食品是能够满足人类正常生命活动并能延续正常寿命的物质,食品安全关系到每个人的身体健康和生命安全,重金属污染是造成食品安全问题的重要原因之一。重金属具有毒性强、难降解的特点,可通过各种途径进入人体,对体内的肾脏等器官、神经系统和血红细胞等产生危害[1]。为了人类的生命安全,因此食品中重金属的检测是十分必要的。
常见的重金属检测方法有原子吸收光谱[2]、电感耦合等离子体质谱[3]、X射线荧光光谱[4]、紫外可见分光光度法[5]等。虽然这些方法可以准确地定性和定量分析重金属离子,灵敏度高,但检测设备价格昂贵,需要进行较复杂的预处理,检测周期长[6]。而电化学检测方法具有设备简单、易于操作、响应速度快、能够应用于现场检测的优点,但在选择性和稳定性方面有待提高[7]。近年来,涌现了许多电化学检测重金属的综述[8-10],均主要从传感器的类型和电极改性的角度进行描述,而系统性介绍特异性识别元件对重金属检测的综述鲜有报道。
特异性识别元件可实现目标物的捕获和识别,经典的特异性识别元件主要是生物体本身如细胞、组织等,或者是从生物体中分离出的酶、抗体等,但重金属离子免疫原性相对较低、抗体制备难度较大以及可特异性识别的细胞种类有限等原因,因此经典的特异性识别元件在重金属检测中存在不足[11]。而新型特异性识别元件如脱氧核酶(deoxyribozyme,DNAzyme)、适配体、分子印迹聚合物等具有可人工合成、选择性强、成本低等优点[12-13]。因此可以利用特异性识别元件结合电化学传感器对重金属离子进行检测。本文主要介绍了DNAzyme、适配体、离子印迹聚合物的性质,并综述基于特异性识别元件的电化学传感器在食品重金属(主要包括Pb2+、As3+、Cd2+、Cr3+/6+)检测中的应用(图1)。
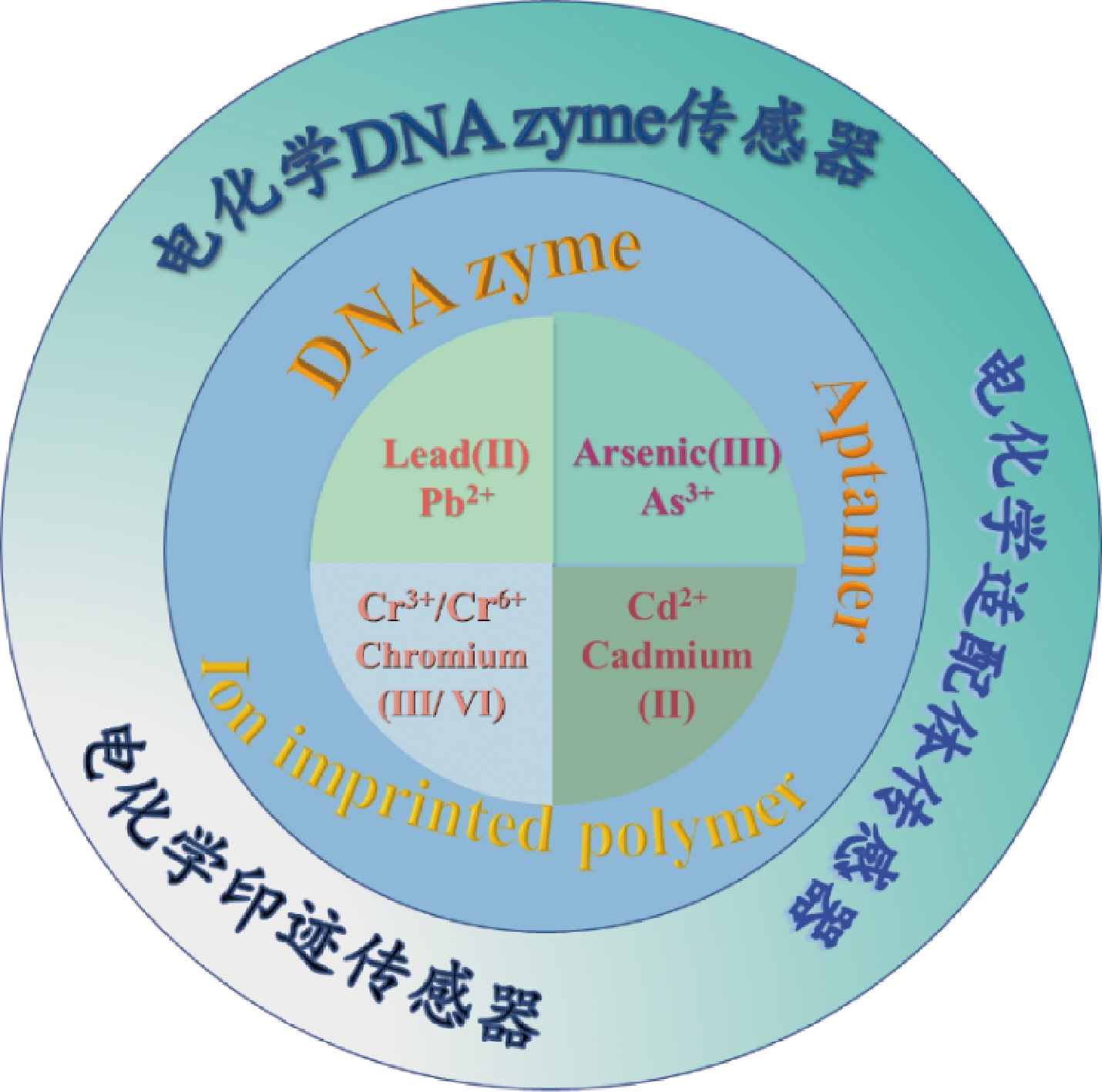
图1 特异性识别元件应用于电化学传感器中检测重金属
Fig.1 Application of specific recognition elements in electrochemical sensors for the detection of heavy metals
1 常见的特异性识别元件
识别元件又称为目标受体,能够特异性识别目标物质。识别元件捕获到目标物,使电极表面的电流或电势发生变化,从而实现目标物的检测。目前在电化学重金属检测中研究最多的识别元件有DNAzyme、适配体和离子印迹聚合物等。
1.1 DNAzyme
DNAzyme是BREAKER等[14]利用体外分子进化技术筛选得到的一类具有催化功能的DNA片段,具有较强的催化活性、易于合成、成本低等优点。根据催化性能,DNAzyme可以分为RNA切割活性的DNAzyme、连接酶活性的DNAzyme、过氧化物酶活性的DNAzyme等,其中以金属离子为辅因子的DNAzyme只有与特定金属离子结合时才显示活性,且酶活性的大小与金属离子的浓度密切相关[15-17],因此DNAzyme可以检测重金属离子。
图2总结了不同重金属检测的DNAzyme。YANG等[18]制备了一种基于沸石咪唑酸框架-8(zeolitic imidazolate framework-8,ZIF-8)和DNAzyme的新型荧光传感平台,用于检测水和鱼样品中的Pb2+,以ZIF-8为荧光猝灭剂,将DNAzyme吸附到ZIF-8上并伴随荧光淬灭,Pb2+存在时DNAzyme被激活并裂解,使荧光恢复,依此对Pb2+检测。YU等[19]以DNAzyme为识别元件开发了一种检测Pb2+的电化学传感器。该传感器线性范围在10-13~10-7 mol/L,检出限为1.74×10-14 mol/L,并成功应用于饮用水检测中。ZHENG等[20]制备了一种依赖化学发光共振能量转移的荧光生物传感器检测Pb2+,该传感器通过DNAzyme与荧光素和血红素/G-四链体偶联模拟辣根过氧化物酶使发光物氧化并发光。在Pb2+存在时,DNAzyme被激活并裂解,荧光素和血红素/G-四链体分离,荧光淬灭。在最优实验条件下,检出限为5 nmol/L。

图2 不同重金属检测的DNAzyme[18-30]
Fig.2 DNAzymes for detection of various heavy metals[18-30]
1.2 适配体
适配体一般是指通过体外指数富集配体的系统进化技术人工筛选得到的寡核苷酸DNA或RNA分子[31]。适配体能够与目标物特异性结合,当适配体与目标物结合后结构会发生变化,利用这个特点将适配体作为识别材料应用于重金属检测中。
表1对近3年来部分检测重金属的适配体传感器进行了总结(主要包括适配体序列、传感器类型、线性范围及检测限)。
表1 检测重金属的适配体传感器
Table 1 Aptasensors for the detection of heavy metals

