干酪的微生物生态系统组成主要由干酪加工过程中添加的中温乳球菌(如Lactococcus lactis subsp.Cremoris,Lactococcus lactis subsp.lactis)为主,或以添加非发酵剂乳酸菌(non-starter lactic acid bacteria,NSLAB)为辅[1-2],如乳杆菌(Lactobacillus)、片球菌(Pediococcus)、双歧杆菌(Bifidobacterium)等,这些附属发酵菌株单独或联合使用,有助于促进干酪中蛋白和脂肪的酶解,产生游离氨基酸、脂肪酸和风味物质,可以改善干酪的口感、风味、质构和营养特性[3-8]。
益生菌干酪是指在干酪加工过程中添加益生菌为附属发酵剂制成的干酪产品[9-10]。益生菌赋予了干酪新的功能,提升干酪品质和安全性并对健康有促进作用。同时,干酪的高脂肪、高蛋白、低含氧量等理化性质是附属发酵剂益生菌的优良载体[11],有利于菌株存活。因此,这类益生菌与干酪完美结合的新型干酪产品成为当前功能食品领域的研究热点。目前,商业上应用的附属发酵剂主要为乳杆菌属和双歧杆菌属的菌株,其他功能性益生菌株应用于干酪的研究较少。筛选具有优良发酵特性和功能作用的益生菌附属发酵剂,对于开发益生菌干酪新产品具有十分重要的意义。本研究团队前期筛选评价获得2株功能益生菌新菌株,其中1株为降血脂和改善脂肪肝作用的植物乳杆菌(Lactobacillus plantarum)NA136[12-13],另1株为具有预防和治疗早期动脉粥样硬化的乳酸片球菌(Pediococcus acidilactici)AS185[14],且2株菌具有较好的耐受、黏附、产酸和生长特性,本研究以这2株益生菌为附属发酵剂加工益生菌切达干酪,评价益生菌对干酪品质提升的作用。
1 材料与方法
1.1 材料与试剂
植物乳杆菌(L. plantarum)NA136由本实验室分离自延吉农家酸菜,现保藏于中国典型培养物保藏中心(CCTCC NO:M 2018112);乳酸片球菌(P. acidilactici)AS185由本实验室分离自延吉农家黄豆酱,现保藏于中国典型培养物保藏中心(CCTCC NO:M 2018114);商品发酵剂R707(由乳酸乳球菌乳酸亚种(Lactococcus lactis.subsp.lactis)和乳酸乳球菌乳脂亚种(Lactococcus lactis.subsp.cremoris)组成)、凝乳酶(CHY-MAX Power NB),丹麦科汉森公司。
1.2 仪器与设备
MLS-3780高压蒸汽灭菌锅,日本Sanyo公司;BCN-1360B无菌超净工作台,哈尔滨东联电子技术开发有限公司;HZQ-Q电热恒温培养箱,上海一恒实验设备有限公司;Sorvall Evolotion RC高速冷冻离心机,美国Thermo公司;Lacto Star乳成分含量分析仪,德国Gerber公司;HYCV-407干酪槽、HYPC-4压榨机,黑龙江赫益乳业科技有限公司;SHP-60匀浆机,上海科学技术大学机电厂;Testo205 pH计,德国Testo公司;TA.XT plus物性分析仪,英国Stable Micro Systems公司;1260高效液相色谱仪、7890B-7000D气相色谱-质谱联用仪,美国Agilent公司。
1.3 培养基
改良MRS培养基(g/L):蛋白胨 10.0,牛肉浸粉 10.0,酵母浸粉 2.0,磷酸氢二钾 2.0,无水乙酸钠5.0,枸橼酸三铵 2.0,硫酸镁 0.2,硫酸锰 0.05,吐温-80 1.0,葡萄糖 20.0,碳酸钙 5.0,琼脂粉 20.0,pH=6.0~6.5,115 ℃高压灭菌20 min。
改良M17培养基(g/L):胰蛋白胨 10.0,牛肉膏 5.0,酵母膏 2.5,磷酸甘油二钠 10.0,硫酸镁 0.25,异抗坏血酸钠 0.5,琼脂粉 20.0,pH=7.1~7.2,115 ℃高压灭菌20 min。
1.4 试验方法
1.4.1 菌悬液制备
植物乳杆菌NA136和乳酸片球菌AS185的冻存菌种经连续传代活化后,分别接种于改良MRS及M17液体培养基中,37 ℃静置培养16 h后,离心(5 000 r/min,4 ℃,10 min),去上清液,用PBS溶液(pH=7.2)洗涤菌体沉淀,再重悬于PBS溶液中,调整菌液浓度为1.0×1010CFU/mL,置于4 ℃冰箱保存备用。
