随着工农业生产的发展,镉广泛存在于环境中,会造成食品的污染,对人类健康造成了极大的威胁[1]。研究表明,镉在体内累积,可导致肾脏、肝脏、睾丸、肺等器官的病理损伤[2-3]。肝脏被认为是镉积聚并发挥其毒性作用的靶器官[4]。以往的研究表明,镉会增加氧化应激,促进炎症和肝功能损伤[5]。此外,各种应激刺激下引起的氧化应激会导致肝细胞脂质代谢紊乱[6-7]。肝脏是脂质代谢的关键器官,接触镉可引起脂质代谢紊乱而导致肝功能障碍[8]。以前的研究表明,镉可引起血脂异常,包括总甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)、低密度脂蛋白-胆固醇(low-density lipoprotein-cholesterol, LDL-C)水平升高[9]。
氨基酸(amino acids, AAs)是构成动物营养所需蛋白质的基本物质,在减少体内过量脂肪方面发挥着重要作用[10]。半胱氨酸,作为一种生物体内常见的氨基酸,在保护雄性大鼠肝脏细胞免受镉毒性中发挥作用[11]。据报道,半胱氨酸在抗氧化、抗炎、促进脂质代谢方面表现出良好的效果[11-12]。有研究显示,半胱氨酸可保护肝脏免受重金属的毒性影响,膳食半胱氨酸影响抗氧化酶活性以及血清和肝脏中的脂质水平[13]。但目前关于不同剂量半胱氨酸对镉暴露所致肝脏损伤保护作用的研究尚不明确。因此,本研究以小鼠为研究对象,通过组织学检查和生化指标分析,研究半胱氨酸对急性镉暴露致肝细胞损伤的保护作用。此外,还评估了肝脏脂质代谢变化,全面阐明半胱氨酸对急性镉暴露的小鼠肝细胞氧化应激、炎症和脂质代谢的保护及作用机制。这些发现可能有助于为半胱氨酸临床应用提供数据支撑。
1 材料和方法
1.1 材料与设备
SPF雄性小鼠(25~35 g),长沙天勤生物科技有限公司;氯化镉(CdCl2,CAS:7790-78-5),汕头西龙化工;L-半胱氨酸(CAS:52-90-4)、Trizol试剂,生工生物工程(上海)股份有限公司;BCA蛋白检测试剂盒,上海碧云天生物技术有限公司;过氧化氢酶(catalase, CAT)试剂盒、超氧化物歧化酶(superoxide dismutase, SOD)试剂盒、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)试剂盒、丙二醛(malondialdehyde, MDA)试剂盒,北京索莱宝科技有限公司;TG试剂盒、TC试剂盒、谷丙转氨酶(alanine aminotransferase,ALT)试剂盒、谷草转氨酶(aspartate aminotransferase,AST)试剂盒,南京建成生物工程研究所;IL-6 ELISA试剂盒、IL-1β ELISA试剂盒、TNF-α ELISA试剂盒,江苏酶免实业有限公司;去基因组DNA反转录预混试剂(StarScript II RT Mix with gDNA Remover),北京康润诚业生物科技有限公司。