目标物适配体序列(5'→3')传感器类型线性范围/(nmol/L)检测限/(nmol/L)参考文献Ag+CTACCCTAGC荧光-10[33]Ag+A24CTACCCTAGC荧光0.694~6.940.694[35]Pb2+CAACGGTGGGTGTGTGGTTGG电化学0.483~48.30.464[32]Hg2+AGAAAGGAGGACTTTCATACCTCGAG-GAGCTGAGG-GTCCTCCTTTC表面增强拉曼散射2×10-7~1.25×10-41.1×10-7[34]Cd2+CTCAGGACGGGTTCACAGTCCGTTGTC场效应晶体管-1.25×10-4[36]Cd2+ACCGACCGTGCTGGACTCTGGACTGTTGT-GGTATTA-TTTTTGGTGGTGCAGTATGAGCGAGCGTTGCG电化学1×10-5~1×1052×10-5[37]Cd2+CTCAGGACGGGGTTCAGTCCGTTGTC比色8.93~3.57×1038.93[38]Ca2+GGGGTTTTGGGGG荧光0~353.77×10-3[39]Co2+GGTAATACGACTCACTAAGGGAGATAC-CAGCTTAT-TCAATTTTACAGAACAC-CAACGTCGCTCGGGTACT-TCTTCATCGAGATAGTAAGTGCAACTT表面增强拉曼散射3.3×10-2~10.02[40]As3+GGTAATACGACTCACTATAGGGAGATAC-CAGCTTA-TTCAATTTTACAGAACAAC-CAACGTCGCTCCGGGT-ACTTCTTCATCGATAGTAAGTGCAATCT荧光1×10-2~1×1020.247[41]
注:-表示无数据(下同)。
DING等[32]合成了一种新型双壳碳纳米笼材料,并研制出了一种适体传感器用于测定水溶液中的铅。该传感器在最优实验条件下对Pb2+具有良好线性关系的范围为0.1~10 μg/L,检出限为0.096 μg/L。JIANG等[33]利用纳米金的光学猝灭性能和双链适配体捕获银离子的能力构建了一种用于检测Ag+的荧光适配体传感器。Ag+不存在时,反应体系具有很强的荧光,而Ag+存在时和适配体中C-C碱基对错配转换为稳定的C-Ag+-C碱基对,荧光淬灭。TIAN等[34]制备了一种用于痕量检测汞离子的新型表面增强拉曼光谱适配体传感器,该传感器通过适配体和具有发夹结构的DNA将表面增强拉曼光谱与核酸信号扩增相结合,在最佳实验条件下,制备的适配体传感器线性范围为0.2~125 fmol/L,检测限为0.11 fmol/L。
1.3 离子印迹聚合物
离子印迹技术是分子印迹技术的重要分支,也是分子印迹技术在金属离子方向的拓展,因存在金属离子配位作用而具有稳定性好、配位键键位的强度可控等优势,受到了广泛的关注[42-44]。离子印迹聚合物是基于离子印迹技术,通过模板离子与功能单体的螯合作用等作用力互相结合形成具有空穴结构的且对模板离子有高选择性的聚合物[45]。表2对近3年来部分检测重金属不同离子印迹聚合物进行了总结(主要包括模板离子、功能单体、检测方法、线性范围及检测限)。
表2 不同离子印迹聚合物检测重金属
Table 2 Detection of heavy metals using different ion-imprinted polymers
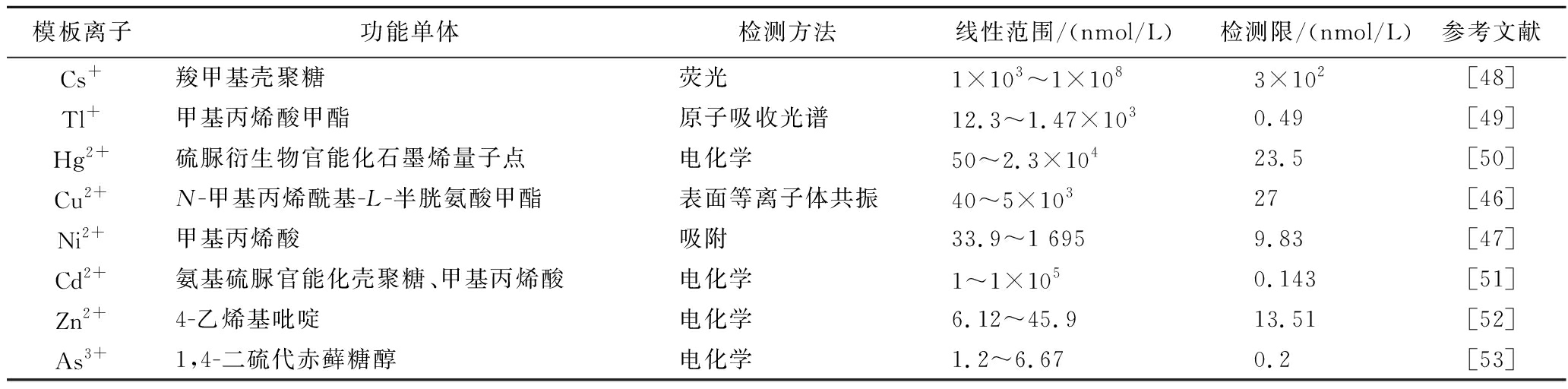
模板离子功能单体检测方法线性范围/(nmol/L)检测限/(nmol/L)参考文献Cs+羧甲基壳聚糖荧光1×103~1×1083×102[48]Tl+甲基丙烯酸甲酯原子吸收光谱12.3~1.47×1030.49[49]Hg2+硫脲衍生物官能化石墨烯量子点电化学50~2.3×10423.5[50]Cu2+N-甲基丙烯酰基-L-半胱氨酸甲酯表面等离子体共振40~5×10327[46]Ni2+甲基丙烯酸吸附33.9~1 6959.83[47]Cd2+氨基硫脲官能化壳聚糖、甲基丙烯酸电化学1~1×1050.143[51]Zn2+4-乙烯基吡啶电化学6.12~45.913.51[52]As3+1,4-二硫代赤藓糖醇电化学1.2~6.670.2[53]
GERDAN等[46]采用氨基酸基功能单体与铜离子聚合,制备了一种用于检测铜离子(Cu2+)的离子印迹聚合物等离子体传感器,此传感器在最优实验条件下展现出良好的检测性能,响应时间为20 s,检测范围为0.04~5.0 μmol/L,检测限为0.027 μmol/L。但离子印迹聚合物在结合目标重金属离子后难以与检测样品分离,又因磁分离技术具有操作简单、反应迅速等特点,因此将离子印迹技术与磁分离技术相结合得到了磁性离子印迹聚合物。KUMAR等[47]以胺官能化的二氧化硅包被四氧化三铁纳米颗粒为核心材料,制备了一种新型镍离子磁性印迹聚合物用于吸附消除工业废水中的有毒镍离子。所制备的聚合物选择性高、可重复性使用,并且在外加磁场下可从检测样品中快速分离。
1.4 不同特异性识别元件对比分析
表3对于不同的特异性识别元件在电化学传感器重金属检测中的检测性能(识别原理、制备工艺的难易程度以及优缺点)进行了比较,由表3可知特异性识别元件的优缺点。
