短链伯醇氧化酶(short-chain primary alcohol oxidase,SPAOX,EC 1.1.3.13)是一种依赖黄素腺嘌呤核苷酸(Flavin adenine dinucleotide,FAD)氧化短链伯醇(C1~C8)生成相应的醛和过氧化氢的氧化还原酶。SPAOX最早由JANSSEN等[1]于1968年从Basidiomycete(多孔菌科担子菌)的菌丝体中分离出来。近年来,SPAOX在甲基营养型微生物等多种真核生物中被发现。而且,SPAOX已经较为广泛地应用于醇类分析、生物转化等场景中。与SPAOX相关的研究逐渐得到众多学者的关注,取得一定进展的同时具有广泛的应用前景。本文对比了不同生物来源的SPAOX,阐述了其催化机制,总结了SPAOX性质的优缺点,并对提高SPAOX稳定性的研究进行了展望,以期为其进一步的应用奠定基础。
1 SPAOX的来源和催化机制
SPAOX已经在博乙丁假丝酵母(Candida boidinii)[2]、汉逊酵母(Hansenula polymorpha[3]、Ogataea angusta[4])、法夫驹形氏酵母(Komagataella phaffii)[5-6]、毕赤酵母(Pichia sp.)[7]等甲基营养型酵母中发现,也在土曲霉(Aspergillus terreus)[8-9]、黄孢原毛平革菌(Phanerochaete chrysosporium)[10]等丝状真菌中存在。利用软件MEGA7[11]对SPAOX的基因进行进化树分析(图1),表明SPAOX有2个分支;其中上分支第一部分中的SPAOX主要来源于甲基营养型酵母菌、曲霉以及癌肿病菌(Lachnellula willkommii)、特异青霉(Penicillium chrysosporium)、立枯丝核菌(Rhizoctonia solani)等真菌;上分支第二部分则来源于褐孢霉(Fulvia fulva)、密粘褶菌(Gloeophyllum trabeum)、朱红栓菌(Trametes cinnabarina)、牛樟芝(Taiwanofungus camphoratus)、原毛平革菌(Phanerochaete sordida)等真菌。而下分支中的SPAOX主要来源于短梗霉(Aureobasidium sp.)、担子菌(Basidiomycete)、炭疽病菌(Colletotrichum chlorophyti)、裂褶菌(Schizophyllum commune)、环红酵母(Rhodotorula toruloides)、炭角菌(Xylariomycetidae sp.)等真菌。两个分支中微生物种类差异较大。例如,上分支中汉逊酵母、博乙丁假丝酵母、法夫驹形氏酵母等甲基营养型酵母菌中SPAOX序列的分支置信度达100。其他真菌类,例如青霉、曲霉、原毛平革菌、牛樟芝、担子菌等的置信度则为13~99。

图1 短链伯醇氧化酶基因的进化树
Fig.1 Phylogenetic tree of SPAOX gene
因为SPAOX在甲基营养型酵母中参与甲醇代谢,将甲醇氧化成甲醛和过氧化氢,所以很多关于SPAOX的研究以甲基营养型酵母中的SPAOX作为研究对象来开展[12-13]。甲基营养型酵母新生成的SPAOX依靠其过氧化物酶体靶向信号(peroxisome targeting signal,PTS),被运输到过氧化物酶体中发挥作用。SPAOX单亚基的分子质量约为65 k~80 kDa,SPAOX的活性功能体是含有8个FAD辅因子和8个亚基的同源八聚体,分子质量约500~700 kDa。SPAOX属于葡萄糖-甲醇-胆碱(glucose-methanol-choline,GMC)家族氧化还原酶,由FAD结合域和底物结合域组成。GMC家族氧化还原酶的FAD结合域的氨基酸序列保守,而由于不同酶的最适底物不同,使得底物结合域的氨基酸序列差异较大[14]。因为GMC氧化还原酶的催化活性中心(组氨酸/组氨酸或组氨酸/天冬酰胺)高度保守,所以其催化机制相似[15]。SPAOX的催化过程分为还原半反应(还原FAD,以及醇类电子供体底物的氧化)和氧化半反应(FADH2被氧化形成H2O2)[16]。其中催化活性中心进行还原半反应,H567与醇底物结合并在催化过程中携带正电荷,由N616催化醇底物并稳定醇盐的负电荷[17]。结合口袋的W566和F98的芳香族侧链限制了底物分子的可用空间,使其最适底物为短链伯醇[3,18]。还原半反应中底物的氢转移到FAD的异四氧嘧啶上,FAD被还原成FADH2。另一部分氧化半反应的FAD结合域E38与FAD的腺嘌呤基团结合,N97与黄素连接环相互作用,FADH2被氧重新氧化成FAD,并释放H2O2[5, 17]。不同于乙醇脱氢酶(alcohol dehydrogenase,ADH,EC 1.1.1.1)在催化反应中不断消耗辅酶烟酰胺腺嘌呤二核苷酸,SPAOX循环利用FAD是一大优势[19]。
表1总结了GMC氧化还原酶家族常见酶的单体分子质量、结构、底物结合口袋等性质。其中,只有SPAOX是八聚体,其他家族中的酶都是单体或二聚体。单体大小除了胆固醇氧化酶(cholesterol oxidase)为36 kDa相对较小,其他都在60 k~80 kDa附近。底物结合口袋中都有H/H或H/N作为底物催化活性中心,而限制底物分子可用空间的氨基酸有所不同。例如SPAOX中的F98在胆碱氧化酶(choline oxidase)中为S101,W566为V464,而F98在芳香醇氧化酶(aryl-alcohol oxidase)中为Y92,在胆固醇氧化酶中为G66,而丝氨酸、缬氨酸、甘氨酸等氨基酸的残基都较小,使得这些氧化酶可催化诸如胆碱、胆固醇等较大醇类底物[5, 17]。
表1 GMC氧化还原酶家族结构的比较
Table 1 Comparing of the GMC family of oxidoreductases structures

