益生菌是一类对宿主健康有益的活性微生物,当摄入足够量时会产生益处,如增强免疫、缓解肠胃不适、改善炎症等[1]。益生菌主要是双歧杆菌和乳杆菌属中的一些种,已被证实其安全性并具有悠久的食用历史[2]。近年来,随着高通量测序和培养组学技术的发展,一些分离自肠道的新型微生物受到广泛关注,研究发现它们对宿主健康具有潜在益处,有望成为下一代益生菌,主要包括Akkermansia muciniphila、Roseburia intestinalis、Faecalibacterium prausnitzii和Bacteroides spp.[3-7]。
除了公认的下一代益生菌外,肠道内还有一些特定种属的肠道菌也与健康密切相关。我们前期调研发现,肠道中布劳特氏属(Blautia)的相对丰度与一些肠道炎症、肥胖、糖尿病等的改善呈正相关[8-11],表明其在缓解炎症性疾病和代谢性疾病方面可能发挥重要作用,也是潜在的肠道益生菌[12]。我们从肠道粪便中分离到了一株Blautia producta D4,发现其能改善脂多糖(lipopolysaccharide,LPS)诱导的急性肝损伤[13],同时也能很好地改善DSS诱导的结肠炎症[14],表明B.producta D4具有较好的益生效果,是一株有潜力的肠道益生菌。
潜在益生菌在应用之前,必须要对其进行安全性评估[15-16]。本研究分析了B.producta D4的毒力因子和抗生素耐药基因,测定了菌株的溶血性和抗生素耐受性,并结合连续灌胃14 d 109和1010 CFU菌株的体内急性毒性实验(包括小鼠体重、饮食饮水情况、血常规、肝功能指标和各器官组织病理切片),综合评估了此菌株的安全性,为后续开发使用此类功能性肠道菌奠定了理论基础。
1 材料与方法
1.1 实验材料
Blautia producta D4(以下简称为D4)菌株分离自健康小鼠粪便,保藏于江南大学食品微生物菌种保藏中心。金黄色葡萄球菌ATCC29213、副干酪乳杆菌ATCC334,购自美国模式菌种保藏中心。
7周龄的无特定病原体级(specific pathogen free,SPF)雌性和雄性C57BL/6J小鼠,购于南京大学模式动物研究所。
无菌脱脂纤维羊血,常德比克曼生物科技有限公司;环丙沙星,上海创赛科技有限公司;卡那霉素、链霉素、新霉素、四环素、红霉素、克林霉素、氨苄青霉素、阿莫西林、甲氧嘧啶、氯霉素、利福平、万古霉素、青霉素、多粘菌素,生工生物工程(上海)股份有限公司。
1.2 实验方法
1.2.1 实验菌株培养
以2%的添加量将冻存的B.producta D4菌株接种于改良的岐阜厌氧培养基(Gifu anaerobic medium,GAM),于37 ℃厌氧工作站培养12~14 h,并将菌株活化3代用于后续实验。GAM培养基配方(g/L):![]() 蛋白胨10,大豆蛋白胨3,酵母提取物5,牛肉膏2.2,牛肝浸粉1.2,消化血清粉13.5,葡萄糖8,磷酸二氢钾2.5,氯化钠3,L-半胱氨酸盐酸盐0.6,并将pH调至7.3±0.1,115 ℃灭菌20 min。
蛋白胨10,大豆蛋白胨3,酵母提取物5,牛肉膏2.2,牛肝浸粉1.2,消化血清粉13.5,葡萄糖8,磷酸二氢钾2.5,氯化钠3,L-半胱氨酸盐酸盐0.6,并将pH调至7.3±0.1,115 ℃灭菌20 min。
取对数期活化3代菌株的细菌培养物,离心(8 000×g,4 ℃)20 min,用无菌磷酸盐缓冲液清洗菌泥2次,然后于脱脂牛奶(100 g/L)中重悬并保存在-80 ℃冰箱备用。灌胃前取出冻存的菌液,待恢复至室温后用平板计数法确定菌体浓度,调整至5×109 CFU/mL和5×1010 CFU/mL,动物实验待用。
1.2.2 毒力基因和抗生素耐受基因概况
从-80 ℃取出冻存的菌液,恢复至室温后接种2%至GAM液体培养基,于厌氧工作站培养24 h,得到第一代活化的菌株。按照同样方法将菌株活化3代,最后一代接种至100 mL GAM液体培养基中,厌氧培养14 h后离心(8 000×g,10 min)收集菌泥,然后用无菌PBS洗涤菌泥,并转移至5 mL EP管中,再次离心,弃上清液得到菌泥沉淀,冷链运输寄至上海美吉生物医药科技有限公司进行菌株基因草图测序。