表3 不同特异性识别元件之间的性能比较
Table 3 Comparison of the performance among various molecular recognition materials

识别元件制备难易程度识别原理优点缺点DNAzyme适中特异性识别和催化作用特异性好、成本低稳定性差适配体适中与相应配体特异性结合热稳定、特异性好、可重复使用筛选复杂离子印迹聚合物复杂与模板离子特异性结合成本低、高耐热性、高选择性、特异性好模板离子不易洗脱
2 基于特异性识别元件的电化学传感器在食品中重金属检测的应用
电化学传感器在重金属检测中具有操作简便、选择性好等优点,但传统电极表面积较小,对重金属的捕获能力有限。利用特异性识别元件可大大提高电化学传感器对重金属离子的选择性,并且由于识别元件的特异性可以实现重金属离子的有效检测。
2.1 基于特异性识别元件的电化学传感器在检测Pb2+中的应用
铅是一种有毒的重金属元素,在自然环境中不能被分解,并会通过生物富集放大生成毒性更大的有机物。铅中毒会严重危害人的健康,可以对神经、心血管、胃肠道、骨骼等各类器官造成影响[54-56]。因此,对铅离子的检测是非常有必要的。表4列出了部分基于特异性识别元件的电化学传感器在检测Pb2+中的应用(主要包括特异性识别元件的种类、线性范围、检测限和实际样品)。
表4 基于特异性识别元件的电化学传感器在检测Pb2+中的应用
Table 4 Electrochemical sensors based on molecular recognition materials for lead(Ⅱ) detection

特异性识别元件线性范围/(nmol/L)检测限/(nmol/L)实际样品参考文献DNAzyme0.5~320.1大鼠、鸡[57]DNAzyme10-2~8×1032.74×10-3饮用水[60]适配体4.83×10-3~0.4831.45×10-3饮用水[58]适配体1×10-3~29.8×10-4自来水[61]离子印迹聚合物0.483~3.86×1020.242自来水、果汁[59]离子印迹聚合物1~5×1040.283饮用水、牛奶[62]
LIU等[57]制备了一种新型检测Pb2+的电化学生物传感器,以DNAzyme为识别元件,对Pb2+具有高度特异性。当Pb2+存在时,DNAzyme在底物链DNA的核糖腺嘌呤(rA)位点被激活,双螺旋结构解开,这种反应增强了Ti3C2Tx Mxenes对DNAzyme的吸附性能,使离子可以更好地嵌入到Ti3C2Tx Mxenes中,并诱导氧化峰电流的增加。该传感器在最佳实验条件下,线性范围为0.5~32 nmol/L,检出限低至0.1 nmol/L。YADAV等[58]先将银和金合金纳米颗粒电沉积到电极上,再将筛选出的Pb2+适配体修饰上电极,制备出一种用于检测Pb2+的电化学适配体传感器。在最优实验条件下,该传感器的线性范围为0.01~10 μg/L,循环伏安法(cyclic voltammetry,CV)、差分脉冲伏安法(differential pulse voltammetry,DPV)的检测限为0.03×10-2 μg/L,电化学阻抗谱(electrochemical impedance spectroscopy,EIS)的检测限为0.04×10-2 μg/L。DAHAGHIN等[59]以2-(2-氨基苯基)-1 h-苯并咪唑为配体,4-乙烯基吡啶为功能单体,合成了具有选择性的新型磁性离子印迹聚合物,制备了一种新型铅离子检测电化学传感器。在最佳实验条件下,线性浓度范围为0.1~80 ng/mL,Pb2+的检出限0.05 ng/mL,并成功应用于测定天然水和果汁中的Pb2+离子。目前,基于特异性识别元件的电化学传感器在检测Pb2+上具有较低的检测限、较宽的线性范围等优点,在Pb2+检测方面具有广阔的应用前景。
2.2 基于特异性识别元件的电化学传感器在检测As3+中的应用
砷(As)是一种类金属有毒物质,因其化学性质与重金属类似,砷也被认为是重金属。砷在环境中广泛存在,特别是地下水中,对人类身体健康有着巨大的威胁[63-65]。因此建立特异性的As3+检测方法是十分重要的。表5列出了部分基于特异性识别元件的电化学传感器在检测As3+中的应用(主要包括特异性识别元件的种类、线性范围、检测限和实际样品)。
表5 基于特异性识别元件的电化学传感器在检测As3+中的应用
Table 5 Electrochemical sensors based on molecular recognition materials for arsenic(Ⅲ) detection

特异性识别元件线性范围/(nmol/L)检测限/(nmol/L)实际样品参考文献适配体 1.33×10-9~1.33×10-41.33×10-9大米 [68]适配体 5.33~1.33×1052.67贝类 [69]离子印迹聚合物2×10-3~97.1×10-3饮用水[66]离子印迹聚合物3.795×10-4~4.51.139×10-4饮用水[67]
