生姜(Zingiber officinale Roscoe)是姜科姜属植物的根茎,广泛作为食品调味品的同时还是重要的中药材,是一种非常典型的药食两用植物,《中国药典》2020版(一部)中收录有生姜、干姜、炮姜,姜炭4个品名,其炮制品制作工艺不同,在功能方面也有所不同。生姜中营养成分很多,目前研究得最多且重要生姜提取物是挥发性及脂溶性的姜精油、姜油和姜油树脂[1-2]。从生姜中提取姜精油、姜油及姜油树脂的最主要工艺技术有传统的水蒸汽蒸馏法[3-4]、溶剂法[5]以及超临界CO2提取法[6-7],将水蒸气蒸馏法与溶剂萃取2个过程结合的同时萃取蒸馏法[8-9]也有一些研究报道。不同生姜提取工艺得到的姜精油、姜油和姜油树脂提取产物在外观形态和化学成分组成上有一定差异,GC-MS和HPLC法是对这些提取物化学成分组成应用最多的分析方法,王颖[10]对水蒸气蒸馏法提取不同产地鲜姜精油用GC-MS分析其成分,裴亚萍[11]用GC-MS分析比较了用超临界CO2法和水蒸气蒸馏法提取山东大姜产物的香气成分,于大胜等[12]对水蒸气蒸馏法、有机溶剂浸提法和超临界CO2萃取法提取得到的生姜中风味物质进行了分析比较;除风味物质外,姜油和姜油树脂中最重要的生物活性成分是以6-姜酚为主的系列化合物[13-14],对其的分析测定均以HPLC法对6-姜酚的定量分析为主[1,15-16]。包括上述研究在内,已有文献研究大都对生姜的单一提取工艺或2种提取工艺进行分析比较,如文献[12]虽然研究了3种不同提取工艺,但主要是用GC-MS从风味物质角度进行分析比较,且不同研究文献之间由于采用的生姜原料不同也不足以充分比较论证各种提取工艺对生姜挥发性及生物活性成分进行提取的特点。本研究对产自同一地的生姜用不同提取工艺进行提取分离,对得到的各类姜精油、姜油及姜油树脂同时采用GC-MS和HPLC法对提取物化学组成和其中主要生物活性成分进行定性、定量检测及分析比较,并结合相关提取工艺技术进行了讨论。
1 材料与方法
1.1 材料与试剂
生姜产于贵州省水城县中科院扶贫项目示范种植地,系贵州当地小黄姜品系。生姜经清洗、切片后,80 ℃干燥至水分含量≤10%,粉碎过40目筛后密封包装贮存于4 ℃冰柜中备用。CO2气体,贵州申建气体股份有限公司(食品级,纯度≥99.5%)。其余试剂均为分析纯,实验用水均为超纯水。
对照品:6-姜酚、6-姜醇、8-姜酚及10-姜酚购自成都德斯特生物技术有限公司,纯度均≥99%(HPLC)。
1.2 仪器与设备
实验室水蒸气蒸馏提取挥发油标准玻璃仪器;SFE HA 120-40-0.5超临界CO2萃取装置,江苏南通华安超临界萃取有限公司;FW 100高速万能粉碎机,天津泰斯特仪器有限公司;BZF 50真空干燥箱,上海博迅医疗生物仪器股份有限公司;GZX-GF 101-3-BS-Ⅱ热风干燥箱,上海贺德实验设备有限公司;RE-2000 A旋转蒸发仪,上海亚荣生化仪器厂;DHS-16 A水分测定仪,宁波力辰科技;1260 HPLC、9000-7000 D GC-MS,美国安捷伦仪器公司。
1.3 实验方法
1.3.1 姜精油的水蒸气蒸馏
参照文献[17]进行,投入50 g 1.1中所述姜粉,提取完成分出精油部分,无水硫酸钠干燥,得浅黄色油状流动性良好液体为水蒸气蒸馏姜精油,称重并计算收率为0.86%。
1.3.2 姜油的溶剂法提取
