有机磷农药(organophosphorus pesticides,OPPs)是一类含有磷原子的有机酯类化合物,因其高效、经济、广谱、易降解等特点而广泛用于农业生产中,主要用于防治植物病虫害[1]。OPPs经使用后残存于环境和植物体内,通过食物链富集进入动物体内,可对生鲜肉造成污染[2]。据报道,OPPs进入人体后,通过抑制胆碱酯酶活性,使机体发生功能性紊乱,导致机体出现一系列神经中毒症状,如出汗、震颤、精神错乱、语言失常等,严重者可导致呼吸麻痹而死亡[3]。因此,我国自2001年起已明令禁止使用对硫磷、久效磷、甲胺磷等高毒有机磷农药,并限制使用毒死蜱、三唑磷、敌敌畏等中毒有机磷农药[4]。世界各国和国际组织也制定了动物源食品中农药最大残留限量(maximum residue limits,MRLs)。例如,国际食品法典委员会(Codex Alimentarius Commission,CAC)规定39种动物源产品中1 021项农药MRLs[5];韩国规定35种动物源产品中7 941项农药MRLs[6];我国GB 2763—2021《食品安全国家标准 食品中农药最大残留限量》规定27种动物源产品中903项农药MRLs。
生鲜肉因脂肪、蛋白含量较高,致使其基质较为复杂。因此,为减少农药检测过程中基质干扰现象,选择合适的样品前处理方法显得尤为重要。迄今,生鲜肉中农药残留检测的前处理方法主要有固相萃取法[7]、加速溶剂萃取法[8]、凝胶渗透色谱净化[9]、QuEChERS(quick,easy,cheap,effective,rugged,safe)[10]等。现有方法虽各有优势,但也存在着缺点和局限性,如凝胶渗透色谱和加速溶剂萃取法虽能准确分离出农药残留组分,但溶剂消耗量大、操作繁琐、易造成二次污染[11]。QuEChERS法作为近年来新兴的样品前处理技术,因其净化过程简单、快速、高效、经济等优点,已广泛应用于食品、环境、生物等领域[12]。因此,本实验选择QuEChERS法对生鲜肉进行前处理。农药残留的检测技术主要有酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)[13]、GC[14]、HPLC[15]、GC-MS、GC-MS/MS[16]、LC-MS/MS[17-18]等。其中,ELISA主要用于大批样品的初步筛查,抗体有效性短,易出现假阳性结果[13];GC与GC-MS检测范围有限,不利于挥发性小、分子质量大的样品检测[16];HPLC与LC-MS/MS常用于挥发性小,热稳定性差的样品检测,检测农药种类少[19]。较之上述方法,GC-MS/MS具有较高灵敏度和抗干扰能力,分析结果准确可靠,适用于痕量物质的检测[20]。因此,本实验基于QuEChERS前处理方法,建立了一种可同时检测生鲜肉中18种OPPs的GC-MS/MS方法。该方法的建立为我国生鲜肉中农药残留的高通量快速检测以及食品安全监管提供科学支持。
1 材料与方法
1.1 材料与试剂
生鲜猪肉、牛肉、羊肉、鸡肉、鸭肉,江苏省南京市超市和农贸市场;阴性畜禽肉,农业农村部肉及肉制品质量监督检验测试中心(南京)。
标准品:敌敌畏(dichlorvos)、虫螨磷(insecticidal phosphorus)、速灭磷(mevinphos)、二嗪磷(diazinon)、三唑磷(triazophos)、灭线磷(ethoprophos)、皮蝇磷(fenchlorphos)、杀螟硫磷(fenitrothion)、毒死蜱(chlorpyrifos)、倍硫磷(fenthion)、对硫磷(parathion)、乙硫磷(ethion)、地虫硫磷(fonofos)、甲拌磷(phorate)、久效磷(monocrotophos)、马拉硫磷(malathion)、乙拌磷(disulfoton)、特丁硫磷(terbufos),质量浓度均为100 μg/mL,上海安谱实验科技股份有限公司。
试剂:乙腈、正己烷、乙酸乙酯,丙酮,均为色谱纯,MgSO4、NaCl、CH3COONa,均为分析纯,上海安谱实验科技股份有限公司;N-丙基乙二胺(primary secondary amine,PSA)、十八烷基键合硅胶(C18),粒径均为40 μm,美国安捷伦科技有限公司;实验所用水均为一级水。
1.2 仪器与设备
TSQ 8000 EVO三重四级杆气质联用仪,赛默飞世尔科技(中国)有限公司;DB-1701色谱柱(30 m×0.25 mm,0.25 μm),美国安捷伦科技有限公司;D-16C高速冷冻离心机、arium® advance EDI纯水仪,德国Sartorius公司;N-EVAP型氮吹仪,美国Organomation公司;Vortex Genius漩涡混匀器,德国IKA公司。
1.3 实验方法
1.3.1 样品前处理
提取:准确称取5.00 g(精确至0.01 g)均质样品于50 mL离心管中,加入10 mL饱和正己烷的乙腈溶液提取,涡旋混匀1 min;加入1 g MgSO4和1.5 g CH3COONa,涡旋混匀1 min,4 ℃下10 000 r/min离心5 min。
