砷是一种有毒类金属,因具有致癌、致畸等特性,被国际癌症研究中心(International Center for Cancer Research,IARC)归为I类致癌物。砷毒性不仅与总量有关,更与其化学形态密切相关。砷分为有机砷和无机砷,无机砷主要有亚砷酸盐(iAsⅢ)和砷酸盐(iAsⅤ),有机砷主要有一甲基砷(monomethyl arsenic,MMA)、二甲基砷(dimethyl arsenic,DMA)、三甲基砷(trimethyl arsenic, TMA)、砷甜菜碱(arsenic betaine,AsB)、砷胆碱(arsenic choline,AsC)、砷糖(arsenic sugar,AsS)和砷脂(arsenic lipid,AsL)等。一般认为无机砷毒性远大于有机砷,若以半数致死量(half lethal dose,LD50)计,则砷化合物的毒性依次为:iAsⅢ>iAsV>MMA>DMA>TMA>AsS>AsC>AsB, 而AsL被认为毒性极低[1]。食用无机砷含量超标的海产品会导致砷中毒,诱发的疾病包括但不限于皮肤癌、肺癌和膀胱癌、心血管疾病、糖尿病、生殖和发育障碍以及神经和认知功能障碍[2]。因此,分析海产品中不同砷形态具有重要意义。
砷普遍存在于海洋环境中,且海产品容易富集砷,并以有机砷为主要形式。因为海洋鱼类和贝类等生物可将吸收的无机砷通过食物链被生物体转化合成AsB等有机砷,而有机砷比无机砷具有更强的食物链传递能力,最终在海产品中富集更高浓度的砷[3]。其中,AsS是海藻类主要的砷形态,AsB是鱼类和甲壳类主要的砷形态,AsS和AsB均是贝类的主要砷形态[4]。人体内约90%的砷来源于海产品,其中海产品中85%~94%砷以低毒或者无毒的AsB等有机砷形式存在[5]。但近年来,研究发现有机砷MMAIII(三价一甲基砷)和DMAⅢ(三价二甲基砷)毒性均比iAsⅢ强[6]。但目前对摄入海产品后不同砷形态在体内代谢途径的研究不够深入,且砷毒性效应的细胞靶点以及分子靶点仍不明确,以及新型砷化合物的检出与结构鉴定仍需进一步改进与完善。
文章对海产品中砷的形态、分布特征、毒性以及对人类健康风险评估进行了论述,并对未来多学科交叉发展下,海产品中砷形态分析、毒性研究及预测防控的发展趋势提出展望。旨在为砷形态毒性和安全性分析的研究提供借鉴与参考。
1 海产品中砷的形态
海产品中砷有无机砷和有机砷2种形态。之前的文献报道中,均未在海产品中检测出砷单质。表1列举了海产品中砷的存在形式。
海产品中无机砷受国家标准限量监控。同时,IARC将砷和无机砷列为I类致癌物,其中,无机砷(iAsⅢ和iAsⅤ)被公认为致癌物,有机砷MMA和DMA也被列为明确的致癌物。不同烹调方式也会影响砷的毒性和含量[9],潜在的健康风险需引起重视。
表1 海产品中砷的存在形式
Table 1 Arsenic species in marine

名称英文存在化学式基础结构式参考文献亚砷酸盐iAsⅢarsenite海藻,羊栖菜As(OH)3[5]砷酸盐iAsⅤarsenate海藻,羊栖菜AsO(OH)3[5]砷甜菜碱(AsB)arsenobetaine鱼,节足动物,软体动物(CH3)3As+CH2COOH[5]砷胆碱(AsC)arsenocholine鱼类,虾类(CH3)3As+CH2CH2OH[5]一甲基砷(MMA)monomethylarsonic acid脂肪型鱼类CH3AsO(OH)2[7]二甲基砷(DMA)dimethylated arsenic绿藻:海松(CH3)2AsO(OH)[7]二甲基砷氧化醋酸(DMAA)dimethylarsinoylacetic acid鳕鱼,扇贝,褐藻(CH3)2As(O)CH2COOH[7]二甲基砷氧化乙醇(DMAE)dimethylarsinoyl ethanol鳕鱼,扇贝,褐藻(CH3)2As(O)CH2CH2OH[7]二甲基砷氧化丙酯(DMAP)dimethylarsinoyl