果梅为蔷薇科植物梅Prunus mume Sieb.et Zucc.的干燥近成熟果实,原产于川、鄂山区[1]。目前,福建、云南、四川、安徽、江苏和浙江等地均有栽培[2]。果梅酸甜、肉质饱满,具有较高的营养价值,富含有机酸、氨基酸、糖类和黄酮等[3-4], 具有抗菌[5]、抗氧化[6]、抗病毒[7]等生物活性,在医药和食品方面用途广泛。且果梅中有机酸类(柠檬酸、苹果酸、绿原酸等)被认为是一类主要的消炎和抗菌的化合物。芹菜素、槲皮素、芦丁和山奈酚等黄酮类化合物中,对于恶性细胞的增殖具有良好的抑制作用。
果梅传统的处理方法用松木熏蒸,在60~80 ℃的温度下熏蒸2~3 d。然而,传统的熏蒸与高时间和人工成本有关,在一些生产地区采用热风干燥方法进行低温干燥。研究发现,热风干燥与松木熏蒸后果实中的颜色和活性成分不同[8]。因此,考察不同的干燥条件对果梅中成分的影响对于指导果梅加工有重要意义。
目前,生物活性成分通常使用高效液相色谱[9]进行分析。然而,在运用高效液相色谱时,多组分含量测定不够准确[10-11]。相比之下,液相色谱-质谱联用技术集成了液相色谱的分离能力和质谱的高灵敏度和选择性,使该方法比高相液相色谱更适合用于与食品相关的多组分复杂体系的分离和分析。因此,建立的超高效液相色谱-串联质谱法(ultra performance liquid chromatography-tandem mass spectrometry, UPLC-MS/MS)方法用于检测不同干燥果梅中16种活性成分,并构建了综合评价模型,旨在为采后果梅果实品质研究提供参考。
1 材料与方法
1.1 材料与试剂
枸橼酸、苹果酸、咖啡酸、富马酸、绿原酸、原儿茶酸、琥珀酸、奎宁酸、芦丁、山奈酚、熊果酸、苦杏仁苷、芹菜素、槲皮素、5-羟甲基糠醛,纯度均≥98%,上海元业生物技术有限公司;甲醇、乙腈,均为质谱级,德国Merck公司。
本研究收集了四川和福建2个产地的样品,详细样品信息见表1。
表1 样品采集信息表
Table 1 Sample collection information table

编号名称加工方式S1果梅熏制S2果梅熏制S3果梅熏制S4果梅熏制S5果梅熏制S6果梅熏制S7果梅热风干燥S8果梅热风干燥S9果梅热风干燥S10果梅热风干燥S11果梅热风干燥S12果梅热风干燥
1.2 仪器与设备
QUAD-4500三重四极杆线性离子阱质谱仪,美国ABSCIEX公司;ZORBAX SB-C18(3.0 mm×100 mm,3.5 μm),Millipore Q超纯水系统,Millipore公司;十万分之一电子天平,瑞士METTLER公司;JK-5200B超声清洗器,合肥机械制造有限公司。
1.3 实验方法
1.3.1 样品的制备
精密称定样品粉末(1 g),置入50 mL离心管,加入25 mL体积分数为80%的甲醇,称重,超声30 min,放冷,补重,离心,过0.22 μm滤膜,取滤液、即得。
1.3.2 对照品溶液的制备