净化:转移上清液,置于装有净化材料(1 g MgSO4、0.15 g PSA、0.15 g C18)离心管中,涡旋振荡1 min,4 ℃下10 000 r/min离心5 min;转移上清液于新的50 mL离心管中,35 ℃氮吹至近干,加入1 mL乙酸乙酯复溶;复溶液过0.22 μm滤膜,供GC-MS/MS分析检测。
1.3.2 标准溶液的配制
标准中间溶液:将18种质量浓度为100.0 mg/L的OPPs标准品,根据其理化性质和测定条件以丙酮为溶剂稀释定容至各农药质量浓度为5.00 mg/L,-20 ℃保存备用。
标准工作溶液:精确移取一定量标准中间溶液,用丙酮稀释定容至质量浓度为1.00 mg/L的标准工作溶液,-20 ℃保存备用。
标准工作曲线:精确移取一定量标准工作溶液,用丙酮稀释至各质量浓度为0.005、0.01、0.02、0.05、0.10、0.20、0.50 mg/L的标准工作曲线溶液,现配现用。
1.3.3 GC-MS/MS方法的建立
气相色谱条件:色谱柱:Agilent DB-1701(30 m×0.25 mm,0.25 μm);载气:高纯氦气(99.999%);流速1.0 mL/min;进样方式:不分流进样;进样量1 μL;进样口温度280 ℃;升温程序:初始温度50 ℃保持2 min,以30 ℃/min升温至180 ℃,再以10 ℃/min升温至260 ℃。
质谱条件:电离方式:电子轰击源(electron impact ion source,EI);电离能量70 eV;离子源温度230 ℃;传输线温度270 ℃;扫描离子范围50~500 u;扫描方式:多反应离子监测(multi-reactive ion monitoring,MRM)。
1.3.4 基质效应
基质效应(matrix effects,ME)是指样品中除分析物以外的其他成分对待测物产生干扰,从而引起待测组分信号抑制或增强[21]。实际工作中ME通常以基质标准曲线与纯溶剂标准曲线两者斜率(k)之比来进行评估,即ME=k基质标准曲线/k纯溶剂标准曲线。当ME>1时,为基质增强效应;ME<1为基质减弱效应;ME=1,则不存在基质干扰现象。一般认为,当ME比值为0.8~1.2时,为弱基质效应;当ME>1.2或<0.8时,ME较强[22]。
1.3.5 线性范围的确定
基于已建立的GC-MS/MS方法,分别以阴性猪肉、牛肉、羊肉、鸡肉、鸭肉为基质,配制7个不同梯度(0.005~0.50 mg/L)的混合基质标准溶液。经检测,最低点浓度出峰状况良好,最高点浓度也在仪器检测范围之内,能够满足本实验对不同组分相关系数的要求。以定量离子质量色谱图峰面积为纵坐标(Y),对应溶液质量浓度(mg/L)为横坐标(X),绘制标准曲线,计算平方相关系数(square of correlation coefficient,R2)。
1.3.6 检出限和定量限
分别以阴性猪肉、牛肉、羊肉、鸡肉、鸭肉的低浓度添加水平(5.00 μg/kg)进行测定,平行测定10份样品。以信噪比S/N=3和S/N=10所对应浓度,分别计算出各组分在5种基质中的检出限(limit of detection,LOD)和定量限(limit of quantitation,LOQ)[23]。
1.3.7 准确度和精密度试验
分别以阴性猪肉、牛肉、羊肉、鸡肉、鸭肉为基质进行低、中、高3个水平(5.00、10.00、20.00 μg/kg)的加标回收试验,每个水平平行测定6次,计算平均加标回收率和相对标准偏差(relative standard deviation,RSD)。
1.3.8 方法的实际应用
选用市售生鲜肉30份,其中猪、牛、羊、鸡、鸭各6份,15份购自超市,15份购自农贸市场,参照1.3.1和1.3.3节进行样品前处理和上机测定,以此评价该方法的实际应用效果。
2 结果与分析
2.1 前处理条件优化
2.1.1 提取溶剂的优化
鉴于18种OPPs的分子极性差异,本试验分别采用乙腈、饱和正己烷的乙腈溶液、V(乙腈)∶V(水)=4∶1、丙酮、乙酸乙酯作为样品提取溶剂。在其他试验条件相同情况下,分别向5种阴性基质中添加20 μg/kg OPPs混合标准溶液,以18种农药回收率为指标,比较5种不同提取剂的提取效果。由表1可知,饱和正己烷的乙腈溶液在5种基质中均呈现出最佳的提取效果,其回收率最高。因此,本试验选择饱和正己烷的乙腈溶液作为样品提取剂。
表1 提取溶剂对18种农药回收率的影响
Table 1 Effect of extraction solvents on the recovery of 18 pesticides
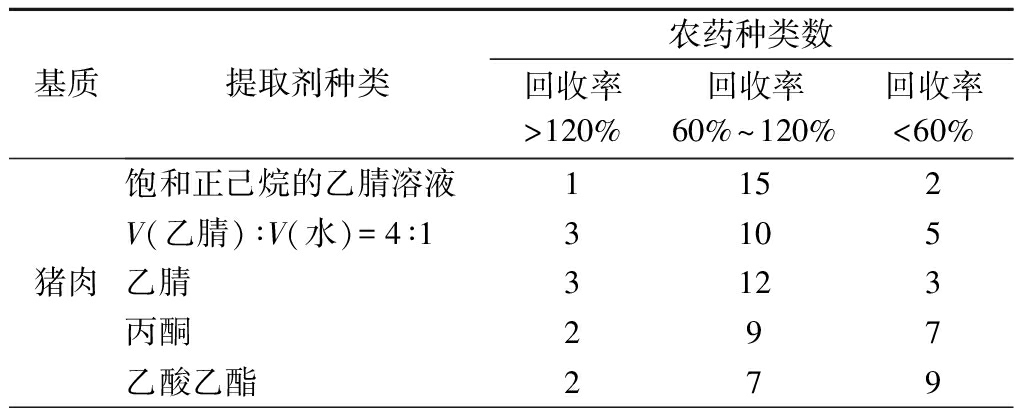
基质提取剂种类农药种类数回收率>120%回收率60%~120%回收率<60%猪肉饱和正己烷的乙腈溶液1152V(乙腈)∶V(水)=4∶13105乙腈3123丙酮297乙酸乙酯279
续表1
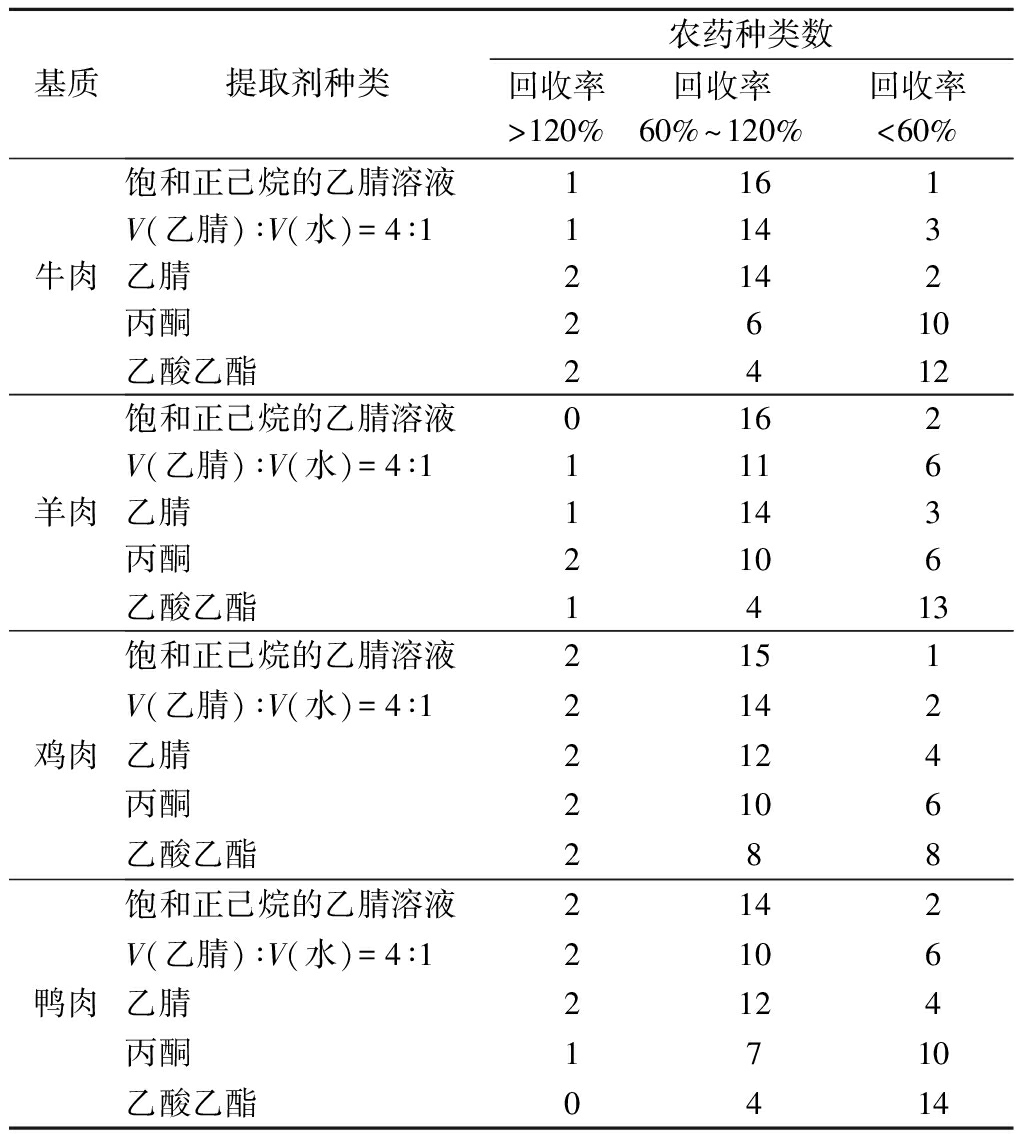
基质提取剂种类农药种类数回收率>120%回收率60%~120%回收率<60%牛肉饱和正己烷的乙腈溶液1161V(乙腈)∶V(水)=4∶11143乙腈2142丙酮2610乙酸乙酯2412羊肉饱和正己烷的乙腈溶液0162V(乙腈)∶V(水)=4∶11116乙腈1143丙酮2106乙酸乙酯1413鸡肉饱和正己烷的乙腈溶液2151V(乙腈)∶V(水)=4∶12142乙腈2124丙酮2106乙酸乙酯288鸭肉饱和正己烷的乙腈溶液2142V(乙腈)∶V(水)=4∶12106乙腈2124丙酮1710乙酸乙酯0414