1.4.2 切达干酪制作与分组
切达干酪加工工艺如下:
原料乳→过滤→巴氏杀菌(63 ℃,30 min)→冷却(37 ℃)→接种商品发酵剂和益生菌(NA136或AS185)→发酵(37 ℃,60 min)→添加氯化钙(0.1 g/L)→添加凝乳酶(0.014 g/L)→凝乳(37 ℃,35~50 min)→切割凝块→搅拌、热烫(30 min内升温至42 ℃)→排乳清→堆酿→破碎凝块→拌盐(2%,质量分数)→预压榨(0.2 MPa,1.5 h)→压榨(0.5 MPa,20 h)→真空包装→贮存成熟(8~12 ℃,相对湿度70%)。
干酪制作分组:分为对照组、NA136组和AS185组,对照组仅接种商品发酵剂(R707 0.03 g/L),NA136组和AS185组接种商品发酵剂的同时分别添加对应的菌悬液,并使其在原料乳中的终浓度达到1.0×108CFU/mL。
1.4.3 理化指标测定
蛋白质含量测定参照GB 5009.5—2016,脂肪含量测定参照GB 5009.6—2016,水分含量测定参照GB 5009.3—2016,pH值测定用Testo205 pH计。
1.4.4 活菌数测定
分别在干酪成熟期的第1、30、60、90天取干酪样品10 g,用90 mL 2%无菌柠檬酸钠溶液(40 ℃)进行均浆处理(2 min),经无菌生理盐水倍比稀释后,分别涂布于改良MRS和M17固体培养基,37 ℃静置培养48 h后进行菌落计数。改良MRS固体培养基用于乳杆菌的分离计数,改良M17固体培养基用于乳球菌的分离计数。
1.4.5 质构测定
分别在干酪成熟期的第1、30、60、90天取干酪样品1.5 cm×1.5 cm×1.5 cm,室温放置2 h后,利用物性分析仪对干酪进行全质构分析。选择P/5探头,设定测量前探头下降速度为1.0 mm/s,测试速度为1.0 mm/s,测量后探头回程速度为5.0 mm/s,下压变形为10.0 mm,触发力为0.2 N。
1.4.6 游离氨基酸测定
分别取不同成熟期(1、30、60、90 d)干酪冻干粉样品30 mg,采用HPLC分析游离氨基酸含量。色谱分析条件:C18色谱柱(4.6 mm×250 mm,5 μm);流动相:A:0.1 mol/L 97%无水乙酸钠乙腈溶液(pH=6.5),B:80%乙腈水溶液;进样量:10 μL;柱温:40 ℃;检测波长:254 nm。
1.4.7 短链脂肪酸测定
分别取不同成熟期(1、30、60、90 d)干酪冻干粉样品30 mg,采用LC/MS分析短链脂肪酸含量。色谱分析条件:HP-INNOWAX色谱柱(25 m×0.20 mm×0.40 μm);升温程序:100 ℃保持5 min,以5 ℃/min升至150 ℃,再以30 ℃/min升至240 ℃,保持30 min;进样口温度:240 ℃;载气流速:1.0 mL/min,不分流;质谱分析条件:离子源温度:200 ℃;传输线温度:250 ℃;质谱:EI源轰击电压:70 eV,单离子扫描模式:定量离子60、73。
1.4.8 感官评定
在干酪成熟90 d时进行感官评定。选取10名对色、味、质属性敏感的资深专家组成评定小组(男∶女=1∶1),对干酪的外型、色泽、纹理图案、滋味、气味和组织状态进行感官评定。评定细则参照RHB 501—2004《切达干酪感官质量评鉴细则》,具体评分标准见表1。
表1 切达干酪感官评定标准
Table 1 Sensory evaluation score of Cheddar cheeses

评价指标特征描述分值/分外型(5分)表皮均匀、细致5表皮粗糙,稍有裂缝4表皮粗糙,裂缝明显<3色泽(5分)乳白色或乳黄色,均匀、有光泽5色泽略有变化4~3色泽有明显变化,不均匀<2纹理图案(15分)有切达干酪特征的“鸡胸纹”图案15纹理图案不清晰14有裂痕13~10有网状结构9~6有孔眼,不密实5~3断面粗糙<3滋味和气味(50分)具有切达干酪特有的滋味和气味,具有奶油味、风味良好 50具有切达干酪特有的滋味和气味,具有奶油味、风味较好49~45滋味和气味良好,但香味较淡44~40滋味和气味合格,但香味淡39~35滋味和气味平淡,无奶香味34~30有明显异味(饲料味、酸异常味、霉味、苦味、氧化味等)<29组织状态(25分)质地紧密、光滑、硬度适度25质地均匀、光滑、硬度适度24质地基本均匀、稍软或稍硬,组织较细腻23组织状态粗糙,较硬22~16组织状态疏松,易碎15~10组织状态呈碎粒状<10