将D4的氨基酸序列分别于毒力因子数据库(Virulence Factor Database,VFDB,http://www.mgc.ac.cn/VFs/main.htm)和综合抗生素抗性数据库(Comprehensive Antibiotic Resistance Database,CARD,http://arpcard.mcmaster.ca/)进行比对,其中相似度(identity)>30%、覆盖度(coverage)>70%及E值(E-value)<0.01的结果视为毒力基因和抗生素耐受基因。
1.2.3 溶血性测定
利用改良的GAM培养基(额外添加0.01%氯化血红素、0.01%的维生素K1和5%无菌脱脂纤维羊血)测定D4的溶血性,实验中阳性对照菌为金黄色葡萄球菌ATCC29213。取稀释至10-6梯度的对数末期的菌液100 μL涂布于GAM平板,37 ℃厌氧培养24 h,观察菌株溶血情况。溶血活性分为β溶血(菌落周围有界限分明且透明的溶血环)、α溶血(菌落周围有草绿色光环)和γ溶血(菌落周围无溶血环)。
1.2.4 抗生素耐受性测定
根据国际标准ISO10932—2010,采用微量肉汤微稀释法测定13种不同抗菌机制的抗生素对菌株的最低抑菌浓度(minimum inhibitory concentration,MIC)。具体方法如下:采用二倍数稀释法,将每种抗生素制为终质量浓度为0.032~2 048 μg/mL的溶液,加入100 μL至无菌96孔板中。刚进入对数末期的菌液用GAM培养基稀释,使最终接种浓度为3×105 CFU/mL。取100 μL稀释后的菌液加至含抗生素溶液的96孔板中,于37 ℃厌氧工作站培养24 h,用酶标仪读取OD625吸光值,将抑制90%菌株生长的抗生素浓度称为MIC。副干酪乳杆菌ATCC334作为本次实验的质控菌株。
参照欧洲食品安全局(European Food Safety Authority,EFSA)标准指南和欧盟委员会(European Commission,EUC)动物营养科学委员会(Scientific Committee on Animal Nutrition,SCAN)的建议,用断点值来判断菌株对抗生素的耐受性,当MIC值≤断点值时,认为菌株对抗生素敏感。实验重复3次用于结果统计。
1.2.5 小鼠体内急性毒性实验
1.2.5.1 动物实验设计
30只SPF级健康雌、雄C57BL/6J小鼠(7周龄,体重约20 g)饲养于江南大学实验动物中心SPF级屏障中,温度为(25±2) ℃,相对湿度40%~60%,严格遵循光照12 h/黑夜12 h循环,所有动物均喂食标准饲料和无菌水,并可自由摄食和饮水,适应1周后开始实验。
30只小鼠分为6组,雌雄各3组,每组5只,分别是空白组、D4菌株109组、D4菌株1010组。空白组灌胃脱脂牛奶(100 g/L),其余组别灌胃相应菌株的活菌悬液,实验持续14 d,每天灌胃时间一致。本实验方案经江南大学伦理委员会批准(JN.No20200630c0300815[125]),且符合2010/63/EU欧盟保护实验动物条例。在整个实验过程中,观察小鼠的精神状态、行为并记录小鼠体重和摄食饮水量。实验结束处死小鼠,收集血液和组织进行后续检测。详细实验设计如表1所示。
表1 动物实验设计
Table 1 Animal experimental design

组别性别灌胃物质灌胃体积/μL空白-雄雄10%脱脂乳200 D4-109-雄雄B.producta D4 (5×109 CFU/mL)200D4-1010-雄雄B.producta D4 (5×1010 CFU/mL)200空白-雌雌10%脱脂乳200D4-109-雌雌B.producta D4 (5×109 CFU/mL)200D4-1010-雌雌B.producta D4 (5×1010 CFU/mL)200