基本信息单体分子质量/kDa亚单位最适底物底物结合口袋参考文献alcohol oxidase EC 1.1.3.1365^80八聚体甲醇M59、F98、W566、H567、C568、N616[5, 17]aryl-alcohol oxidase EC 1.1.3.770^78单体2-萘乙醇Y92、F367、F501、H502、H546[15, 20]choline oxidase EC 1.1.3.1760二聚体胆碱H99、S101、E312、H351、V464、H466[21]cholesterol oxidase EC 1.1.3.636单体胆固醇H69、G66、H121、W130、R304、E305、P561[22]glucose oxidase EC 1.1.3.480二聚体β-D-葡萄糖Y73、F418、W430、R516、N518、H520、H563[23]pyridoxine 4-oxidase EC 1.1.3.1267^68单体吡哆醇H167、Y169、D542、F454、Y456、H548、N593[16]
SPAOX在过氧化物酶体中组装成八聚体,得益于外界条件的影响,促使亚基相互接触[17]。由于八聚体结构组装较困难,因此SPAOX的稳定性较差,容易受到环境的影响而降解。
2 SPAOX的性质
2.1 底物特异性
SPAOX的底物主要为甲醇、乙醇、正丙醇、正丁醇。随着醇类底物碳链的增长,SPAOX的Km值逐渐变大,活力也逐渐下降(表2)。甲基营养型酵母中多形汉逊酵母(Hansenula polymorpha DL-1)SPAOX的Km值最小,为0.23 mmol/L。其他类型真菌中只有茯苓(Poria contigua)SPAOX的Km为0.2 mmol/L,小于甲基营养型酵母SPAOX的Km值。而且,茯苓SPAOX的最大反应速率为12.8 μmol/(min·nmol),高于目前报道的其他SPAOX的最大反应速率[24]。对SPAOX进行突变可以改变其催化底物的性质。SPAOX对甲醇和乙醇表现出良好的催化活性,对其他底物的低活性限制了SPAOX的进一步应用。SPAOX的蛋白质工程改造使其适配多种底物,将拓宽SPAOX的应用范围。DMYTRUK等[25]对多形汉逊酵母SPAOX进行突变,得到对甲醇Km值从0.62 mmol/L提高到1.1~2.48 mmol/L,拓宽了多形汉逊酵母SPAOX突变体的应用范围。虽然大部分SPAOX也展现出较弱的氧化甲醛的活力(表2),但是KJELLANDER等[26]将毕赤酵母SPAOX固定到纳米多孔氧化铝膜后,固定化SPAOX对甲醛表现出与游离酶对甲醇的102%相对活力,说明固定化技术为保护SPAOX不受甲醛的损伤提供了有力保障。
表2 不同SPAOX的底物特异性比较
Table 2 Comparing of the substrate specificity of SPAOX

来源参数甲醇乙醇正丙醇正丁醇甲醛参考文献BasidiomyceteKm/(mmol/L)1.521054.6133\相对活力/%100285.32.1\[1]Candida methanosorbosaKm/(mmol/L)2.438.23\\\相对活力/%10097.877.552.2\[27]Hansenula polymorpha DL-1Km/(mmol/L)0.234.414402.6Vmax/(U/mol)7.543.763.342.411相对活力/%10049.844.231.90.13[3]Hansenula polymorpha C-105Km/(mmol/L)0.46.6\\10.5kcat/s-16058\\32[18]Pichia sp.Km/(mmol/L)0.5\\\3.5相对活力/%10092745215[7]Pichia pastorisKm/(mmol/L)0.470.751.232.791.59Vmax/(pmol/s)759617485607778相对活力/%10081.363.980102[26]Pichia pastorisKm/(mmol/L)0.67.921.727.5\kcat/Km [L·(mmol/min)]57542149\[5]Ogataea thermosmethanolicaKm/(mmol/L)0.275.4124.91\\Vmax/(nmol/min)0.240.410.37\\相对活力/%10080.352.922.6\[28]Kloeckera sp.Km/(mmol/L)0.442.55.79.12.4Vmax/(U/mol)5.746.14.543.973.18相对活力/%100106796955.4[3]Peniophora giganteanKm/(mmol/L)1.82.9\\\Vmax/[μmol/(min·mg)]9.48.73.42.7\相对活力/%10092.636.228.7\[29]Thermoascus aurantiacus NBRC 31693Km/(mmol/L)相对活力/%Km/(mmol/L)相对活力/%胞内胞外13.515.8\\\10044145\0.510.2\\\100846028\[30]Gloeophyllum trabeumKm/(mmol/L)2.3\\\\kcat/Km/[L/(s·mol)]6.81.10.260.07\相对活力/%100947354\[31]Paecilomyces variotiiKm/(mmol/L)1.93.8\\4.9相对活力/%10010633\28[32]Penicillium purpurescens AIU 063Km/(mmol/L)19.321.5\\Vmax/[μmol/(min·mg)]4.355.122.921.951.28相对活力/%86100573825[33]Poria contiguaKm/(mmol/L)0.218.321.36.1Vmax/[μmol/(min·nmol)]12.8129.4\1.9相对活力/%10093.773.4\14.8[24]
