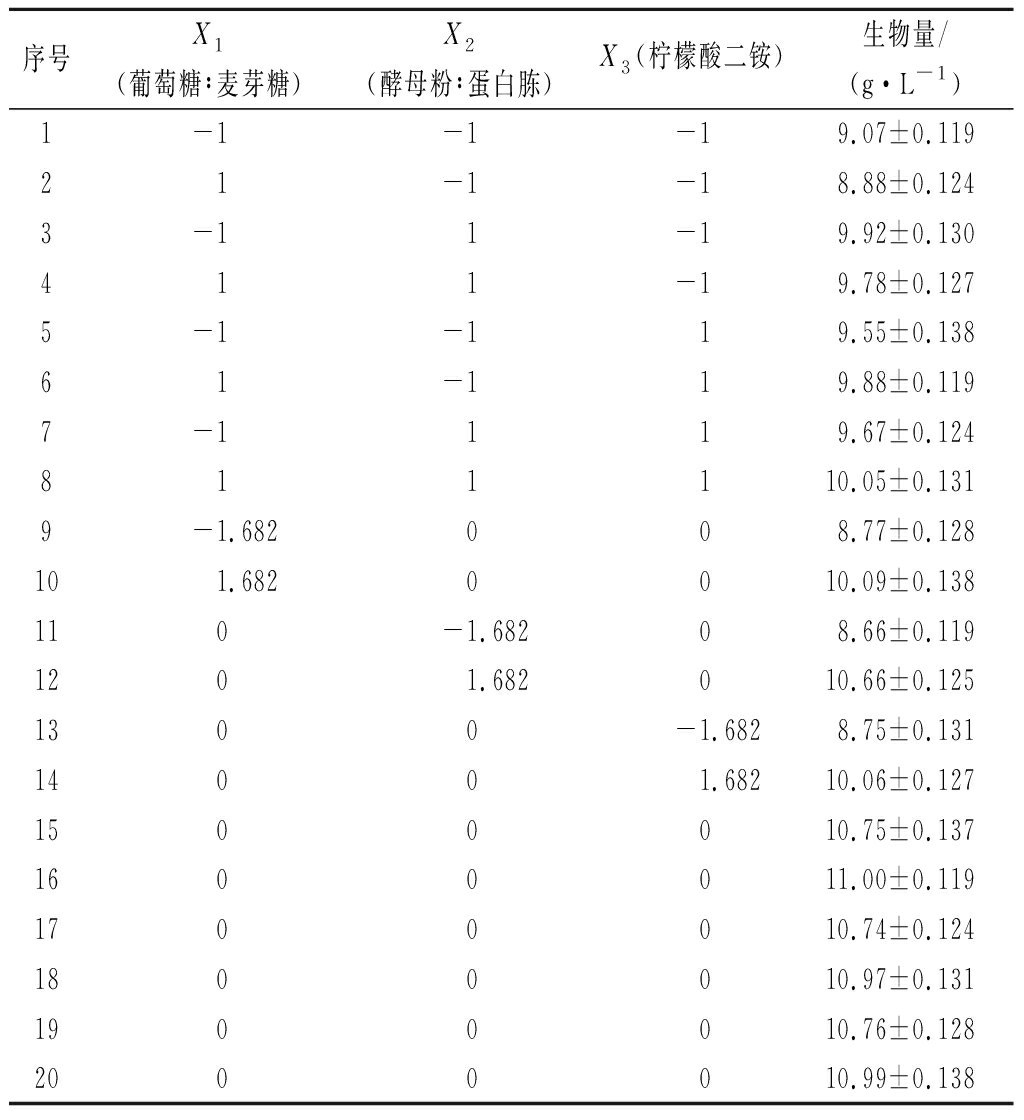
序号X1(葡萄糖∶麦芽糖)X2(酵母粉∶蛋白胨)X3(柠檬酸二铵)生物量/(g·L-1)1-1-1-19.07±0.11921-1-18.88±0.1243-11-19.92±0.130411-19.78±0.1275-1-119.55±0.13861-119.88±0.1197-1119.67±0.124811110.05±0.1319-1.682008.77±0.128101.6820010.09±0.138110-1.68208.66±0.1191201.682010.66±0.1251300-1.6828.75±0.13114001.68210.06±0.1271500010.75±0.1371600011.00±0.1191700010.74±0.1241800010.97±0.1311900010.76±0.1282000010.99±0.138

a-单隐藏层BP神经网络MSE;b-双隐藏层BP神经网络MSE;c-单隐藏层和双隐藏层BP神经网络实际值与预测值比较
图2 ANN模型的确定
Fig.2 Determination of the ANN model
2.5 发酵动力学
2.5.1 发酵过程动力学曲线
如图3-c所示为植物乳杆菌在优化培养基条件下细胞干重、残糖量、pH和乳酸变化情况。在0~4 h,菌体处于滞后期,由于初始糖含量较高,基质消耗多用于菌体自身的乳酸代谢,导致细胞干重变化较小,发酵液pH迅速降至4.3左右。在4~20 h,菌体处于对数生长期,糖含量降至4 g/L,pH为3.7左右,细胞干重迅速增加。在培养30 h后,获得最大细胞干重10.59 g/L,乳酸含量增至19.78 g/L。可以看到,发酵液乳酸含量和残糖量在16 h趋于稳定,菌体对数期最大生长速率为0.292 g/(L·h)。

a-BP神经网络结构模型;b-GA最优值和平均值迭代优化;c-发酵动力学曲线
图3 ANN-GA模型优化和发酵动力学曲线
Fig.3 ANN-GA model optimization and fermentation kinetic curve
2.5.2 动力学参数拟合
根据公式(2),对生物量进行模型拟合,得到生长动力学拟合参数X0=0.512 g/L,Xm=10.63 g/L,μm=0.292 g/(L·h)。如图4-a所示,R2=0.995,说明Logistic方程可以很好地描述该发酵条件下的生长动力学。乳酸菌发酵通过碳水化合物代谢产生乳酸作为主要的代谢最终产物,乳酸的积累是抑制其自身生长的主要因素[25]。根据公式(3),对产物生成进行模型拟合,得到产物生成动力学拟合参数P0=0.51 g/L,Pm=19.768 g/L,μm1=0.394 g/(L·h)。如图4-b所示,R2=0.998,说明该方程对植物乳杆菌产物生成拟合较好。在微生物发酵过程中,底物主要转化为细胞质量、产物和维持细胞基本生命活动[26]。根据公式(4),对底物消耗进行模型拟合,得到底物消耗动力学拟合参数S0=33.967 g/L,YX/S=1.755 g/g,YP/S=0.753 g/g。如图4-c所示,R2=0.993,Luedeking-Piret方程很好地描述了底物消耗动力学。
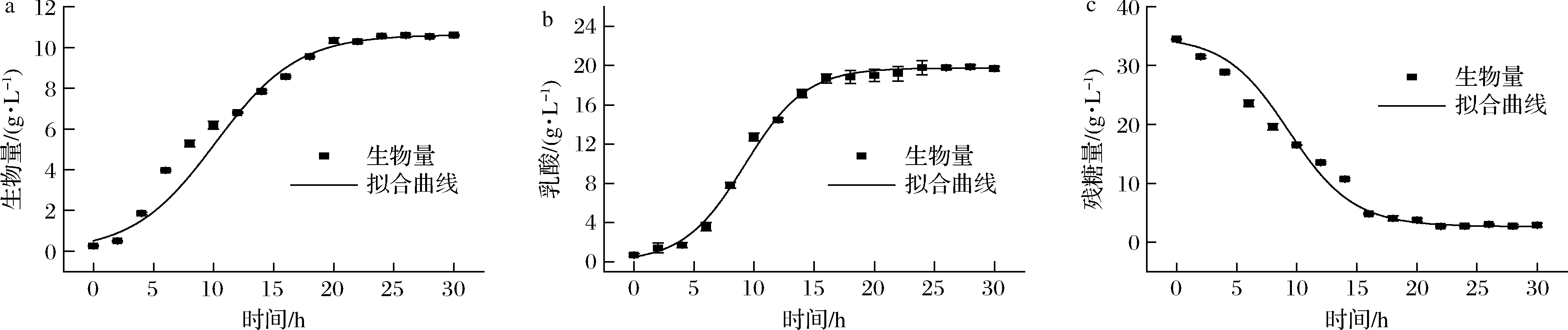
a-生长动力学模型拟合曲线;b-产物生成动力学模型拟合曲线;c-底物消耗动力学模型拟合曲线
图4 植物乳杆菌发酵动力学方程拟合
Fig.4 Fermentation kinetic equation fitting of L.plantarum