propy-lester鳕鱼,扇贝,褐藻(CH3)2As(O)CH2CH2COOH[7]三甲基砷(TMA)trimethylarsine鱼类(CH3)3AsO[8]三甲基氧化砷(TMAO)trimethylarsine oxide鱼类,海螺(CH3)3AsO[8]四甲基砷离子(TETRA)tetramethylarsoniumion贝类,海葵等(CH3)4As+[8]砷糖AsSarsenosugars褐藻:昆布,海带,裙带菜,洋栖菜,石枝藻等(CH3)2AsOCH2C4H4(OH)2OROxo-Gly:R1=OHOxo-PO4:R2=OPO3CH2CHOOxo-SO3:R3=SO3Oxo-SO4:R4=SO4[5]砷脂AsLarsenolipids脂肪型鱼类,海藻,甲壳类动物[7]
2 海产品中砷的分布特征
2.1 海产品中砷的含量与形态
海产品中总砷含量和砷形态的分布如表2所示。海产品中总砷含量依次为:底栖贝类>底层鱼类>中上层鱼类>甲壳类,其中贝类总砷含量比中上层鱼类高5~7倍[10]。与TAYLOR等[11]的实验结果一致,并发现海洋食物网中海藻的总砷含量最高。海产品中无机砷含量依次为:贝类>甲壳类>鱼类[12]。海藻中,羊栖菜的总砷和无机砷较高[3],其中紫菜总砷平均值达到17.0 mg/kg[12],羊栖菜无机砷含量较高[ω(AsⅤ)>60 mg/kg][8],但海藻中主要的砷形态为AsS[4]。贝类中,缢蛏的总砷和无机砷均高于其他贝类[13],以藻类和浮游植物为食物的象拔蚌(底栖贝类)其砷含量是鲑鱼(中上层鱼类)的6~10倍[4]。经鱼类中砷形态分析发现海水鱼中砷含量明显高于淡水鱼,鱼体内重金属富集的次序为:肉食性鱼类>杂食性鱼类>植物食性鱼类[14]。一般认为鱼类中主要砷形态为AsB,但日本学者发现[15],沙丁鱼中DMA浓度高于AsB,占砷总量的16%~24%。甲壳类中,野生捕虾的总砷含量几乎是养殖虾的100倍[4],同时陆奕娜等[16]研究显示,养殖虾的AsB含量明显高于非养殖虾,但AsC含量却呈现相反结果[16]。总结发现海产品中砷形态以AsB为主,约占总砷85%~94%;砷形态的含量次序为ω(AsB)>ω(DMA)>ω(iAsⅤ)>ω(iAsⅢ)>ω(MMA)[10],无机砷约占10%[17]。
表2 海产品中总砷含量和所检出砷形态(以干重计)
Table 2 Total arsenic content and speciation of arsenic
in seafood (on dry basis)
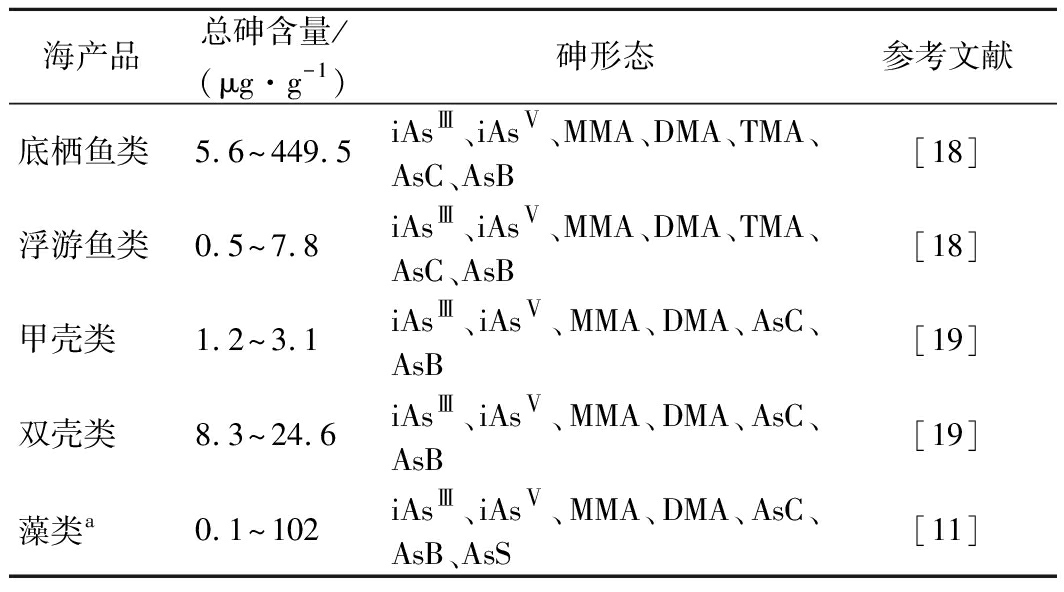
海产品总砷含量/(μg·g-1)砷形态参考文献底栖鱼类5.6~449.5iAsⅢ、iAsⅤ、MMA、DMA、TMA、AsC、AsB[18]浮游鱼类0.5~7.8iAsⅢ、iAsⅤ、MMA、DMA、TMA、AsC、AsB[18]甲壳类1.2~3.1iAsⅢ、iAsⅤ、MMA、DMA、AsC、AsB[19]双壳类8.3~24.6iAsⅢ、iAsⅤ、MMA、DMA、AsC、AsB[19]藻类a0.1~102iAsⅢ、iAsⅤ、MMA、DMA、AsC、AsB、AsS[11]
注:a按湿重算
2.2 不同组织中砷的分布
海产品不同组织中总砷和形态砷的富集研究发现砷累积量与初始暴露期(约0~10 d)呈正相关;达到稳定期后,砷在组织中积累顺序为:胃肠道>肝脏>鳃>肌肉[20]。同时,90%的砷在鱼体内转化为有机形式,且AsB是砷代谢的最终产物[20]。有机砷在不同组织中的分布为:肌肉>鳃>肝脏>胃肠道,且占总砷95.5%~99.5%[21]。鱼类研究发现尼罗罗非鱼中无机砷的积累顺序为:肝脏>胃>鳃>肌肉[22];从北波斯湾采集的鱼类,除了杜氏真鲨外,其他鱼类肝脏组织的砷含量明显高于肌肉[23]。鱼类的生物蓄积表明,肝脏和胃对砷的吸收和蓄积起着重要作用[22]。甲壳类动物中,巨型红虾肌肉的砷含量[(16.3±0.8) mg/kg干重]是头胸[(32.7±1.1) mg/kg干重]的一半[24]。调查中国主要产区螃蟹中重金属(含砷)发现,雌性褐蟹肉的饮食风险高于雄性可食组织[25];海蟹中性腺(雌)组织的总砷含量同样高于其他可食用部分,可见性腺组织是积累砷的主要组织[26]。贝类中砷积累顺序为:贝类组织鳃>性腺>足部肌肉>套膜[27]。综合鱼类、甲壳类和贝类组织中砷富集情况,发现内脏相比肌肉组织更容易富集砷。