2.2 SPAOX活力
由于SPAOX的八聚体结构,易受到环境因素的影响,因此,很多学者对多种SPAOX的影响因素pH、温度、化合物等进行考察。
2.2.1 环境的影响
表3汇总了pH和温度分别对不同SPAOX活力的影响。SPAOX的最适pH可以低至5,也可以高达9。部分菌株来源的SPAOX具有相同的最适pH,例如Candida 25-A和Komagataella pastoris的最适pH为7.5。Ogataea angusta、Aspergillus terreus和Penicillium purpurescens AIU 063的最适pH为8.5。Phanerochaete chrysosporium、Pichia sp.、Phanerochaete chrysosporium和Ogataea thermomethanolica的最适pH为9。相比pH性质的较为相似性,不同SPAOX的最适温度差异较大。例如Aspergillus ochraceus[34]、Candida methanosorbosa[27]、Thermoascus aurantiacus[30]、Ogataea angusta[4]、Phanerochaete chrysosporium[10]、Ogataea thermomethanolica[28]的SPAOX均有较好的热稳定性,最适温度为45~55 ℃左右;而其他SPAOX的最适温度均为25~40 ℃左右。环境因素除了pH和温度,静水压力也会影响SPAOX的活力[35-36]。高静水压力会稳定SPAOX的热失活,尤其是酶的不稳定组分,使得结构更加稳定。高静水压力可以使SPAOX在高温下保持活性并获得更快的反应速度。
表3 不同SPAOX性质的比较
Table 3 Comparing of SPAOX properties

来源pH温度/℃最适活性范围最适活性范围参考文献Aspergillus ochraceus5.5^76.5^8.550^5540[34]Paecilomyces variotii6^105^10<50[32]Gloeophyllum trabeum6^107^11[31]Candida methanosorbosa M-20036^9 6^8.550<50[27]Basidiomycetes6.5^97^925[1]Thermoascus aurantiacus NBRC 316938^115^1130^65[30]Peniophora gigantea7.3-96^935[29]Candida 25-A7.56^837.5<60[37]Komagataella pastoris7.57^837<38[4]Ogataea angustaAspergillus terreus8.58^1045<488.56^11304^4096^1132[9][8]Penicillium purpurescens AIU 0638.55.5^9.535^4020^50[33]Phanerochaete chrysosporium97^1050[10]Pichia sp.9[7]Phanerochaete chrysosporium97^10[38]Ogataea thermomethanolica96^95030^50[28]
2.2.2 化合物的影响
SPAOX溶液中有机、无机化合物、金属离子等物质会影响SPAOX活性(表4、表5)。其中联吡啶对SPAOX活性没有显著影响。羟基喹啉、氨基脲、Cu2+等因为羰基作用对SPAOX活性有一定的抑制[33]。羰基试剂诸如苯肼、羟胺以及肼等也对SPAOX活性有显著抑制作用[32,34]。Cd2+、Hg2+等金属离子对Candida、Ogataea polymorpha、Paecilomyces variotii的SPAOX活性有显著抑制作用,而其他金属离子对SPAOX活性无显著影响[7,29-30,34,39-40]。这也使得金属螯合剂乙二胺四乙酸(ethylene diamine tetraacetic acid, EDTA)、邻菲咯啉对SPAOX活性也无显著抑制作用[39]。叠氮化钠是甲醇的可逆竞争性抑制剂,结合位点位于SPAOX的活性中心区域,与FAD作用,从而改变SPAOX二级结构[18]。此外,SPAOX在环丙酮中20 ℃、pH 7.5条件下孵育2 h后完全失去活性[41]。但是在隔绝氧气情况下能有效抑制失活。由于SPAOX活性位点附近有巯基,因此,对氯苯甲酸汞(p-chloromercuribenzoate,PCMB)、Cu2+、Hg2+等对巯基有封闭作用的化合物对SPAOX有显著抑制作用。这表明SPAOX中半胱氨酸残基对于维持结构的作用必不可少[24,27,32]。此外,可以通过在Hg2+处理后的SPAOX中添加硫醇或在Cu2+处理后的SPAOX中添加EDTA进行去抑制[40]。过量的β-巯基乙酸可恢复由PCMB引起的失活,表明抑制由有机汞与巯基结合而引起[40]。因此,巯基的抑制作用是可逆竞争性抑制。
表4 化合物对SPAOX活力的影响
Table 4 Effects of chemical compounds on SPAOX activity
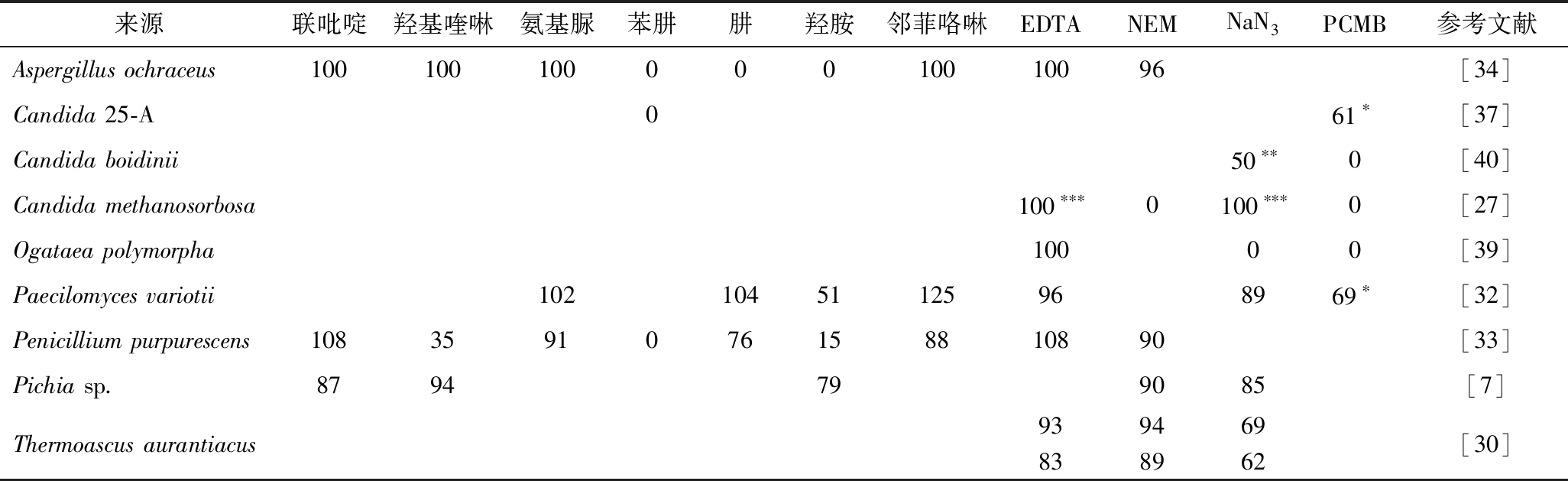
注:表格中的数据描述的是SPAOX在与抑制剂共存时的相对活力(%),其中抑制剂浓度均为1 mmol/L,除*为0.1 mmol/L,**为1.8 mmol/L,***为10 mmol/L;T. aurantiacus中上行为胞内SPAOX,下行为胞外SPAOX。