1.2.5.2 脏器指数测定
小鼠处死后,称取心脏、肝、肺、肾、脾和结肠的质量,并确定脏器指数,计算如公式(1)所示:

1.2.5.3 血常规及生化指标测定
处死小鼠后,取新鲜的全血装于含肝素的抗凝管中,在0.5 h内用全自动血液细胞分析仪测定小鼠白细胞、中性粒细胞、淋巴细胞、单核细胞、嗜酸性粒细胞、嗜碱性粒细胞、红细胞以及血红蛋白浓度等血常规项目。
取小鼠血清,用全自动生化分析仪和相关的试剂测定葡萄糖、总胆固醇、甘油三酯等基本生化指标以及肝功能相关的丙氨酸转氨酶、碱性磷酸酶、天冬氨酸转氨酶、总蛋白和白蛋白等指标。
1.2.5.4 小鼠脏器形态学观察
小鼠处死后收集小块肝脏、肾脏、脾脏和结肠,置于4%多聚甲醛固定液中,过夜浸泡后经脱水透明、浸蜡包埋于切片机上制成5 μm厚的切片,苏木精-伊红(hematoxylin-eosin staining,HE)染色后,使用切片扫描仪对组织的形态进行扫描和拍照。
1.2.6 数据统计与分析
所有结果均以各组的平均值±标准差(mean±SD)表示,采用Origin 2017以及Adobe photoshop CS6进行图像处理。SPSS 22.0用于数据的统计学检验,采用单因素方差(One-Way ANOVA)或独立样本t检验进行显著性分析。
2 结果与分析
2.1 B.producta D4的毒力基因分析
来源于微生物并引起宿主产生特定疾病的物质被称为毒力因子,可作为评价菌株是否安全的重要指标[17]。基于VFDB数据库对D4中的毒力基因进行鉴定,结果如表2所示。存在于D4中的毒力基因类别丰富,不同种类的毒力基因调控着不同的功能。PhoP-PhoQ是细菌中重要的双组分调节系统之一,其中phoP作为反应调节剂,可以帮助细菌提高对外环境的适应性[18]。RelA、FbpABC、FeoAB、HitABC等和铁离子摄取有关,均不具备致病能力。FbpA、Hsp60、Hyaluronic acid capsule、PI-2a、LPS、Lap等与黏附有关,而Dot/Icm和HSI-I和分泌系统有关。同时,D4中还鉴定出与防御相关的毒力基因,主要有负责抗吞噬作用的alginate、capsule和capsule I[19]以及与应激蛋白相关的clpC、clpP、katA、msrAB。
表2 B.producta D4鉴定的毒力基因
Table 2 Virulence genes identified in B.producta D4

基因类别基因功能基因名称调控型毒力基因调控phoP,relA无特异致病型毒力基因铁离子摄取系统fbpABC,feoAB,hitABC入侵flagella进攻型毒力基因分泌系统HSI-I,Dot/Icm黏附LPS,CBPs,fbpA,hsp60,hyaluronic acid capsule,il-pA,lap,PI-2a,Type IV pili防御型毒力基因应激蛋白clpC,clpP,katA,msrAB抗吞噬作用alginate,capsule,capsule I
2.2 B.producta D4的抗生素耐受性评价
近年来,抗生素耐药性的增加对全球健康产生了巨大影响。EFSA指南指出,对于拟供食用的细菌菌株需要进行抗生素敏感性测定,以证明其安全性[20]。不同抗生素对B.producta D4和副干酪乳杆菌ATCC 334的MIC测定结果如表3所示,四环素、阿莫西林和氨苄青霉素对D4的MIC较小,分别是0.125、0.5、1 μg/mL;链霉素、甲氧苄啶和环丙沙星对D4的MIC一致,均为32 μg/mL;卡那霉素对D4的MIC最大,为256 μg/mL。结合断点值可以看出,D4对链霉素、新霉素、四环素、氨苄青霉素、阿莫西林、甲氧苄啶、氯霉素、利福平和万古霉素敏感,对卡那霉素、红霉素、克林霉素和环丙沙星表现出耐药性。
表3 不同抗生素对B.producta D4的最低抑制浓度