2.3 不同海域中砷的分布
收集不同海域海产品砷含量数据见表3,并根据meta分析,发现我国软体动物中砷含量由北向南呈下降趋势[28]。北波斯湾的海产品砷含量均高于地中海和欧洲大西洋,其中软体动物砷含量分别为欧洲大西洋、地中海的12和6倍。我国从北至南不同鱼种的砷含量变化范围较广(2.09~134 μg/g)[29],青岛地区存在相对偏高的现象[13]。我国贝类中,深圳市近江牡蛎砷较高,As含量(按湿重算)为2.24~95.5 mg/kg[17]。砷水平存在地理差异,这可能是由微量元素生物累积量以及工业和农业污染情况差异造成的[29]。
表3 不同海域海产品中砷含量 单位:mg/kg
Table 3 Arsenic content in seafood in from different sea areas

海域软体动物鱼类甲壳类动物参考文献胶州湾18.68±2.519.31±1.4528.84±4.95[28]黄海12.32±9.295.65±4.9617.51±3.43[28]东海7.65±8.754.43±3.7118.78±7.06 (三门湾)30.95±3.58 (象山湾)[28]南海6.28±3.565.04±3.609.13 (广东省)17.04±5.59 (大亚湾)[28, 30]地中海a3.99±3.64中上层:6.47±7.15底层:5.06±5.350.40±0.03[31]欧洲大西洋a2.04±0.75中上层:4.19±5.78底层:4.72±5.27—[31]北波斯湾a24.90±8.5010.87±3.1320.85±7.40[23]
注:a以湿重计,其余均以干重计
3 海产品中砷的毒性
海产品是砷暴露的主要途径,大量存在于海产品中的AsB无需代谢可直接随尿液排出,其他砷化合物如AsS、AsL和iAs的尿液代谢物为DMA。然而,最近的研究显示,某些有机砷及其中间代谢产物具有细胞毒性[11],说明需要对有机砷的代谢途径和毒性进行下一步研究。图1显示海产品中砷形态在人体内的代谢情况[8]。

图1 海产品中不同形态砷在人体内的代谢
Fig.1 Metabolism of arsenic species from seafood in humans
3.1 致癌性
有学者提出,随着砷暴露时间的增加或累积,会导致细胞生长、凋亡行为和基因组信号通路的改变,从而引发癌症[32]。关于剂量-反应关系,暴露在等于或低于参考剂量的砷浓度下均会致癌[33]。怀孕小鼠砷暴露后,会增加其子代的癌症易感性[34]。流行病学研究已证实,砷暴露在200 μg/L或更高水平时,膀胱癌、肺癌和皮肤癌的风险增加[35]。砷诱发的不是癌症,而是多种毒性,如砷中毒、皮肤病变、支气管炎或尿路上皮毒性,这些毒性导致再生性增殖,最终间接诱发癌症[2]。砷的致癌机制与miRNA失调有关,但诱发癌症病因中的机制和确切作用仍不明确[32]。无机砷癌变是通过几种表观遗传机制:DNA甲基化、组蛋白翻译后修饰、组蛋白变体和miRNA[36]。有机砷(MMA和DMA)可以抑制线粒体呼吸,导致氧活性物质的形成,从而DNA发生突变,诱发癌症和细胞死亡[22]。其中MMAⅢ和DMAⅢ均可诱导试验鼠发生肿瘤[37]。在动物研究中,DMAV可促进膀胱、肾脏、肝脏和甲状腺致癌[12]。图2为摄入无机砷后,砷在体内的代谢情况。
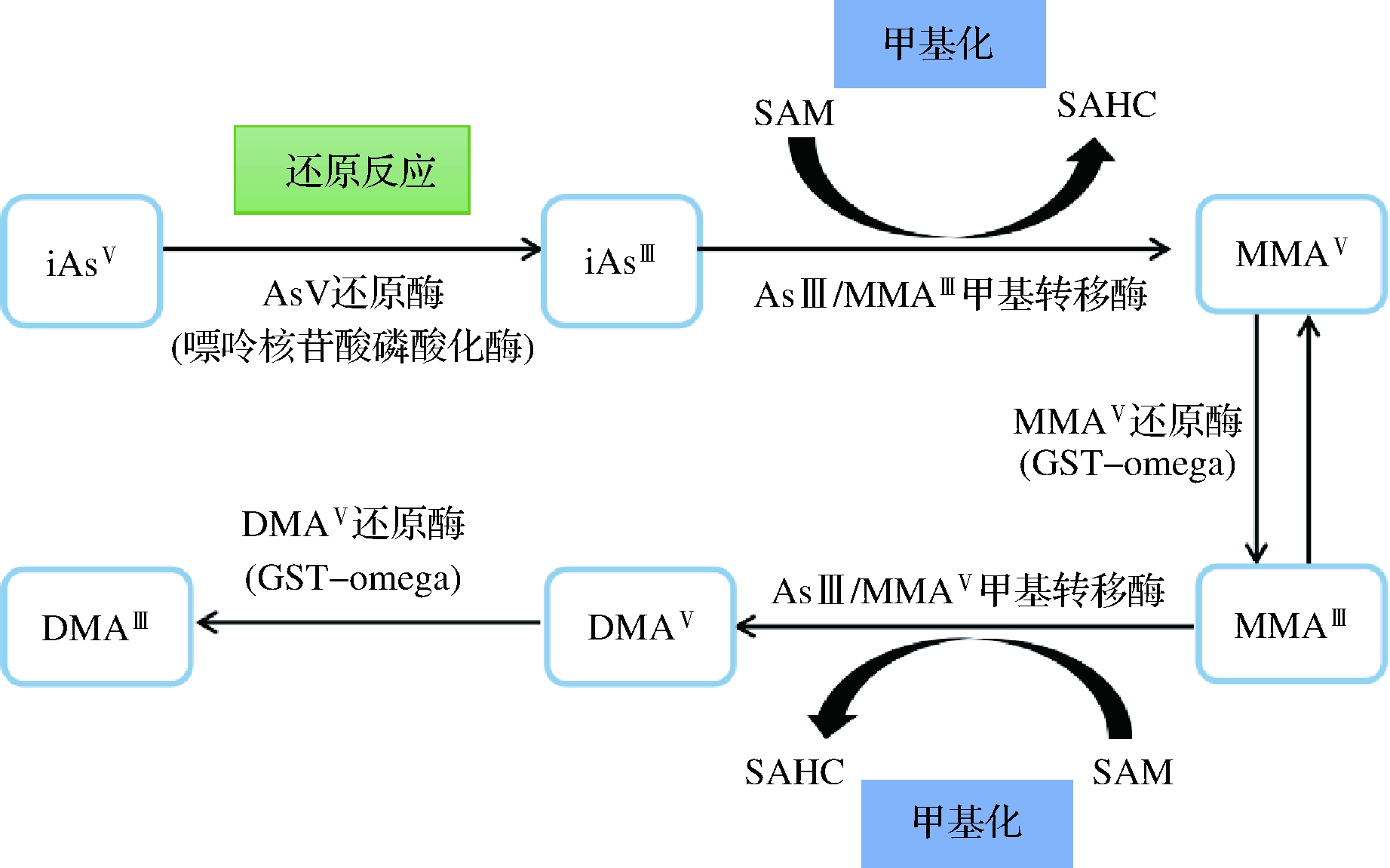
SAM:S-腺苷甲硫氨酸合成酶;SAHC:S-腺苷同型半胱氨酸