1.5 数据分析
每组实验重复3次,结果以平均值±标准差(SD)表示。采用SPSS 19.0 统计软件对数据进行差异显著性分析,采用GraphPad Prism 6.0软件作图,采用CANOCO 4.5软件对质构、游离氨基酸和短链脂肪酸数据进行冗余分析(redundancy analysis,RDA)。
2 结果与分析
2.1 益生菌对干酪成熟期间基本理化指标的影响
由表2可知,在干酪成熟期间,3组干酪中的蛋白质、水分含量及pH值之间存在差异(P<0.05),脂肪含量无差异(P>0.05);同一成熟期内各指标无差异,组分变化符合切达干酪成分含量标准(蛋白质20%~30%,脂肪20%~34%,水分<39%,pH<5.3)(质量分数)。这与ONG等[15]和段翠翠等[16]的研究结果一致,即添加附属发酵剂乳酸菌对干酪的基本理化指标无影响。说明这2株菌可作为附属发酵剂用于切达干酪的生产。
2.2 益生菌对干酪成熟期间活菌数的影响
益生菌干酪在成熟期内保持较高的活菌数,同时益生菌对干酪的品质无不良影响,是生产益生菌干酪对附属发酵剂益生菌的基本要求[17]。由图1可知,益生菌干酪因添加了L.plantarum NA136或 P.acidilactici AS185而使其乳杆菌数(图1-a)或乳球菌数(图1-b)明显高于对照组。各组的活菌数随干酪成熟逐渐降低;在成熟90 d时,益生菌组活菌数均高于对照组,且益生菌数均在7.5 lgCFU/g以上,符合益生菌食品的活菌数要求(活菌数≥107 CFU/g)[17-18]。此外,由图1-a发现,在第1天时,对照组和AS185组中已检测到了少量的乳杆菌存在,且随成熟期延长,乳杆菌含量在增加并于后期趋于稳定。这与GARDE等[19]研究结果一致,即这些乳杆菌可能来源于加工环境。
表2 干酪成熟期间基本理化指标变化
Table 2 Changes in physicochemical composition during cheese ripening
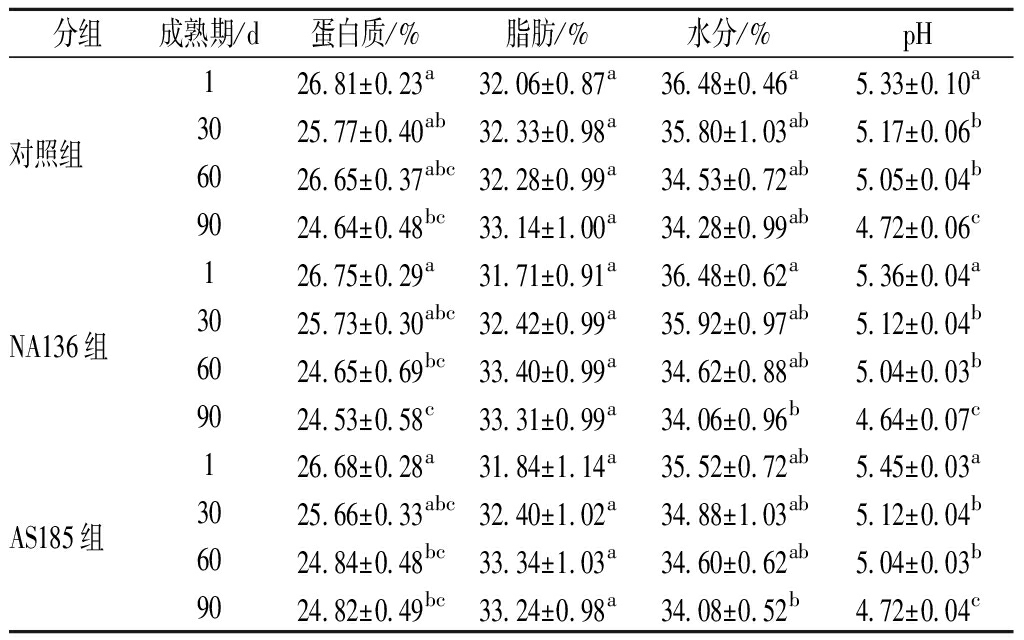
分组成熟期/d蛋白质/%脂肪/%水分/%pH1 26.81±0.23a32.06±0.87a36.48±0.46a5.33±0.10a对照组30 25.77±0.40ab32.33±0.98a35.80±1.03ab5.17±0.06b60 26.65±0.37abc32.28±0.99a34.53±0.72ab5.05±0.04b90 24.64±0.48bc33.14±1.00a34.28±0.99ab4.72±0.06c1 26.75±0.29a31.71±0.91a36.48±0.62a5.36±0.04aNA136组30 25.73±0.30abc32.42±0.99a35.92±0.97ab5.12±0.04b60 24.65±0.69bc33.40±0.99a34.62±0.88ab5.04±0.03b90 24.53±0.58c33.31±0.99a34.06±0.96b4.64±0.07c1 26.68±0.28a31.84±1.14a35.52±0.72ab5.45±0.03aAS185组30 25.66±0.33abc32.40±1.02a34.88±1.03ab5.12±0.04b60 24.84±0.48bc33.34±1.03a34.60±0.62ab5.04±0.03b90 24.82±0.49bc33.24±0.98a34.08±0.52b4.72±0.04c
注:同一列不同字母表示差异显著(P<0.05)(下同)
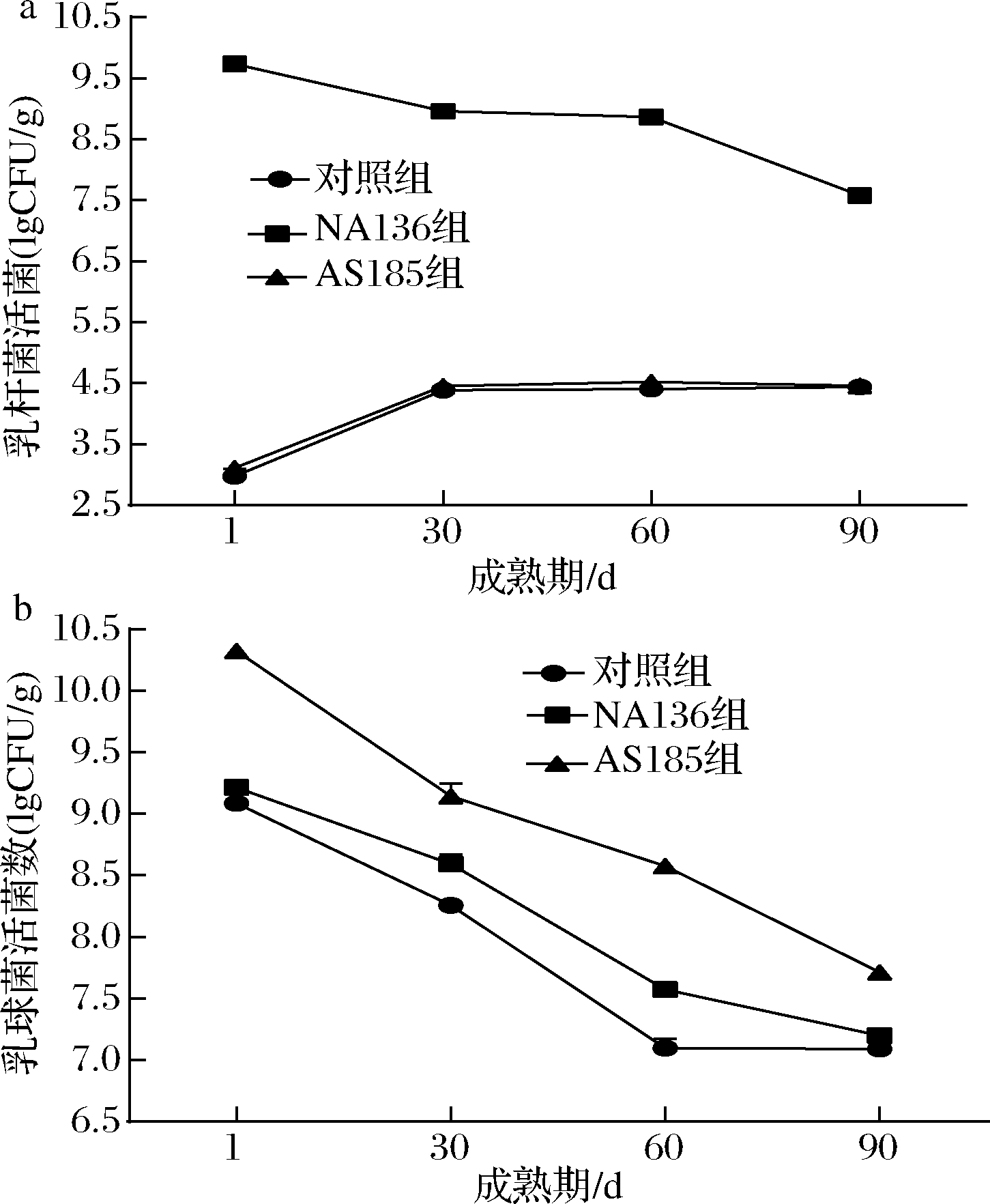
a-乳杆菌活菌数;b-乳球菌活菌数
图1 干酪成熟期间活菌数的变化
Fig.1 Changes in viable counts during cheese ripening
2.3 益生菌对干酪成熟期间质构的影响
应用物性分析仪对干酪的硬度、咀嚼性、黏聚性、回复性进行分析,可反应干酪的质构特性[20]。由表3可知,随成熟期延长,各组干酪的硬度和咀嚼度在逐渐增加,黏聚性和回复性在逐渐降低(P<0.05);在同一成熟期内,益生菌组干酪的硬度均低于对照组,可能是益生菌添加促进了蛋白质水解,使酪蛋白胶束结构变松散,干酪硬度下降、质地变软[21]。与对照组相比,益生菌对干酪咀嚼度、黏聚性和回复性无显著影响,这与添加L.plantarum或P.acidilactici 菌株为附属发酵剂的益生菌干酪分析结果一致[5, 16],益生菌添加对干酪咀嚼度、黏聚性和回复性影响不显著。