Table 3 The MIC of different antibiotics on B.producta D4
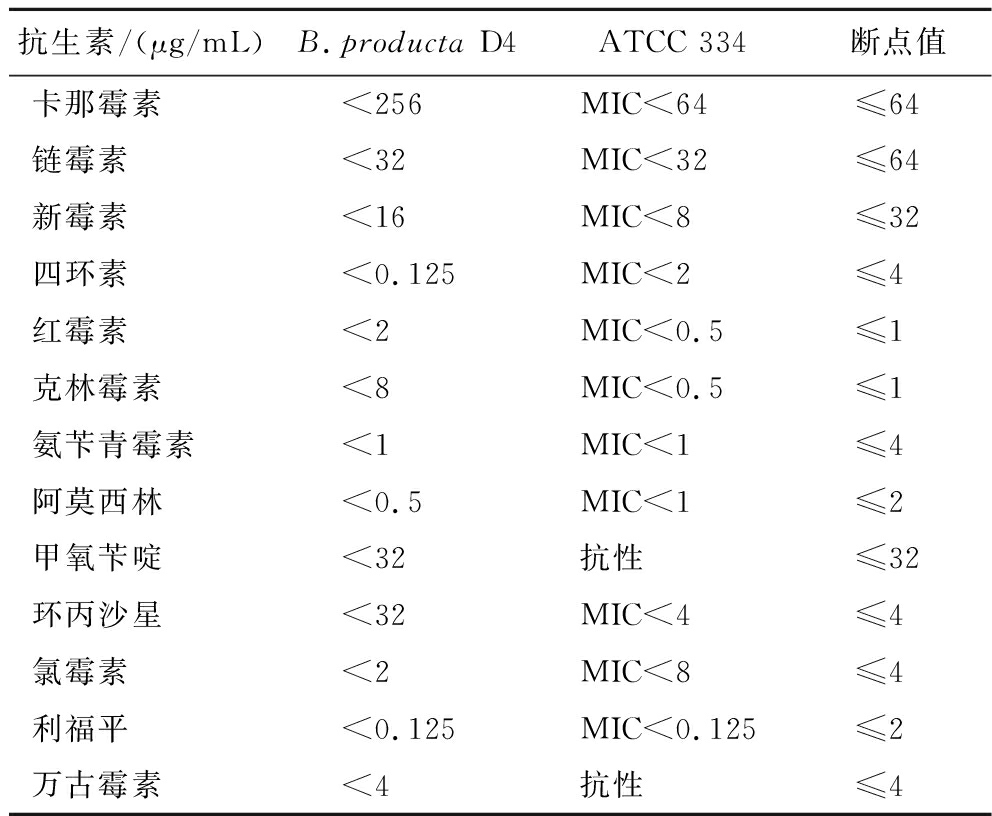
抗生素/(μg/mL)B.producta D4ATCC 334断点值卡那霉素<256MIC<64≤64链霉素<32MIC<32≤64新霉素<16MIC<8≤32四环素<0.125MIC<2≤4红霉素<2MIC<0.5≤1克林霉素<8MIC<0.5≤1氨苄青霉素<1MIC<1≤4阿莫西林<0.5MIC<1≤2甲氧苄啶<32抗性≤32环丙沙星<32MIC<4≤4氯霉素<2MIC<8≤4利福平<0.125MIC<0.125≤2万古霉素<4抗性≤4
由CARD注释结果可知(表4),D4具有大环内酯类、氟喹诺酮类、氨基糖苷类、糖肽类、四环素类和肽类抗生素的相关耐药基因(表4)。结合MIC测定结果,D4对环丙沙星(氟喹诺酮类抗生素)具有耐药性,其耐药机制为抗生素外排[21];D4对卡那霉素(氨基糖苷类抗生素)具有耐药性,被认为是乳酸菌的内在特性,菌株会产生多种酶来降解抗生素,使其失活[22],基因分析与表型结果一致。此外,D4基因中鉴定出万古霉素耐受相关基因(如vanHA、vanHB、vanRN、vanSA和vanTG,),但与表型结果相反。
表4 B.producta D4中的抗生素耐药基因
Table 4 Genes related to antibiotic resistance in B.producta D4
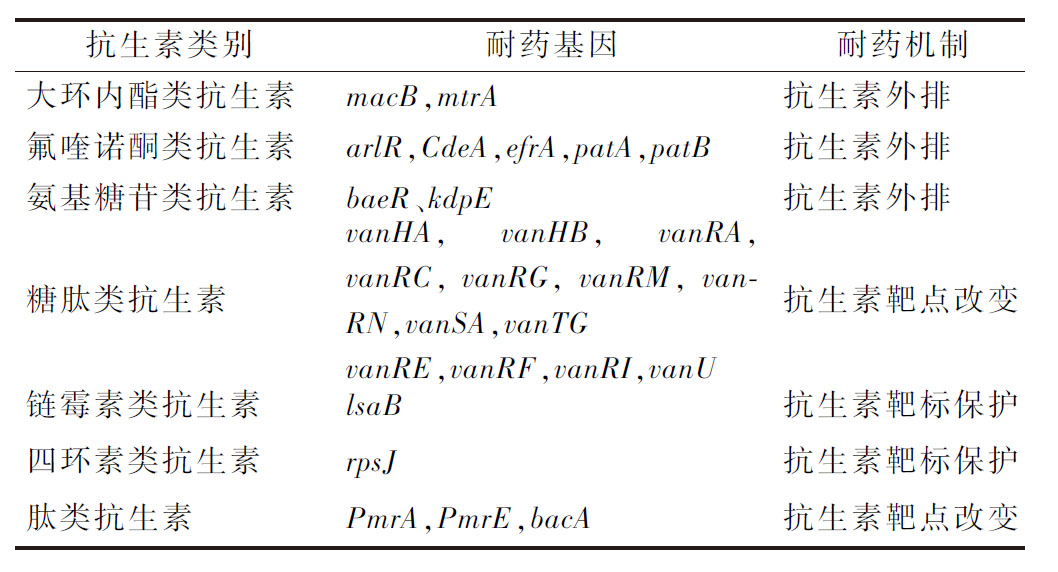
抗生素类别耐药基因耐药机制大环内酯类抗生素macB,mtrA抗生素外排氟喹诺酮类抗生素arlR,CdeA,efrA,patA,patB抗生素外排氨基糖苷类抗生素baeR、kdpE抗生素外排糖肽类抗生素vanHA,vanHB,vanRA,vanRC,vanRG,vanRM,vanRN,vanSA,vanTGvanRE,vanRF,vanRI,va-nU抗生素靶点改变链霉素类抗生素lsaB抗生素靶标保护四环素类抗生素rpsJ抗生素靶标保护肽类抗生素PmrA,PmrE,bacA抗生素靶点改变
2.3 B.producta D4的溶血性
微生物的溶血性表明其具有潜在的致病能力,根据FAO/WHO安全评估指南,溶血性可以作为评价益生菌安全性的重要证据[23]。如图1所示,阳性对照金黄色葡萄球菌ATCC29213菌株周围有界限分明的金黄色溶血环,表现出明显的溶血现象。当把D4涂布在含有5%(体积分数)绵羊血的平板上37 ℃厌氧孵育48 h后,菌落呈现圆型,灰白色,表面光滑,没有观察到溶血现象(γ-溶血),表明D4是安全的。
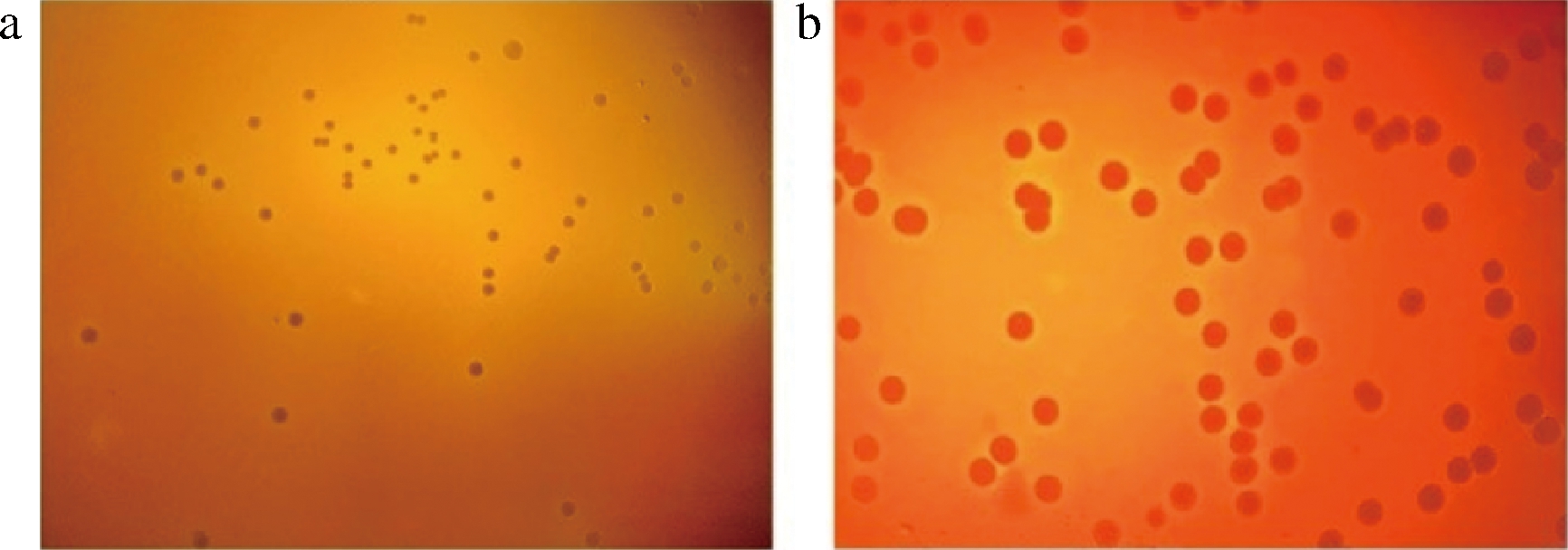
a-Blautia producta D4;b-Staphylococcus aureus ATCC29213
图1 B.producta D4的溶血性
Fig.1 Hemolysis of B.producta D4
2.4 B.producta D4对小鼠体内急性毒性实验分析
2.4.1 B.producta D4对小鼠体重、摄食和饮水量的影响
实验期间,小鼠精神状态正常,未出现脱毛、腹泻和死亡现象。每2 d记录1次小鼠的体重、摄食量和饮水量,监测小鼠生长情况。由图2可知,所有组别的小鼠体重均呈上升趋势,灌胃D4组与空白组小鼠无显著性差异。雌、雄小鼠每日摄食量在3 g左右,饮水量在5 g左右,不同处理组之间无显著差异,说明D4菌株干预不会对小鼠产生不利影响。

a-雌性小鼠体重;b-雄性小鼠体重;c-摄食量;d-饮水量
图2 不同组别小鼠体重、饮水量和摄食量
Fig.2 Body weight, water intake, and food intake of mice in different groups
2.4.2 B.producta D4对小鼠器官指数的影响