MA等[66]以As3+作为模板离子,邻苯二胺作功能单体,制得离子印迹聚合物,构建了As3+检测传感器。通过优化实验条件,采用CV法对As3+进行了定量检测,其线性范围为2.0×10-11~9.0×10-9 mol/L,检测限为7.1×10-12 mol/L。该传感器的检出限远低于10 μg/L,符合世界卫生组织设定的饮用水标准,并且该传感器具有制备步骤简单、良好的重现性和稳定性。GIULIO等[67]构建了一种检测As3+的电化学印迹传感器,利用离子印迹技术提高传感器的选择性,壳聚糖稳定的银纳米粒子为电极表面带来更多的活性位点。在最优实验条件下采用DPV法检测As3+,检出限为11.39 pmol/L。当前,基于特异性识别元件的电化学传感器在检测As3+中具有特异性强、灵敏度高等优点,但DNAzyme在检测As3+中的应用不如适配体和印迹聚合物流行。未来还需对检测As3+的DNAzyme以及检测方法的抗干扰能力进一步分析。
2.3 基于特异性识别元件的电化学传感器在检测Cd2+中的应用
镉(Cd)是一种有毒重金属,也是一类有毒有害水污染物,对人体有严重危害。镉中毒会损伤肾脏、对骨骼和神经造成影响,最著名的镉中毒事件是日本的“痛痛病”[70]。因此建立高效、快捷、特异性的镉离子痕量检测体系是很有必要的。表6列出了部分基于特异性识别元件的电化学传感器在检测Cd2+中的应用(主要包括特异性识别元件的种类、线性范围、检测限和实际样品)。
表6 基于特异性识别元件的电化学传感器在检测Cd2+中的应用
Table 6 Electrochemical sensors based on molecular recognition materials for cadmium (Ⅱ) detection

特异性识别元件线性范围/(nmol/L)检测限/(nmol/L)实际样品参考文献适配体 1×10-5~1×1052×10-5镉污染水[37]适配体 0.1~1×1060.275自来水[71]离子印迹聚合物8.93~4461.16饮用水[72]离子印迹聚合物4.46~4.46×1022.05生菜、橙、桃[73]
RABAI等[37]将结合金纳米颗粒、碳纳米管和壳聚糖优点的纳米复合材料用作镉适配体的固定载体,制备了一种用于水中镉检测的无标签电化学适配体传感器。该生物传感器检出限为0.02 pmol/L,线性范围为10-13~10-4 mol/L,为水溶液中Cd2+的检测提供了一种简单而灵敏的方法,在实际样品中痕量重金属的监测中具有广阔的应用前景。RABAI等[71]首先用羧酸电化学还原重氮盐对金电极进行修饰,然后将镉适配体通过碳二亚胺反应固定在电极上,开发了一种用于检测水中镉(Cd2+)的适配体传感器。采用EIS对Cd2+检测,在最优实验条件下,检测限为2.75×10-10 mol/L,对Cd2+具有较高的选择性。WANG等[72]利用电聚合离子印迹聚邻苯二胺[poly (o-phenylenediamine),PoPD]制备了一种电化学传感器。以oPD为功能单体,以Cd2+为模板,电聚合得到印迹后的离子印迹聚合物,去除模板后得到的传感器对目标Cd2+表现出良好的选择性。在最优实验条件下,该传感器的方波阳极汽提伏安法溶出峰值与Cd(II)质量浓度在1~50 ng/mL内呈良好的线性关系,检测限为0.13 ng/mL。总体而言,本文中用于检测Cd2+的电化学传感器大多检测限低于国标中生活饮用水卫生标准的毒理指标,在Cd2+的检测中表现出良好的应用潜力。
2.4 基于特异性识别元件材料的电化学传感器在检测Cr3+/Cr6+中的应用
铬(Cr)在自然环境中以多种氧化态的形式出现,其中Cr3+和Cr6+是自然界中最常见的稳定价态,Cr3+是人体所必需的微量元素,而Cr6+具有毒性作用[74-76]。由于其毒性,Cr6+的测定特别重要,且Cr3+和Cr6+之间可相互转化,因此,Cr3+和Cr6+的检测都是十分重要的。
ZHANG等[77]首先在Cu基金属有机框架(metal-organic framework,MOF)上原位生长二维酞菁基共价有机框架(covalent-organic framework,COF),形成的MOF@COF结构能够将DNA链紧密的固定,构建了特异性识别Cr3+的光电化学-电化学双模式生物传感器。在最优实验条件下,该生物传感器的线性范围为0.1 pmol/L~100 nmol/L,光电化学的检测限为1.45×10-5 nmol/L,电化学的检测限为2.29×10-5 nmol/L。BOJDI等[78]构建了一种检测Cr6+的印迹传感器,首先在甲醇中沉淀聚合功能单体、引发剂、交联剂、铬结合配体和铬离子(模板离子)生成离子印迹聚合物纳米颗粒(IP-NPs),聚合后,从聚合物纳米颗粒中浸出Cr6+,形成Cr6+孔。在最优实验条件下此传感器的检测限为30 pmol/L,并成功用于环境水样中Cr6+痕量的检测。目前用于检测铬离子的特异性识别元件种类仍然偏少,加强特异性识别元件的研究,拓宽特异性识别元件在铬离子检测中的实际应用。