2.1.2 缓冲体系的优化
样品经提取后,向提取液中加入缓冲体系能使其整体状态更加均一,同时利用盐析作用,经离心后可使水相、有机相更易分层。本试验分别考察了4种缓冲体系组合对提取效果的影响,在其他试验条件相同情况下,分别向5种阴性基质中添加20 μg/kg OPPs混合标准溶液,以18种农药回收率为指标,比较不同缓冲体系的萃取效果。由表2可知:缓冲体系(1 g MgSO4+1.5 g CH3COONa)在5种基质中的萃取效果最佳,回收率最高。因此,本试验选择1 g MgSO4和1.5 g CH3COONa作为缓冲体系。
表2 缓冲体系对18种农药回收率的影响
Table 2 Effect of buffer system on the recovery of 18 pesticides
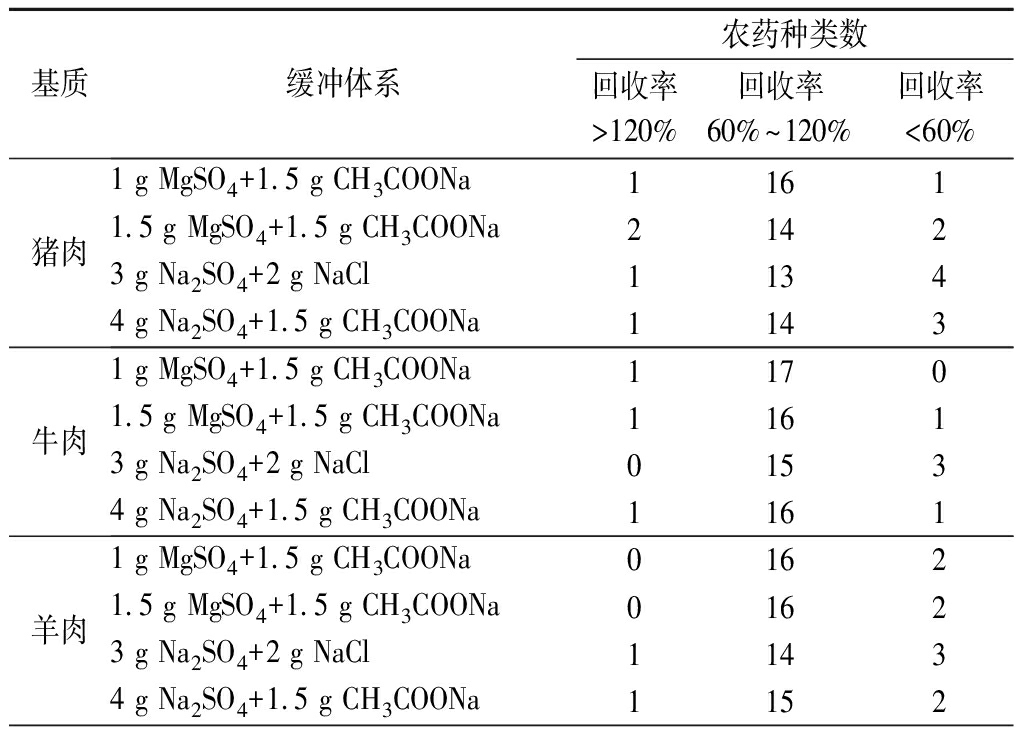
基质缓冲体系农药种类数回收率>120%回收率60%~120%回收率<60%猪肉1 g MgSO4+1.5 g CH3COONa11611.5 g MgSO4+1.5 g CH3COONa21423 g Na2SO4+2 g NaCl11344 g Na2SO4+1.5 g CH3COONa1143牛肉1 g MgSO4+1.5 g CH3COONa11701.5 g MgSO4+1.5 g CH3COONa11613 g Na2SO4+2 g NaCl01534 g Na2SO4+1.5 g CH3COONa1161羊肉1 g MgSO4+1.5 g CH3COONa01621.5 g MgSO4+1.5 g CH3COONa01623 g Na2SO4+2 g NaCl11434 g Na2SO4+1.5 g CH3COONa1152
续表2
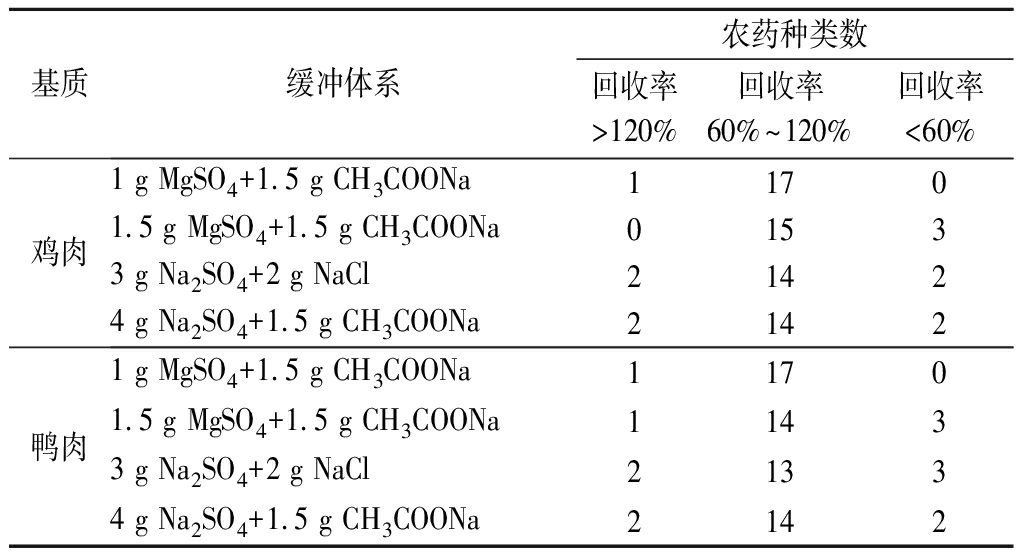
基质缓冲体系农药种类数回收率>120%回收率60%~120%回收率<60%鸡肉1 g MgSO4+1.5 g CH3COONa11701.5 g MgSO4+1.5 g CH3COONa01533 g Na2SO4+2 g NaCl21424 g Na2SO4+1.5 g CH3COONa2142鸭肉1 g MgSO4+1.5 g CH3COONa11701.5 g MgSO4+1.5 g CH3COONa11433 g Na2SO4+2 g NaCl21334 g Na2SO4+1.5 g CH3COONa2142
2.1.3 吸附剂用量的优化
生鲜肉种类繁多,其基质也较为复杂,因此需根据样品基质类型,选择合适配比的净化材料。本试验分别考察了4种吸附剂组合对净化效果的影响,在其他试验条件相同情况下,分别向5种阴性基质中添加20 μg/kg OPPs混合标准溶液,以18种农药回收率为指标,比较不同吸附剂的净化效果。由表3可知,吸附剂(0.15 g PSA+0.15 g C18+1 g MgSO4)在5种基质中的净化效果最佳,回收率均为60%~120%。因此,本试验选择吸附剂(0.15 g PSA+0.15 gC18+1 g MgSO4)组合对样品进行净化。
表3 吸附剂组合对18种农药回收率的影响
Table 3 Effect of adsorbent combinations on the recovery of 18 pesticides
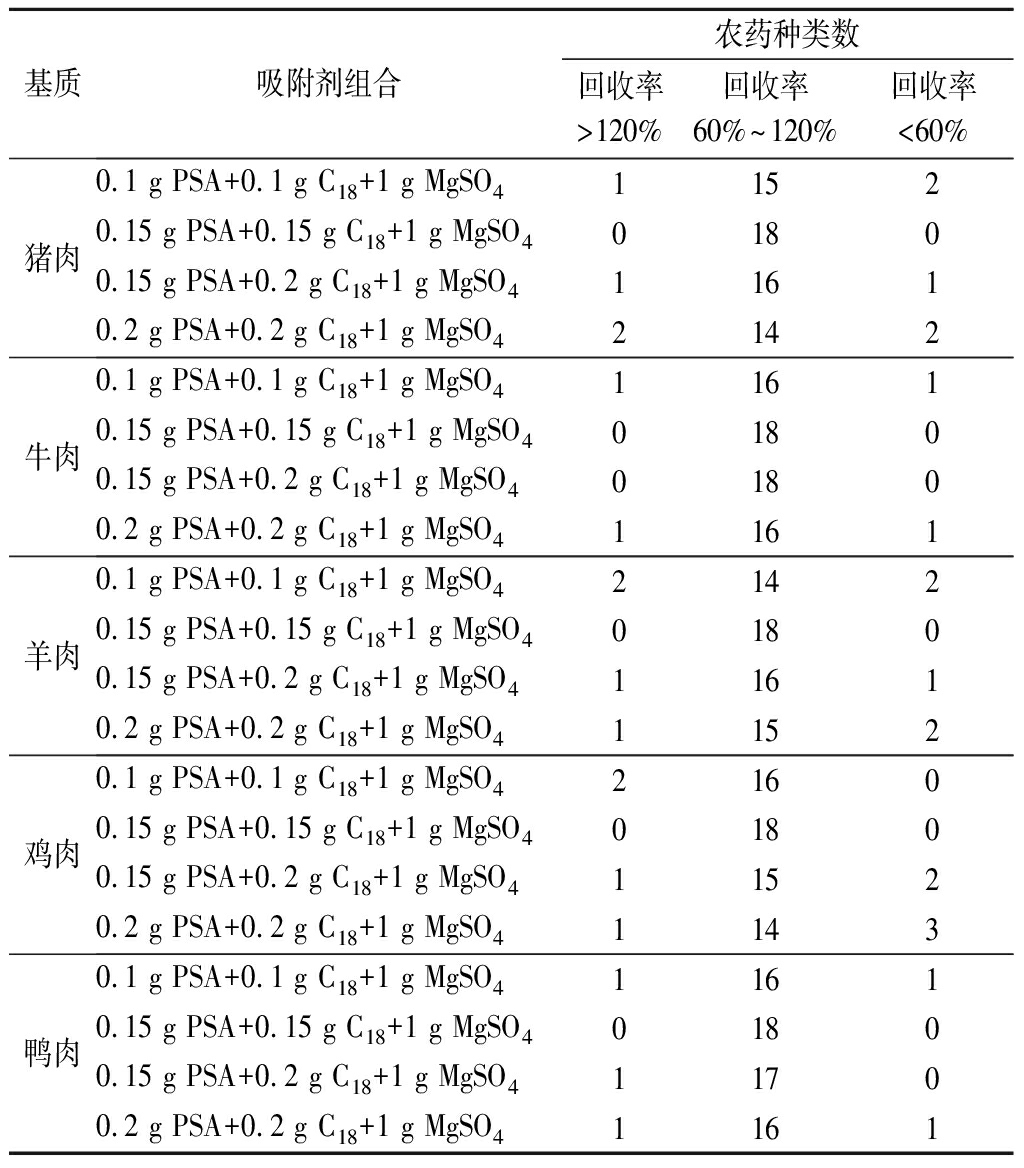
基质吸附剂组合农药种类数回收率>120%回收率60%~120%回收率<60%猪肉0.1 g PSA+0.1 g C18+1 g MgSO411520.15 g PSA+0.15 g C18+1 g MgSO401800.15 g PSA+0.2 g C18+1 g MgSO411610.2 g PSA+0.2 g C18+1 g MgSO42142牛肉0.1 g PSA+0.1 g C18+1 g MgSO411610.15 g PSA+0.15 g C18+1 g MgSO401800.15 g PSA+0.2 g C18+1 g MgSO401800.2 g PSA+0.2 g C18+1 g MgSO41161羊肉0.1 g PSA+0.1 g C18+1 g MgSO421420.15 g PSA+0.15 g C18+1 g MgSO401800.15 g PSA+0.2 g C18+1 g MgSO411610.2 g PSA+0.2 g C18+1 g MgSO41152鸡肉0.1 g PSA+0.1 g C18+1 g MgSO421600.15 g PSA+0.15 g C18+1 g MgSO401800.15 g PSA+0.2 g C18+1 g MgSO411520.2 g PSA+0.2 g C18+1 g MgSO41143鸭肉0.1 g PSA+0.1 g C18+1 g MgSO411610.15 g PSA+0.15 g C18+1 g MgSO401800.15 g PSA+0.2 g C18+1 g MgSO411700.2 g PSA+0.2 g C18+1 g MgSO41161