2.4 益生菌对干酪成熟期间游离氨基酸含量的影响
在干酪成熟期间,益生菌附属发酵剂与主发酵剂菌株共同组成干酪的微生物群落,益生菌产生的多种蛋白酶促进了游离氨基酸的产生和干酪风味的形成[22]。由表4可以知,3组干酪中均检测出17种游离氨基酸,且随成熟期延长,游离氨基酸总量逐渐增加。我们的研究结果进一步证实CIOCIA等[23]和STEFANOVIC等[24]的实验结论,他们的研究发现,添加植物乳杆菌或副干酪乳杆菌,显著影响干酪中二级蛋白质的水解并促进游离氨基酸的产生。
表3 干酪成熟期间质构指标变化
Table 3 Changes in texture characteristics during cheese ripening

分组成熟期/d硬度/g咀嚼度黏聚性回复性/(g·s)1 475.69±4.11f524.83±3.97fg0.95±0.05a0.47±0.06a对照组30 582.50±1.51e581.27±3.78d0.92±0.03abc0.45±0.03a60 678.54±6.34c638.37±3.26b0.90±0.02abc0.44±0.02ab90 700.81±4.69a642.17±6.43b0.89±0.02bc0.41±0.08abcd1 468.16±2.95f529.92±4.57ef0.95±0.04ab0.44±0.03abcNA136组30 581.57±4.03e583.93±8.36d0.94±0.03ab0.37±0.04bcd60 689.28±7.18b643.68±4.34b0.89±0.01bc0.37±0.04bcd90 691.74±5.74ab656.83±1.46a0.87±0.05c0.36±0.03cd1 452.24±3.84g517.69±4.10g0.93±0.02abc0.40±0.03abcdAS185组30 572.89±8.16e534.01±4.59e0.91±0.03abc0.37±0.02bcd60 626.13±4.89d623.70±4.67c0.89±0.04bc0.36±0.02cd90 635.16±8.89d637.31±4.56b0.87±0.02c0.35±0.04d
表4 干酪成熟期间游离氨基酸含量变化 单位:mg/kg
Table 4 Changes in contents of individual free amino acids during cheese ripening
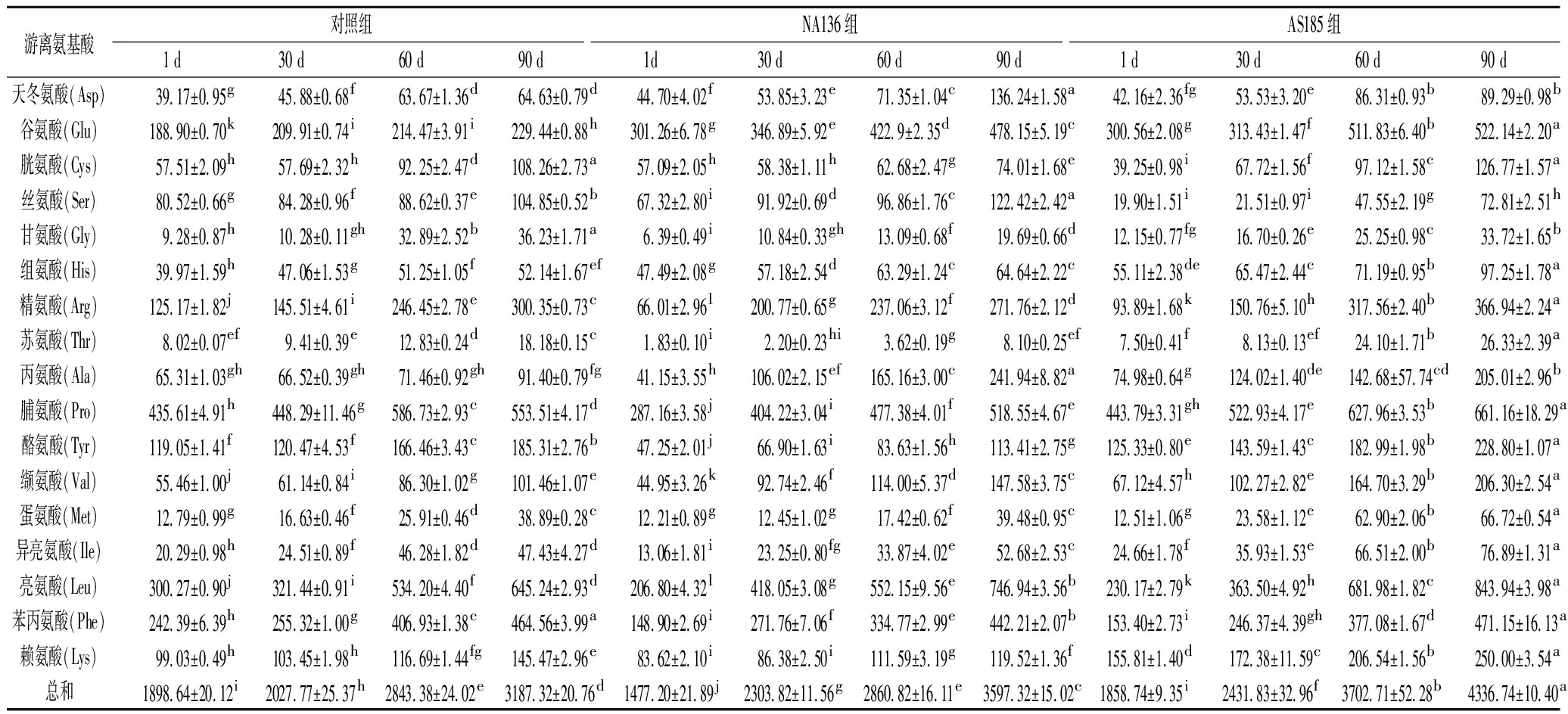
游离氨基酸 对照组NA136组AS185组1 d30 d60 d90 d1d30 d60 d90 d1 d30 d60 d90 d天冬氨酸(Asp)39.17±0.95g45.88±0.68f63.67±1.36d64.63±0.79d44.70±4.02f53.85±3.23e71.35±1.04c136.24±1.58a42.16±2.36fg53.53±3.20e86.31±0.93b89.29±0.98b谷氨酸(Glu)188.90±0.70k209.91±0.74i214.47±3.91i229.44±0.88h301.26±6.78g346.89±5.92e422.9±2.35d478.15±5.19c300.56±2.08g313.43±1.47f511.83±6.40b522.14±2.20a胱氨酸(Cys)57.51±2.09h57.69±2.32h92.25±2.47d108.26±2.73a57.09±2.05h58.38±1.11h62.68±2.47g74.01±1.68e39.25±0.98i67.72±1.56f97.12±1.58c126.77±1.57a丝氨酸(Ser)80.52±0.66g84.28±0.96f88.62±0.37e104.85±0.52b67.32±2.80i91.92±0.69d96.86±1.76c122.42±2.42a19.90±1.51i21.51±0.97i47.55