3 结论与展望
重金属污染是目前食品安全的一大威胁,对人体健康有着巨大的危害。电化学传感器具有高响应、操作简便等优点,结合特异性识别元件易获取、高选择性等特点,因此在检测食品中重金属得到了广泛应用。
本文介绍了近五年来在电化学重金属检测中常用的特异性识别元件,着重讨论了特异性识别元件在电化学传感器检测重金属的应用。特异性识别元件在重金属检测的应用中展现出了优越的性能,主要归因于:a)DNAzyme和适配体具有较强的选择性、易修饰、较高的特异性以及对金属离子有较高的亲和力;b)因离子印迹聚合物与金属离子存在配位作用而具有稳定性好、特异性强等优点。但特异性识别元件在研究中仍存在一些不足:a)依赖金属离子的DNAzyme一般是裂解RNA,而RNA极不稳定易被分解,因此检测重金属离子对环境要求高;b)适配体筛选过程复杂;c)离子印迹聚合物由于功能单体、聚合方法等具有局限性,导致合成的离子印迹聚合物结合目标物的能力一般。
为了使特异性识别元件在重金属检测中有更好的应用前景,笔者提出两点建议:a)改进DNAzyme和适配体的筛选技术;b)研发适用性强的印迹聚合物的制备方法。随着新技术的应用和对特异性识别元件的深入研究,基于特异性识别元件的电化学传感器将在食品安全检测领域展现出更广阔的应用前景。
[1] GUO W F, ZHANG C X, MA T T, et al. Advances in aptamer screening and aptasensors′ detection of heavy metal ions[J]. Journal of Nanobiotechnology, 2021, 19(1):166.
[2] UZCAN F, SHAH S N, SOYLAK M. Magnetic dispersive solid phase extraction of lead(II) as dithizone chelates in food and environmental samples on Fe3O4@XAD-8 prior to its flame atomic absorption spectrometric detection[J]. International Journal of Environmental Analytical Chemistry, 2022, 102(19):7767-7778.
[3] XU C, HE M, CHEN B B, et al. Magnetic porous coordination networks for preconcentration of various metal ions from environmental water followed by inductively coupled plasma mass spectrometry detection[J]. Talanta, 2022, 245:123470.
[4] SILVA C C, DA CRUZ DE OLIVEIRA G, DO AMARAL CARVALHO H R, et al. Detection and quantification of heavy metals in blood and milk of Amazon River dolphin (Inia geoffrensis) (Cetartiodactyla: Iniidae) using wavelength-dispersive X-ray fluorescence spectrometry[J]. X-Ray Spectrometry, 2023, 52(3):121-129.
[5] DOGRU S, YILMAZ E, GUNDUZ S B, et al. An easy and green amine-based microextraction strategy combined UV-Vis spectrophotometric detection for mercury in natural water samples[J]. Journal of the Iranian Chemical Society, 2021, 18(11):3069-3075.
[6] YANG M, LI P H, CHEN S H, et al. Nanometal oxides with special surface physicochemical properties to promote electrochemical detection of heavy metal ions[J]. Small, 2020, 16(25):2001035.
[7] SAIKRITHIKA S, SENTHIL KUMAR A. Electrochemical detections of tea polyphenols: A review[J]. Electroanalysis, 2020, 32(11):2343-2360.