2.2 GC-MS/MS条件优化
通过优化GC条件,18种OPPs用DB-1701色谱柱均能实现较好地分离,且响应高,无杂峰。因此,本试验在1.3.3节选定的色谱条件下,对每种农药进行全扫描分析,得到18种OPPs标准工作溶液总离子流图(图1)。从总离子流图中确定每种农药的保留时间,并选择质荷比较大、丰度高的特征离子作为母离子。对选定的母离子用选择离子方式进行扫描,从二级质谱图中选择1~2个离子作为子离子,在MRM模式下,进行碰撞能量优化,优化结果见增强出版附表1。
附表1 18种OPPs保留时间、定量离子、定性离子
Appendix 1 Retention time,quantitative ion and qualitative ion of 18 OPPs
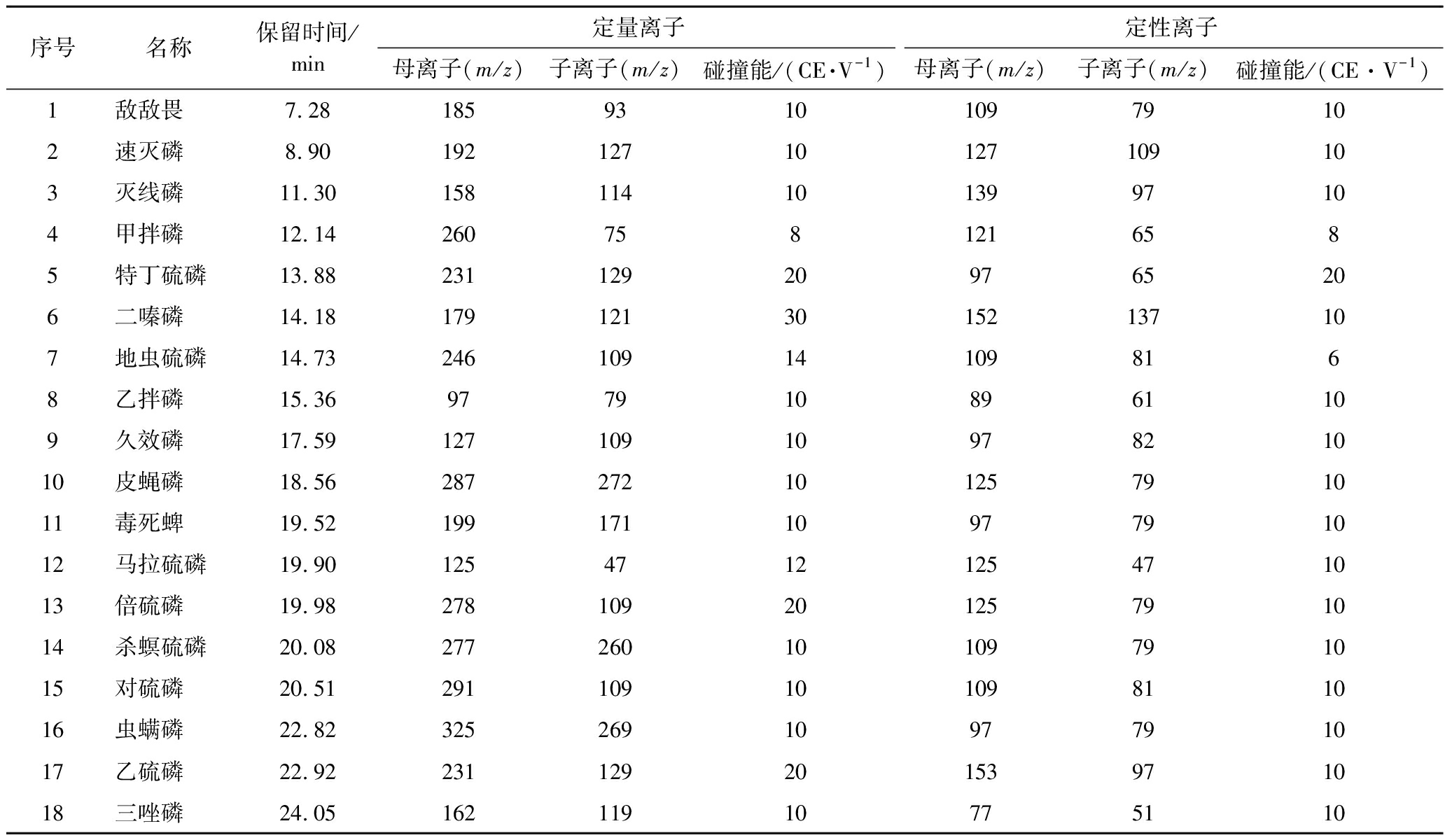
序号名称保留时间/min 定量离子定性离子母离子(m/z)子离子(m/z)碰撞能/(CE·V-1)母离子(m/z)子离子(m/z)碰撞能/(CE·V-1)1敌敌畏7.28185931010979102速灭磷8.9019212710127109103灭线磷11.301581141013997104甲拌磷12.142607581216585特丁硫磷13.88231129209765206二嗪磷14.1817912130152137107地虫硫磷14.73246109141098168乙拌磷15.369779108961109久效磷17.591271091097821010皮蝇磷18.5628727210125791011毒死蜱19.521991711097791012马拉硫磷19.901254712125471013倍硫磷19.9827810920125791014杀螟硫磷20.0827726010109791015对硫磷20.5129110910109811016虫螨磷22.823252691097791017乙硫磷22.9223112920153971018三唑磷24.0516211910775110
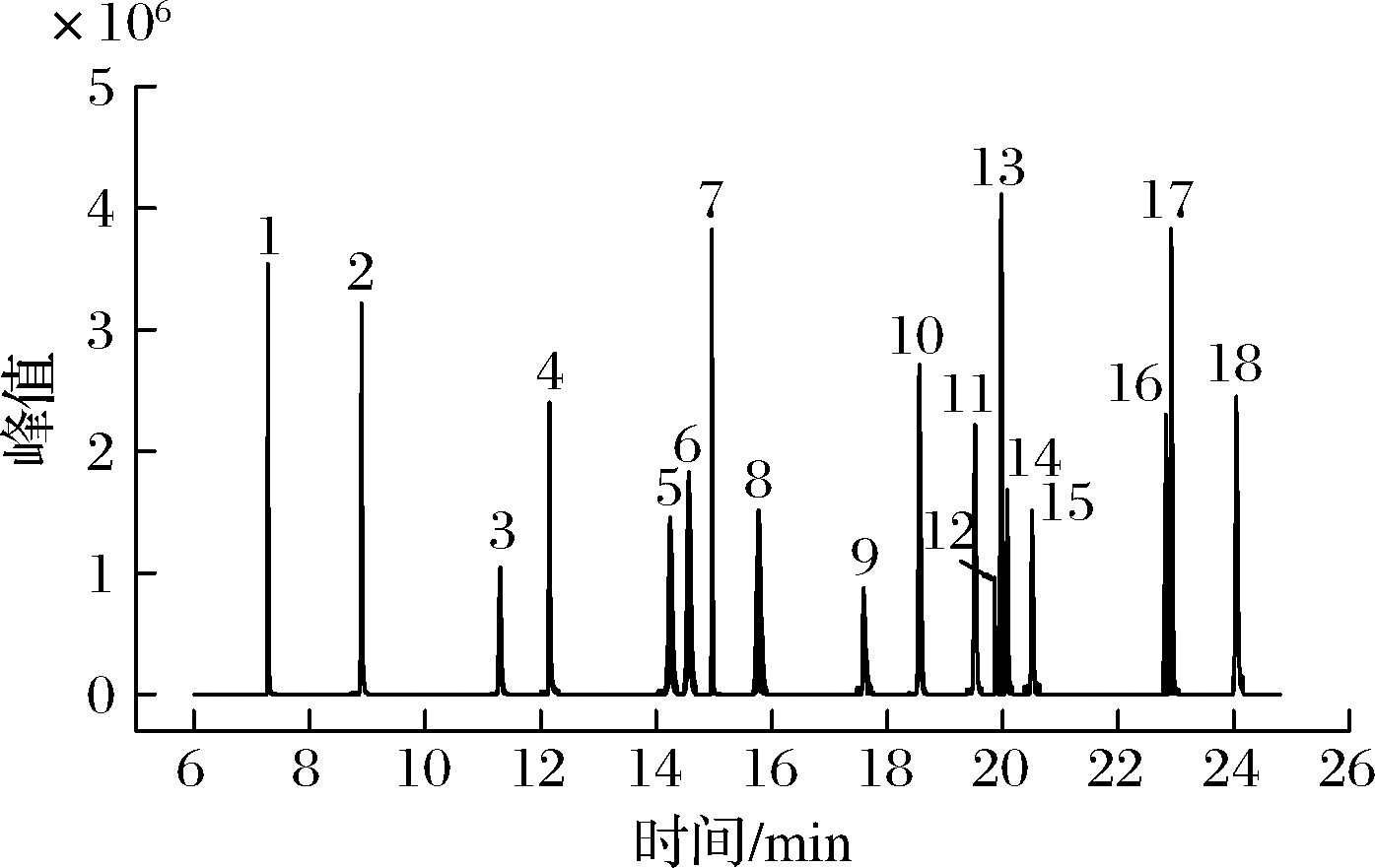
图1 18种OPPs标准工作溶液总离子流图
Fig.1 Total ion chromatogram of 18 OPPs standard working solution
注:1-敌敌畏;2-速灭磷;3-灭线磷;4-甲拌磷;5-特丁硫磷;6-二嗪磷;7-地虫硫磷;8-乙拌磷;9-久效磷;10-皮蝇磷;11-毒死蜱;12-马拉硫磷;13-倍硫磷;14-杀螟硫磷;15-对硫磷;16-虫螨磷;17-乙硫磷;18-三唑磷
2.3 基质效应
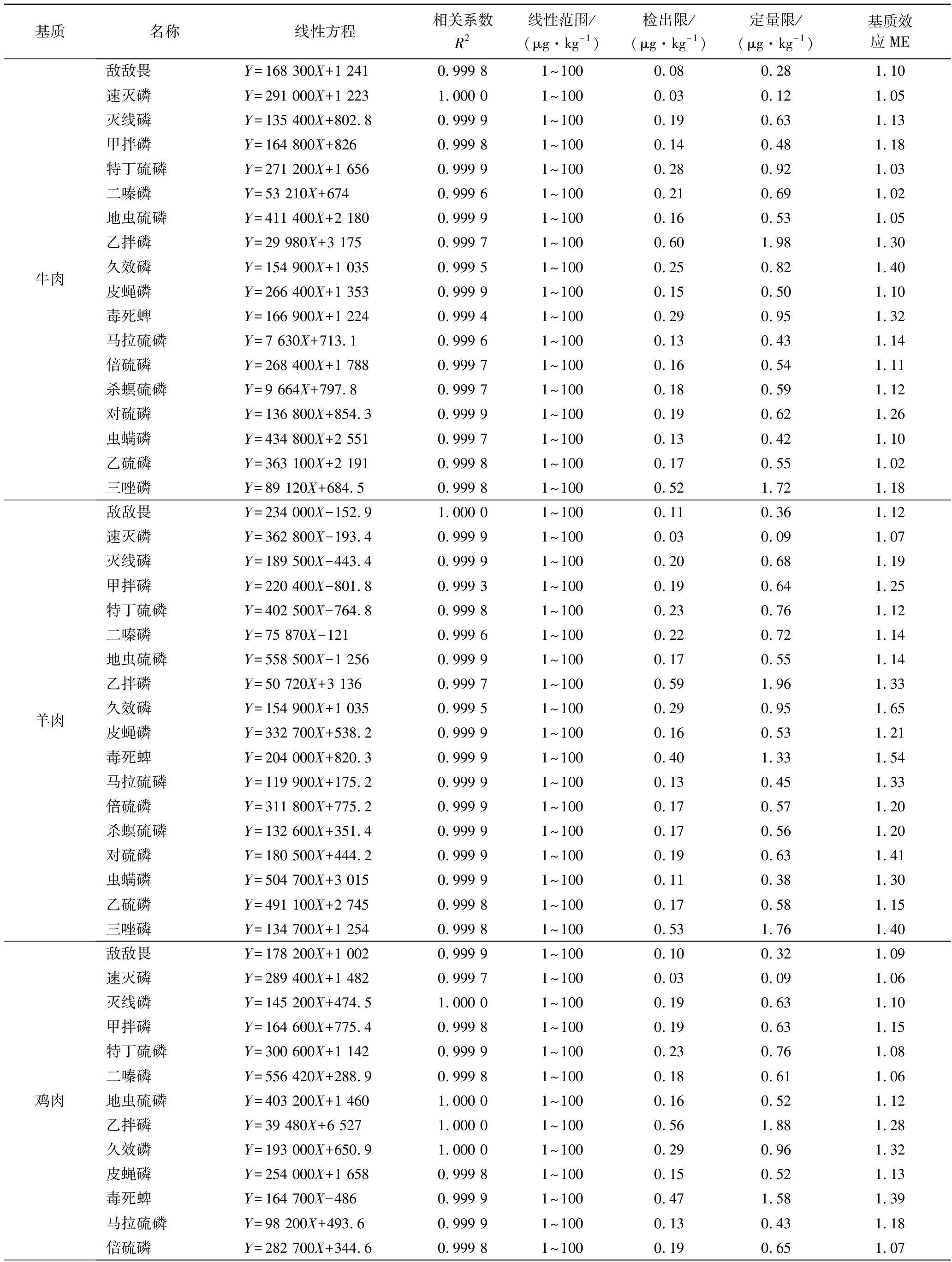
由增强出版附表2可知,18种农药在5种基质中均呈现出基质增强效应。本实验虽通过优化前处理方法减少了其基质干扰,但仍有部分农药ME较强(ME>1.2)。因此,为克服基质效应和确保分析方法的准确性,本试验最终采用基质匹配标准溶液进行校正。
附表2 18种OPPs的线性方程、相关系数及检出限、定量限、基质效应
Appendix 2 Linear equations,correlation coefficients and detection limits,quantification limits,and matrix effects of 18 OPPs
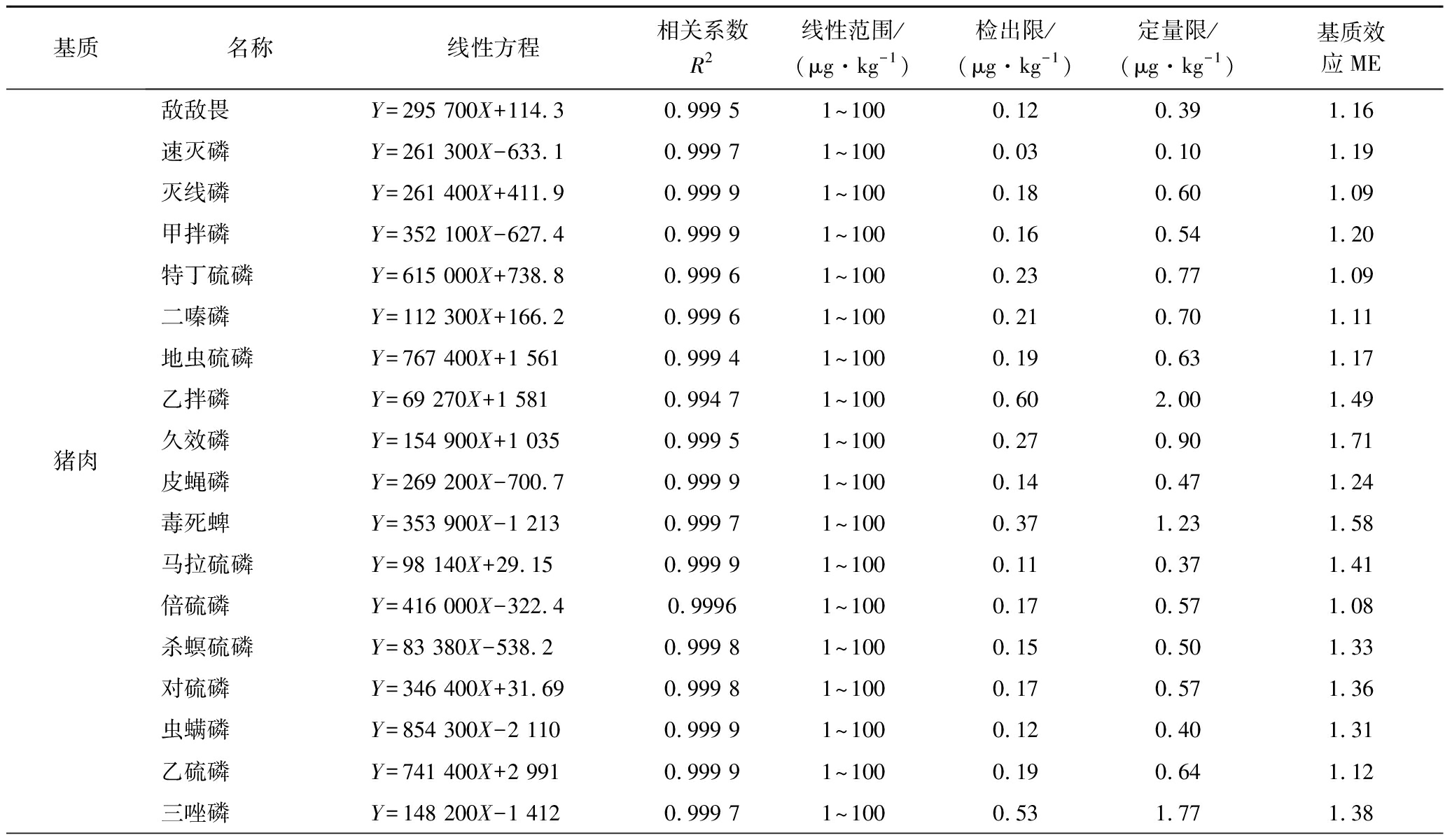
基质名称线性方程相关系数R2线性范围/(μg·kg-1)检出限/(μg·kg-1)定量限/(μg·kg-1)基质效应ME猪肉敌敌畏Y=295 700X+114.30.999 51~1000.120.391.16速灭磷Y=261 300X-633.10.999 71~1000.030.101.19灭线磷Y=261 400X+411.90.999 91~1000.180.601.09甲拌磷Y=352 100X-627.40.999 91~1000.160.541.20特丁硫磷Y=615 000X+738.80.999 61~1000.230.771.09二嗪磷Y=112 300X+166.20.999 61~1000.210.701.11地虫硫磷Y=767 400X+1 5610.999 41~1000.190.631.17乙拌磷Y=69 270X+1 5810.994 71~1000.602.001.49久效磷Y=154 900X+1 0350.999 51~1000.270.901.71皮蝇磷Y=269 200X-700.70.999 91~1000.140.471.24毒死蜱Y=353 900X-1 2130.999 71~1000.371.231.58马拉硫磷Y=98 140X+29.150.999 91~1000.110.371.41倍硫磷Y=416 000X-322.40.99961~1000.170.571.08杀螟硫磷Y=83 380X-538.20.999 81~1000.150.501.33对硫磷Y=346 400X+31.690.999 81~1000.170.571.36虫螨磷Y=854 300X-2 1100.999 91~1000.120.401.31乙硫磷Y=741 400X+2 9910.999 91~1000.190.641.12三唑磷Y=148 200X-1 4120.999 71~1000.531.771.38