±2.19g72.81±2.51h甘氨酸(Gly)9.28±0.87h10.28±0.11gh32.89±2.52b36.23±1.71a6.39±0.49i10.84±0.33gh13.09±0.68f19.69±0.66d12.15±0.77fg16.70±0.26e25.25±0.98c33.72±1.65b组氨酸(His)39.97±1.59h47.06±1.53g51.25±1.05f52.14±1.67ef47.49±2.08g57.18±2.54d63.29±1.24c64.64±2.22c55.11±2.38de65.47±2.44c71.19±0.95b97.25±1.78a精氨酸(Arg)125.17±1.82j145.51±4.61i246.45±2.78e300.35±0.73c66.01±2.96l200.77±0.65g237.06±3.12f271.76±2.12d93.89±1.68k150.76±5.10h317.56±2.40b366.94±2.24a苏氨酸(Thr)8.02±0.07ef9.41±0.39e12.83±0.24d18.18±0.15c1.83±0.10i2.20±0.23hi3.62±0.19g8.10±0.25ef7.50±0.41f8.13±0.13ef24.10±1.71b26.33±2.39a丙氨酸(Ala)65.31±1.03gh66.52±0.39gh71.46±0.92gh91.40±0.79fg41.15±3.55h106.02±2.15ef165.16±3.00c241.94±8.82a74.98±0.64g124.02±1.40de142.68±57.74cd205.01±2.96b脯氨酸(Pro)435.61±4.91h448.29±11.46g586.73±2.93c553.51±4.17d287.16±3.58j404.22±3.04i477.38±4.01f518.55±4.67e443.79±3.31gh522.93±4.17e627.96±3.53b661.16±18.29a酪氨酸(Tyr)119.05±1.41f120.47±4.53f166.46±3.43c185.31±2.76b47.25±2.01j66.90±1.63i83.63±1.56h113.41±2.75g125.33±0.80e143.59±1.43c182.99±1.98b228.80±1.07a缬氨酸(Val)55.46±1.00j61.14±0.84i86.30±1.02g101.46±1.07e44.95±3.26k92.74±2.46f114.00±5.37d147.58±3.75c67.12±4.57h102.27±2.82e164.70±3.29b206.30±2.54a蛋氨酸(Met)12.79±0.99g16.63±0.46f25.91±0.46d38.89±0.28c12.21±0.89g12.45±1.02g17.42±0.62f39.48±0.95c12.51±1.06g23.58±1.12e62.90±2.06b66.72±0.54a异亮氨酸(Ile)20.29±0.98h24.51±0.89f46.28±1.82d47.43±4.27d13.06±1.81i23.25±0.80fg33.87±4.02e52.68±2.53c24.66±1.78f35.93±1.53e66.51±2.00b76.89±1.31a亮氨酸(Leu)300.27±0.90j321.44±0.91i534.20±4.40f645.24±2.93d206.80±4.32l418.05±3.08g552.15±9.56e746.94±3.56b230.17±2.79k363.50±4.92h681.98±1.82c843.94±3.98a苯丙氨酸(Phe)242.39±6.39h255.32±1.00g406.93±1.38c464.56±3.99a148.90±2.69i271.76±7.06f334.77±2.99e442.21±2.07b153.40±2.73i246.37±4.39gh377.08±1.67d471.15±16.13a赖氨酸(Lys)99.03±0.49h103.45±1.98h116.69±1.44fg145.47±2.96e83.62±2.10i86.38±2.50i111.59±3.19g119.52±1.36f155.81±1.40d172.38±11.59c206.54±1.56b250.00±3.54a总和1898.64±20.12i2027.77±25.37h2843.38±24.02e3187.32±20.76d1477.20±21.89j2303.82±11.56g2860.82±16.11e3597.32±15.02c1858.74±9.35i2431.83±32.96f3702.71±52.28b4336.74±10.40a