[8] SONG H J, HUO M Z, ZHOU M M, et al. Carbon nanomaterials-based electrochemical sensors for heavy metal detection[J]. Critical Reviews in Analytical Chemistry, 2022, 12(4):1-20.
[9] GE S P, MA X H. Electrochemical aptamer-based sensors for the detection of heavy metals[J]. International Journal of Electrochemical Science, 2022, 17(9):220926.
[10] WANG L Y, PENG X L, FU H J, et al. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food[J]. Biosensors &Bioelectronics, 2020, 147:111777.
[11] MORALES M A, HALPERN J M. Guide to selecting a biorecognition element for biosensors[J]. Bioconjugate Chemistry, 2018, 29(10):3231-3239.
[12] SANDE M G, RODRIGUES J L, FERREIRA D, et al. Novel biorecognition elements against pathogens in the design of state-of-the-art diagnostics[J]. Biosensors, 2021, 11(11):418.
[13] CHEN Y F, SUN Y F, WANG R Q, et al. One-pot synthesis of a novel conductive molecularly imprinted gel as the recognition element and signal amplifier for the selective electrochemical detection of amaranth in foods[J]. Biosensors &Bioelectronics, 2023, 228:115185.
[14] BREAKER R R, JOYCE G F. A DNA enzyme that cleaves RNA[J]. Chemistry &Biology, 1994, 1(4):223-229.
[15] PONCE-SALVATIERRA A, BOCCALETTO P, BUJNICKI J M. DNAmoreDB, a database of DNAzymes[J]. Nucleic Acids Research, 2021, 49(D1): D76-D81.
[16] MA X Y, DING W, WANG C, et al. DNAzyme biosensors for the detection of pathogenic bacteria[J]. Sensors and Actuators B: Chemical, 2021, 331:129422.
[17] HUANG P J J, LIU J W. In vitro selection of chemically modified DNAzymes[J]. ChemistryOpen, 2020, 9(10):1046-1059.
[18] YANG C Y, YU P T, LI Y, et al. Platform formed from ZIF-8 and DNAzyme: Turn-on fluorescence assay for simple, high-sensitivity, and high-selectivity detection of Pb2+[J]. Journal of Agricultural and Food Chemistry, 2022, 70(30):9567-9576.
[19] YU Z S, LI N, HU X S, et al. Highly efficient electrochemical detection of lead ion using metal-organic framework and graphene as platform based on DNAzyme[J]. Synthetic Metals, 2019, 254:164-171.
[20] ZHENG J, WAI J L, LAKE R J, et al. DNAzyme sensor uses chemiluminescence resonance energy transfer for rapid, portable, and ratiometric detection of metal ions[J]. Analytical Chemistry, 2021, 93(31):10834-10840.
[21] ZHU L J, MIAO M, SHAO X L, et al. A universal electrochemical biosensor using nick-HCR nanostructure as molecular gate of nanochannel for detecting chromium(III) ions and microRNA[J]. Analytical Chemistry, 2019, 91(23):14992-14999.
[22] PAVADAI R, PERUMAL P. An innovative trimetallic-MOF mediated catalytic cleavage activity of FAM tagged Ag10/T-rich DNAzyme as an ultra-sensitive and selective fluorescent biosensor for subsequent recognition of Ag+ and Hg2+ ions[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2022, 429:113901.
[23] XING S G, LIN Y, CAI L Y, et al. Detection and quantification of tightly bound Zn2+ in blood serum using a photocaged Chelator and a DNAzyme fluorescent sensor[J]. Analytical Chemistry, 2021, 93(14):5856-5861.
[24] LI D W, LING S, CHENG X R, et al. Development of a DNAzyme-based colorimetric biosensor assay for dual detection of Cd2+ and Hg2+[J]. Analytical and Bioanalytical Chemistry, 2021, 413(28):7081-7091.
[25] GUAN H Q, YANG S L, ZHENG C, et al. DNAzyme-based sensing probe protected by DNA tetrahedron from nuclease degradation for the detection of lead ions[J]. Talanta, 2021, 233:122543.
[26] ZHANG X L, XU J G, PENG Y B, et al. Dual-palindrome chained assembly regulates the formation of palindromic DNAzyme wire transducers empowering sensitized and one-step copper ion-dependent assay[J]. Sensors and Actuators B: Chemical, 2022, 370:132471.
[27] PAVADAI R, AMALRAJ A, SUBRAMANIAN S, et al. High catalytic activity of fluorophore-labeled Y-shaped DNAzyme/3D MOF-MoS2NBs as a versatile biosensing platform for the simultaneous detection of Hg2+, Ni2+, and Ag+ ions[J]. ACS Applied Materials &Interfaces, 2021, 13(27):31710-31724.
[28] ZHAI B Q, HUANG K L, WANG H T, et al. Highly sensitive and selective copper (II)-catalyzed dual-DNAzyme colorimetric biosensor based on exonuclease III-mediated cyclical assembly[J]. Catalysts, 2021, 11(11):1352.
[29] HWANG K, MOU Q B, LAKE R J, et al. Metal-dependent DNAzymes for the quantitative detection of metal ions in living cells: Recent progress, current challenges, and latest results on FRET ratiometric sensors[J]. Inorganic Chemistry, 2019, 58(20):13696-13708.
[30] WANG H, ZHENG S S, NAN X M, et al. Non-specific DNAzyme-based biosensor with interfering ions for the Cd2+ determination in feed[J]. Sensors and Actuators B: Chemical, 2021, 329:129139.
[31] CHEN Z L, XIE M J, ZHAO F G, et al. Application of nanomaterial modified aptamer-based electrochemical sensor in detection of heavy metal ions[J]. Foods, 2022, 11(10):1404.
[32] DING J N, ZHANG D W, LIU Y, et al. An electrochemical aptasensor for Pb2+ detection based on metal-organic-framework-derived hybrid carbon[J]. Biosensors, 2020, 11(1):1.