附表续表2
基质名称线性方程相关系数R2线性范围/(μg·kg-1)检出限/(μg·kg-1)定量限/(μg·kg-1)基质效应ME牛肉敌敌畏Y=168 300X+1 2410.999 81~1000.080.281.10速灭磷Y=291 000X+1 2231.000 01~1000.030.121.05灭线磷Y=135 400X+802.80.999 91~1000.190.631.13甲拌磷Y=164 800X+8260.999 81~1000.140.481.18特丁硫磷Y=271 200X+1 6560.999 91~1000.280.921.03二嗪磷Y=53 210X+6740.999 61~1000.210.691.02地虫硫磷Y=411 400X+2 1800.999 91~1000.160.531.05乙拌磷Y=29 980X+3 1750.999 71~1000.601.981.30久效磷Y=154 900X+1 0350.999 51~1000.250.821.40皮蝇磷Y=266 400X+1 3530.999 91~1000.150.501.10毒死蜱Y=166 900X+1 2240.999 41~1000.290.951.32马拉硫磷Y=7 630X+713.10.999 61~1000.130.431.14倍硫磷Y=268 400X+1 7880.999 71~1000.160.541.11杀螟硫磷Y=9 664X+797.80.999 71~1000.180.591.12对硫磷Y=136 800X+854.30.999 91~1000.190.621.26虫螨磷Y=434 800X+2 5510.999 71~1000.130.421.10乙硫磷Y=363 100X+2 1910.999 81~1000.170.551.02三唑磷Y=89 120X+684.50.999 81~1000.521.721.18羊肉敌敌畏Y=234 000X-152.91.000 01~1000.110.361.12速灭磷Y=362 800X-193.40.999 91~1000.030.091.07灭线磷Y=189 500X-443.40.999 91~1000.200.681.19甲拌磷Y=220 400X-801.80.999 31~1000.190.641.25特丁硫磷Y=402 500X-764.80.999 81~1000.230.761.12二嗪磷Y=75 870X-1210.999 61~1000.220.721.14地虫硫磷Y=558 500X-1 2560.999 91~1000.170.551.14乙拌磷Y=50 720X+3 1360.999 71~1000.591.961.33久效磷Y=154 900X+1 0350.999 51~1000.290.951.65皮蝇磷Y=332 700X+538.20.999 91~1000.160.531.21毒死蜱Y=204 000X+820.30.999 91~1000.401.331.54马拉硫磷Y=119 900X+175.20.999 91~1000.130.451.33倍硫磷Y=311 800X+775.20.999 91~1000.170.571.20杀螟硫磷Y=132 600X+351.40.999 91~1000.170.561.20对硫磷Y=180 500X+444.20.999 91~1000.190.631.41虫螨磷Y=504 700X+3 0150.999 91~1000.110.381.30乙硫磷Y=491 100X+2 7450.999 81~1000.170.581.15三唑磷Y=134 700X+1 2540.999 81~1000.531.761.40鸡肉敌敌畏Y=178 200X+1 0020.999 91~1000.100.321.09速灭磷Y=289 400X+1 4820.999 71~1000.030.091.06灭线磷Y=145 200X+474.51.000 01~1000.190.631.10甲拌磷Y=164 600X+775.40.999 81~1000.190.631.15特丁硫磷Y=300 600X+1 1420.999 91~1000.230.761.08二嗪磷Y=556 420X+288.90.999 81~1000.180.611.06地虫硫磷Y=403 200X+1 4601.000 01~1000.160.521.12乙拌磷Y=39 480X+6 5271.000 01~1000.561.881.28久效磷Y=193 000X+650.91.000 01~1000.290.961.32皮蝇磷Y=254 000X+1 6580.999 81~1000.150.521.13毒死蜱Y=164 700X-4860.999 91~1000.471.581.39马拉硫磷Y=98 200X+493.60.999 91~1000.130.431.18倍硫磷Y=282 700X+344.60.999 81~1000.190.651.07