2.5 益生菌对干酪成熟期间短链脂肪酸含量的影响
干酪成熟期间,短链脂肪酸因感官阈值较低直接贡献干酪的芳香味道,同时也是甲基酮、醇、内酯、醛和酯等其他重要风味化合物的前体[25]。由表5可知,3组干酪中,随成熟期延长,短链脂肪酸含量变化存在统计学差异(P<0.05),总体比较,乳酸片球菌AS185对脂质降解的程度要好于植物乳杆菌NA136。各组乙酸含量显著高于其他短链脂肪酸,且与对照组相比,NA136组和AS185组乙酸含量先增后降,这可能是由于益生菌在干酪成熟前期活性较强,促进乳酸代谢产生乙酸,后期含量降低可能与乙酸作为前体合成其他风味物质有关[26]。在ONG等[15]和EUGSTER等[5]的研究中也证实,益生菌对干酪中乙酸的增加有促进作用。在成熟90 d时,NA136组和AS185组丁酸含量显著高于对照组(P<0.05)。ATASOY等[27]研究指出,丁酸具有典型的奶酪味,风味较好的切达干酪中丁酸含量在4.5~5.0 mg/kg。本研究中益生菌干酪中的丁酸含量在这一范围内,说明益生菌对干酪的风味形成有促进作用。另外,其他种类的短链脂肪酸含量较低,这也与MOREIRA等[28]的研究结果一致。
表5 干酪成熟期间短链脂肪酸含量变化 单位:mg/kg
Table 5 Changes in contents of short-chain fatty acid during cheese ripening
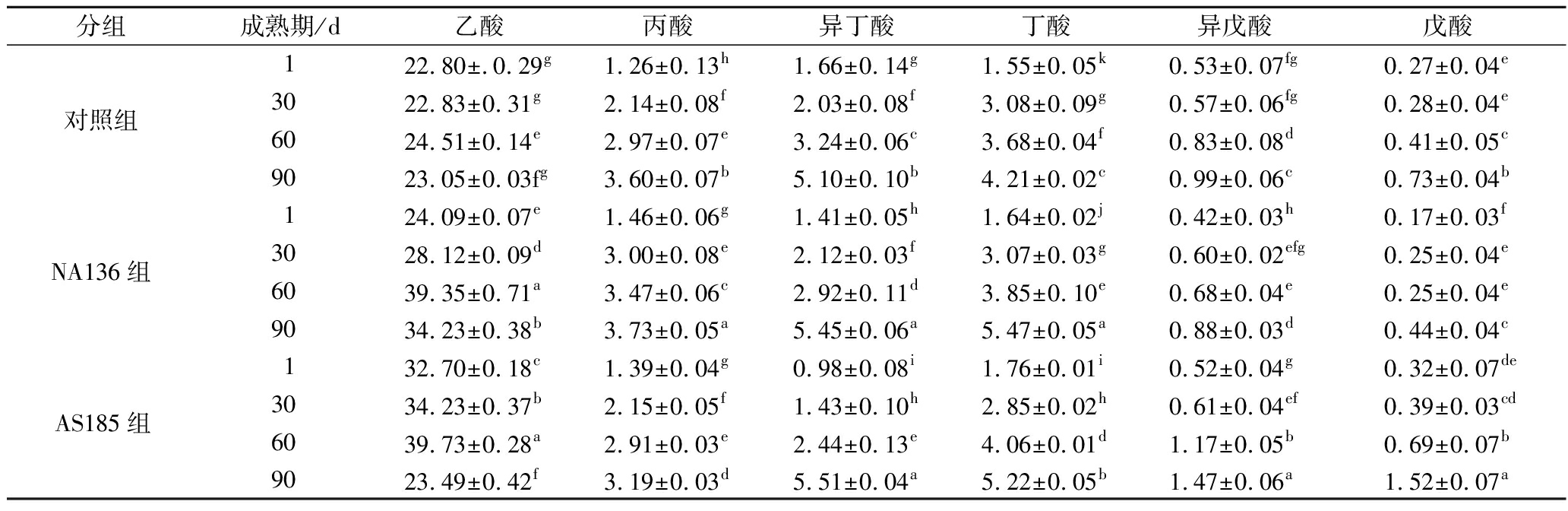
分组成熟期/d乙酸丙酸异丁酸丁酸异戊酸戊酸1 22.80±.0.29g1.26±0.13h1.66±0.14g1.55±0.05k0.53±0.07fg0.27±0.04e对照组30 22.83±0.31g2.14±0.08f2.03±0.08f3.08±0.09g0.57±0.06fg0.28±0.04e60 24.51±0.14e2.97±0.07e3.24±0.06c3.68±0.04f0.83±0.08d0.41±0.05c90 23.05±0.03fg 3.60±0.07b5.10±0.10b4.21±0.02c0.99±0.06c0.73±0.04b1 24.09±0.07e1.46±0.06g1.41±0.05h1.64±0.02j0.42±0.03h0.17±0.03fNA136组30 28.12±0.09d3.00±0.08e2.12±0.03f3.07±0.03g0.60±0.02efg0.25±0.04e60 39.35±0.71a3.47±0.06c2.92±0.11d3.85±0.10e0.68±0.04e0.25±0.04e90 34.23±0.38b3.73±0.05a5.45±0.06a5.47±0.05a0.88±0.03d0.44±0.04c1 32.70±0.18c1.39±0.04g0.98±0.08i1.76±0.01i0.52±0.04g0.32±0.07deAS185组30 34.23±0.37b 2.15±0.05f1.43±0.10h2.85±0.02h0.61±0.04ef0.39±0.03cd60 39.73±0.28a2.91±0.03e2.44±0.13e4.06±0.01d1.17±0.05b0.69±0.07b90 23.49±0.42f 3.19±0.03d5.51±0.04a5.22±0.05b1.47±0.06a1.52±0.07a
2.6 益生菌与干酪质构、游离氨基酸和短链脂肪酸的关系
采用RDA对成熟90 d干酪中游离氨基酸、短链脂肪酸含量与质构的关系进行了分析(图2)。NA136组干酪的咀嚼度与天冬氨酸、丝氨酸、丙氨酸、谷氨酸、丙酸和丁酸有一定的相关性,说明NA136对上述的生成有促进作用(图2-a)。NA136也影响了丙酸和丁酸的产生(图2-b),并与干酪的咀嚼度有关。另外,AS185更有利于干酪中苯丙氨酸、酪氨酸、赖氨酸、脯氨酸、精氨酸及异丁酸、异戊酸、戊酸等风味物质的形成,但对干酪的质构无影响。