XS105DU型十万分之一分析天平, 上海梅特勒-托利多仪器有限公司;Varioskan LUX多功能酶标仪, 美国赛默飞世尔科技有限公司;DMI4000B倒置荧光显微镜, 徕卡(Leica)生物系统有限公司;CFX96荧光定量PCR系统, 美国伯乐(biorad)。
1.2 实验方法
1.2.1 动物分组与处理
24只体重相近的雄性小鼠,将所有动物饲养在含木屑的塑料笼中,并在22~25 ℃的房间中饲养,12 h 光照/夜间循环,自由获取标准实验室饲料和水。预饲养1周后,随机分为4组,每组6只。对照组:每天腹腔注射0.9% NaCl,每天1次;镉暴露组(Cd):小鼠经0.9%(质量分数)NaCl预处理后腹腔注射CdCl2剂量为5 mg/(kg·d)[14];低剂量半胱氨酸治疗组(Cd+L-Cys):在腹腔注射5 mg/(kg·d) CdCl2的基础上,每日灌胃0.04 mmol/(g·d)的L-半胱氨酸;高剂量半胱氨酸治疗组(Cd+H-Cys):在腹腔注射5 mg/(kg·d) CdCl2的基础上,每日灌胃0.08 mmol/(g·d)的L-半胱氨酸[15]。
1.2.2 动物分组与处理
小鼠自由饮水,禁食15 h后,眼球采血,3 500 r/min离心10 min,收集血清[16],于4 ℃保存备用,用于生化指标评估。7 d结束时,断颈处死小鼠,取肝脏,并于-80 ℃保存待测。实验得到了广东海洋大学动物伦理委员会的批准(批号:GDOU-LAE-2020-009)。
1.2.3 小鼠AST和ALT的活性测定
血液样本是通过小鼠眼球采集的。采用全自动酶标仪和检测试剂盒分别测定血清AST和ALT的活性。
1.2.4 组织病理学分析
将肝脏置于4%多聚甲醛溶液中室温固定24 h,无水乙醇脱水后,石蜡包埋并进行切片,采用苏木精和伊红(hematoxylin-eosin,HE)染色对肝脏进行常规染色,利用倒置荧光显微镜在200倍下观察肝组织。
1.2.5 生化指标测定
将肝匀浆离心以获得上清液(3 500 r/min,4 ℃离心10 min),根据试剂盒说明书测定TG、TC、SOD、CAT和MDA水平。
1.2.6 炎症指标测定
将每组的肝脏组织用裂解缓冲液匀浆。将匀浆在4 ℃下以3 500 r/min离心10 min。总蛋白浓度采用BCA蛋白检测试剂盒测定。通过使用ELISA试剂盒检测肝脏组织中的IL-6、IL-1β和TNF-α浓度,严格按照试剂盒要求操作。
1.2.7 实时荧光定量PCR