附表续表2

基质名称线性方程相关系数R2线性范围/(μg·kg-1)检出限/(μg·kg-1)定量限/(μg·kg-1)基质效应ME鸡肉杀螟硫磷Y=107 600X+471.70.999 91~1000.150.491.16对硫磷Y=189 500X+8340.999 91~1000.190.621.31虫螨磷Y=459 600X+2 8231.000 01~1000.120.391.04乙硫磷Y=465 000X+3 7090.999 81~1000.210.691.06三唑磷Y=115 700X+2 7780.999 71~1000.662.211.15鸭肉敌敌畏Y=250 700X-107.10.999 91~1000.090.291.16速灭磷Y=399 500X-5681.000 01~1000.020.081.19灭线磷Y=204 300X+113.90.999 71~1000.160.541.15甲拌磷Y=225 600X+7260.998 81~1000.200.671.30特丁硫磷Y=457 500X-179.80.99961~1000.210.721.17二嗪磷Y=91 030X-11.850.999 91~1000.190.641.09地虫硫磷Y=606 700X+257.50.999 81~1000.160.521.18乙拌磷Y=79 840X+3 3610.999 91~1000.501.681.38久效磷Y=3 602X-4.1540.997 81~1000.260.871.62皮蝇磷Y=354 900X+477.60.999 71~1000.150.501.25毒死蜱Y=166 900X+1 2240.999 41~1000.391.301.49马拉硫磷Y=139 300X+1270.999 91~1000.130.421.30倍硫磷Y=351 300X-202.71.000 01~1000.170.551.16杀螟硫磷Y=144 500X+142.30.999 81~1000.130.431.31对硫磷Y=219 400X+398.20.999 41~1000.200.661.28虫螨磷Y=605 900X+1 7470.999 51~1000.100.331.26乙硫磷Y=645 800X+940.20.999 61~1000.160.531.09三唑磷Y=191 200X+915.70.999 21~1000.742.481.32
2.4 方法学验证
2.4.1 方法的线性范围、检出限和定量限
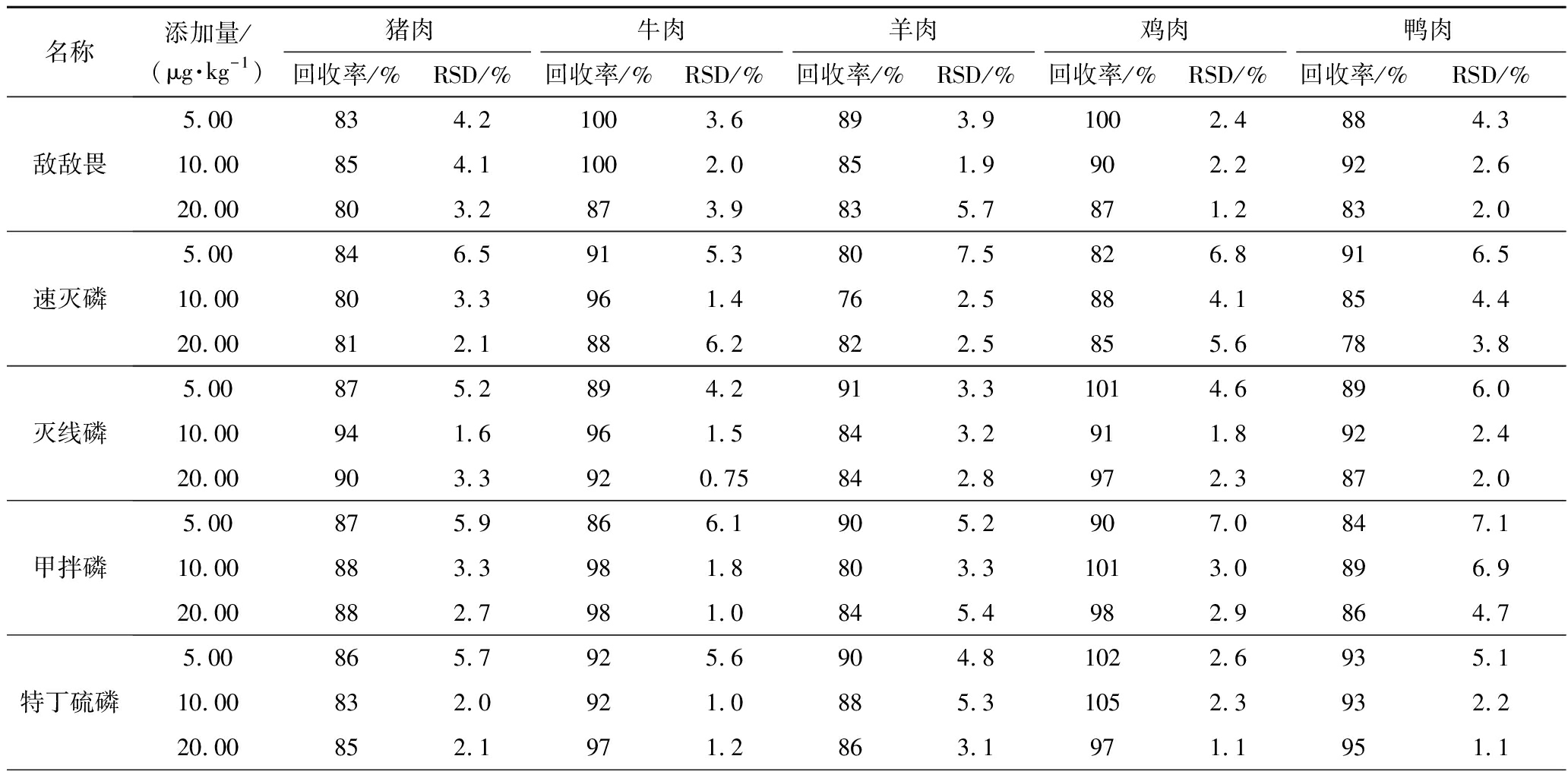
本试验采用空白基质提取液配制基质匹配标准工作溶液,共设置7个浓度点,绘制标准曲线。由增强出版附表3可见,每种农药在1~100 μg/kg均呈现良好线性关系(R2>0.990 0),检出限为0.02~0.74 μg/kg,定量限为0.08~2.48 μg/kg。
附表3 18种OPPs的加标回收率和RSD(n=6)
Appendix 3 The recovery rate and relative standard deviation of 18 OPPs(n=6)
名称添加量/(μg·kg-1)猪肉牛肉羊肉鸡肉鸭肉回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%敌敌畏5.00834.21003.6893.91002.4884.310.00854.11002.0851.9902.2922.620.00803.2873.9835.7871.2832.0速灭磷5.00846.5915.3807.5826.8916.510.00803.3961.4762.5884.1854.420.00812.1886.2822.5855.6783.8灭线磷5.00875.2894.2913.31014.6896.010.00941.6961.5843.2911.8922.420.00903.3920.75842.8972.3872.0甲拌磷5.00875.9866.1905.2907.0847.110.00883.3981.8803.31013.0896.920.00882.7981.0845.4982.9864.7特丁硫磷5.00865.7925.6904.81022.6935.110.00832.0921.0885.31052.3932.220.00852.1971.2863.1971.1951.1
附表续表3
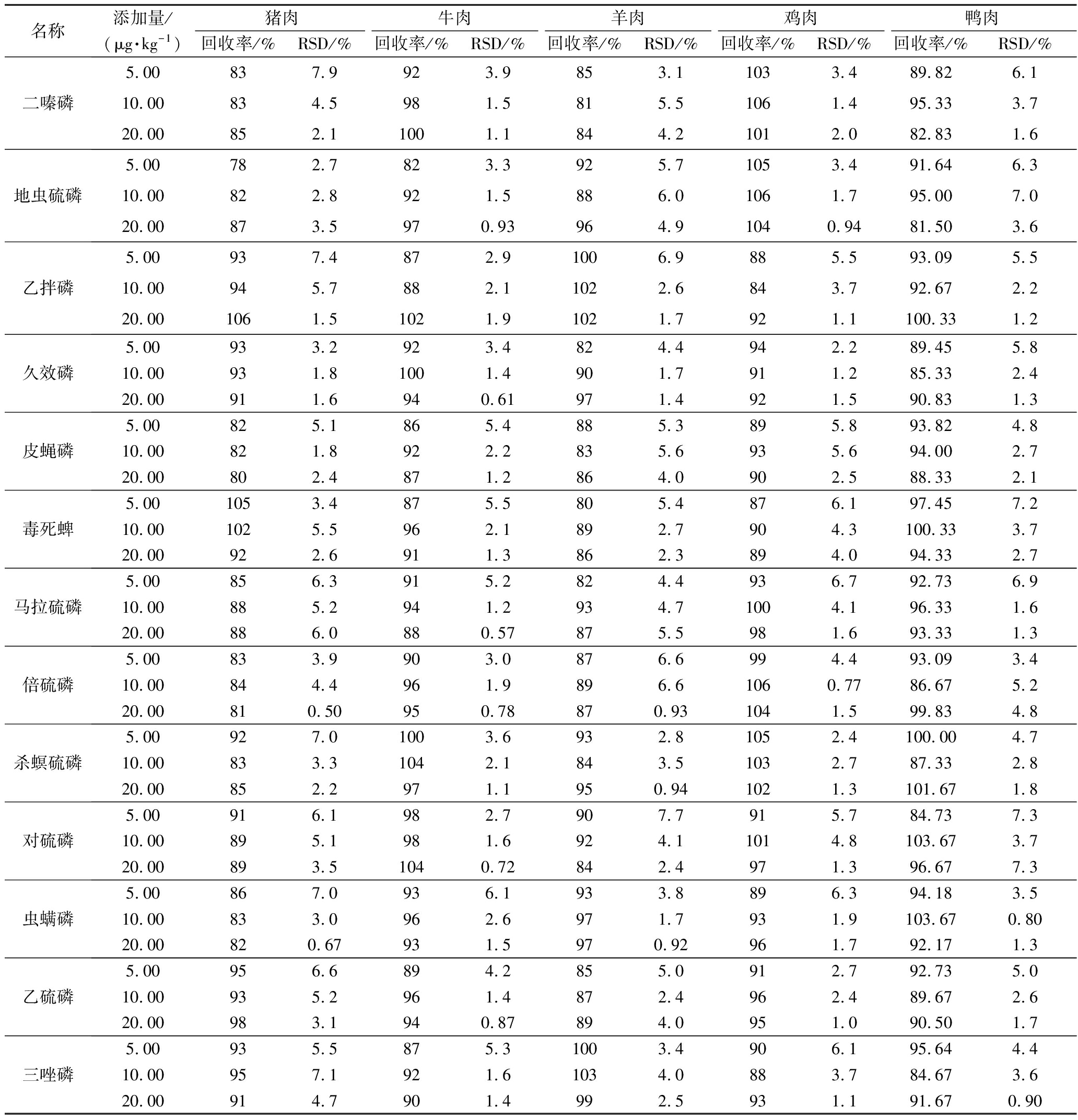