图2 无机砷在人体内的代谢
Fig.2 Metabolism of inorganic arsenic
3.2 致畸性
动物实验表明,iAsⅤ对斑点蝾螈胚胎发育有致畸作用,表现为腹部水肿、尾部扭结、鳃和面部畸形以及异常弯曲,其严重程度随着砷(iAsⅤ)浓度的增加而增加[38]。研究斑马鱼胚胎暴露在0.8 mmol/L砷的致畸性,结果为卵凝固、发育迟缓、水肿形成、孵化成功、脊柱侧凸[39]。
3.3 生殖毒性
砷和无机砷对生殖系统有毒性作用。As2O3处理的小鼠中,睾酮生物合成显著降低,精子运动性、活力、膜完整性也出现异常[40]。研究发现砷可通过抑制睾酮、黄体生成素(luteinizing hormone,LH)、卵泡刺激素(follicle-stimulating hormone, FSH)的合成来抑制精子的发生和成熟[40]。OMMATI等[41]研究发现,氧化应激诱导的线粒体损伤和自噬细胞死亡,通过AMPK/TSC/mTOR和LC3相关途径诱导生殖系统毒性。
3.4 神经毒性
长期接触砷会损害中枢神经系统和外周神经系统。较高的砷暴露[1 mg As/(kg·d)或更多]通常会导致脑病,并伴有幻觉、癫痫、精神混乱、嗜睡、头痛,有时还会昏迷[34]。在儿童发育阶段接触砷会改变他们的神经发育。例如,儿童砷暴露与低智商和神经认知功能有关[42]。对体外血脑屏障模型的研究中发现,含砷碳氢化合物(arsenic hydrocarbons,AsHCs)对完全分化的人脑细胞具有潜在的神经毒性[43]。吸入砷还会导致人类的神经系统产生缺陷,如感觉和运动神经元的周围神经病变,导致反射丧失和肌肉无力[32]。
3.5 细胞毒性
砷通过不同途径诱导活性氧,激活蛋白JNK产生细胞毒性,JNK是有丝分裂活化蛋白激酶的相关亚组之一,具有介导细胞凋亡、分化和增殖等细胞功能,并刺激JNK肿瘤坏死因子[34]。细胞毒性的顺序为DMAⅢ、DMMTAV>iAsⅢ、iAsV>MMMTAⅤ(一甲基单硫砷酸)>MMAⅤ、DMAV[42]。有研究发现小鼠食用海产品后,在其尿液和粪便中发现一种有毒的代谢物DMMTAV(硫代砷酸),该物质对人肺和膀胱癌细胞的细胞毒性作用比iAsⅢ更明显[6]。比较iAsⅢ或MMAⅢ在人脑细胞中的细胞毒性,结果发现MMAⅢ对神经元的毒性显著高于iAsⅢ[44]。砷脂中,AsHC发挥了最强的细胞毒性作用,其毒性比iAsⅢ高出5倍;其中AsHC 360的毒性最高[43]。
3.6 遗传毒性
砷的本质上是非诱变的,但可通过DNA损伤、染色体畸变、姐妹染色单体交换和人体系统微核形成引起显著的细胞遗传学损伤[34]。长期暴露于细胞中的砷可通过细胞中S-腺苷蛋氨酸缺失、导致基因组不稳定的DNA低甲基化和DNA甲基化的整体丢失而诱发遗传毒性[45]。经胎盘砷暴露会导致男性胎儿肝脏DNA甲基化变化和基因表达异常,且可改变胎儿肝脏的miRNA表达[34]。DMAⅤ对培养的哺乳动物细胞具有遗传毒性作用[8]。
综述毒性研究中,均未发现食用海产品导致急性毒性的迹象,但长期低剂量暴露于砷,会引起多器官衰竭、癌症、皮肤色素沉着、角化亢进、生殖并发症、神经和行为障碍[2]。砷化合物氧化数越低,毒性越高,甲基化越高,毒性就越低[4]。无机砷甲基化过程中的中间产物MMAⅢ和DMAⅢ,比无机砷表现出更强的细胞毒性、遗传毒性和酶抑制力[6]。
4 人类健康风险评估
海产品中砷通过食物链进入人体,从而对人类健康构成危害。此外,砷在代谢过程中会产生其他未知形态的中间产物[46],因此,海产品中砷形态的分析和毒性研究对健康风险评估起重要作用。