精密称取苯甲酸、槲皮素、咖啡酸、富马酸、绿原酸、原儿茶酸、琥珀酸、奎宁酸、芦丁、枸橼酸、苹果酸、山奈酚、熊果酸、苦杏仁苷、芹菜素、5-羟甲基糠醛对照品适量,分别置于25 mL容量瓶中,加入超纯水配制成0.11、0.12、1.24、2.66、1.25、1.35、2.47、2.65、1.39、12.37、6.18、0.43、2.21、0.65、2.58、0.97 mg/mL 的对照品储备液。
1.3.3 UPLC-MS/MS条件
流动相条件:采用ZORBAX SB-C18(3.0 mm×100 mm,3.5 μm)色谱柱。流动相:水(含0.2%体积分数甲酸)(A)和乙腈(含体积分数0.2%甲酸)(B)。梯度洗脱:0~3 min, 10%~30% A;3~7 min, 30%~50% A;7~10 min, 50%~80% A;10~14.5 min,80%~10% A;14.5~17 min, 10% A。柱温30 ℃,流速0.2 mL/min,进样量1 μL。
质谱条件:采用电喷雾离子源( electrospray ionization, ESI),采取多反应监测(multiple reaction monitoring,MRM)检测,离子化温度550 ℃,喷雾电压5.5 kV,离子化温度550 ℃,Gas1为379.2 kPa(55 psi),Gas2为379.2 kPa(55 psi),气帘气(N2)241.3 kPa(35 psi)。监测16种成分离子对,去簇电压(declustering potential,DP)和碰撞能量(collision energy,CE)见表2。被测成分的提取MRM模式色谱图见图1。
表2 化合物的保留时间和相关质谱(MS)数据
Table 2 Retention times and related mass spectrometry (MS) data of the target compounds

No.名称 分子式 保留时间/[M+H]+[M-H]-MRMminm/zm/z定量离子对定性离子对DP/VCE/V1苯甲酸C6H6O310.43120.92120.5-76.9120.5-76.9、120.5-59.1-56-162槲皮素C21H20O119.79447.81447.2-299.8447.2-299.8、447.2-165.3-145-353咖啡酸C9H8O49.74178.53178.5-134.6178.5-134.6、178.5-117.4-68-224富马酸C4H4O47.22115.01114.5-70.9114.5-70.9、114.5-87.6-57-135绿原酸C16H18O96.02353.11352.8-190.7352.8-179.2、352.8-299.4-79-206原儿茶酸C7H6O46.27152.95152.6-108.7152.6-108.7、152.6-135.31-59-227琥珀酸C4H6O44.87116.82116.3-72.8116.3-72.8、116.3-99.1-28-178奎尼酸C7H12O65.47190.52190.5-85.0190.5-85.0、190.52-135.4-60-279芦丁C27H30O168.87610.55609.0-300.6609.0-300.6