器官指数是评估外源性有毒物质对动物健康状况的一个可靠指标,通常情况下,各器官与体重的比例相对稳定,但如果相应器官受损,器官指数也会发生变化。实验结束后处死小鼠并解剖,取出心脏、肝脏、肾脏、脾脏、肺、结肠进行称重。如图3所示,与空白组相比,灌胃109及1010 CFU的D4处理组未显示出与空白组之间的显著性差异。
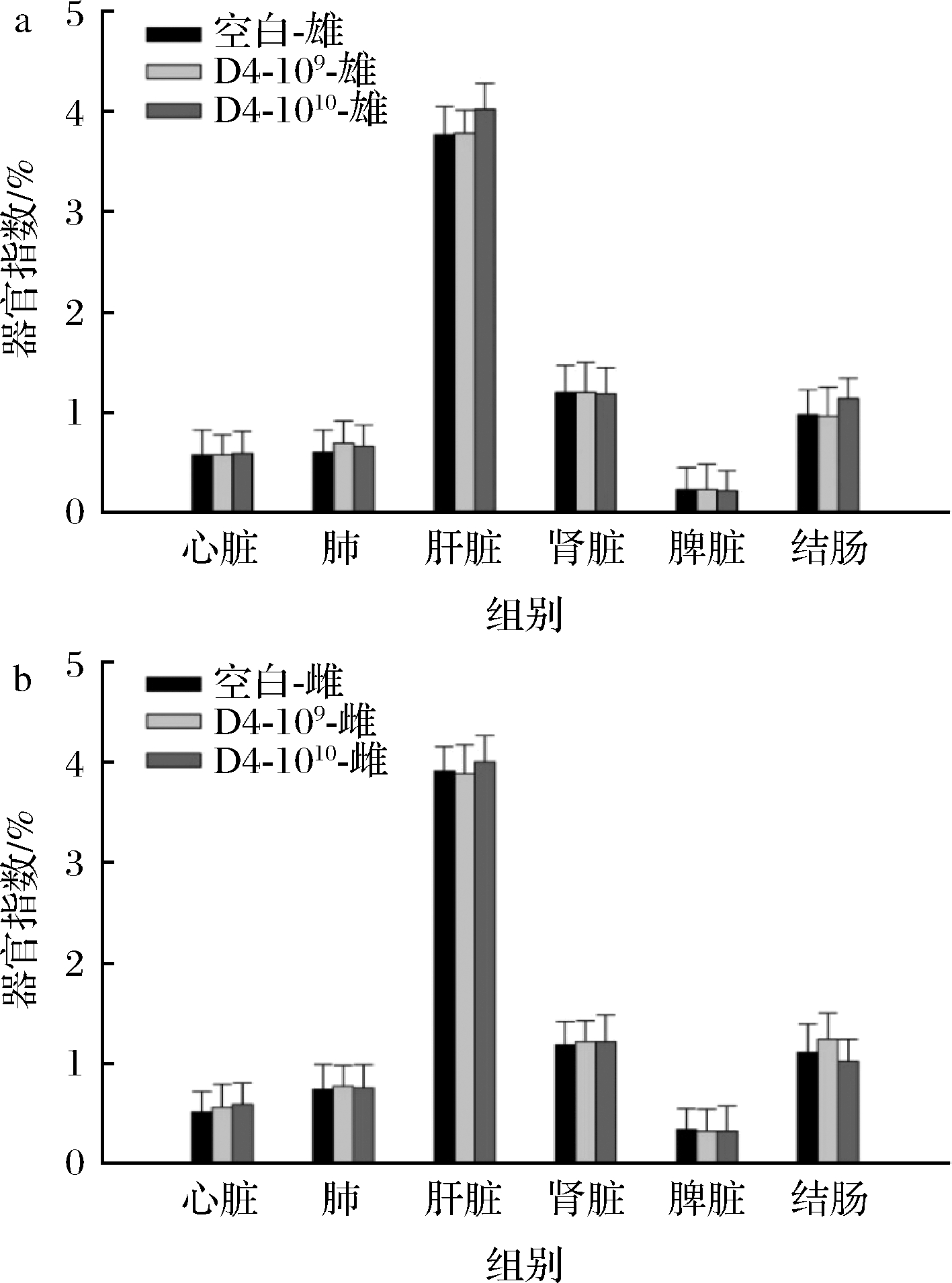
a-雄性小鼠;b-雌性小鼠
图3 不同组别小鼠器官指数
Fig.3 Organ index of mice in different groups
2.4.3 B.producta D4对小鼠血常规的影响
对小鼠的血常规项目进行检测,结果表明(表5),无论雌雄,灌胃不同剂量的D4与空白组小鼠血液指标无显著性差异。研究表明,血细胞的数量和比例在许多疾病中可作为慢性炎症标志物和预后的重要指标,可反映机体的健康状况[24]。红细胞、白细胞和血小板可在一定程度上反映宿主生理病理状态[25],实验结果显示D4处理后小鼠血液指标无异常,证明灌胃109和1010CFU的D4对小鼠健康状况无毒副影响。
表5 B.producta D4对雄性和雌性小鼠血常规指标的影响
Table 5 The effects of B.producta D4 on hematological parameters of male and female mice

血常规指标空白-雄D4-109-雄D4-1010-雄空白-雌D4-109-雌D4-1010-雌WBC/(109/L)2.58±0.462.40±0.802.74±0.612.40±0.472.43±0.912.84±0.79Neu #/(109/L)0.25±0.060.29±0.040.31±0.10.24±0.130.17±0.070.25±0.13Lym #/(109/L)1.91±0.232.04±0.822.30±0.572.26±0.352.16±0.892.51±0.69Mon #/(109/L)0.03±0.010.05±0.040.10±0.080.05±0.070.07±0.070.03±0.03Eos #/(109/L)0.04±0.030.02±0.010.03±0.010.03±0.010.03±0.010.05±0.01Bas #/(109/L)0.00±0.000.00±0.000.00±0.010.00±0.000.00±0.000.00±0.01Neu/%11.82±2.4913.06±5.1511.32±2.1511.44±3.039.98±2.6010.64±3.04Lym/%80.54±10.0983.14±6.2483.36±4.0083.82±5.7387.92±4.5288.24±3.82Mon/%2.70±0.702.48±2.332.80±3.522.70±3.193.44±3.742.62±0.90Eos/%1.40±0.141.12±0.411.26±0.301.28±0.221.46±0.461.82±0.73Bas/%0.14±0.150.20±0.100.26±0.150.24±0.090.20±0.100.28±0.13RBC/(1012/L)7.84±0.277.48±0.597.27±0.307.91±0.597.27±0.237.49±0.32HGB/(g/L)105.80±8.38112.8±8.53113.00±6.44104.00±17.83115.00±4.58116.6±4.98HCT/%39.19±1.9834.62±2.6933.64±1.5940.14±4.4433.8±1.0234.68±1.54MCV/fL41.90±1.7046.32±0.246.26±0.4743.34±0.7746.56±0.1146.32±0.11MCH/pg15.30±0.2615.06±0.3615.54±0.3115.70±0.2915.82±0.1615.54±0.27MCHC/(g/L)330.72±5.