目前主要采用目标危害商数(target hazard quotient,THQ)和致癌风险(cancer risk,CR)进行健康风险评估。表4列举了不同海域的风险评估数据。其中,北波斯湾的THQ和CR均超于安全阈值,给人体健康带来风险,其中成人食用贝类的THQ高达8.50。贝类是滤食性动物,易从栖息地摄取和积累重金属,对人体健康构成潜在威胁,是研究重金属污染的重点对象。有研究表明,食用南海海湾扇贝、栉孔扇贝对儿童构成非致癌风险,并提示食用该地区近江牡蛎、方斑东风螺、竹蛏极大可能存在健康风险,建议该沿海居民减少贝类的摄入[47]。一般而言,食用商业鱼类和甲壳类海产品的CR在可接受的范围内,不构成重大的健康风险[48]。但在相同的暴露剂量下,儿童比成人面临更高的风险。这可能是由于儿童的体重相对较低,对非致癌和致癌健康影响更敏感。
表4 不同海域海产品的THQ和CR
Table 4 THQ and CR of seafood in different sea areas
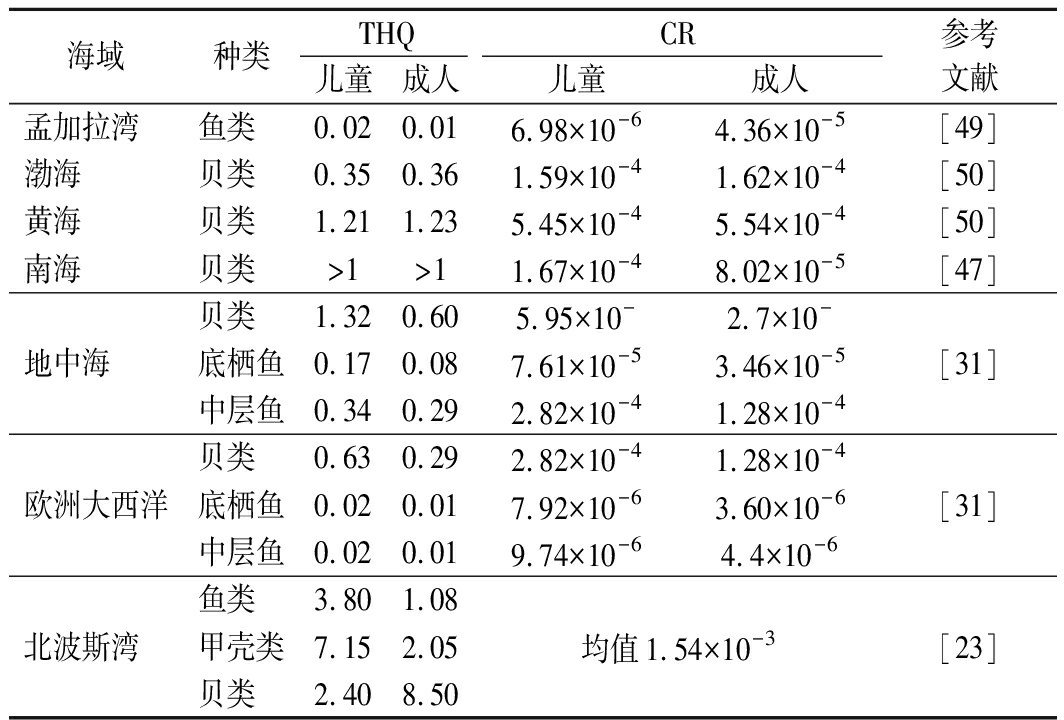
海域种类THQCR儿童成人儿童成人参考文献孟加拉湾鱼类0.020.016.98×10-64.36×10-5[49]渤海贝类0.350.361.59×10-41.62×10-4[50]黄海贝类1.211.235.45×10-45.54×10-4[50]南海贝类>1>11.67×10-48.02×10-5[47]地中海贝类1.320.605.95×10-2.7×10-底栖鱼0.170.087.61×10-53.46×10-5中层鱼0.340.292.82×10-41.28×10-4[31]欧洲大西洋贝类0.630.292.82×10-41.28×10-4底栖鱼0.020.017.92×10-63.60×10-6中层鱼0.020.019.74×10-64.4×10-6[31]北波斯湾鱼类3.801.08甲壳类7.152.05贝类2.408.50均值1.54×10-3[23]
注:THQ<1为无明显健康风险,THQ≥1表明极大可能存在健康风险;CR>1×10-6为存在致癌风险,>10-4为不可接受的限值,10-6~10-4为可接受范围
风险评估结果表明,幼童、孕妇或备孕女性等特殊人群应避免一次性大量或长期食用无机砷含量较高的海产品,应采取有效措施减少有害元素向海洋环境的排放。
5 结语
海产品是人体摄入砷的主要来源之一。目前国内外对海产品中无机砷的分析趋于成熟,但对有机砷形态的分析方法仍未建立标准。GB 5009.11—2014《食品安全国家标准 食品中总砷及无机砷的测定》中只提及食品中总砷及无机砷的测定,同时,缺少应用于海产品中不同砷形态的标准品。在砷化合物的毒性研究中,有机砷的毒性作用机制未明确。
因此,对海产品中砷的形态分析和毒性研究,不应只停留于其含量,还应在以下几方面深入研究:在原有检测技术上结合分子生物学等技术,对砷进行长期追踪,为海产品中砷形态转化及污染情况提供数据支撑;探究不同砷形态在人体内的毒作用机制和代谢途径,例如砷毒性效应的细胞靶点以及分子靶点;结合预防医学相关知识,建立预测风险模型,为科学指导海产品的摄入和砷中毒的防控提供依据。相信在不同学科碰撞下,海产品中砷形态及其毒性的研究更深入、更全面,并对人类健康风险提供科学依据。
[1] MA Z L, LIN L D, WU M J, et al.Total and inorganic arsenic contents in seaweeds:Absorption, accumulation, transformation and toxicity[J].Aquaculture, 2018, 497:49-55.
[2] ZHOU Q, XI S H.A review on arsenic carcinogenesis:Epidemiology, metabolism, genotoxicity and epigenetic changes[J].Regulatory Toxicology and Pharmacology, 2018, 99:78-88.
[3] 张伟, 黄良民.海洋生物体内砷含量及其形态研究进展[J].生态毒理学报, 2019, 14(1):41-53.