、609.0-463.2-162-4810枸橼酸C6H8O76.82190.32190.3-74.8190.3-74.8、190.3-172.2-202-2511苹果酸C4H6O56.03132.93132.5-114.0132.5-114.0、132.5-78.5-69-1312山奈酚C15H10O613.28283.85282.9-246.8282.9-246.8、282.9-255.4-120-3513熊果酸C30H48O310.89457.37457.3-411.2457.3-411.2、457.3-351.61164014苦杏仁苷C20H27NO116.45458.41458.3-163.0458.3-163.0、458.3-145.5502015芹菜素C15H10O512.76274.59274.0-87.9274.0-87.9、274.0-115.47932165-羟甲基糠醛C6H6O36.66109.0109.0-53.0109.0-53.0、109.0-78.18721
1.3.4 检测限、定量限和线性关系考察
取“1.3.2”项下不同浓度的对照品浓度及混合对照品储备液1 μL,梯度稀释。按照“1.3.3”项下色谱-质谱条件进样测定,平行3次,以质量浓度X(μg/mL)为横坐标与峰面积(y)绘制标准曲线,得出回归方程,线性范围,相关系数(r)。以信噪比(S/N)约为3计算各成分的检测限(limit of detection,LOD),以信噪比(S/N)约为10,计算各成分的定量限(limit of quantitation,LOQ)。结果见表3,表明16种成分在各自的线性范围内关系良好。
1.3.5 方法学考察
精密度试验:精密吸取混合对照品溶液1 μL,进样6次,通过测定各对照品的峰面积并计算16种对照品峰面积的相对标准偏差(relative standard deviation,RSD)。
重复性试验:取S1样品6份,每份1.0 g,按照“1.3.1”项下方法制备供试品溶液,进样测定,计算16种目标成分质量分数的RSD。
稳定性试验:取S1样品的溶液,分别在0、2、4、8、12、24 h时,进样测定,计算16种目标成分峰面积的RSD。

图1 十六种组分的MRM色谱图
Fig.1 MRM chromatogram of 16 components
表3 十六种化学成分的线性关系考察
Table 3 linear relationship of 16 chemical components
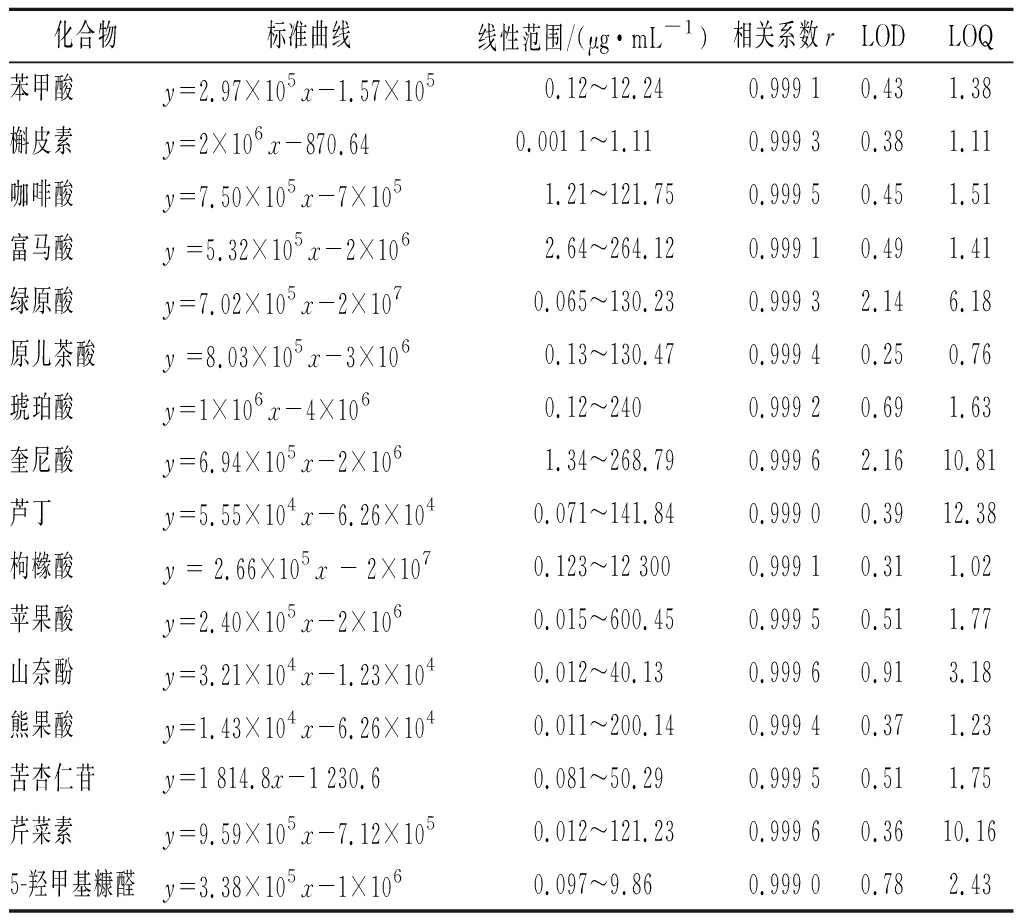
化合物标准曲线线性范围/(μg·mL-1)相关系数rLODLOQ苯甲酸y=2.97×105x-1.57×1050.12~12.240.999 10.431.38槲皮素y=2×106x-870.640.001 1~1.110.999 30.381.11咖啡酸y=7.50×105x-7×1051.21~121.750.999 50.451.51富马酸y =5.32×105x-2×1062.64~264.120.999 