2.7 益生菌干酪感官评定
成熟期为90 d的3组干酪感官评定结果如图3所示。可以看出,益生菌对干酪的外型、色泽没有任何影响,但对干酪的纹理图案、滋味、气味和组织状态产生了一些影响。益生菌干酪的纹理图案和组织状态得分略低于对照组,而滋味和气味的得分略高于对照组,综合得分略低于対照组,但无统计学差异(P>0.05)。这可能是因为益生菌能够促进蛋白及脂肪的水解,使得干酪的质地变软,而导致切达干酪中固有的“鸡胸纹”图案纹理不够清晰,但同时又产生了一些风味物质,促进干酪风味的形成[28]。也有研究指出,添加益生菌可轻微改变干酪的感官特性,但不会对产品的销售产生不利影响[5, 15],本研究结果也得到了证实。

1~3-对照组;4~6-NA136组;7~9-AS185组 a-游离氨基酸含量与质构的关系;b-短链脂肪酸含量 与质构的关系
图2 干酪中游离氨基酸和短链脂肪酸含量与质构的关系
Fig.2 Associations of texture and free amino acids and short-chain fatty acids in cheese
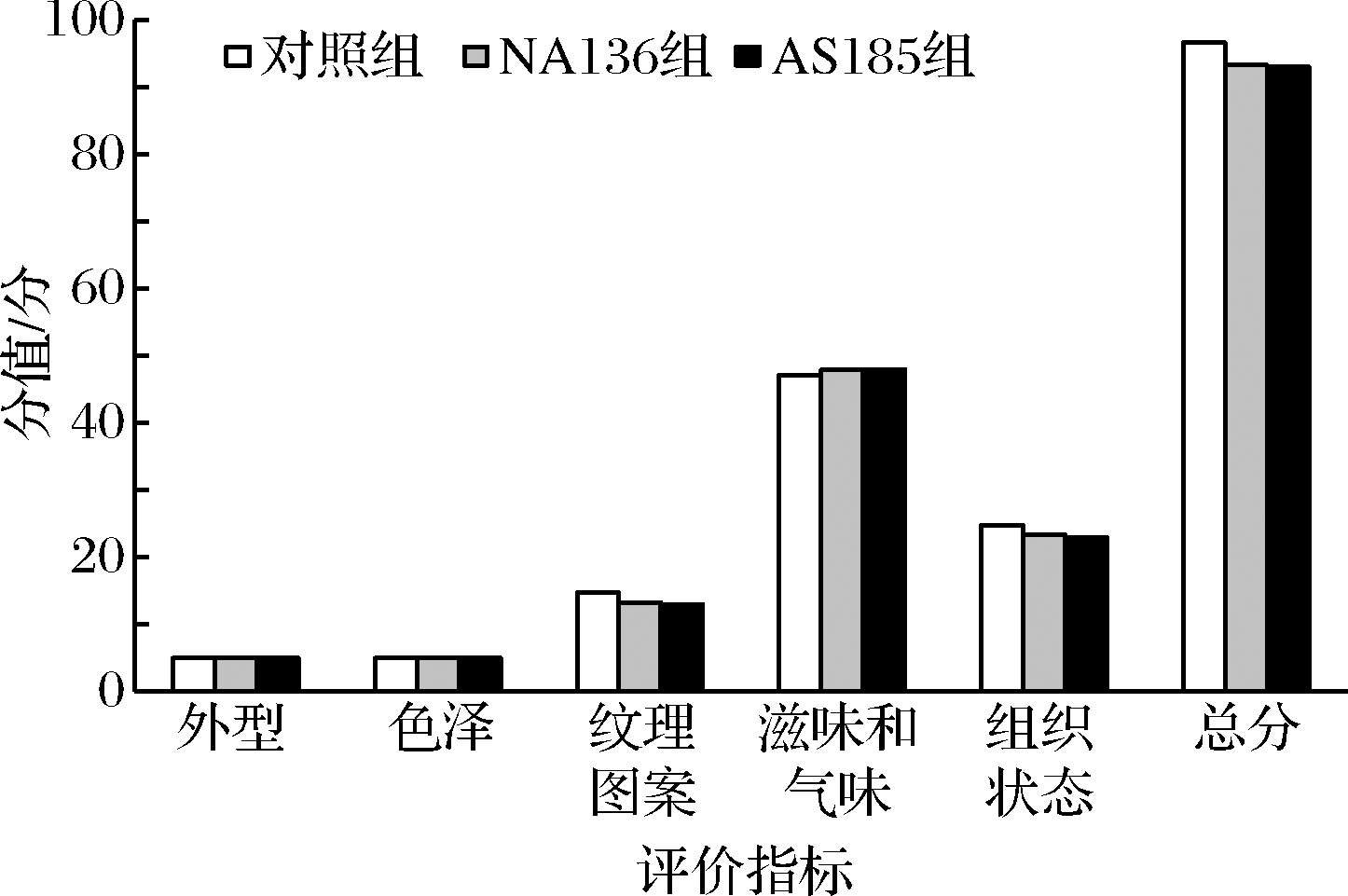
图3 成熟90 d干酪感官评分
Fig.3 Sensory score of cheeses ripened after 90 days
3 结论
本研究以自主分离鉴定的植物乳杆菌NA136和乳酸片球菌AS185为附属发酵剂应用于切达干酪。益生菌对切达干酪的理化指标和质构无不良影响,并促进了干酪中的蛋白质的水解、游离氨基酸和短链脂肪酸的生成,对干酪风味的形成和品质的提升起到了促进作用。综上所述,植物乳杆菌NA136和乳酸片球菌AS185可作为附属发酵剂用于益生菌干酪的生产。
[1] SETTANNI L, MOSCHETTI G.Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits[J].Food Microbiology, 2010, 27(6):691-697.
[2] CROW V L, COOLBEAR T, GOPAL P K, et al.The role of autolysis of lactic acid bacteria in the ripening of cheese[J].International Dairy Journal, 1995, 5(8):855-875.
[3] ORTAKCI F, BROADBENT J R, OBERG C J, et al.Growth and gas production of a novel obligatory heterofermentative Cheddar cheese nonstarter lactobacilli species on ribose and galactose[J].Journal of Dairy Science, 2015, 98(6):3 645-3 654.
[4] PERALTA G H, WOLF I V, PEROTTI M C, et al.Formation of volatile compounds, peptidolysis and carbohydrate fermentation by mesophilic lactobacilli and streptoccocci cultures in a cheese extract[J].Dairy Science and Technology, 2016, 96(5):603-621.
[5] EUGSTER E, FUCHSMANN P, SCHLICHTHERLE-CERNY H, et al.Formation of alanine, α-aminobutyrate, acetate, and 2-butanol during cheese ripening by Pediococcus acidilactici FAM18098[J].International Dairy Journal, 2019, 96:21-28.