ZHANG W, HUANG L M.Advances of arsenic contents and different species in marine organisms[J].Asian Journal of Ecotoxicology, 2019, 14(1):41-53.
[4] LUVONGA C, RIMMER C A, YU L L, et al.Determination of total arsenic and hydrophilic arsenic species in seafood[J].Journal of Food Composition and Analysis, 2021, 96:103729.
[5] RAHMAN M A, HASEGAWA H, PETER LIM R.Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain[J].Environmental Research, 2012, 116:118-135.
[6] KOBAYASHI Y, HIRANO S.Distribution and excretion of arsenic metabolites after oral administration of seafood-related organoarsenicals in rats[J].Metals, 2016, 6(10):231.
[7] LUVONGA C, RIMMER C A, YU L L, et al.Organoarsenicals in seafood:Occurrence, dietary exposure, toxicity, and risk assessment considerations:A review[J].Journal of Agricultural and Food Chemistry, 2020, 68(4):943-960.
[8] MOLIN M, ULVEN S M, MELTZER H M, et al.Arsenic in the human food chain, biotransformation and toxicology:Review focusing on seafood arsenic[J].Journal of Trace Elements in Medicine and Biology, 2015, 31:249-259.
[9] ALVES R N, MAULVAULT A L, BARBOSA V L, et al.Oral bioaccessibility of toxic and essential elements in raw and cooked commercial seafood species available in European markets[J].Food Chemistry, 2018, 267:15-27.
[10] 宋冬冬, 熊海燕, 张伟.广州市售海产品中砷质量安全与健康风险评估[J].食品安全质量检测学报, 2019, 10(19):6 704-6 711.
SONG D D, XIONG H Y, ZHANG W.Quality safety and health risk assessment of arsenic in seafood sold in Guangzhou[J].Journal of Food Safety & Quality, 2019, 10(19):6 704-6 711.
[11] TAYLOR V, GOODALE B, RAAB A, et al.Human exposure to organic arsenic species from seafood[J].Science of the Total Environment, 2017, 580:266-282.