[33] JIANG L Y, XU X P, QIN Z R, et al. A fluorescence aptasensor for detecting silver ion concentration in aqueous environment[J]. Spectroscopy and Spectral Analysis, 2021, 41(4): 1066-1071.
[34] TIAN C, ZHAO L, ZHU J, et al. Ultrasensitive detection of trace Hg2+ by SERS aptasensor based on dual recycling amplification in water environment[J]. Journal of Hazardous Materials, 2021, 416:126251.
[35] REN L, CHEN G, PENG Z, et al. Design of a fluorescence-enhanced aptasensor for sensitive detection of silver ions[J]. Journal of Applied Spectroscopy, 2022, 89(5):984-991.
[36] WANG H, HAO Z, HUANG C, et al. Monitoring Cd2+ in oily wastewater using an aptamer-graphene field-effect transistor with a selective wetting surface[J]. Nanoscale Advances, 2022, 5(5):1416-1424.
[37] RABAI S, TENIOU A, CATANANTE G, et al. Fabrication of AuNPs/MWCNTS/chitosan nanocomposite for the electrochemical aptasensing of cadmium in water[J]. Sensors, 2021, 22(1):105.
[38] XU L, LIANG J, WANG Y H, et al. Highly selective, aptamer-based, ultrasensitive nanogold colorimetric smartphone readout for detection of Cd(II)[J]. Molecules, 2019, 24(15):2745.
[39] GHOSH S, CHEN Y H, GEORGE A, et al. Fluorescence resonant energy transfer-based quantum dot sensor for the detection of calcium ions[J]. Frontiers in Chemistry, 2020, 8:594.
[40] WEN G Q, XIAO Y, CHEN S X, et al. A nanosol SERS/RRS aptamer assay of trace cobalt(Ⅱ) by covalent organic framework BtPD-loaded nanogold catalytic amplification[J]. Nanoscale Advances, 2021, 3(13):3846-3859.
[41] SONI G K, WANGOO N, COKCA C, et al. Ultrasensitive aptasensor for arsenic detection using quantum dots and guanylated poly(methacrylamide)[J]. Analytica Chimica Acta, 2022, 1209:339854.
[42] EL OUARDI Y, GIOVE A, LAATIKAINEN M, et al. Benefit of ion imprinting technique in solid-phase extraction of heavy metals, special focus on the last decade[J]. Journal of Environmental Chemical Engineering, 2021, 9(6):106548.
[43] SALA A, BRISSET H, MARGAILLAN A, et al. Electrochemical sensors modified with ion-imprinted polymers for metal ion detection[J]. TrAC Trends in Analytical Chemistry, 2022, 148:116536.
[44] ZHOU X Y, WANG B Q, WANG R. Insights into ion-imprinted materials for the recovery of metal ions: Preparation, evaluation and application[J]. Separation and Purification Technology, 2022, 298:121469.
[45] FRANCISCO J E, FEITEIRA F N, DA SILVA W A, et al. Synthesis and application of ion-imprinted polymer for the determination of mercury II in water samples[J]. Environmental Science and Pollution Research International, 2019, 26(19):19588-19597.
[46] GERDAN Z, SAYLAN Y, ![]() M, et al. Ion-imprinted polymer-on-a-sensor for copper detection[J]. Biosensors, 2022, 12(2):91.
M, et al. Ion-imprinted polymer-on-a-sensor for copper detection[J]. Biosensors, 2022, 12(2):91.
[47] KUMAR S, BALOUCH A, ![]() E, et al. Fabrication of nickel-tagged magnetic imprinted polymeric network for the selective extraction of Ni(II) from the real aqueous samples[J]. Environmental Science and Pollution Research International, 2021, 28(29):40022-40034.
E, et al. Fabrication of nickel-tagged magnetic imprinted polymeric network for the selective extraction of Ni(II) from the real aqueous samples[J]. Environmental Science and Pollution Research International, 2021, 28(29):40022-40034.
[48] WANG L, WANG Z M, ZHOU C, et al. Potentiometric microsensor based on ion-imprinted polymer for the trace determination of cesium(I) ions[J]. Journal of Dispersion Science and Technology, 2020, 41(7):1095-1103.
[49] DARROUDI A, CHAMSAZ M, ZAVAR M, et al. Application of ion-imprinted polymer synthesized as new sorbent for preconcentration and separation of thallium (I) and its determination by electrothermal atomic absorption spectroscopy[J]. Iranian Journal of Chemistry \& Chemical Engineering-International English Edition, 2020, 39:59-66.
[50] SOMAN S, ASWATHY P V, KALA R. Covalently modified graphene quantum dot using a thiourea based imprinted polymer for the selective electrochemical sensing of Hg(II) ions[J]. Journal of Polymer Research, 2021, 28(9):1-12.
[51] HUANG W H, LIU Y M, WANG N W, et al. A sensitive electrochemical sensor based on ion imprinted polymers with gold nanoparticles for high selective detecting Cd (II) ions in real samples[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2021, 31(5):2043-2053.
[52] KHAIRNAR N A, JIRIMALI H D, PATIL K P, et al. Zinc ion-imprinted polymer based on silica particles modified carbon paste electrodes for highly selective electrochemical determination of zinc ions[J]. Polymer-Plastics Technology and Materials, 2020, 59(15):1698-1714.
[53] YANG H Z, YIN F Q, MO Y L, et al. An ion imprinted electrochemical sensor based on flower-like gold nanoparticles for the detection of trace arsenic(III) ions in water and food samples[J]. Electroanalysis, 2023: e202200513.
[54] S NCHEZ-MATEOS S, PÉREZ L V, C
NCHEZ-MATEOS S, PÉREZ L V, C RDOVA SU
RDOVA SU REZ M A, et al. Heavy metal contamination in the Cotopaxi and Tungurahua Rivers: A health risk[J]. Environmental Earth Sciences, 2020, 79(6):1-14.