名称添加量/(μg·kg-1)猪肉牛肉羊肉鸡肉鸭肉回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%二嗪磷5.00837.9923.9853.11033.489.826.110.00834.5981.5815.51061.495.333.720.00852.11001.1844.21012.082.831.6地虫硫磷5.00782.7823.3925.71053.491.646.310.00822.8921.5886.01061.795.007.020.00873.5970.93964.91040.9481.503.6乙拌磷5.00937.4872.91006.9885.593.095.510.00945.7882.11022.6843.792.672.220.001061.51021.91021.7921.1100.331.2久效磷5.00933.2923.4824.4942.289.455.810.00931.81001.4901.7911.285.332.420.00911.6940.61971.4921.590.831.3皮蝇磷5.00825.1865.4885.3895.893.824.810.00821.8922.2835.6935.694.002.720.00802.4871.2864.0902.588.332.1毒死蜱5.001053.4875.5805.4876.197.457.210.001025.5962.1892.7904.3100.333.720.00922.6911.3862.3894.094.332.7马拉硫磷5.00856.3915.2824.4936.792.736.910.00885.2941.2934.71004.196.331.620.00886.0880.57875.5981.693.331.3倍硫磷5.00833.9903.0876.6994.493.093.410.00844.4961.9896.61060.7786.675.220.00810.50950.78870.931041.599.834.8杀螟硫磷5.00927.01003.6932.81052.4100.004.710.00833.31042.1843.51032.787.332.820.00852.2971.1950.941021.3101.671.8对硫磷5.00916.1982.7907.7915.784.737.310.00895.1981.6924.11014.8103.673.720.00893.51040.72842.4971.396.677.3虫螨磷5.00867.0936.1933.8896.394.183.510.00833.0962.6971.7931.9103.670.8020.00820.67931.5970.92961.792.171.3乙硫磷5.00956.6894.2855.0912.792.735.010.00935.2961.4872.4962.489.672.620.00983.1940.87894.0951.090.501.7三唑磷5.00935.5875.31003.4906.195.644.410.00957.1921.61034.0883.784.673.620.00914.7901.4992.5931.191.670.90
2.4.2 方法添加回收率和精密度
根据GB 2763—2021规定,生鲜肉中农药残留限量一般在10 μg/kg。因此,本试验选取1/2限量、限量及2倍限量对每种基质进行低、中、高3个水平(5、10、20 μg/kg)的加标回收试验。由表6可知,样品平均回收率为76%~106%,RSD为0.50%~7.9%。
2.5 方法实际应用评价
为证实本方法的实际应用价值,分别在超市和农贸市场随机选取30份生鲜肉作为研究对象,采用本试验建立的方法进行检测。由表4可知,在1份牛肉中检出马拉硫磷(0.8 μg/kg),1份羊肉中检出倍硫磷(0.6 μg/kg)。
表4 市售产品中农药检测结果 单位:μg/kg
Table 4 Detection results of pesticides in commercial products
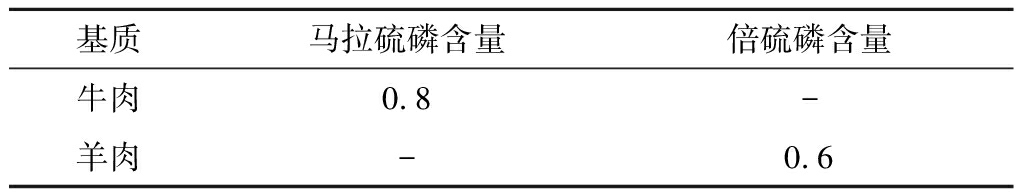
基质马拉硫磷含量倍硫磷含量牛肉0.8-羊肉-0.6
注:“-”未检出
3 讨论
GC-MS/MS法具有灵敏度好、准确度高,定性性能强等优点,能够实现多种农药残留的高通量快速检测。本文采用GC-MS/MS法检测生鲜肉中18种OPPs残留,所有检测目标物分离良好,重现性好,并基于优化的QuEChERS法进行样品前处理,可有效降低畜禽肉中脂肪、蛋白质及色素等基质干扰,提高目标物回收率,其中猪肉、羊肉因脂肪含量高,基质效应强,其回收率略低于其他肉类。
本研究建立了一种QuEChERS结合GC-MS/MS检测生鲜肉中18种OPPs残留的分析方法。18种OPPs的检出限可达0.02~0.74 μg/kg,定量限0.08~2.48 μg/kg,均低于文献[24-26]的报道。低、中、高3个浓度水平的加标回收率在76%~106%,RSD为0.50%~7.9%,方法的回收率和精密度符合GB/T 27404—2008《实验室质量控制规范 食品理化检测》对回收率和精密度的要求。该方法在实际应用中简单、快速、高效,可满足生鲜肉中18种OPPs残留的高通量快速检测,对加强生鲜肉在整个流通环节的追踪和监控以及保障我国食品安全具有重大意义。
[1] 李雯雯,王岩,王盛男,等.植物源性食品中有机磷农药残留检测前处理技术的研究进展[J].食品安全质量检测学报,2020,11(6):1 852-1 858.