10.491.41绿原酸y=7.02×105x-2×1070.065~130.230.999 32.146.18原儿茶酸y =8.03×105x-3×1060.13~130.470.999 40.250.76琥珀酸y=1×106x-4×1060.12~2400.999 20.691.63奎尼酸y=6.94×105x-2×1061.34~268.790.999 62.1610.81芦丁y=5.55×104x-6.26×1040.071~141.840.999 00.3912.38枸橼酸y = 2.66×105x - 2×1070.123~12 3000.999 10.311.02苹果酸y=2.40×105x-2×1060.015~600.450.999 50.511.77山奈酚y=3.21×104x-1.23×1040.012~40.130.999 60.913.18熊果酸y=1.43×104x-6.26×1040.011~200.140.999 40.371.23苦杏仁苷y=1 814.8x-1 230.60.081~50.290.999 50.511.75芹菜素y=9.59×105x-7.12×1050.012~121.230.999 60.3610.165-羟甲基糠醛y=3.38×105x-1×1060.097~9.860.999 00.782.43
加样回收率试验:取S1样品6份,每份1.0 g,分别加入与1.0 g样品中各待测成分含量相当的对照品,照“1.3.1”项下方法制备供试溶液,进样。
基质效应(matrix effect, ME):精密称取已知含量的果梅内容物约4.0 g,共15份,按1.3.2项下方法配制的对照品溶液吸取1 μL稀释成3个浓度水平的对照品溶液,配制成加标溶液,每个浓度水平5份,作为A样, 测得含量为A。未添加对照品溶液的已知含量的果梅为B样, 测得含量为B。空白对照品溶液为C样, 测得含量为C。按公式(1)计算基质效应[11]:
(5)
考察结果见表4,各成分的日内和日间精密度、稳定性和重复性分别为1.28%~2.79%, 1.78%~2.79%, 1.41%~2.96%和1.74%~2.89%,平均加样回收率为95%~104%,RSD为2.01%~3.69%,表明该方法良好。在本次实验中,16种成分分离良好, 基质效应为98.5%~102.15%, 基质成分不干扰16种成分的测定。可能是由于待测组分与样品中的基质成分在雾滴表面离子化过程不存在竞争,所以基质对于所测定成分的定量影响并不大,因此可以不考虑基质效应对本方法定量的影响[12]。
1.3.6 样品测定
按照“1.3.1”项下方法制备供试品溶液,按照“1.3.3”项下色谱-质谱条件进样分析,测定相应的峰面积,根据对应的标准曲线计算出样品中16种化学成分的含量,结果见表5。
表4 十六种化学成分的方法学考察
Table 4 Methodology investigation of 16 chemical constituents
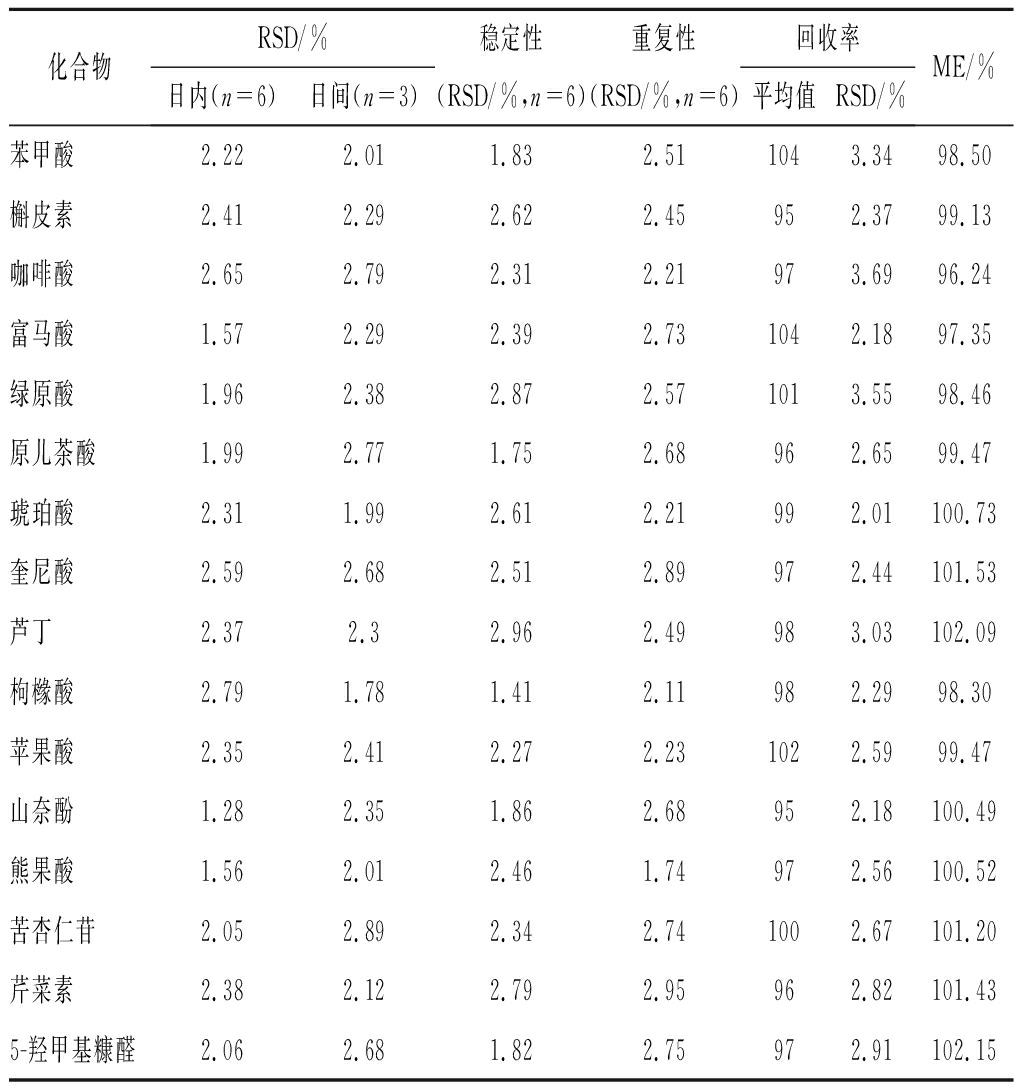