71325.20±6.46335.80±3.63333.4±9.29339.8±3.11335.8±6.53RDW-CV/%12.50±0.1612.40±0.2212.60±0.2912.40±0.2212.50±0.2312.50±0.16RDW-SD/fL27.66±0.4926.92±0.4627.20±0.5127.52±0.4927.08±0.6327.04±0.38PLT/(109/L)819.51±91.73958.80±50.55978.00±89.56972.22±51.26808.20±90.46852.2±61.62MPV/fL5.04±0.964.44±0.054.46±0.134.70±0.144.46±0.114.50±0.10PDW/%12.54±0.1714.96±0.0914.98±0.0812.66±0.0515.02±0.0415.02±0.11PCT/%0.37±0.030.43±0.020.44±0.030.46±0.050.36±0.040.34±0.02
注:WBC,white blood cell,白细胞;Neu,Neutrophil,中性粒细胞;Lym,lymphocyte,淋巴细胞;Mon,monocytes,单核细胞;Eos,eosinophil,嗜酸性粒细胞;Bas,basophil,嗜碱性粒细胞;RBC,red blood cell,红细胞;HGB,hemoglobin concentration,血红蛋白浓度;HCT,hematocrit value,血细胞比容值;MCV,mean corpuscular volume,平均红细胞体积;MCH,mean corpuscular hemoglobin,平均红细胞血红蛋白;MCHC,mean corpuscular hemoglobin concentration,平均红细胞血红蛋白浓度;RDW,Red blood cell distribution width,红细胞分布宽度;CV,coefficient of variation,变异系数;SD,standard deviation,标准偏差;PLT,platelet,血小板;MPV,mean platelet volume,平均血小板体积;PDW,platelet distribution width,血小板分布宽度;PCT,plateletcrit,血小板压积。
2.4.4 B. producta D4对小鼠血清生化指标的影响
为探究109和1010 CFU两种剂量的D4在灌胃期间是否会引起小鼠潜在的全身毒性,检测了小鼠血清甘油三酯(triglyceride, TG)、总胆固醇(total cholesterol, TC)、空腹血糖(glucose, Glu),以及肝功能相关指标谷丙转氨酶(alanine transaminase, ALT)、谷草转氨酶(aspartate transaminase, AST)、碱性磷酸酶(alkaline phosphatase, ALP)、血清白蛋白(serum albumin, ALB)、肌酸激酶(creatine kinase, CK)、乳酸脱氢酶(lactate dehydrogenase, LDH)等指标(图4)。TC和TG的升高是脂质代谢异常的表现,可能伴随着脂肪肝和心血管疾病的发生,对宿主健康构成潜在威胁[26]。与空白组相比,D4处理后小鼠血清TC和TG无显著差异,表明D4不会影响小鼠的脂质和碳水化合物代谢。血清中ALT被认为是细胞和肝脏损伤的初期迹象,肝脏的任何损害都会导致该指标的升高。相比于空白组,109 CFU和1010 CFU的D4组肝功能相关指标正常,与正常小鼠组无显著差异。

a-TC;b-TG;c-Glu;d-ALT;e-AST;f-ALP;g-ALB;h-CK;i-LDH
图4 B.producta D4对小鼠生化指标的影响
Fig.4 The effect of B.producta D4 on the biochemical parameters of mice