按照说明书,使用Trizol试剂从肝脏组织中提取总RNA。根据制造商的方案,使用去基因组DNA反转录预混试剂(StarScript Ⅱ RT Mix with gDNA Remover)合成第一链cDNA。使用 CFX96荧光定量PCR系统(Bio-Rad,USA)对 cDNA 样品进行实时荧光定量PCR,使用的引物如下[17-18]:SREBP-1(正向:5′-GCAGTCTGCTTTGGAACCTC-3′;反向:5′-CCACAAAGAAACGGTGACCT-3′);ACC(正向:5′-GTTGCACAAAAGGATTTCA-3′;反向:5′-CGCATTACCATGCTCCGC-3′);FAS(正向:5′-TACCAGTGCCACAGGAGTTCCA-3′;反向:5′-TAAACACCTCGTCGATTT-CGTTC-3′);SCD1(正向5′-CTGCCTCTTCGGGAT-TTTCTACT-3′;反向:5′-GCCCATTCGTACACGTGATTC-3′)和β-actin(正向:5′-AGAGGGGAATCGTGCGTGAC-3′;反向:5′-AGGAAGAGGATGCGGCAGTG-3′),所有样本进行3个重复分析,采用2-ΔΔCt法分析基因表达水平。
1.3 统计学方法
数据使用JMP Pro 13进行显著性分析,使用GraphPad Prism(美国,GraphPad软件)进行绘图。数据采用平均数±标准差表示,组间比较采用方差分析。P<0.05表示差异显著,P<0.01表示差异极显著。
2 结果与分析
2.1 半胱氨酸对镉暴露小鼠血清肝功能酶活性的影响
在药物中毒性肝细胞坏死时,ALT和AST大量释放入血中,因此是诊断中毒性肝炎的重要指标。小鼠血清ALT和AST活性检测结果见图1。与对照组相比,镉暴露组ALT,AST活性极显著升高(P<0.01);与镉暴露组相比,不同浓度的半胱氨酸处理组ALT,AST活性均极显著降低(P<0.01)。
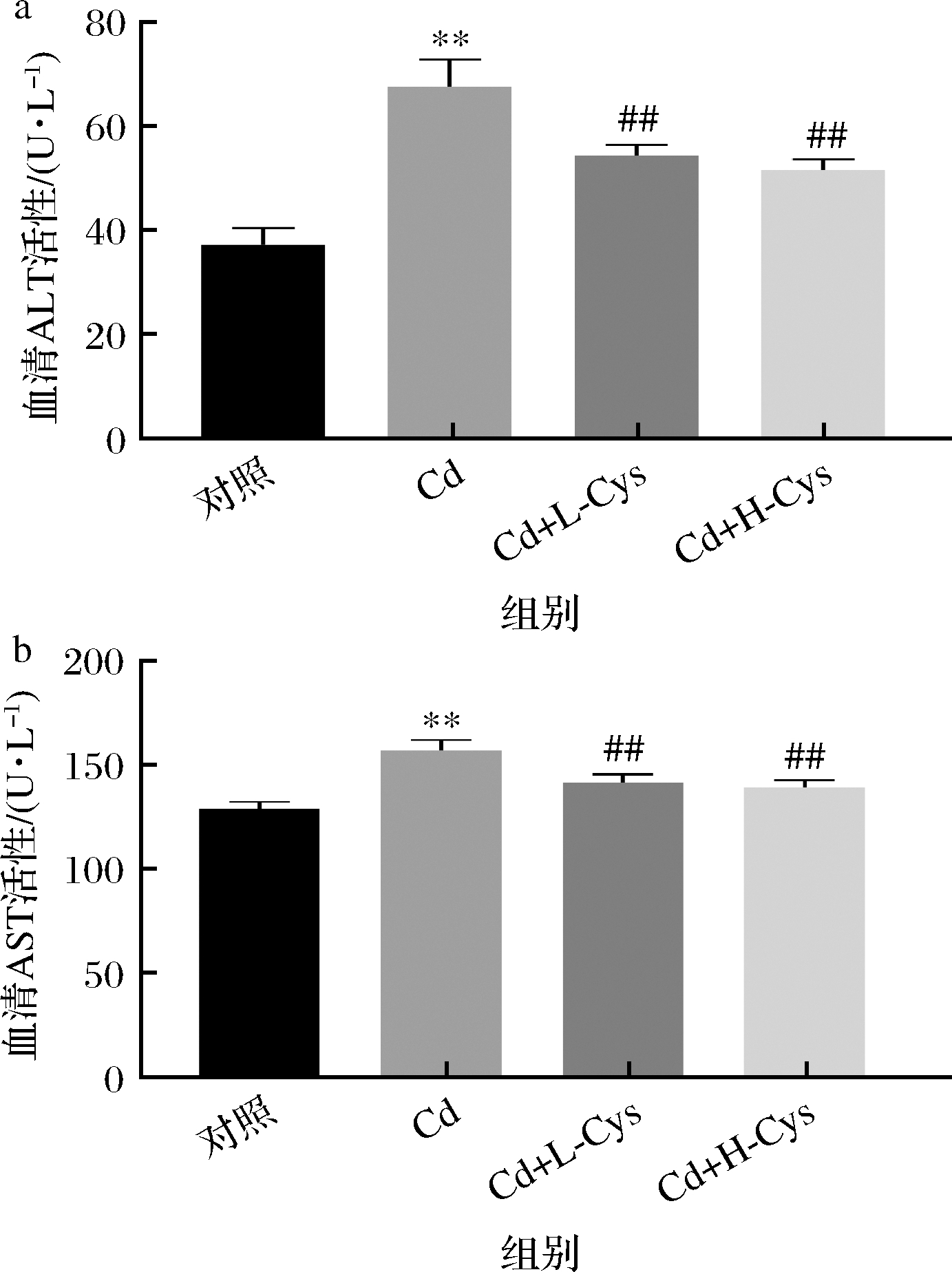
a-ALT活性;b-AST活性
图1 半胱氨酸对镉暴露小鼠血清肝功能酶活性的影响
Fig.1 Effect of cysteine on the activity of serum liver function enzymes in cadmium-exposed mice
注:与空白对照组相比,**表示P<0.01;与镉暴露组相比,##表示P<0.01(下同)
2.2 半胱氨酸对镉暴露小鼠肝组织病理变化的影响
为了进一步评估半胱氨酸对Cd诱导的小鼠肝损伤的保护作用,本文评估了肝脏组织学变化。对照组中肝索细胞有序排列(图2-a);镉暴露组小鼠肝脏中央静脉可见大量出血现象,中央静脉周围可见少量炎性细胞浸润(图2-b);低剂量和高剂量半胱氨酸组的肝脏病理变化较镉组有所缓解(图2-c、图2-d)。这表明半胱氨酸缓解了镉引起的肝脏组织损伤。

a-对照组;b-Cd组;c-Cd+L-Cys组;d-Cd+H-Cys组
图2 半胱氨酸对镉中毒小鼠肝组织病理变化的影响(200×)
Fig.2 Effect of cysteine on histopathological changes in the liver of cadmium-exposed mice(200×)
2.3 半胱氨酸对镉暴露小鼠肝脏总TG和TC水平的影响