化合物RSD/%稳定性重复性回收率日内(n=6)日间(n=3)(RSD/%,n=6)(RSD/%,n=6)平均值RSD/%ME/%苯甲酸2.222.011.832.511043.3498.50槲皮素2.412.292.622.45952.3799.13咖啡酸2.652.792.312.21973.6996.24富马酸1.572.292.392.731042.1897.35绿原酸1.962.382.872.571013.5598.46原儿茶酸1.992.771.752.68962.6599.47琥珀酸2.311.992.612.21992.01100.73奎尼酸2.592.682.512.89972.44101.53芦丁2.372.32.962.49983.03102.09枸橼酸2.791.781.412.11982.2998.30 苹果酸2.352.412.272.231022.5999.47山奈酚1.282.351.862.68952.18100.49熊果酸1.562.012.461.74972.56100.52苦杏仁苷2.052.892.342.741002.67101.20芹菜素2.382.122.792.95962.82101.435-羟甲基糠醛2.062.681.822.75972.91102.15
2 结果与分析
2.1 质谱和液相条件
采用全扫描质谱方法以2种ESI+/-模式检查目标化合物,以获得最佳的质谱条件。结果表明,熊果酸、苦杏仁苷、芹菜素和5-羟甲基糠醛标准品在正离子模式下的灵敏度和强度高于负离子模式,其余标准品在负离子模式下较好。根据质谱的稳定性和离子响应,自动选择了2个前体/产物对,在MRM模式下进行进一步分析,以提高分析的选择性和灵敏度。
2.2 含测结果
本节建立UPLC-MS/MS技术同时测定12份果梅样品的16种成分(10个有机酸类、4个黄酮类、苦杏仁苷和5-羟甲基糠醛),结果表明样品中有机酸组成和含量有一定差异,枸橼酸含量为13.28%~16.85%,均高于药典标准(含量不得少于12%),苹果酸、富马酸、奎尼酸、琥珀酸含量次之,原儿茶酸和苯甲酸含量较低。在黄酮类化合物中以芹菜素含量最高,槲皮素含量最低。此外,果梅中还测定了苦杏仁苷和5-羟甲基糠醛,含量分别为1.59%和1.60%。
表5 样品含量测定结果 单位:μg/g
Table 5 Samples content determination results
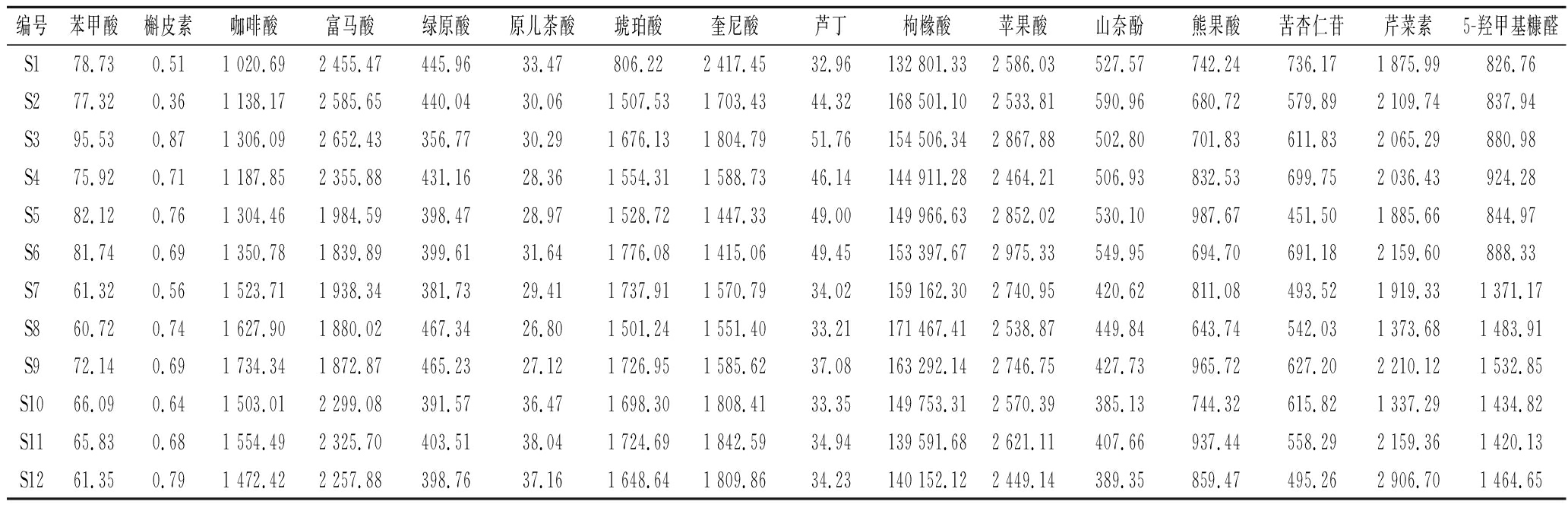
编号苯甲酸槲皮素咖啡酸富马酸绿原酸原儿茶酸琥珀酸奎尼酸芦丁枸橼酸苹果酸山奈酚熊果酸苦杏仁苷芹菜素5-羟甲基糠醛S178.730.511 020.692 455.47445.9633.47806.222 417.4532.96132 801.332 586.03527.57742.24736.171 875.99826.76 S277.320.361 138.172 585.65440.0430.061 507.531 703.4344.32168 501.102 533.81590.96680.72579.892 109.74837.94 S395.530.871 306.092 