LI W W,WANG Y,WANG S N,et al.Research progress of pretreatment technology for detection of organophosphorus pesticide residues in plant-derived foods[J].Journal of Food Safety & Quality,2020,11(6):1 852-1 858.
[2] GRILO A,MOREIRA A,CARRAPIÇO B,et al.Epidemiological study of pesticide poisoning in domestic animals and wildlife in Portugal:2014-2020[J].Frontiers in Veterinary Science,2020,7:616293.
[3] 沙鸥,姚佳伟,许丽,等.有机磷农药中毒患者体内有机磷检测研究进展[J].化学试剂,2021,43(7):857-864.
SHA O,YAO J W,XU L,et al.Research progress on organophosphorus detection in patients with organophosphorus pesticide poisoning in diagnosis[J].Chemical Reagents,2021,43(7):857-864.
[4] 刘仁杰,赵悦,王玉华,等.有机磷农药残留现状及去除方法的研究进展[J].食品工业,2019,40(9):299-302.
LIU R J,ZHAO Y,WANG Y H,et al.Research progress of organophosphorus pesticide residues status and removal methods[J].The Food Industry,2019,40(9):299-302.
[5] 张峰祖,朴秀英,李富根.国际食品法典委员会动物源性食品中农药最大残留限量制定现状[J].农药学学报,2021,23(1):22-30.
ZHANG F Z,PIAO X Y,LI F G.Current situation on setting of pesticide maximum residue limits in food commodities of animal origin in Codex Alimentarius Commission[J].Journal of Pesticide Science,2021,23(1):22-30.
[6] 穆兰,朴秀英,陈晓初,等.韩国农药残留肯定列表制度对我国农产品出口贸易的影响[J].农药科学与管理,2019,40(4):12-15.
MU L,PIAO X Y,CHEN X C,et al.Influence on agricultural product export from China bysouth Korea PLS[J].Pesticide Science and Administration,2019,40(4):12-15.
[7] DALLEGRAVE A,PIZZOLATO T M,BARRETO F,et al.Methodology for trace analysis of 17 pyrethroids and chlorpyrifos in foodstuff by gas chromatography-tandem mass spectrometry[J].Analytical and Bioanalytical Chemistry,2016,408(27):7 689-7 697.
[8] MADEJ K,KALENIK T K,PIEKOSZEWSKI W.Sample preparation and determination of pesticides in fat-containing foods[J].Food Chemistry,2018,269:527-541.
[9] PANG G F,CAO Y Z,ZHANG J J,et al.Validation study on 660 pesticide residues in animal tissues by gel permeation chromatography cleanup/gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry[J].Journal of Chromatography.A,2006,1125(1):1-30.
[10] CASTILLO M,GONZ LEZ C,MIRALLES A.An evaluation method for determination of non-polar pesticide residues in animal fat samples by using dispersive solid-phase extraction clean-up and GC-MS[J].Analytical and Bioanalytical Chemistry,2011,400(5):1 315-1 328.
LEZ C,MIRALLES A.An evaluation method for determination of non-polar pesticide residues in animal fat samples by using dispersive solid-phase extraction clean-up and GC-MS[J].Analytical and Bioanalytical Chemistry,2011,400(5):1 315-1 328.
[11] HU S P,ZHAO M,MAO Q Q,et al.Rapid one-step cleanup method to minimize matrix effects for residue analysis of alkaline pesticides in tea using liquid chromatography-high resolution mass spectrometry[J].Food Chemistry,2019,299:125146.
[12] ISLAM A,NOH HH,RO J H,et al.Optimization and validation of a method for the determination of acidic pesticides in cabbage and spinach by modifying QuEChERS procedure and liquid chromatography-tandem mass spectrometry[J].Journal of Chromatography B,2021,1173:122667.
[13] 郭思依,孙明娜,董旭,等.免疫分析技术在农药残留快速检测中的应用及研究进展[J].中国农学通报,2021,37(23):106-112.
GUO S Y,SUN M N,DONG X,et al.Application and research progress of immunoassay technology in rapid detection of pesticide residues[J].Chinese Agricultural Science Bulletin,2021,37(23):106-112.
[14] YANG L X,LI H L,ZENG F G,et al.Determination of 49 organophosphorus pesticide residues and their metabolites in fish,egg,and milk by dual gas chromatography-dual pulse flame photometric detection with gel permeation chromatography cleanup[J].Journal of Agricultural and Food Chemistry,2012,60(8):1 906-1 913.
[15] 段税优,卢专,杨昌彪,等.QuEChERS结合GC-MS法测定新鲜鸡蛋中15种农药残留[J].现代食品,2019(14):123-128.
DUAN S Y,LU Z,YANG C B,et al.Determination of 15 pesticide residues in fresh eggs byQuEChERS combined with GC-MS[J].Modern Food,2019(14):123-128.
[16] 吕青骎,王玮,梁丽姣,等.QuEChERS结合超高效液相色谱质谱法测定禽蛋及其制品中42种农药及其代谢物[J].食品与发酵工业,2020,46(5):255-262.
LV Q Q,WANG W,LIANG L J,et al.Determination of 42 pesticides and their metabolites in poultry eggs and products by QuEChERS-UPLC-MS/MS[J].Food and Fermentation Industries,2020,46(5):255-262.
[17] KILJANEK T,NIEWIADOWSKA A,MA YSIAK M,et al.Miniaturized multiresidue method for determination of 267 pesticides,their metabolites and polychlorinated biphenyls in low mass beebread samples by liquid and gas chromatography coupled with tandem mass spectrometry[J].Talanta,2021,235:122721.
YSIAK M,et al.Miniaturized multiresidue method for determination of 267 pesticides,their metabolites and polychlorinated biphenyls in low mass beebread samples by liquid and gas chromatography coupled with tandem mass spectrometry[J].Talanta,2021,235:122721.
[18] BARCI P E P,ALVES L D S,AVELLAR  A S,et al.ModifiedQuEChERS method for multiresidue determination of pesticides in pecan nuts by liquid chromatography tandem mass spectrometry[J].Food Analytical Methods,2020,13(3):793-801.
A S,et al.ModifiedQuEChERS method for multiresidue determination of pesticides in pecan nuts by liquid chromatography tandem mass spectrometry[J].Food Analytical Methods,2020,13(3):793-801.
[19] 李德青,石桂珍.有机磷农药残留检测方法研究进展与分析[J].煤炭与化工,2020,43(2):146-150.
LI D Q,SHI G Z.Research progress and analysis of organophosphorus pesticide residue detection methods[J].Coal and Chemical Industry,2020,43(2):146-150.
[20] SONG L,HAN Y T,YANG J,et al.Rapid single-step cleanup method for analyzing 47 pesticide residues in pepper,chili peppers and its sauce product by high performance liquid and gas chromatography-tandem mass spectrometry[J].Food Chemistry,2019,279:237-245.
[21] WALORCZYK S.Validation and use of a QuEChERS-based gas chromatographic-tandem mass spectrometric method for multiresidue pesticide analysis in blackcurrants including studies of matrix effects and estimation of measurement uncertainty[J].Talanta,2014,120:106-113.
[22] GIACINTI G,RAYNAUD C,CAPBLANCQ S,et al.Evaluation and prevention of the negative matrix effect of terpenoids on pesticides in apples quantification by gas chromatography-tandem mass spectrometry[J].Journal of Chromatography A,2017,1 483:8-19.
[23] 李敏青,安文佳,李菊,等.固相萃取/气相色谱法测定禽蛋中19种农药残留[J].分析测试学报,2020,39(4):520-525.
LI M Q,AN W J,LI J,et al.Determination of 19 pesticides residues in eggs by gas chromatography with solid phase extraction[J].Journal of Analytical Testing,2020,39(4):520-525.
[24] 吴卫东,吴凤琪,万志刚,等.在线凝胶渗透色谱-串联气质联用法测定植物油和猪肉中55种农药残留[J].食品安全质量检测学报,2014,5(11):3 400-3 409.
WU W D,WU F Q,WAN Z G,et al.Determination of 55 pesticide residues in vegetable oil and animal tissues using on-line gel permeation chromatography coupled with gas chromatography-mass spectrometry[J].Journal of Food Safety and Quality,2014,5(11):3 400-3 409.
[25] HAMADAMIN A Y,HASSAN K I.Gas chromatography-mass spectrometry based sensitive analytical approach to detect and quantify non-polar pesticides accumulated in the fat tissues of domestic animals[J].Saudi Journal of Biological Sciences,2020,27(3):887-893.
[26] KANG H S,KIM M,KIM E J,et al.Determination of 66 pesticide residues in livestock products using QuEChERS and GC-MS/MS[J].Food Science and Biotechnology,2020,29(11):1 573-1 586.