[12] 李子孟, 王范盛, 朱剑.浙江省沿海常见海产品总砷含量水平调查分析[J].安徽农业科学, 2021, 49(1):186-187;193.
LI Z M, WANG F S, ZHU J.Investigation and analysis of total arsenic content of common seafood in Zhejiang Province[J].Journal of Anhui Agricultural Sciences, 2021, 49(1):186-187;193.
[13] 张荣昶, 宋扬, 于红卫, 等.青岛市市售海产品砷污染状况及无机砷暴露风险评估[J].现代预防医学, 2020, 47(6):1 016-1 019;1 027.
ZHANG R C, SONG Y, YU H W, et al.Arsenic pollution and risk assessment of inorganic arsenic from seafood in Qingdao[J].Modern Preventive Medicine, 2020, 47(6):1 016-1 019;1 027.
[14] 欧阳静茹, 邵昭明, 戚慕怡, 等.佛山市禅城区食用鱼中汞和砷形态分析及食用安全性评价[J].食品安全质量检测学报, 2020, 11(18):6 575-6 580.
OUYANG J R, SHAO Z M, QI M Y, et al.Species analysis and safety evaluation of mercury and arsenic in edible fish in Chancheng district in Foshan City[J].Journal of Food Safety & Quality, 2020, 11(18):6 575-6 580.
[15] ALI M M, ALI M L, PROSHAD R, et al.Assessment of trace elements in the demersal fishes of a coastal river in Bangladesh:A public health concern[J].Thalassas:an International Journal of Marine Sciences, 2020, 36(2):641-655.
[16] 陆奕娜, 魏建华, 许慨, 等.高效液相色谱-电感耦合等离子体质谱法同时测定海产品中9种砷形态化合物[J].理化检验-化学分册, 2017, 53(9):1 087-1 093.
LU Y N, WEI J H, XU K, et al.Simultaneous determination of nine arsenic species in marine products by HPLC-ICP-MS[J].Physical Testing and Chemical Analysis (Part B:Chemical Analysis), 2017, 53(9):1 087-1 093.
[17] LIU S, LIU Y L, YANG D F, et al.Trace elements in shellfish from Shenzhen, China:Implication of coastal water pollution and human exposure[J].Environmental Pollution, 2020, 263:114582.
[18] 杜森, 张黎.砷在海洋食物链中的生物放大潜力及发生机制探讨[J].生态毒理学报, 2019, 14(1):54-66.
DU S, ZHANG L.Biomagnification potential and the mechanisms of arsenic in marine food chains[J].Asian Journal of Ecotoxicology, 2019, 14(1):54-66.
[19] ZMOZINSKI A V, LLORENTE-MIRANDES T, L PEZ-S
PEZ-S NCHEZ J F, et al.Establishment of a method for determination of arsenic species in seafood by LC-ICP-MS[J].Food Chemistry, 2015, 173:1 073-1 082.
NCHEZ J F, et al.Establishment of a method for determination of arsenic species in seafood by LC-ICP-MS[J].Food Chemistry, 2015, 173:1 073-1 082.
[20] PEI J, ZUO J X, WANG X Y, et al.The bioaccumulation and tissue distribution of arsenic species in tilapia[J].International Journal of Environmental Research and Public Health, 2019, 16(5):757.
[21] AVIGLIANO E, SCHLOTTHAUER J, DE CARVALHO B M, et al.Inter-and intra-stock bioaccumulation of anionic arsenic species in an endangered catfish from South American estuaries:Risk assessment through consumption[J].Journal of Food Composition and Analysis, 2020, 87:103404.
[22] FERREIRA N S, OLIVEIRA L H B, AGRELLI V, et al.Bioaccumulation and acute toxicity of as(III) and as(V) in Nile tilapia (Oreochromis niloticus)[J].Chemosphere, 2019, 217:349-354.
[23] SOLTANI N, MARENGO M, KESHAVARZI B, et al.Occurrence of trace elements (TEs) in seafood from the North Persian Gulf:Implications for human health[J].Journal of Food Composition and Analysis, 2021, 97:103754.
[24] SOULTANI G, SELE V, RASMUSSEN R R, et al.Elements of toxicological concern and the arsenolipids’ profile in the giant-red Mediterranean shrimp, Aristaeomorpha foliacea[J].Journal of Food Composition and Analysis, 2021, 97:103786.
[25] WANG Q, FAN Z H, QIU L P, et al.Occurrence and health risk assessment of residual heavy metals in the Chinese mitten crab (Eriocheir sinensis)[J].Journal of Food Composition and Analysis, 2021, 97:103787.
[26] 常家琪. 海产品中多元素及砷形态检测技术开发与应用[D].舟山:浙江海洋大学, 2018.
CHANG J Q.Development and application of multi-element and arsenic speciation detection technologies in aquatic products[D].Zhoushan:Zhejiang Ocean University, 2018.
[27] ARUMUGAM A, LI J, KRISHNAMURTHY P, et al.Investigation of toxic elements in Carassius gibelio and Sinanodonta woodiana and its health risk to humans[J].Environmental Science and Pollution Research International, 2020, 27(16):19 955-19 969.
[28] WANG Z X, GU X, WEI O Y, et al.Trophodynamics of arsenic for different species in coastal regions of the Northwest Pacific Ocean:In situ evidence and a meta-analysis[J].Water Research, 2020, 184:116186.