脂质代谢紊乱已被证明在肝损伤中起着关键作用。本文检测了肝脏样本中的TC和TG水平(图3)。结果表明,镉可提高肝脏中的TG和TC水平(P<0.01);给予半胱氨酸后TC水平极显著降低(P<0.01),TG水平显著降低(P<0.05)。这些结果表明,半胱氨酸对镉干扰的脂质代谢具有缓解作用。
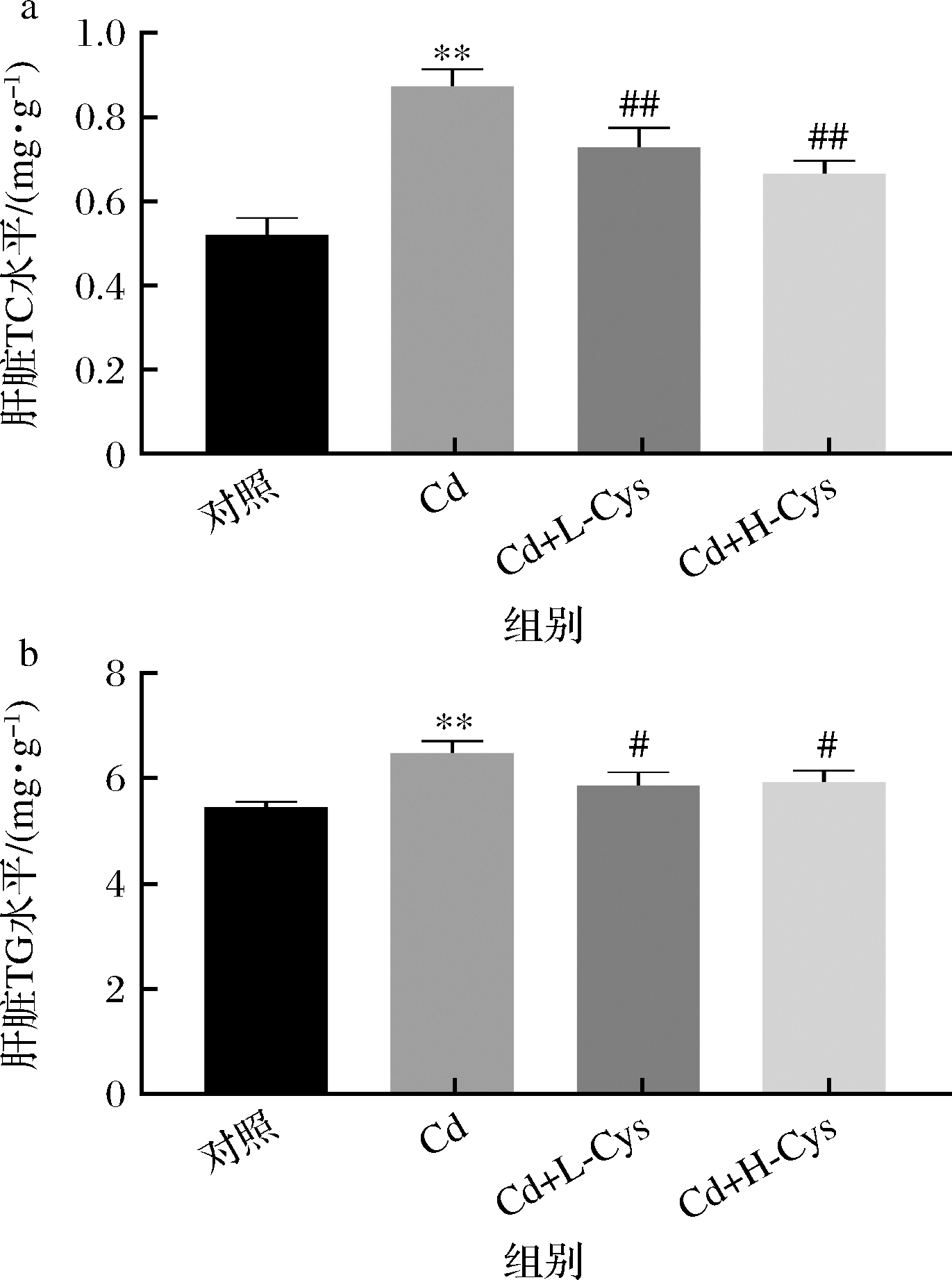
a-肝脏TC水平;b-肝脏TG水平
图3 半胱氨酸对镉暴露小鼠肝脏总TG和TC水平的影响
Fig.3 Effect of cysteine on the levels of total TG and TC in the liver of cadmium-exposed mice
注:#表示P<0.05
2.4 半胱氨酸对镉暴露小鼠肝脏MDA、SOD、CAT变化的影响
众所周知,ROS过量产生会导致肝脏脂质代谢紊乱。为了确定半胱氨酸是否增强了参与抗氧化防御系统的酶的活性,本文研究了主要的抗氧化水平。如图4和图5所示,镉暴露后抗氧化酶SOD、CAT活性极显著降低(P<0.01),脂质过氧化MDA水平极显著升高(P<0.01);而给予半胱氨酸后,SOD、CAT活性得到恢复;低剂量半胱氨酸组与镉暴露组相比MDA水平显著降低(P<0.05);高剂量半胱氨酸组与镉暴露组相比MDA水平极显著降低(P<0.01)。这些结果表明,镉处理小鼠肝脏中脂质过水平升高可能是由于自由基的过度产生和镉对抗氧化防御的侵蚀。通过半胱氨酸干预镉暴露小鼠可以将这些氧化应激标志物的水平恢复到接近正常水平,表明半胱氨酸具有抗脂质过氧化和抗氧化作用。

图4 半胱氨酸对镉暴露小鼠肝脏MDA水平的影响
Fig.4 Effect of cysteine on the level of MDA in the liver of cadmium-exposed mice

a-SOD活性;b-CAT活性
图5 半胱氨酸对镉暴露小鼠肝脏SOD、CAT活性的影响
Fig.5 Effect of cysteine on the activities of SOD and CAT enzymes in the liver of cadmium-exposed mice
2.5 半胱氨酸对镉暴露小鼠肝脏IL-1β、IL-6、TNF-α含量的影响
小鼠肝脏IL-1β、IL-6和TNF-α水平测定结果见图6。由图6可知,与对照组相比,镉暴露组小鼠肝脏中IL-1β、IL-6和TNF-α水平极显著升高(P<0.01);然而,半胱氨酸组处理促使这些标记物的水平显著降低(P<0.05或P<0.01)。这些结果表明镉可能通过增加炎症细胞因子如TNF-α和IL-1β的产生而促进脂质的产生。在镉中毒的小鼠中,用半胱氨酸预处理被证明可以使升高的炎症水平正常化。半胱氨酸可以通过降低促炎细胞因子的水平来保护免受镉诱导的炎症反应,从而有助于减轻肝损伤。