2.6 发酵条件优化
采用单因素试验研究了不同初始pH、发酵温度和接种量对植物乳杆菌生物量的影响。由图5-a可知,在优化的培养基条件下,培养温度为35 ℃时,植物乳杆菌生长效果最好。在35 ℃时,初始pH和接种量对植物乳杆菌生长影响显著。最适初始pH因菌种而异,乳酸菌有一定的耐酸性,如图5-b所示,在pH=5.0时有最高的菌体量,随着培养基初始pH升高,菌体生长也受到抑制。此外,接种量也是乳酸菌生长的重要参数,在培养过程中应保持在最优值。由图5-c可知,接种量为4%时,菌体生长效果最好。当接种量为5%时,菌体量下降,可能是由于接种量过大,培养基营养物质不能满足大量菌体同时生长。
2.7 分批补料优化
分批补料是发酵工业中提高生产效率最常用的方法之一。菌体在对数生长后期,对碳源的需求量较大,可通过补加碳源以提高产量。乳酸菌在生长过程中产生有机酸快速降低环境pH,影响细胞膜渗透性和胞内酶活性,流加中和剂以保持良好的发酵条件[19]。如图6-a所示,NH3·H2O能显著提高培养液中的菌体密度。本实验研究了补加不同浓度的葡萄糖对乳酸菌生物量的影响,由图6-b可知,过高的葡萄糖导致菌体产量降低,当补加葡萄糖至30 g/L时,菌体生物量最大。同时对葡萄糖补料次数进行了优化,如图6-c所示,单次补料生物量最大。可以发现,多次补加葡萄糖并未增加菌体生物量,一些研究表明,在分批补料发酵过程中,乳酸菌菌体产量主要受到产物抑制的影响[27]。因此,植物乳杆菌通过补入葡萄糖和NH3·H2O,菌体干重可达12.64 g/L。如图6-d所示,分批补料的菌体产量较未补料提高了19%。

a-不同温度对植物乳杆菌生物量的影响;b-不同初始pH对植物乳杆菌生物量的影响;c-不同接种量对植物乳杆菌生物量的影响
图5 植物乳杆菌发酵条件优化
Fig.5 Optimization of fermentation conditions for L.plantarum

a-不同中和剂对生物量的影响;b-葡萄糖浓度优化;c-补料方式对生物量的影响;d-补料培养对生长曲线的影响
图6 植物乳杆菌分批补料优化
Fig.6 Fed-batch optimization of L.plantarum
3 结论
本实验对植物乳杆菌发酵动力学及高密度培养进行了研究,通过单因素试验,确定植物乳杆菌生长所需的最佳碳源、氮源和缓冲盐,利用ANN-GA模型对培养基组成进行了优化,培养基优化结果为碳源36.64 g/L(葡萄糖-麦芽糖质量比2∶3)、氮源47.83 g/L(酵母粉-蛋白胨质量比1∶1)、柠檬酸二铵33.27 g/L。根据植物乳杆菌发酵动力学曲线得到菌体生长、产物生成及底物消耗动力学模型参数,相关系数R2分别为0.995、0.996、0.996,表明建立的模型能很好地预测植物乳杆菌发酵过程变化情况。基于最佳培养基配方,对植物乳杆菌培养条件和补料方式进行了优化,菌体产量可达12.64 g/L。
[1] MELGAR-LALANNE G, RIVERA-ESPINOZ Y, HERN NDEZ-S
NDEZ-S NCHEZ H.Lactobacillus plantarum:An overview with emphasis in biochemical and healthy properties[J].Lactobacillus:Classification, Uses and Health Implications, 2012:1-31.