[29] ZHANG W, WANG W X.Large-scale spatial and inter species differences in trace elements and stable isotopes in marine wild fish from Chinese waters[J].Journal of Hazardous Materials, 2012, 215-216:65-74.
[30] PALASH M A U, ISLAM M S, BAYERO A S, et al.Evaluation of trace metals concentration and human health implication by indigenous edible fish species consumption from Meghna River in Bangladesh[J].Environmental Toxicology and Pharmacology, 2020, 80:103440.
[31] FERRANTE M, NAPOLI S, GRASSO A, et al.Systematic review of arsenic in fresh seafood from the Mediterranean Sea and European Atlantic coasts:A health risk assessment[J].Food and Chemical Toxicology, 2019, 126:322-331.
[32] SUDHAKAR S.Arsenic in Drinking Water and Food [M].Singapore:Springer, 2020.
[33] WALLACE D R, BUHA DJORDJEVIC A.Heavy metal and pesticide exposure:A mixture of potential toxicity and carcinogenicity[J].Current Opinion in Toxicology, 2020, 19:72-79.
[34] LIU J, GUNEWARDENA S, YUE CUI J L, et al.Transplacental arsenic exposure produced 5-methylcytosine methylation changes and aberrant microRNA expressions in livers of male fetal mice[J].Toxicology, 2020, 435:152409.
[35] LAMM S H, BOROJE I J, FERDOSI H, et al.A review of low-dose arsenic risks and human cancers[J].Toxicology, 2021, 456:152768.
[36] SAINTILNORD W N, FONDUFE-MITTENDORF Y.Arsenic-induced epigenetic changes in cancer development[J].Seminars in Cancer Biology, 2021, 76:195-205.
[37] 刘香丽, 汪倩, 宋超, 等.不同砷形态在水产品中的毒理及转化研究进展[J].农学学报, 2019, 9(12):33-38.
LIU X L, WANG Q, SONG C, et al.Arsenic forms in aquatic products:Progress research on toxicology and transformation[J].Journal of Agriculture, 2019, 9(12):33-38.
[38] GARDNER S, CLINE G, MWEBI N, et al.Developmental and interactive effects of arsenic and chromium to developing Ambystoma maculatum embryos:Toxicity, teratogenicity, and whole-body concentrations[J].Journal of Toxicology and Environmental Health, Part A, 2017, 80(2):91-104.
[39] ADEYEMI J A, DA CUNHA MARTINS- A Jr, BARBOSA F Jr.Teratogenicity, genotoxicity and oxidative stress in zebrafish embryos (Danio rerio) co-exposed to arsenic and atrazine[J].Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2015, 172-173:7-12.
[40] OMMATI M M, HEIDARI R, MANTHARI R K, et al.Paternal exposure to arsenic resulted in oxidative stress, autophagy, and mitochondrial impairments in the HPG axis of pubertal male offspring[J].Chemosphere, 2019, 236:124325.
[41] OMMATI M M, MANTHARI R K, TIKKA C, et al.Arsenic-induced autophagic alterations and mitochondrial impairments in HPG-S axis of mature male mice offspring (F1-generation):A persistent toxicity study[J].Toxicology Letters, 2020, 326:83-98.
[42] CHEN J, GARBINSKI L D, ROSEN B, et al.Organoarsenical compounds:Occurrence, toxicology and biotransformation[J].Critical Reviews in Environmental Science and Technology, 2020, 50(3):217-243.
[43] MÜLLER S M, EBERT F, RABER G, et al.Effects of arsenolipids on in vitro blood-brain barrier model[J].Archives of Toxicology, 2018, 92(2):823-832.
[44] YOSHINAGA-SAKURAI K, SHINDE R, RODRIGUEZ M, et al.Comparative cytotoxicity of inorganic arsenite and methylarsenite in human brain cells[J].ACS Chemical Neuroscience, 2020, 11(5):743-751.
[45] ALI W, ZHANG H, JUNAID M, et al.Insights into the mechanisms of arsenic-selenium interactions and the associated toxicity in plants, animals, and humans:A critical review[J].Critical Reviews in Environmental Science and Technology, 2021, 51(7):704-750.
[46] CUI D, ZHANG P, LI H P, et al.The dynamic changes of arsenic biotransformation and bioaccumulation in muscle of freshwater food fish crucian carp during chronic dietborne exposure[J].Journal of Environmental Sciences, 2021, 100:74-81.
[47] GONG Y, CHAI M W, DING H, et al.Bioaccumulation and human health risk of shellfish contamination to heavy metals and As in most rapid urbanized Shenzhen, China[J].Environmental Science and Pollution Research International, 2020, 27(2):2 096-2 106.
[48] LI P, HUANG Y Y, ZENG J, et al.Health risk assessment of heavy metals in shellfish collected from Fujian, China[J].Human and Ecological Risk Assessment:an International Journal, 2020, 26(3):621-635.
[49] AHMED A S S, SULTANA S, HABIB A, et al.Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults[J].PLoS One, 2019, 14(10):e0219336.
[50] LI P, PAN Y S, FANG Y, et al.Concentrations and health risks of inorganic arsenic and methylmercury in shellfish from typical coastal cities in China:A simultaneous analytical method study[J].Food Chemistry, 2019, 278:587-592.