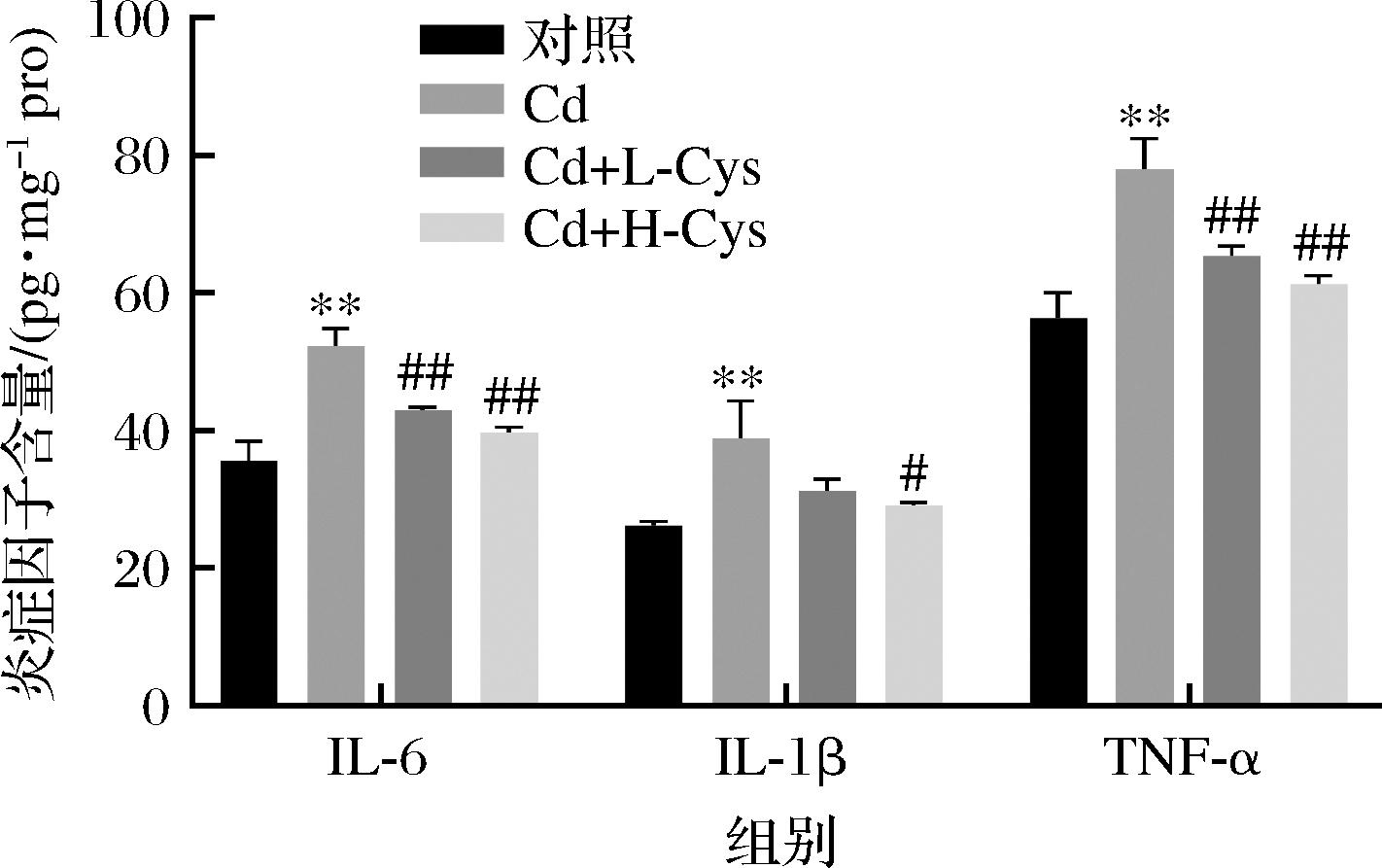
图6 半胱氨酸对镉暴露小鼠肝脏IL-1β、IL-6、TNF-α含量的影响
Fig.6 Effect of cysteine on the content of IL-1β, IL-6, and TNF-α in the liver of cadmium-exposed mice
2.6 半胱氨酸对镉暴露小鼠肝脏脂类代谢相关基因表达的影响
为了确定半胱氨酸改善镉导致脂质代谢异常的机制,本文进一步通过实时荧光定量PCR检测参与脂质代谢的基因表达水平。如图7所示,与对照组相比,镉组的SREBP-1、ACC、FAS和SCD1等脂质合成基因表达水平显著升高(P<0.01)。然而低剂量半胱氨酸(Cd+L-Cys)和高剂量半胱氨酸(Cd+H-Cys)处理组ACC和SCD1基因表达水平显著低于镉暴露组(P<0.05或P<0.01);高剂量半胱氨酸处理组SREBP-1和FAS基因表达水平显著低于镉暴露组(P<0.05),低剂量组差异不显著。总之,半胱氨酸减弱了Cd干扰脂质代谢相关基因的表达,从而改善了脂质积累,有助于肝损伤的恢复。
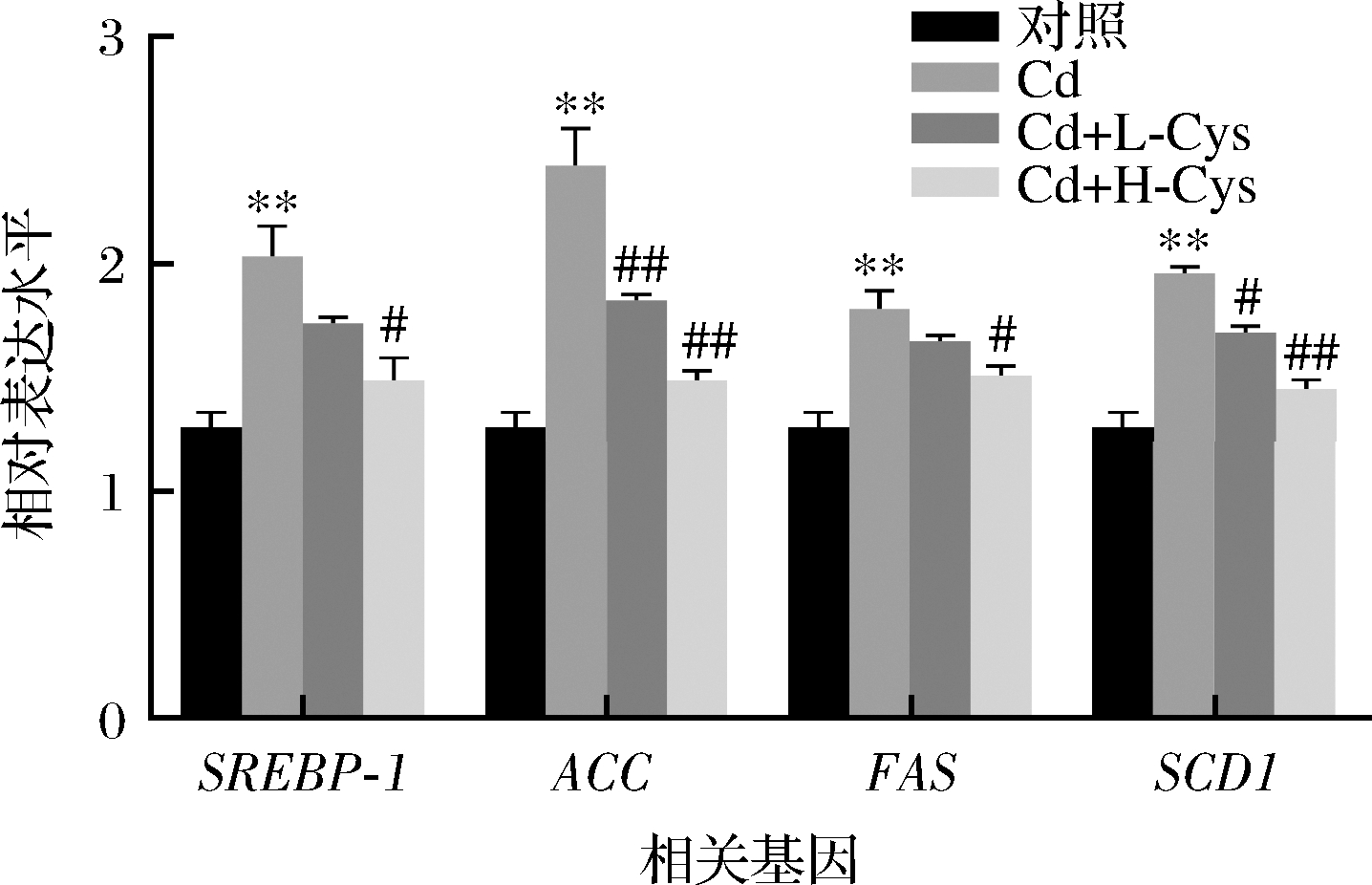
图7 半胱氨酸对镉暴露小鼠肝脏脂类代谢相关基因表达的影响
Fig.7 Effect of cysteine on the expression of genes related to lipid metabolism in the liver of cadmium-exposed mice
3 讨论
有研究表明,镉暴露可抑制肝细胞增殖能力,改变肝细胞形态,破坏肝细胞结构,最终引起细胞死亡[19]。先前的研究已经报道,镉诱导的氧化应激和炎症会导致肝脏损伤[20]。研究表明,镉可诱导小鼠肝脏中抗氧化酶活性显著降低和MDA水平的升高[21-22]。已知L-半胱氨酸等含硫氨基酸具有抗氧化作用[23]。以前的研究发现膳食L-半胱氨酸降低了砷诱导大鼠肝脏氧化应激水平[24]。本研究旨在评估L-半胱氨酸摄入对急性镉暴露小鼠肝毒性的保护作用及机制。
ALT和AST是肝损伤的特异性酶标记物,与对照组相比,镉可使血清中的肝功能酶活性(ALT和AST)水平升高。此外,镉可引起肝脏病理改变,包括肝索排列不规则、胞浆空泡化等。氧化应激被定义为组织内促氧化剂/抗氧化剂平衡的紊乱[24]。在这里,L-半胱氨酸和氯化镉联合治疗通过降低MDA水平以及恢复SOD和CAT活性来减轻肝组织中的氧化反应。除了氧化损伤外,炎症反应在肝损伤中也起着关键作用。本实验结果显示,镉暴露小鼠肝脏中IL-1β、IL-6和TNF-α水平极显著升高,而不同剂量组L-半胱氨酸治疗阻断了镉诱导的炎症反应。有研究发现乙酰半胱氨酸可通过减少促炎标志物(IL-6、IL-1β、TNF-α)来有效改善肝功能损伤[25]。