NCHEZ H.Lactobacillus plantarum:An overview with emphasis in biochemical and healthy properties[J].Lactobacillus:Classification, Uses and Health Implications, 2012:1-31.
[2] ISLAM M Z, UDDIN M E, RAHMAN M T, et al.Isolation and characterization of dominant lactic acid bacteria from raw goat milk:Assessment of probiotic potential and technological properties[J].Small Ruminant Research, 2021, 205:106532.
[3] XIA A N, MENG X S, TANG X J, et al.Probiotic and related properties of a novel lactic acid bacteria strain isolated from fermented rose jam[J].LWT, 2021, 136:110327.
[4] QIN X S, GAO Q Y, LUO Z G.Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles[J].Food Hydrocolloids, 2021, 116:106658.
[5] DONG Z X, GU L, ZHANG J, et al.Optimisation for high cell density cultivation of Lactobacillus salivarius BBE 09-18 with response surface methodology[J].International Dairy Journal, 2014, 34(2):230-236.
[6] RABIYA R, SEN R.Artificial intelligence driven advanced optimization strategy vis-à-vis response surface optimization of production medium:Bacterial exopolysaccharide production as a case-study[J].Biochemical Engineering Journal, 2022, 178:108271.
[7] HOSU A, CRISTEA V M, CIMPOIU C.Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines:Prediction of antioxidant activities and classification of wines using artificial neural networks[J].Food Chemistry, 2014, 150:113-118.
[8] AUNG T, KIM S J, EUN J B.A hybrid RSM-ANN-GA approach on optimisation of extraction conditions for bioactive component-rich laver (Porphyra dentata) extract[J].Food Chemistry, 2022, 366:130689.