652.43356.7730.291 676.131 804.7951.76154 506.342 867.88502.80701.83611.832 065.29880.98 S475.920.711 187.852 355.88431.1628.361 554.311 588.7346.14144 911.282 464.21506.93832.53699.752 036.43924.28 S582.120.761 304.461 984.59398.4728.971 528.721 447.3349.00149 966.632 852.02530.10987.67451.501 885.66844.97 S681.740.691 350.781 839.89399.6131.641 776.081 415.0649.45153 397.672 975.33549.95694.70691.182 159.60888.33 S761.320.561 523.711 938.34381.7329.411 737.911 570.7934.02159 162.302 740.95420.62811.08493.521 919.331 371.17 S860.720.741 627.901 880.02467.3426.801 501.241 551.4033.21171 467.412 538.87449.84643.74542.031 373.681 483.91 S972.140.691 734.341 872.87465.2327.121 726.951 585.6237.08163 292.142 746.75427.73965.72627.202 210.121 532.85 S1066.090.641 503.012 299.08391.5736.471 698.301 808.4133.35149 753.312 570.39385.13744.32615.821 337.291 434.82 S1165.830.681 554.492 325.70403.5138.041 724.691 842.5934.94139 591.682 621.11407.66937.44558.292 159.361 420.13 S1261.350.791 472.422 257.88398.7637.161 648.641 809.8634.23140 152.122 449.14389.35859.47495.262 906.701 464.65
2.3 主成分分析及综合评价
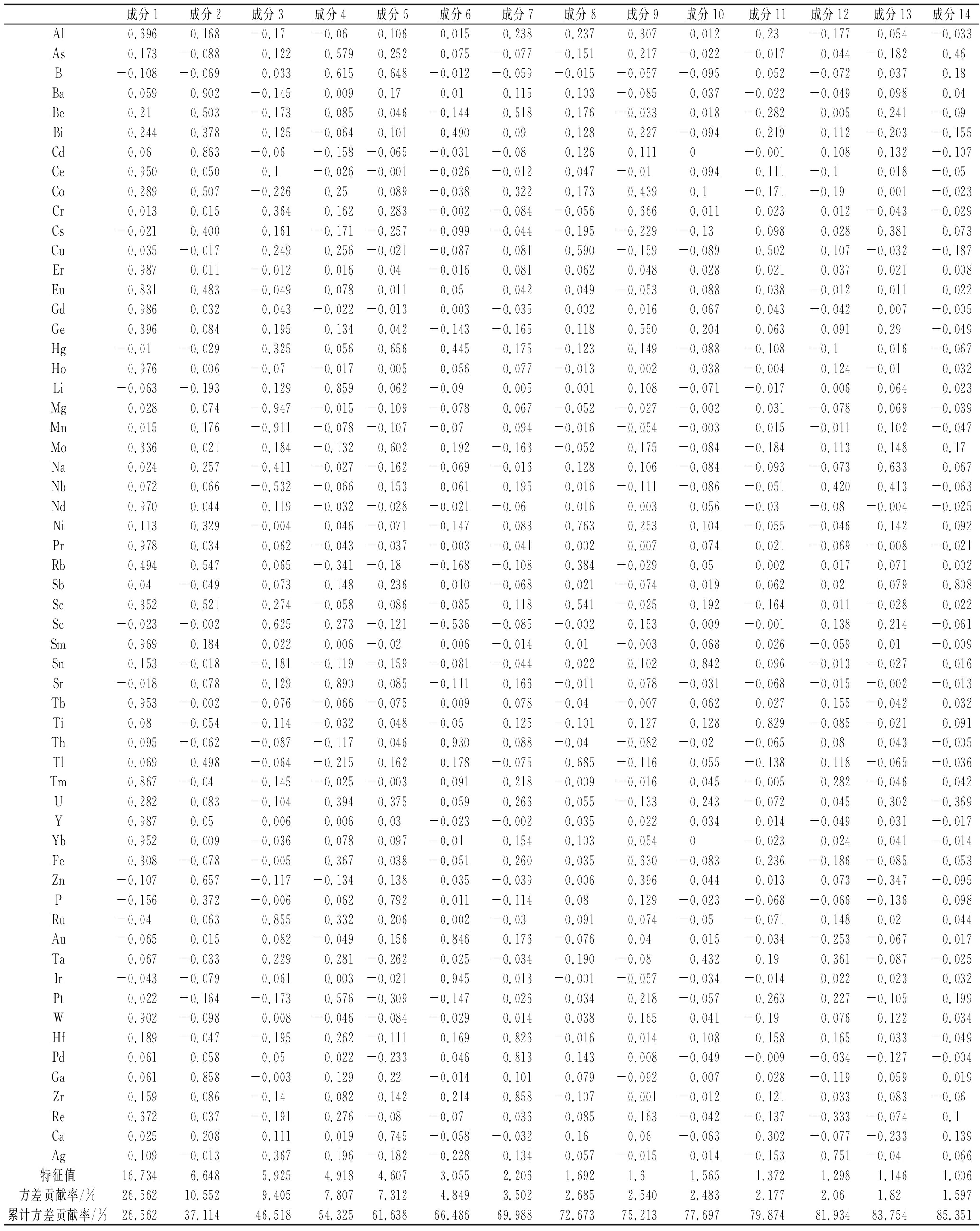
将12份样品中测定的含量导入SPSS 24.0软件,进行主成分分析,根据特征值大于1的原则,筛选出5个主成分,总贡献率达到84.38%。结果见表6。采用SPSS 24.0进行主成分分析时,得到5个主成分因子,分别为F1、F2、F3、F4、F5。采用综合评价函数为F=0.307 9F1+0.228 8F2+0.165 0F3+0.076 9F4+0.065 8F5,得到果梅样品总因子得分值F及排名,具体见表7。由表7可知,熏制的果梅样品综合品质表较好。