2.4.5 B.producta D4对小鼠器官组织形态的影响
肝脏作为负责新陈代谢的主要器官,可以反映机体的健康状况;脾脏作为人体最大的免疫器官,参与体液和细胞的免疫反应;肠黏膜在防止潜在病原体和毒素等物质入侵方面起着重要作用。空白组和D4菌株处理组的小鼠各脏器形态,由图5可知,所有组别的肝组织结构清晰,肝窦和肝索排列整齐,未观察到明显的肝细胞变性,凋亡或坏死。肾脏细胞排列整齐,结构完整,细胞核清晰,胞质染色均匀;空白组和不同处理组的脾脏组织清晰,红髓和白髓结构完整且轮廓分明,脾索和鼻窦整齐排列。另外所有组别的小鼠结肠形态良好,隐窝结构完整,富含杯状细胞。

图5 不同组别小鼠组织切片HE染色
Fig.5 HE staining of tissue sections in different groups of mice
注:肝脏、脾脏、肾脏的比例尺为100 μm,结肠的比例尺为200 μm。
总体而言,灌胃109 CFU和1010 CFU的D4未对小鼠脏器造成明显的组织病理学损害,表明D4是安全的,与我们之前研究的B.producta DSM2950是类似的[27]。
3 结论
本文通过对B.producta D4开展基因层面分析(鉴定毒力因子、抗生素耐受基因)、表型实验(测定溶血性和抗生素耐受性)及体内急性毒性实验(测定小鼠体重、摄食饮水量、器官指数、血常规、血清肝功能相关生化指标及器官组织病理结果),综合评价该菌株的安全性,主要结论如下:
在基因层面分析中可知,D4的毒力基因大部分与铁离子摄取和调控有关,不具备致病能力,同时鉴定出8种不同类别的抗生素耐受基因;D4在血琼脂平板上无溶血现象,对链霉素、新霉素、四环素、氨苄青霉素、阿莫西林、甲氧苄啶、氯霉素、利福平和万古霉素敏感,对卡那霉素、红霉素、克林霉素和环丙沙星表现出耐药性;体内急性毒性实验结果表明,连续灌胃14 d 109 CFU和1010 CFU的D4菌株对小鼠的体重、摄食、饮水情况、器官指数均无影响,血常规指标、代谢和肝功能相关的指标与空白组无显著性差异,D4处理组的脏器形态组织正常、无病变。由此可见,B.producta D4对小鼠健康无毒副作用,初步表明B.producta D4是安全的。未来,还需要进一步研究D4的其他安全特性,为功能性肠道菌的开发与利用提供更全面的理论依据和支撑。
[1] RIJKERS GT, DE VOS WD, BRUMMER R, et al. Health benefits and health claims of probiotics:Bridging science and marketing[J].British Journal of Nutrition, 2011,106(9):1291-1296.
[2] CUNNINGHAM M, AZCARATE-PERIL M A, BARNARD A, et al.Shaping the future of probiotics and prebiotics[J].Trends in Microbiology, 2021, 29(8):667-685.
[3] O’TOOLE P W, MARCHESI J R, HILL C.Next-generation probiotics:The spectrum from probiotics to live biotherapeutics[J].Nature Microbiology, 2017, 2(5):17057.
[4] CANI P D, DEPOMMIER C, DERRIEN M, et al.Akkermansia muciniphila:Paradigm for next-generation beneficial microorganisms[J].Nature Reviews Gastroenterology &Hepatology, 2022, 19(10):625-637.