[9] YAHYA H S M, ABBAS T, AMIN N A S.Optimization of hydrogen production via toluene steam reforming over Ni-Co supported modified-activated carbon using ANN coupled GA and RSM[J].International Journal of Hydrogen Energy, 2021, 46(48):24 632-24 651.
[10] SIROHI R, PANDEY J P, TARAFDAR A, et al.Tailoring a hybrid intelligent model to predict fermentable sugar production from enzyme-catalyzed hydrolysis of damaged wheat grains[J].Food Bioscience, 2021, 43:101299.
[11] MU W M, LIU F L, JIA J H, et al.3-Phenyllactic acid production by substrate feeding and pH-control in fed-batch fermentation of Lactobacillus sp.SK007[J].Bioresource Technology, 2009, 100(21):5 226-5 229.
[12] SHARMA V, MISHRA H N.Unstructured kinetic modeling of growth and lactic acid production by Lactobacillus plantarum NCDC 414 during fermentation of vegetable juices[J].LWT-Food Science and Technology, 2014, 59(2):1 123-1 128.
[13] YANG X Z, HU W Z, JIANG A L, et al.Effect of salt concentration on quality of Chinese northeast sauerkraut fermented by Leuconostoc mesenteroides and Lactobacillus plantarum[J].Food Bioscience, 2019, 30:100421.
[14] BORSHCHEVSKAYA L N, GORDEEVA T L, KALININA A N, et al.Spectrophotometric determination of lactic acid[J].Journal of Analytical Chemistry, 2016, 71(8):755-758.
[15] YANG J, HUANG Y, XU H Y, et al.Optimization of fungi co-fermentation for improving anthraquinone contents and antioxidant activity using artificial neural networks[J].Food Chemistry, 2020, 313:126138.
[16] YANG Y, GAO M, YU X D, et al.Optimization of medium composition for two-step fermentation of vitamin C based on artificial neural network-genetic algorithm techniques[J].Biotechnology & Biotechnological Equipment, 2015, 29(6):1 128-1 134.
[17] KHEZRI V, YASARI E, PANAHI M, et al.Hybrid artificial neural network-genetic algorithm-based technique to optimize a steady-state gas-to-liquids plant[J].Industrial & Engineering Chemistry Research, 2020, 59(18):8 674-8 687.
[18] PHUKOETPHIM N, SALAKKAM A, LAOPAIBOON P, et al.Kinetic models for batch ethanol production from sweet sorghum juice under normal and high gravity fermentations:Logistic and modified Gompertz models[J].Journal of Biotechnology, 2017, 243:69-75.
[19] WANG T, LU Y Y, YAN H, et al.Fermentation optimization and kinetic model for high cell density culture of a probiotic microorganism:Lactobacillus rhamnosus LS-8[J].Bioprocess Biosystems Engineering, 2020, 43(3):515-528.
[20] MEBANE W R Jr, SEKHON J S.Genetic optimization using derivatives:The rgenoud package for R[J].Journal of Statistical Software, 2011, 42(11):1-26.
[21] BECK M W.NeuralNetTools:Visualization and analysis tools for neural networks[J].Journal of Statistical Software, 2018,85(11):1-20.
[22] GIVRY S, DUCHIRO F.Optimization of culture medium and growth conditions for production of L-arabinose isomerase and D-xylose isomerase by Lactobacillus bifermentans[J].Mikrobiologiia, 2008, 77(3):324-330.
[23] CUI S M, ZHAO J X, ZHANG H, et al.High-density culture of Lactobacillus plantarum coupled with a lactic acid removal system with anion-exchange resins[J].Biochemical Engineering Journal, 2016, 115:80-84.
[24] ZHENG Z Y, GUO X N, ZHU K X, et al.Artificial neural network-genetic algorithm to optimize wheat germ fermentation condition:Application to the production of two anti-tumor benzoquinones[J].Food Chemistry, 2017, 227:264-270.
[25] OTHMAN M, ARIFF A B, RIOS-SOLIS L, et al.Extractive fermentation of lactic acid in lactic acid bacteria cultivation:A review[J].Frontiers in Microbiology, 2017, 8:2285.
[26] THAKUR A, PANESAR P S, SAINI M S.Optimization of process parameters and estimation of kinetic parameters for lactic acid production by Lactobacillus casei MTCC 1423[J].Biomass Conversion and Biorefinery, 2019, 9(2):253-266.
[27] OTHMAN M, ARIFF A B, WASOH H, et al.Strategies for improving production performance of probiotic Pediococcus acidilactici viable cell by overcoming lactic acid inhibition[J].AMB Express, 2017, 7(1):1-14.