REZ M A, et al. Heavy metal contamination in the Cotopaxi and Tungurahua Rivers: A health risk[J]. Environmental Earth Sciences, 2020, 79(6):1-14.
[55] MUNIR N, JAHANGEER M, BOUYAHYA A, et al. Heavy metal contamination of natural foods is a serious health issue: A review[J]. Sustainability, 2021, 14(1):161.
[56] ZAANOUNI N, GHARSSALLAOUI M, ELOUSSAIEF M, et al. Heavy metals transfer in the olive tree and assessment of food contamination risk[J]. Environmental Science and Pollution Research, 2018, 25(19):18320-18331.
[57] LIU Y Y, QIU R H, ZHANG Z C, et al. Label-free electrochemical biosensor based on GR5 DNAzyme/Ti3C2Tx Mxenes for Pb2+ detection[J]. Journal of Electroanalytical Chemistry, 2022, 905:115979.
[58] YADAV R, BERLINA A N, ZHERDEV A V, et al. Rapid and selective electrochemical detection of Pb2+ ions using aptamer-conjugated alloy nanoparticles[J]. SN Applied Sciences, 2020, 2(12):1-11.
[59] DAHAGHIN Z, KILMARTIN P A, MOUSAVI H Z. Novel ion imprinted polymer electrochemical sensor for the selective detection of lead(II)[J]. Food Chemistry, 2020, 303:125374.
[60] ZHANG D, YU X, WU L N, et al. Ultrasensitive electrochemical detection of Pb2+ based on DNAzyme coupling with exonuclease III-assisted target recycling[J]. Journal of Electroanalytical Chemistry, 2021, 882:114960.
[61] GAO F, ZHAN F P, LI S L, et al. Dual signal-based electrochemical aptasensor for simultaneous detection of lead(II) and mercury(II) in environmental water samples[J]. Biosensors and Bioelectronics, 2022, 209:114280.
[62] WU S P, LI K H, ZHANG Z H, et al. Synthesis of imprinted chitosan/AuNPs/graphene-coated MWCNTs/Nafion film for detection of lead ions[J]. New Journal of Chemistry, 2020, 44(33):14129-14135.
[63] MAO K, ZHANG H, WANG Z L, et al. Nanomaterial-based aptamer sensors for arsenic detection[J]. Biosensors and Bioelectronics, 2020, 148:111785.
[64] KEMPAHANUMAKKAGARI S, DEEP A, KIM K H, et al. Nanomaterial-based electrochemical sensors for arsenic-A review[J]. Biosensors &Bioelectronics, 2017, 95:106-116.
[65] XU X C, NIU X H, LI X, et al. Nanomaterial-based sensors and biosensors for enhanced inorganic arsenic detection: A functional perspective[J]. Sensors and Actuators B: Chemical, 2020, 315:128100.
[66] MA W W, CHANG Q G, SHI X W, et al. Novel electrochemical sensor based on integration of nanoporous gold with molecularly imprinted polymer for detection of arsenic ion(III)[J]. Journal of Electrochemistry, 2020, 26:900.
[67] GIULIO A M C, AGNIHOTRI A S, Varghese A, et al. Ion-imprinted chitosan-stabilized biogenic silver nanoparticles for the electrochemical detection of arsenic (III) in water samples[J]. New Journal of Chemistry, 2023, 47(11): 5179-5192.
[68] UDA M N A, GOPINATH S C B, HASHIM U, et al. Silica and graphene mediate arsenic detection in mature rice grain by a newly patterned current-volt aptasensor[J]. Scientific Reports, 2021, 11:14688.
[69] ZHANG W, CHEN Z Y, GUAN Y F, et al. Aptamer-functionalized screen-printed electrode coupled with graphene oxide and methylene blue nanocomposite as enhanced signal label for total arsenic determination in shellfish[J]. Sensors and Actuators B: Chemical, 2021, 335:129383.
[70] GENCHI G, SINICROPI M S, LAURIA G, et al. The effects of cadmium toxicity[J]. International Journal of Environmental Research and Public Health, 2020, 17(11):3782.
[71] RABAI S, BENOUNIS M, CATANANTE G, et al. Development of a label-free electrochemical aptasensor based on diazonium electrodeposition: Application to cadmium detection in water[J]. Analytical Biochemistry, 2021, 612:113956.
[72] WANG J Y, HU J F, HU S W, et al. A novel electrochemical sensor based on electropolymerized ion imprinted PoPD/ERGO composite for trace Cd(II) determination in water[J]. Sensors, 2020, 20(4):1004.
[73] YIN F Q, MO Y L, LIU X T, et al. An ultra-sensitive and selective electrochemical sensor based on GOCS composite and ion imprinted polymer for the rapid detection of Cd2+ in food samples[J]. Food Chemistry, 2023, 410:135293.
[74] MURTHY M K, KHANDAYATARAY P, PADHIARY S, et al. A review on chromium health hazards and molecular mechanism of chromium bioremediation[J]. Reviews on Environmental Health, 2022 May 11.
[75] WAKEEL A, XU M. Chromium Morpho-phytotoxicity[J]. Plants, 2020, 9(5):564.
[76] MUSHTAQ F, CHEN X Z, VECIANA A, et al. Magnetoelectric reduction of chromium(VI) to chromium(III)[J]. Applied Materials Today, 2022, 26:101339.
[77] ZHANG S, CHEN K, ZHU L, et al. Direct growth of two-dimensional phthalocyanine-based COF on Cu-MOF to construct a photoelectrochemical-electrochemical dual-mode biosensing platform for high-efficiency determination of Cr(III)[J]. Dalton Transactions, 2021, 50(40):14285-14295.
[78] BOJDI M K, BEHBAHANI M, FEYZABADI Z B. Material design of a chromium imprinted polymer and its application as a highly selective electrochemical sensor for determining chromium ion at trace levels[J]. ChemistrySelect, 2021, 6(43):11939-11947.