[5] NIE K, MA K J, LUO W W, et al.Roseburia intestinalis:A beneficial gut organism from the discoveries in genus and species[J].Frontiers in Cellular and Infection Microbiology, 2021, 11:757718.
[6] MIQUEL S, MART N R, ROSSI O, et al.Faecalibacterium prausnitzii and human intestinal health[J].Current Opinion in Microbiology, 2013, 16(3):255-261.
N R, ROSSI O, et al.Faecalibacterium prausnitzii and human intestinal health[J].Current Opinion in Microbiology, 2013, 16(3):255-261.
[7] SUN F T, ZHANG Q S, ZHAO J X, et al.A potential species of next-generation probiotics? The dark and light sides of Bacteroides fragilis in health[J].Food Research International, 2019, 126:108590.
[8] DE MOOIJ C E M, VAN GROINGEN L F J, MOLENDIJK E B D, et al.Blautia abundance and mucosal barrier injury:A complex play of cause and effect[J].Clinical Infectious Diseases, 2023, 76(6):1152-1153.
[9] HOSOMI K, SAITO M, PARK J, et al.Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota[J].Nature Communications, 2022, 13:4477.
[10] OZATO N, YAMAGUCHI T, MORI K T, et al.Two Blautia species associated with visceral fat accumulation:A one-year longitudinal study[J].Biology-Basel, 2022, 11(2):318.
[11] OZATO N, SAITO S, YAMAGUCHI T, et al.Blautia genus associated with visceral fat accumulation in adults 20-76 years of age[J].NPJ Biofilms and Microbiomes, 2019, 5:28.
[12] LIU X M, MAO B Y, GU J Y, et al.Blautia-a new functional genus with potential probiotic properties?[J] Gut Microbes, 2021,13(1):1-21.
[13] MAO B, GUO W, LIU X, et al.Potential probiotic properties of Blautia producta against lipopolysaccharide-induced acute liver injury[J].Probiotics and Antimicrobial Proteins, 2023, 15(3): 785-796.
[14] MAO B, GUO W, CUI S, et al.Blautia producta displays potential probiotic properties against dextran sulfate sodium-induced colitis in mice[J].Food Science and Human Wellness, 2024, 13.
[15] PRADHAN D, MALLAPPA R H, GROVER S.Comprehensive approaches for assessing the safety of probiotic bacteria[J].Food Control, 2020, 108:106872.
[16] CRUZ NETO J P R, OLIVEIRA A M, DE OLIVEIRAK R, et al.Safety evaluation of a novel potentially probiotic Limosilactobacillus fermentum in rats[J].Probiotics and Antimicrobial Proteins, 2023. DOI:10.1007/s12602-023-10077-3.
R, et al.Safety evaluation of a novel potentially probiotic Limosilactobacillus fermentum in rats[J].Probiotics and Antimicrobial Proteins, 2023. DOI:10.1007/s12602-023-10077-3.
[17] CHEN L H, XIONG Z H, SUN L L, et al.VFDB 2012 update:toward the genetic diversity and molecular evolution of bacterial virulence factors[J].Nucleic Acids Research, 2012,40(D1):D641-D645.
[18] AYELÉN C M, ASQUITH CHRISTOPHER R M, TUOMO L, et al.Quinazoline-based antivirulence compounds selectively target Salmonella PhoP/PhoQ signal transduction system[J].Antimicrobial Agents and Chemotherapy, 2019, 64(1):e01744-19.
[19] CAI L, ZHENG S W, SHEN Y J, et al.Complete genome sequence provides insights into the biodrying-related microbial function of Bacillus thermoamylovorans isolated from sewage sludge biodrying material[J].Bioresource technology, 2018, 260:141-149.
[20] TERAI T, KATO K, ISHIKAWA E, et al.Safety assessment of the candidate oral probiotic Lactobacillus crispatus YIT 12319:Analysis of antibiotic resistance and virulence-associated genes[J].Food and Chemical Toxicology, 2020, 140:111278.
[21] DAS D J, SHANKAR A, JOHNSON J B, et al.Critical insights into antibiotic resistance transferability in probiotic Lactobacillus[J].Nutrition, 2020,69:110567.
[22] FENG C, ZHANG F X, WANG B N, et al.Evaluation of kanamycin and neomycin resistance in Lactobacillus plantarum using experimental evolution and whole-genome sequencing[J].Food Control, 2019, 98:262-267.
[23] KOIRALA S, ANAL A K.Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims[J].Future Foods, 2021, 3:100013.
[24] ZHANG X P, WNG B B, LI W X, et al.In vivo safety assessment, biodistribution and toxicology of polyvinyl alcohol microneedles with 160-day uninterruptedly applications in mice[J].European Journal of Pharmaceutics and Biopharmaceutics, 2021, 160:1-8.
[25] LI F, WANG L P, CAI Y L, et al.Safety assessment of desaminotyrosine:Acute, subchronic oral toxicity, and its effects on intestinal microbiota in rats[J].Toxicology and Applied Pharmacology, 2021, 417:115464.
[26] RU Y, CHEN X, WANG J, et al.Polysaccharides from Tetrastigma hemsleyanum Diels et Gilg:Extraction optimization, structural characterizations, antioxidant and antihyperlipidemic activities in hyperlipidemic mice[J].International Journal of Biological Macromolecules, 2019, 125:1033-1041.
[27] LIU X, GUO W, CUI S, et al.A comprehensive assessment of the safety of Blautia producta DSM 2950[J].Microorganisms, 2021, 9(5):908.