表6 主成分分析特征值及其贡献率
Table 6 Principal component analysis eigenvalues and their contribution rate

主成分特征值方差贡献率/%累计方差贡献率/%14.9230.7930.7923.6622.8853.6132.6416.5070.1141.237.6977.8051.056.5884.38
表7 果梅样品综合评价结果
Table 7 Comprehensive evaluation results of the samples
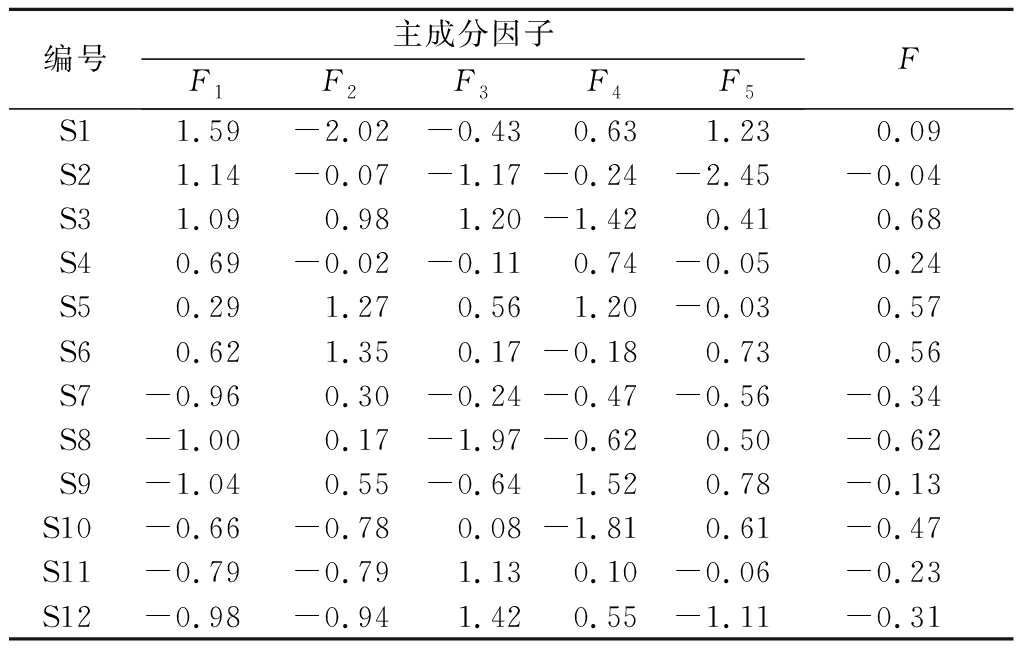
编号主成分因子F1F2F3F4F5FS11.59-2.02-0.430.631.230.09S21.14-0.07-1.17-0.24-2.45-0.04S31.090.981.20-1.420.410.68S40.69-0.02-0.110.74-0.050.24S50.291.270.561.20-0.030.57S60.621.350.17-0.180.730.56S7-0.960.30-0.24-0.47-0.56-0.34S8-1.000.17-1.97-0.620.50-0.62S9-1.040.55-0.641.520.78-0.13S10-0.66-0.780.08-1.810.61-0.47S11-0.79-0.791.130.10-0.06-0.23S12-0.98-0.941.420.55-1.11-0.31
3 讨论与结论
为了确定最佳的提取条件,根据课题组前期条件[11],选择了3个因素,并对每个组分的性质进行了研究和优化:提取溶剂(水,体积分数50%甲醇,体积分数80%甲醇)、溶剂与样品的比例(15∶1、25∶1和35∶1,体积比)、提取时间(20、30、40 min)对提取效率的影响。结果表明,使用80%甲醇, 溶剂与样品的比例为25∶1,超声时间为30 min时提取效率最优。在初步实验中研究了色谱条件,如柱型、流动相组成和pH,以获得理想的色谱剖面,并优化了保留时间和峰形,没有明显的峰尾。比较了2种柱型,BEH C18柱(100 mm×2.1 mm,1.7 μm)和ZORBAX SB-C18(3.0 mm×100 mm,3.5 μm)色谱柱,以优化目标分析物的保留时间。结果表明,在相同条件下,ZORBAX SB-C18色谱柱对所有分析物的保留能力强、分辨率好、分析时间短。至于流动相,与甲醇相比,乙腈具有更好的洗脱能力、分离选择性和峰形。本文考察比较了乙腈/水和甲醇/水2种流动相组成中,相比之下,在乙腈(含体积分数0.2%甲酸)/0.2%甲酸水流动相体系下,能在最短的时间内产生最佳的峰。
本研究成功地建立了一种快速、灵敏的UPLC-QUAD-MS/MS方法,并在20 min内测定了果梅中16种活性成分,为果梅药效物质基础研究提供理论依据。
[1] 张永春,包满珠.梅树品种分类研究进展[J].北京林业大学学报,1998, 20(2):90-94.