来源联吡啶羟基喹啉氨基脲苯肼肼羟胺邻菲咯啉EDTANEMNaN3PCMB参考文献Aspergillus ochraceus10010010000010010096[34]Candida 25-A061∗[37]Candida boidinii50∗∗0[40]Candida methanosorbosa100∗∗∗0100∗∗∗0[27]Ogataea polymorpha10000[39]Paecilomyces variotii10210451125968969∗[32]Penicillium purpurescens1083591076158810890[33]Pichia sp.8794799085[7]Thermoascus aurantiacus939469838962[30]
表5 金属离子对SPAOX活力的影响
Table 5 Effects of metal ions on SPAOX activity

注:表格中的数据描述的是SPAOX在与金属离子共存时的相对活力(%)及相关离子的存在形式,其中抑制剂浓度均为1 mmol/L,除*为10 mmol/L,**为2 mmol/L,***为0.1 mmol/L;T.aurantiacus中上行为胞内SPAOX,下行为胞外SPAOX。
来源Cu2+Cd2+Hg2+Ca2+Co2+Mg2+Mn2+Ni2+Zn2+参考文献Aspergillus ochraceus91(CuCl2)100(CoCl2)102(MgCl2)100(MnCl2)102(NiCl2)[34]Candida 25-A14520∗∗∗[37]Candida boidinii595∗∗0[40]Candida methanosorbosa0(CuSO4)100(CaCl2)∗[27]Ogataea polymorpha000[39]Paecilomyces variotii100(CuSO4)56(HgCl2)[32]Penicillium purpurescens0(CuCl2)94(CoCl2)70(MgCl2)85(MnCl2)80(NiCl2)98(ZnCl2)[33]Peniophora gigantea0(CuSO4)100(CaCl2)100(CoCl2)100(MgSO4)100(MnSO4)100(NiCl2)100(ZnSO4)[29]Pichia sp.50(CuSO4)[7]Thermoascus aurantiacus1054989795891002029376888688[30]
此外,碘乙酸可以使SPAOX可逆失活[40]。炔醇底物使SPAOX不可逆失活,炔醇氧化后产物醛的羟基再与SPAOX的活性中心发生亲电结合,使底物醇不能与SPAOX结合,所以炔醇底物是自杀性底物[2]。此外,底物和产物对SPAOX的稳定性也有影响。在没有过氧化氢酶存在的情况下,SPAOX会被大量产生的过氧化氢可逆抑制[4]。过氧化氢将SPAOX活性中心的巯基可逆氧化。加入巯基乙醇或二硫苏糖醇等还原剂可使SPAOX活性恢复[42]。
2.3 SPAOX的稳定性
SPAOX稳定性的相关研究一直受到广泛关注。将SPAOX储存于4 ℃可保持活性数周至数月[8],储存于-20或-80 ℃可保持活性数年[24]。虽然低温保存对活性的影响较小,但反复冻融会使SPAOX快速失活[29]。Basidiomycota的SPAOX可在0.05 mol/L的磷酸缓冲液中保持至少5个月活性[1]。添加500 g/L蔗糖可使Pichia putida的SPAOX的稳定性提高10倍,在300~500 g/L蔗糖、-20或-80 ℃下SPAOX活性可保持数年[43]。固定化技术可显著提高SPAOX的活性范围,有利于酶保持稳定性。ZHAO等[44]将SPAOX固定在静电纺丝纤维上后,由于纤维表面的生物相容性和亲水性使得最适温度提高至50 ℃,最适pH范围扩大至6.5~7.5,在40 ℃孵育2 h后SPAOX活性仅降低10%。CHUNG等[45]将SPAOX固定在活性纤维素载体上,SPAOX的最适温度提高至65 ℃,在80 ℃仍有25%活性,pH范围扩大至4~10,并在25 ℃下保持稳定10周以上。KJELLANDER等[26]将SPAOX固定在多孔纳米氧化铝上后,室温下反应50 h后仍能保持50%以上的活性。稳定性在SPAOX作为传感器元件中也发挥了重要作用,固定在含有聚中性红(poly neutral red,PNR)介质的碳膜电极上的SPAOX作为传感器,使用2周后传感器的灵敏度下降0.8%,在pH 7、0.1 mol/L磷酸缓冲液中4 ℃保存6周后,传感器的灵敏度仅下降12%[46]。
3 SPAOX的应用
SPAOX的底物广泛、反应的不可逆性和辅因子循环使用的优势使得SPAOX已经在多个方面成功应用。图2从SPAOX的催化机制出发总结了SPAOX的主要应用场景,例如基于固定化SPAOX构建的生物反应器用于醇醛类物质分析检测、醇醛物质的生物转化、生物修复方面的醛类物质降解等。

图2 SPAOX的应用
Fig.2 Applications of SPAOX
3.1 醇类物质的分析检测
固定化的SPAOX常被用于食品中短链醇、醛的检测。根据检测技术分类,可分为光学方法和电化学方法。
光学分析方法基于SPAOX和辣根过氧化物酶(horseradish peroxidase,HRP)耦合,氧化显色物质发生显色反应,进而测定吸光度的变化分析目的物的含量。例如SPAOX、HRP和显色剂混合于两层滤纸之间构成酒精检测试纸,可以快速检测血液或唾液乙醇,其检测范围达10~1 200 mg/L[47]。将二茂铁包埋的SPAOX和包覆HRP的溶胶-凝胶壳聚糖膜逐层固定在多壁碳纳米管(Multi-walled carbon nanotube,MWCNT)修饰的玻碳电极上,制成检测乙醇的生物传感器[48],其检测范围达5~3 000 μmol/L。此外,AHMAD等[49]报道了将SPAOX包埋在含有尼罗蓝(Nile blue chromoionophore,NBCM)的聚丙烯酸酯薄膜上,用于甲醛检测。其原理为SPAOX与甲醛反应的产物甲酸提供的氢离子与NBCM发生离子转移反应,生成深蓝色络合物HNBCM+,进而通过吸光度的变化分析甲醛的含量,其检测范围达10-3~103 mmol/L。基于显色分析的方法操作简单、高效,线性的准确度高,但存在重复性较差的缺点。