氧化应激和慢性炎症刺激导致肝细胞脂质代谢紊乱[26-27]。脂质代谢指生物体内脂肪,在各种相关酶的帮助下,消化吸收、合成与分解的过程,受脂肪生成转录因子[包括固醇调节元件结合蛋白-1(SREBP-1)]、脂肪生成酶(包括FAS和ACC)基因的控制[18]。FAS和ACC是脂肪酸合成的两个关键限速酶,SREBP是一种调节脂肪酸合成酶表达的转录因子,而SCD1主要促成单不饱和脂肪酸的形成[28]。前期的研究发现,镉暴露导致脂质积累并改变参与细胞死亡或存活调节途径[29]。LARREGLE等[9]研究发现镉暴露直接或间接改变血清脂质含量和肝脏脂质代谢。本文的研究结果表明,镉暴露可提高肝脏中的TG和TC水平。肝脏中SREBP-1、ACC、FAS和SCD1等脂肪酸合成相关基因表达水平升高,而补充L-半胱氨酸可通过下调脂肪酸合成相关基因(SREBP-1、ACC和FAS)表达预防血脂异常和肝脂肪变性,这表明L-半胱氨酸可以缓解镉导致的脂质代谢紊乱。
4 结论
本研究通过评估半胱氨酸对镉暴露小鼠肝功能酶活性、肝组织损伤、氧化损伤、炎症及脂质代谢的影响发现,镉诱导的氧化应激和炎症可能触发肝脏脂质积聚,导致小鼠肝脏损伤。高剂量半胱氨酸具有更好的抗氧化和抗炎效果,主要通过抑制氧化应激和炎症减轻镉诱导的小鼠肝脏脂质代谢紊乱。
[1] 杨自军. 镉的污染及对动物的危害与防治[J].中国动物保健, 2008,10(5):55-60.
YANG Z J.Cadmium pollution and its harm to animals and its control[J].China Animal Health, 2008,10(5):55-60.
[2] 张人俊, 马萍, 嵇辛勤, 等.重金属镉的毒性研究进展[J].贵州畜牧兽医, 2016, 40(4):27-33.
ZHANG R J, MA P, JI X Q, et al.The review of recent studies of the toxicity of heavy metal cadmium[J].Guizhou Journal of Animal Husbandry &Veterinary Medicine, 2016, 40(4):27-33.
[3] 谭茂云. 维生素E对亚慢性镉中毒大鼠肝脏氧化损伤的保护作用研究[D].雅安:四川农业大学, 2020.
TAN M Y.The protective effect of vitamin E on oxidative damage of liver in subchronic cadmium poisoning rats[D].Ya′an:Sichuan Agricultural University, 2020.