ZHANG Y C, BAO M Z.Advances in classification for cultivars of Prunus mume[J].Journal of Beijing Forestry University, 1998,20(2):90-94.
[2] 刘兴艳,蒲彪,刘云,等.大邑果梅基础营养成分含量的测定和研究[J].食品研究与开发, 2007,28(6):146-148.
LIU X Y, PU B, LIU Y, et al.Determination of the basic nutritional ingredient of plums (Prunus mume Sieb.et Zucc) in Dayi[J].Food Research and Development, 2007, 28(6):146-148.
[3] 潘惠慧,吴晓琴,陆柏益,等.高效液相色谱法分析和检测青梅花、枝、叶中有机酸的种类和含量[J].科技通报,2008,24(3):350-354.
PAN H H, WU X Q, LU B Y, et al.Separation and determination of organic acids in flower, branch and leaf extract of Prunus mume by HPLC[J].Bulletin of Science and Technology, 2008,24 (3):350-354.
[4] 陈战国,恩伯提,张志琪.RP-HPLC同时测定乌梅中8种有机酸含量[J].中国中药杂志,2006, 31(21):1 783-1 786.
CHEN Z G, EN B T, ZHANG Z Q.Simultaneous determination of eight organic acids in Fructus Mume by RP-HPLC[J].China Journal of Chinese Materia Medica, 2006, 31(21):1 783-1 786.
[5] 安苗,黎雄,赵亚,等.HPLC-PDA同时测定乌梅肉中3种特征成分[J].中国实验方剂学杂志,2017,23(23):52-56.
AN M, LI X, ZHAO Y, et al.Simultaneous determination of three main components in mume fructus pulp by HPLC-PDA[J].Chinese Journal of Experimental Traditional Medical Formulae, 2017,23(23):52-56.
[6] 周茜,韩雪,韩晓梅,等.响应面试验优化乌梅熊果酸提取工艺及其对大肠杆菌的抑制作用[J].食品科学,2016,37(8):67-73.
ZHOU Q, HAN X, HAN X M, et al.Optimization of ursolic acid extraction from fructus mume and evaluation of its antibacterial activity against Escherichia coli[J].Food Science, 2016,37 (8):67-73.
[7] 宋爽. 梅果实提取液抑菌、抗氧化活性成分鉴定与功能分析[D].南京:南京农业大学,2016.
SONG S.Identification and function analysis of Japanese apricot fruit extraction's bacteriostatic and antioxidative active ingredient[D].Nanjing:Nanjing Agricultural University, 2016.
[8] 张飞,李劲松.果梅的研究进展[J].海峡药学,2006(4):21-24.
ZHANG F, LI J S.Research progress in Umei [J].Straits Pharmacy, 2006(4):21-24.
[9] 刘友平,陈鸿平,万德光,等.乌梅的研究进展[J].中药材,2004(6):459-462.
LIU Y P, CHEN H P, WAN D G, et al.Research progress in Umei [J].Journal of Chinese Medicinal Materials, 2004 (6):459-462.
[10] 李昕,王瑞,李肖莉,等.不同加工方法乌梅UPLC特征图谱及模式识别研究[J].中国中医药信息杂志,2020,27(11):76-81.
LI X, WANG R, LI X L, et al.Study on UPLC fingerprints and pattern recognition of mume fructus with different processing methods[J].Chinese Journal of Information on Traditional Chinese Medicine, 2020,27 (11):76-81.
[11] OU J M, WANG R, LI X L, et al.Comparative analysis of free amino acids and nucleosides in different varieties of mume fructus based on simultaneous determination and multivariate statistical analyses.[J].International Journal of Analytical Chemistry, 2020,2020:4767605.
[12] 甘源,贺丽迎,赵舰,等.超高效液相色谱高分辨质谱法同时测定蜂蜜中的5种雷公藤类物质[J].中国卫生检验杂志,2021,31(6):650-653;658.
GAN Y, HE L Y, ZHAO J, et al.Simultaneous determination of 5 kinds of Tripterygium substances in honey by liquid chromatography high resolution mass spectrometry[J].Chinese Journal of Health Laboratory Technology, 2021, 31(6):650-653;658.