电化学分析基于溶解氧含量的测定。例如将SPAOX与纳米金颗粒结合,氧化醇生成的H2O2将苯胺氧化聚合成聚苯胺(polyaniline,PANI),再将PANI包裹的SPAOX-AuNPs组装在玻碳电极上,所制备的生物传感器可用于醇的分析检测,检测范围达10~4 700 μmmol/L[50]。此外,电化学分析方法也可以测定H2O2分解而产生的电流。例如,将SPAOX固定在以氯化高铁血红素和金纳米粒子(CF-H-Au)修饰的碳微纤维而制备成纳米酶,其对H2O2的亲和力比溶液中的氯化血红素高2.6倍,对乙醇的检出限为5 μmol/L,检测范围达0.01~0.15 mmol/L[51]。HOODA等[52]将SPAOX共价固定在聚氯乙烯烧杯上,同时将HRP、Nafion、MWCNT、壳聚糖和金纳米颗粒固定在电极上,检测乙醇的范围为0.01~42 mmol/L。采用电化学分析方法,检测灵敏度高,重复性好,然而生物传感器的搭建较为复杂。
此外,还可以通过气体压力变化检测氧气。ZHANG等[53]将两亲性气凝胶结合Pd @ Pt核壳纳米颗粒,形成密封设备,乙醇经SPAOX和过氧化氢酶得到终产物氧气,通过便携式压力传感器来定量乙醇含量,检出限为0.5 mmol/L。该方法的样品制备和检测反应时间短,且不受醇类的干扰,也为乙醇检测提供了一种新的信号转导途径。
3.2 醇醛物质的生物转化
SPAOX可用于生产甲醛、H2O2、复杂有机物的中间产物[54]。例如,DIENYS等[55]将SPAOX固定在戊二醛活化后的大孔纤维素载体上,用于合成杂环化合物。SPAOX固定在纳米多孔氧化铝膜上,生产H2O2的生产效率远高于葡萄糖氧化酶[26]。为了克服SPAOX稳定性差的问题,MANGKORN等[56]将SPAOX固定在钡铁氧体磁性微粒(BaFe12O19)上不仅提高了SPAOX的热稳定性和催化效率,并且可以固定化SPAOX,在合成醛方面具有良好的工业应用前景。
3.3 环境修复
SPAOX固定于海藻酸钙凝胶中,开发了用于检测或生物修复空气中甲醛的连续生物反应器,能清除90%以上甲醛[57]。DAS等[58]将SPAOX用于燃料电池,链接漆酶,从甲醇基质中发电,用于环境修复中。SPAOX以新颖的生物发电的方式用于环境修复中,开发了SPAOX更大的应用价值。
4 展望
SPAOX的结构和催化原理已经明晰。SPAOX也被成功固定化和应用。但是相对较低的底物亲和力和较差的稳定性限制了SPAOX的应用。未来可以从3个方面克服SPAOX的不足,提高SPAOX在应用中的催化效率和便捷性:
(1)提高SPAOX对特定底物的亲和力。SPAOX氨基酸序列的突变是提高其底物亲和力的有效措施[25]。部分SPAOX由于来自特定物种或是经过改造可以有不同的最适底物,如甘油[10]。着重研究SPAOX的序列及结构,尤其针对底物结合口袋,找到改变底物特异性的关键残基,有利于改变底物结合口袋,控制SPAOX的最适底物,有助于检测应用和特定化合物的生产。
(2)提高SPAOX的环境稳定性。来自O.angusta、A.ochraceus、T.aurantiacus NBRC 31693的SPAOX有较好的pH和热稳定性[4,30,34]。对这些SPAOX的序列进行研究,有利于在后续进一步提高SPAOX的稳定性,使SPAOX在实际检测应用中的损耗更低,重复性更高。研究不同环境条件对SPAOX活性的影响,从氨基酸序列和蛋白质结构2方面分析环境对SPAOX影响的原因,找到更加合适的反应条件,提高SPAOX的反应速率,使其在生产应用中的生物转化效率更高,产量更大。
(3)开发不同的定量方法,以及将生物转化应用于更多领域。优化传统的光学或电化学分析检测定量方法,并开发诸如气体压力检测等非传统的定量方法,使SPAOX可以在不同应用场景中对短链伯醇类物质进行检测。SPAOX循环利用辅酶FAD的特点,可以在各种场景中随时、长时间反应,使其不仅可以用于生产,还可用于环境修复、生物发电等更多领域,提高了SPAOX的应用价值。
[1] JANSSEN F W, RUELIUS H W.Alcohol oxidase, a flavoprotein from several basidiomycetes species[J].Biochimica et Biophysica Acta (BBA) - Enzymology, 1968, 151(2):330-342.
[2] NICHOLS C S, CROMARTIE T H.Irreversible inactivation of the flavoenzyme alcohol oxidase with acetylenic alcohols[J].Biochemical and Biophysical Research Communications, 1980, 97(1):216-221.
[3] KATO N, OMORI Y, TANI Y, et al.Alcohol oxidases of Kloeckera sp.and Hansenula polymorpha. catalytic properties and subunit structures[J].European Journal of Biochemistry, 1976, 64(2):341-350.