[4] MORADKHANI S, REZAEI-DEHGHANZADEH T, NILI-AHMADABADI A.Rosa persica hydroalcoholic extract improves cadmium-hepatotoxicity by modulating oxidative damage and tumor necrosis factor-alpha status[J].Environmental Science and Pollution Research, 2020, 27(25):31 259-31 268.
[5] FOUAD A A, EL-REHANY M A A, MAGHRABY H K.The hepatoprotective effect of carnosine against ischemia/reperfusion liver injury in rats[J].European Journal of Pharmacology, 2007, 572(1):61-68.
[6] WAN X M, CHEN J, WANG M, et al.Puerarin attenuates cadmium-induced hepatic lipid metabolism disorder by inhibiting oxidative stress and inflammation in mice[J].Journal of Inorganic Biochemistry, 2021, 222:111521.
[7] PRABU S M, SHAGIRTHA K, RENUGADEVI J.Amelioration of cadmium-induced oxidative stress, impairment in lipids and plasma lipoproteins by the combined treatment with quercetin and α-tocopherol in rats[J].Journal of Food Science, 2010, 75(7):T132-T140.
[8] 李自发, 张浩, 任萌, 等.槲皮素对镉致小鼠肝脏脂质代谢紊乱模型的保护效应[J].实验动物与比较医学, 2021, 41(4):305-312.
LI Z F, ZHANG H, REN M, et al.Protective effect of quercetin on lipid metabolism disorder in mice livers caused by cadmium[J].Laboratory Animal and Comparative Medicine, 2021, 41(4):305-312.
[9] LARREGLE E V, VARAS S M, OLIVEROS L B, et al.Lipid metabolism in liver of rat exposed to cadmium[J]. Food and Chemical Toxicology, 2008, 46(5):1 786-1 792.