[6] SPERANZA B, CAMPANIELLO D, MONACIS N, et al.Functional cream cheese supplemented with Bifidobacterium animalis subsp.lactis DSM 10140 and Lactobacillus reuteri DSM 20016 and prebiotics[J].Food Microbiology, 2018, 72:16-22.
[7] HEO S, LEE J H, JEONG D W.Food-derived coagulase-negative Staphylococcus as starter cultures for fermented foods[J].Food Science and Biotechnology, 2020, 29(8):1 023-1 035.
[8] CARAFA I, CLEMENTI F, TUOHY K, et al.Microbial evolution of traditional mountain cheese and characterization of early fermentation cocci for selection of autochtonous dairy starter strains[J].Food Microbiology, 2016, 53:94-103.
[9] STANTON C, GARDINER G, LYNCH P B, et al.Probiotic cheese[J].International Dairy Journal, 1998, 8(5-6):491-496.
[10] 王辑, 杨贞耐.益生菌干酪成熟过程中微生态变化的研究进展[J].食品安全质量检测学报, 2014, 5(4):990-994.
WANG J, YANG Z N.Advance of microecological changes of probiotic cheese during ripening[J].Journal of Food Safety and Quality, 2014, 5(4):990-994.
[11] BANKS J M, WILLIAMS A G.The role of the nonstarter lactic acid bacteria in Cheddar cheese ripening[J].International Journal of Dairy Technology, 2004, 57(2-3):145-152.
[12] ZHAO Z J, CHEN L, ZHAO Y J, et al.Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation[J].Applied Microbiology and Biotechnology, 2020, 104(12):5 273-5 282.
[13] ZHAO Z J, WANG C, ZHANG L, et al.Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway[J].Applied Microbiology and Biotechnology, 2019, 103(14):5 843-5 850.
[14] WANG C, WANG H, ZHAO Z J, et al.Pediococcus acidilactici AS185 attenuates early atherosclerosis development through inhibition of lipid regulation and inflammation in rats[J].Journal of Functional Foods, 2019, 60:103424.
[15] ONG L, HENRIKSSON A, SHAH N P.Proteolytic pattern and organic acid profiles of probiotic Cheddar cheese as influenced by probiotic strains of Lactobacillus acidophilus, Lb.paracasei, Lb.casei or Bifidobacterium sp.[J].International Dairy Journal, 2007, 17(1):67-78.
[16] 段翠翠, 赵子健, 赵玉娟, 等.植物乳杆菌S72对高达干酪品质的影响[J].中国乳品工业, 2018, 46(8):21-24;36.
DUAN C C, ZHAO Z J, ZHAO Y J, et al.Effects of Lactobacillus plantarum S72 on the quality of Gouda cheese[J].China Dairy Industry, 2018, 46(8):21-24;36.
[17] KARIMI R, MORTAZAVIAN A M, CRUZ A G.Viability of probiotic microorganisms in cheese during production and storage:A review[J].Dairy Science and Technology, 2011, 91(3):283-308.
[18] 李军训,罗学刚, 高洁, 等.益生菌的分类、生理功能与有效性评价研究进展[J].中国农业科技导报, 2010, 12(6):49-55.
LI J X, LUO X G, GAO J, et al.Research progress on classification, physiological function and validity evaluation of probiotics[J].Journal of Agricultural Science and Technology, 2010, 12(6):49-55.
[19] GARDE S, G MEZ-TORRES N, DELGADO D, et al.Influence of reuterin-producing Lactobacillus reuteri coupled with glycerol on biochemical, physical and sensory properties of semi-hard ewe milk cheese[J].Food Research International, 2016, 90:177-185.
MEZ-TORRES N, DELGADO D, et al.Influence of reuterin-producing Lactobacillus reuteri coupled with glycerol on biochemical, physical and sensory properties of semi-hard ewe milk cheese[J].Food Research International, 2016, 90:177-185.
[20] MITCHELL J. Food texture and viscosity: Concept and measurement[J]. International Journal of Food Science & Technology, 2003, 38(7): 839-840.
[21] VOIGT D D, CHEVALIER F, DONAGHY J A, et al.Effect of high-pressure treatment of milk for cheese manufacture on proteolysis, lipolysis, texture and functionality of Cheddar cheese during ripening[J].Innovative Food Science and Emerging Technologies, 2012, 13:23-30.
[22] ANTONSSON M, MOLIN G, ARDÖ Y.Lactobacillus strains isolated from Danbo cheese as adjunct cultures in a cheese model system[J].International Journal of Food Microbiology, 2003, 85(1-2):159-169.
[23] CIOCIA F, MCSWEENEY P L H, PIRAINO P, et al.Use of dairy and non-dairy Lactobacillus plantarum, Lactobacillus paraplantarum and Lactobacillus pentosus strains as adjuncts in Cheddar cheese[J].Dairy Science and Technology, 2013, 93(6):623-640.
[24] STEFANOVIC E, KILCAWLEY K N, ROCES C et al.Evaluation of the potential of Lactobacillus paracasei adjuncts for flavor compounds development and diversification in short-aged Cheddar cheese[J].Frontiers in Microbiology, 2018, 9:1 506.
[25] LECLERCQ-PERLAT M N, LATRILLE E, CORRIEU G, et al.Controlled production of Camembert-type cheeses.part II.changes in the concentration of the more volatile compounds[J].Journal of Dairy Research, 2004, 71(3):355-366.
[26] CHEN L S, CUI J, DING Q B, et al.The effect of yeast species from raw milk in China on proteolysis and aroma compound formation in Camembert-type cheese[J].Food and Bioprocess Technology, 2012, 5(6):2 548-2 556.
[27] ATASOY A F, ![]() H.Lipolysis in Urfa cheese produced from raw and pasteurized goats’ and cows’ milk with mesophilic or thermophilic cultures during ripening[J].Food Chemistry, 2009, 115(1):71-78.
H.Lipolysis in Urfa cheese produced from raw and pasteurized goats’ and cows’ milk with mesophilic or thermophilic cultures during ripening[J].Food Chemistry, 2009, 115(1):71-78.
[28] MOREIRA G M M, COSTA R G B, TEODORO V A M, et al.Effect of ripening time on proteolysis, free amino acids, bioactive amines and texture profile of Gorgonzola-type cheese[J].LWT, 2018, 98:583-590.