[4] COUDERC R, BARATTI J.Oxidation of methanol by the yeast, Pichia pastoris.purification and properties of the alcohol oxidase[J].Agricultural and Biological Chemistry, 1980, 44(10):2279-2289.
[5] KOCH C, NEUMANN P, VALERIUS O, et al.Crystal structure of alcohol oxidase from Pichia pastoris[J].PLoS One, 2016, 11(2):e0149846.
[6] KURTZMAN C P.Description of Komagataella phaffii sp.nov.and the transfer of Pichia pseudopastoris to the methylotrophic yeast genus Komagataella[J].International Journal of Systematic and Evolutionary Microbiology, 2005, 55(2):973-976.
[7] PATEL R N, HOU C T, LASKIN A I, et al.Microbial oxidation of methanol:Properties of crystallized alcohol oxidase from a yeast, Pichia sp.[J].Archives of Biochemistry and Biophysics, 1981, 210(2):481-488.
[8] KUMAR A K, GOSWAMI P.Purification and properties of a novel broad substrate specific alcohol oxidase from Aspergillus terreus MTCC 6324[J].Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2008, 1784(11):1552-1559.
[9] KUMAR A K, GOSWAMI P.Functional characterization of alcohol oxidases from Aspergillus terreus MTCC 6324[J].Applied Microbiology and Biotechnology, 2006, 72(5):906-911.
[10] LINKE D A, LEHNERT N, NIMTZ M, et al.An alcohol oxidase of Phanerochaete chrysosporium with a distinct glycerol oxidase activity[J].Enzyme and Microbial Technology, 2014, 61-62:7-12.
[11] KUMAR S, STECHER G, TAMURA K.MEGA7:Molecular evolutionary genetics analysis version 7.0 for bigger datasets[J].Molecular Biology and Evolution, 2016, 33(7):1870-1874.
[12] WANG Y Y, LI J W, ZHAO F G, et al.Methanol oxidase from Hansenula polymorpha shows activity in peroxisome-deficient Pichia pastoris[J].Biochemical Engineering Journal, 2022, 180:108369.
[13] CAI H L, DOI R, SHIMADA M, et al.Metabolic regulation adapting to high methanol environment in the methylotrophic yeast Ogataea methanolica[J].Microbial Biotechnology, 2021, 14(4):1512-1524.
[14] SÜTZL L, FOLEY G, GILLAM E M J, et al.The GMC superfamily of oxidoreductases revisited:Analysis and evolution of fungal GMC oxidoreductases[J].Biotechnology for Biofuels, 2019, 12(1):1-18.
[15] HERN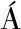 NDEZ-ORTEGA A, FERREIRA P, MART
NDEZ-ORTEGA A, FERREIRA P, MART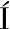 NEZ A T.Fungal aryl-alcohol oxidase:A peroxide-producing flavoenzyme involved in lignin degradation[J].Applied Microbiology and Biotechnology, 2012, 93(4):1395-1410.
NEZ A T.Fungal aryl-alcohol oxidase:A peroxide-producing flavoenzyme involved in lignin degradation[J].Applied Microbiology and Biotechnology, 2012, 93(4):1395-1410.