[10] NIE C X, HE T, ZHANG W J, et al.Branched chain amino acids:Beyond nutrition metabolism[J].International Journal of Molecular Sciences,2018, 19(4):954-969.
[11] RAHMANI TALATAPPEH N, RANJI N, BEIGI HARCHEGANI A.The effect of N-acetyl cysteine on oxidative stress and apoptosis in the liver tissue of rats exposed to cadmium[J].Archives of Environmental &Occupational Health, 2021, 76(8):518-525.
[12] YIN J, REN W K, YANG G, et al.L-Cysteine metabolism and its nutritional implications[J].Molecular Nutrition &Food Research, 2016, 60(1):134-146.
[13] LEE S, HAN K H, NAKAMURA Y, et al.Dietary L-cysteine improves the antioxidative potential and lipid metabolism in rats fed a normal diet[J].Bioscience, Biotechnology, and Biochemistry, 2013, 77(7):1 430-1 434.
[14] AUGUSTINE N, ANI C, EZE W, et al.The effect of aqueous extract of zest of citrus sinensis (AEZCs) on cadmium chloride induced liver toxicity in wistar rats[J].African Journal of Biochemistry Research, 2020, 14(1):5-17.
[15] 黄琳茹. 苏氨酸对镉暴露酵母、小鼠的保护作用及机制分析[D].湛江:广东海洋大学, 2021.
HUANG L R.The protective effect and mechanism of threonine on cadmium-exposed yeast and mice abstract[D].Zhanjiang:Guangdong Ocean University, 2021.
[16] 朱根生, 夏苏干, 佘进进, 等.白藜芦醇对玉米赤霉烯酮致小鼠肝脏氧化损伤及炎症的保护作用[J].中国畜牧兽医, 2021, 48(11):4 254-4 261.
ZHU G S, XIA S G, SHE J J, et al.Protective effects of resveratrol on zearalenone-induced liver oxidative damage and inflammation in mice[J].China Animal Husbandry &Veterinary Medicine, 2021, 48(11):4 254-4 261.
[17] HU S, YIN S, JIANG X, et al.Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis[J].European Journal of Pharmacology, 2009, 616(1-3):287-292.
[18] MA Q Q, ZHOU X B, SUN Y C, et al.Threonine, but not lysine and methionine, reduces fat accumulation by regulating lipid metabolism in obese mice[J].Journal of Agricultural and Food Chemistry, 2020, 68(17):4 876-4 883.
[19] 努扎艾提·艾比布, 张艳慧, 阿斯娅·克里木, 等.不同Zn、Cu水平对香根草(Vetiveria zizanioides)体内Cd积累及生理指标的影响[J].内蒙古大学学报(自然科学版), 2012, 43(5):543-550.
NUZAHAT H, ZHANG Y H, ASIYA K, et al. Influence of different zinc and copper levels on cadmium accumulation and physiological characterization in Vetiveria zizanioides L. Journal of Inner Mongolia University (Natural Science Edition), 2012, 43(5):543-550.
[20] ALMEER R S, ALARIFI S, ALKAHTANI S, et al.The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression[J].Biomedicine &Pharmacotherapy, 2018, 106:1 490-1 498.
[21] YANG Z J, HE Y Q, WANG H F, et al.Protective effect of melatonin against chronic cadmium-induced hepatotoxicity by suppressing oxidative stress, inflammation, and apoptosis in mice[J].Ecotoxicology and Environmental Safety, 2021, 228:112947.
[22] ATMACA G.Antioxidant effects of sulfur-containing amino acids[J].Yonsei Medical Journal, 2004, 45(5):776-788.
[23] NANDI D, PATRA R C, SWARUP D.Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats[J].Toxicology, 2005, 211(1-2):26-35.
[24] YALÇINKAYA S, ÜNLÜÇERÇI Y, GIRI M, et al.Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet:Protective effect of taurine[J].Nutrition, 2009, 25(4):436-444.
M, et al.Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet:Protective effect of taurine[J].Nutrition, 2009, 25(4):436-444.
[25] DLUDLA P V, NKAMBULE B B, MAZIBUKO-MBEJE S E, et al.N-acetyl cysteine targets hepatic lipid accumulation to curb oxidative stress and inflammation in NAFLD:A comprehensive analysis of the literature[J].Antioxidants, 2020, 9(12):1283.
[26] LIAN C Y, ZHAI Z Z, LI Z F, et al.High fat diet-triggered non-alcoholic fatty liver disease:A review of proposed mechanisms[J].Chemico-Biological Interactions, 2020, 330:109199.
[27] SAMARGHANDIAN S, AZIMI-NEZHAD M, SHABESTARI M M, et al.Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats[J].Interdisciplinary Toxicology, 2015, 8(3):151-154.
[28] ZHANG J, WANG Y, FU L, et al.Subchronic cadmium exposure upregulates the mRNA level of genes associated to hepatic lipid metabolism in adult female CD1 mice[J].Journal of Applied Toxicology, 2018, 38(7):1 026-1 035.
[29] GO Y M, SUTLIFF R L, CHANDLER J D, et al.Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice[J].Toxicological Sciences, 2015, 147(2):524-534.