[16] WONGNATE T, CHAIYEN P.The substrate oxidation mechanism of pyranose 2-oxidase and other related enzymes in the glucose-methanol-choline superfamily[J].FEBS Journal, 2013, 280(13):3009-3027.
[17] VONCK J, PARCEJ D N, MILLS D J.Structure of alcohol oxidase from Pichia pastoris by cryo-electron microscopy[J].PLoS One, 2016, 11(7):e0159476.
[18] SHLEEV S V, SHUMAKOVICH G P, NIKITINA O V, et al.Purification and characterization of alcohol oxidase from a genetically constructed over-producing strain of the methylotrophic yeast Hansenula polymorpha[J].Biochemistry (Moscow), 2006, 71(3):245-250.
[19] CONTENTE M L, MARZUOLI I, IDING H, et al.Screening methods for enzyme-mediated alcohol oxidation[J].Scientific Reports, 2022, 12:3019.
[20] VI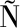 A-GONZALEZ J, ALCALDE M.Directed evolution of the aryl-alcohol oxidase:Beyond the lab bench[J].Computational and Structural Biotechnology Journal, 2020, 18:1800-1810.
A-GONZALEZ J, ALCALDE M.Directed evolution of the aryl-alcohol oxidase:Beyond the lab bench[J].Computational and Structural Biotechnology Journal, 2020, 18:1800-1810.
[21] GADDA G.Hydride transfer made easy in the reaction of alcohol oxidation catalyzed by flavin-dependent oxidases[J].Biochemistry, 2008, 47(52):13745-13753.
[22] CALDINELLI L, IAMETTI S, BARBIROLI A, et al.Dissecting the structural determinants of the stability of cholesterol oxidase containing covalently bound flavin[J].Journal of Biological Chemistry, 2005, 280(24):22572-22581.
[23] WITT S, WOHLFAHRT G, SCHOMBURG D, et al.Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of β-D-glucose[J].Biochemical Journal, 2000, 347(2):553-559.
[24] BRINGER S, SPREY B, SAHM H.Purification and properties of alcohol oxidase from Poria contigua[J].European Journal of Biochemistry, 1979, 101(2):563-570.
[25] DMYTRUK K V, SMUTOK O V, RYABOVA O B, et al.Isolation and characterization of mutated alcohol oxidases from the yeast Hansenula polymorpha with decreased affinity toward substrates and their use as selective elements of an amperometric biosensor[J].BMC Biotechnology, 2007, 7:33.
[26] KJELLANDER M, GÖTZ K, LILJERUHM J, et al.Steady-state generation of hydrogen peroxide:Kinetics and stability of alcohol oxidase immobilized on nanoporous alumina[J].Biotechnology Letters, 2013, 35(4):585-590.
[27] SUYE S I.Purification and properties of alcohol oxidase from Candida methanosorbosa M-2003[J].Current Microbiology, 1997, 34(6):374-377.
[28] MANGKORN N, KANOKRATANA P, ROONGSAWANG N, et al.Purification, characterization, and stabilization of alcohol oxidase from Ogataea thermomethanolica[J].Protein Expression and Purification, 2018, 150:26-32.
[29] DANNEEL H J, REICHERT A, GIFFHORN F.Production, purification and characterization of an alcohol oxidase of the ligninolytic fungus Peniophora gigantea[J].Journal of Biotechnology, 1994, 33(1):33-41.
[30] KO H S, YOKOYAMA Y, OHNO N, et al.Purification and characterization of intracellular and extracellular, thermostable and alkali-tolerant alcohol oxidases produced by a thermophilic fungus, Thermoascus aurantiacus NBRC 31693[J].Journal of Bioscience and Bioengineering, 2005, 99(4):348-353.
[31] DANIEL G, VOLC J, FILONOVA L, et al.Characteristics of Gloeophyllum trabeum alcohol oxidase, an extracellular source of H2O2 in brown rot decay of wood[J].Applied and Environmental Microbiology, 2007, 73(19):6241-6253.
[32] KONDO T, MORIKAWA Y, HAYASHI N.Purification and characterization of alcohol oxidase from Paecilomyces variotii isolated as a formaldehyde-resistant fungus[J].Applied Microbiology and Biotechnology, 2008, 77(5):995-1002.
[33] ISOBE K, TAKAHASHI T, OGAWA J, et al.Production and characterization of alcohol oxidase from Penicillium purpurescens AIU 063[J].Journal of Bioscience and Bioengineering, 2009, 107(2):108-112.
[34] ISOBE K, KATO A, OGAWA J, et al.Characterization of alcohol oxidase from Aspergillus ochraceus AIU 031[J].The Journal of General and Applied Microbiology, 2007, 53(3):177-183.
[35] BUCHHOLZ-AFARI M I, HALALIPOUR A, YANG D Y, et al.Increased stability of alcohol oxidase under high hydrostatic pressure[J].Journal of Food Engineering, 2019, 246:95-101.
[36] YANG D Y, REYES-DE-CORCUERA J I.Increased activity of alcohol oxidase at high hydrostatic pressure[J].Enzyme and Microbial Technology, 2021, 145:109751.
[37] YAMADA H, SHIN K C, KATO N, et al.Purification and characterization of alcohol oxidase from Candida 25-A[J].Agricultural and Biological Chemistry, 1979, 43(4):877-878.
[38] NGUYEN Q T, ROMERO E, DIJKMAN W P, et al.Structure-based engineering of Phanerochaete chrysosporium alcohol oxidase for enhanced oxidative power toward glycerol[J].Biochemistry, 2018, 57(43):6209-6218.
[39] KLEPACH H M, ZAKALSKIY A E, ZAKALSKA O M, et al. Alcohol oxidase from the methylotrophic yeast Ogataea polymorpha: Isolation, purification, and bioanalytical application[J]. Methods in Molecular Biology, 2021, 2280:231-248.
[40] CROMARTIE T H.Sulfhydryl and histidinyl residues in the flavoenzyme alcohol oxidase from Candida boidinii[J].Biochemistry, 1981, 20(19):5416-5423.
[41] CROMARTIE T H.Irreversible inactivation of the flavoenzyme alcohol oxidase by cyclopropanone[J].Biochemical and Biophysical Research Communications, 1982, 105(2):785-790.
[42] LIU Y P, PAN J F, WEI P L, et al.Efficient expression and purification of recombinant alcohol oxidase in Pichia pastoris[J].Biotechnology and Bioprocess Engineering, 2012, 17(4):693-702.
[43] GVOZDEV A R, TUKHVATULLIN I A, GVOZDEV R I.Purification and properties of alcohol oxidase from Pichia putida[J].Biochemistry (Moscow), 2010, 75(2):242-248.
[44] ZHAO L, LIU Q J, YAN S L, et al.Multimeric immobilization of alcohol oxidase on electrospun fibers for valid tests of alcoholic saliva[J].Journal of Biotechnology, 2013, 168(1):46-54.
[45] CHUNG H J, CHO H Y, KONG K H.Immobilization of Hansenula polymorpha alcohol oxidase for alcohol biosensor applications[J].Bulletin of the Korean Chemical Society, 2009, 30(1):57-60.
[46] BARSAN M M, BRETT C M A.An alcohol oxidase biosensor using PNR redox mediator at carbon film electrodes[J].Talanta, 2008, 74(5):1505-1510.
[47] THEPCHUAY Y, SONSA-ARD T, RATANAWIMARNWONG N, et al.Paper-based colorimetric biosensor of blood alcohol with in-situ headspace separation of ethanol from whole blood[J].Analytica Chimica Acta, 2020, 1103:115-121.
[48] CHINNADAYYALA S R, KAKOTI A, SANTHOSH M, et al.A novel amperometric alcohol biosensor developed in a 3rd generation bioelectrode platform using peroxidase coupled ferrocene activated alcohol oxidase as biorecognition system[J].Biosensors and Bioelectronics, 2014, 55:120-126.
[49] AHMAD M, HENG L Y, TAN L L.Optical enzymatic formaldehyde biosensor based on alcohol oxidase and pH-sensitive methacrylic-acrylic optode membrane[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2022, 267:120535.
[50] CHINNADAYYALA S R, SANTHOSH M, SINGH N K, et al.Alcohol oxidase protein mediated in-situ synthesized and stabilized gold nanoparticles for developing amperometric alcohol biosensor[J].Biosensors and Bioelectronics, 2015, 69:155-161.
[51] SMUTOK O, KAVETSKYY T, PROKOPIV T, et al.New micro/nanocomposite with peroxidase-like activity in construction of oxidases-based amperometric biosensors for ethanol and glucose analysis[J].Analytica Chimica Acta, 2021, 1143:201-209.
[52] HOODA V, GAHLAUT A, HOODA V.A novel amperometric biosensor for rapid detection of ethanol utilizing gold nanoparticles and enzyme coupled PVC reaction cell[J].Environmental Technology, 2021, 42(21):3318-3328.
[53] ZHANG Y, LIU Q Y, MA C B, et al.Point-of-care assay for drunken driving with Pd@Pt core-shell nanoparticles-decorated ploy(vinyl alcohol) aerogel assisted by portable pressure meter[J].Theranostics, 2020, 10(11):5064-5073.
[54] SAGIROGLU A, ALTAY V.Bioconversion of methanol to formaldehyde.II.by purified methanol oxidase from modified yeast, Hansenula polymorpha[J].Preparative Biochemistry &Biotechnology, 2006, 36(4):321-332.
[55] DIENYS G, JARMALAVI IUS S, BUDRIEN S, et al.Alcohol oxidase from the yeast Pichia pastoris—A potential catalyst for organic synthesis[J].Journal of Molecular Catalysis B:Enzymatic, 2003, 21(1-2):47-49.
IUS S, BUDRIEN S, et al.Alcohol oxidase from the yeast Pichia pastoris—A potential catalyst for organic synthesis[J].Journal of Molecular Catalysis B:Enzymatic, 2003, 21(1-2):47-49.
[56] MANGKORN N, KANOKRATANA P, ROONGSAWANG N, et al.Synthesis and characterization of Ogataea thermomethanolica alcohol oxidase immobilized on Barium ferrite magnetic microparticles[J].Journal of Bioscience and Bioengineering, 2019, 127(3):265-272.
[57] SIGAWI S, SMUTOK O, DEMKIV O, et al.Immobilized formaldehyde-metabolizing enzymes from Hansenula polymorpha for removal and control of airborne formaldehyde[J].Journal of Biotechnology, 2011, 153(3-4):138-144.
[58] DAS M, BARBORA L, DAS P, et al.Biofuel cell for generating power from methanol substrate using alcohol oxidase bioanode and air-breathed laccase biocathode[J].Biosensors and Bioelectronics, 2014, 59:184-191.