参照文献[18]进行,在250 mL烧杯中投入10 g 1.1中所述姜粉,加入固液比为1∶10(g∶mL)的80%(体积分数)乙醇溶液,于50 ℃恒温水浴中不时搅拌下温浸提取2 h,提取完成后真空抽滤,所得溶液低温真空旋转挥发去乙醇,无水硫酸钠干燥,得黄色至综黄色油状液体为乙醇溶剂姜油,称重并计算收率为3.56%。
1.3.3 姜油、姜精油及姜油树脂的超临界CO2萃取
参照专利[19]进行:(1)姜油的提取:在萃取釜中投入8 kg 40目姜粉,萃取压力30 MPa,萃取温度不变,萃取时间110 min,在分离釜中一次性得到姜油;(2)姜精油和姜油树脂的一次性选择提取:在萃取釜中投入8 kg 40目姜粉,萃取压力10 MPa,萃取温度50 ℃,萃取时间50 min后在分离Ⅱ中得到姜精油;姜油树脂在萃取完姜精油后调整萃取压力至30 MPa,萃取温度不变,继续萃取60 min,在分离Ⅰ中得到姜油树脂;以上分离釜温度均为50 ℃,分离压力均为5 MPa,CO2流量均为15 L/h。以上各提取物均用无水硫酸钠干燥后,得到黄色至棕黄色具流动性油状液体的姜油、黄色流动性良好姜精油和棕黄色流动性较差姜油树脂,称重并计算收率分别为:超临界CO2姜油为4.78%,超临界CO2姜精油部分为1.27%,超临界CO2姜油树脂部分为3.39%。
1.4 GC-MS仪器条件及方法
1.4.1 气相色谱
色谱柱HP-5MS(30 m×0.25 mm×0.25 μm);载气为高纯He 0.8 mL/min;柱箱温度为程序升温,即75 ℃保持1 min;75~150 ℃,2 ℃/min;150~280 ℃,5 ℃/min,继续保持5 min;进样方式为进样口260 ℃,分流比100∶1,直接进样,进样量1 μL。
1.4.2 质谱
离子源温度230 ℃;MS传输线260 ℃;扫描范围10~800 amu。
1.4.3 计算及图谱检测方法
软件Masshunter;谱库NIST MS Search 2.2。
1.5 HPLC仪器条件及方法
1.5.1 仪器条件
色谱柱Diamonsil C18(250 mm×4.6 mm,5 μm);流动相乙腈(A)~水(B),程序梯度变化(体积比)为0 min,55%A;0~6 min,55%A~80%A;30~35 min,80%A~90%A,35~40 min,90%A~55%A;柱温30 ℃;流速1.0 mL/min;波长280 nm;进样量10 μL。
1.5.2 样品前处理及姜酚类物质测定
参照文献[20]测定。
2 结果与分析
2.1 GC-MS总离子流色谱图及化学组成分析
不同提取工艺下所得提取物的GC-MS分析总离子流色谱图和化学组成的归一化法分析结果分别如图1和表1所示。GC-MS共检出50种化合物,其中百分含量最高的是α-姜烯,除超临界CO2姜油树脂外,其余4种提取物中α-姜烯都在30%以上;对提取物中21种相对百分含量1%以上的主要化学成分进行统计,其占全部检出化合物的比例分别为水蒸气蒸馏法姜精油90.5%、超临界CO2姜精油89.24%、乙醇溶剂法姜油92.12%、超临界CO2姜油91.29%及超临界CO2姜油树脂91.84%。生姜中重要生物活性化合物6-姜酚在超临界CO2姜油树脂中含量最高,水蒸气蒸馏法姜精油中含量最低,由6-姜酚脱水转化生成的另一种生物活性化合物6-姜醇[21]同样也是在超临界CO2姜油树脂中最高,水蒸气蒸馏姜精油中则未检出。
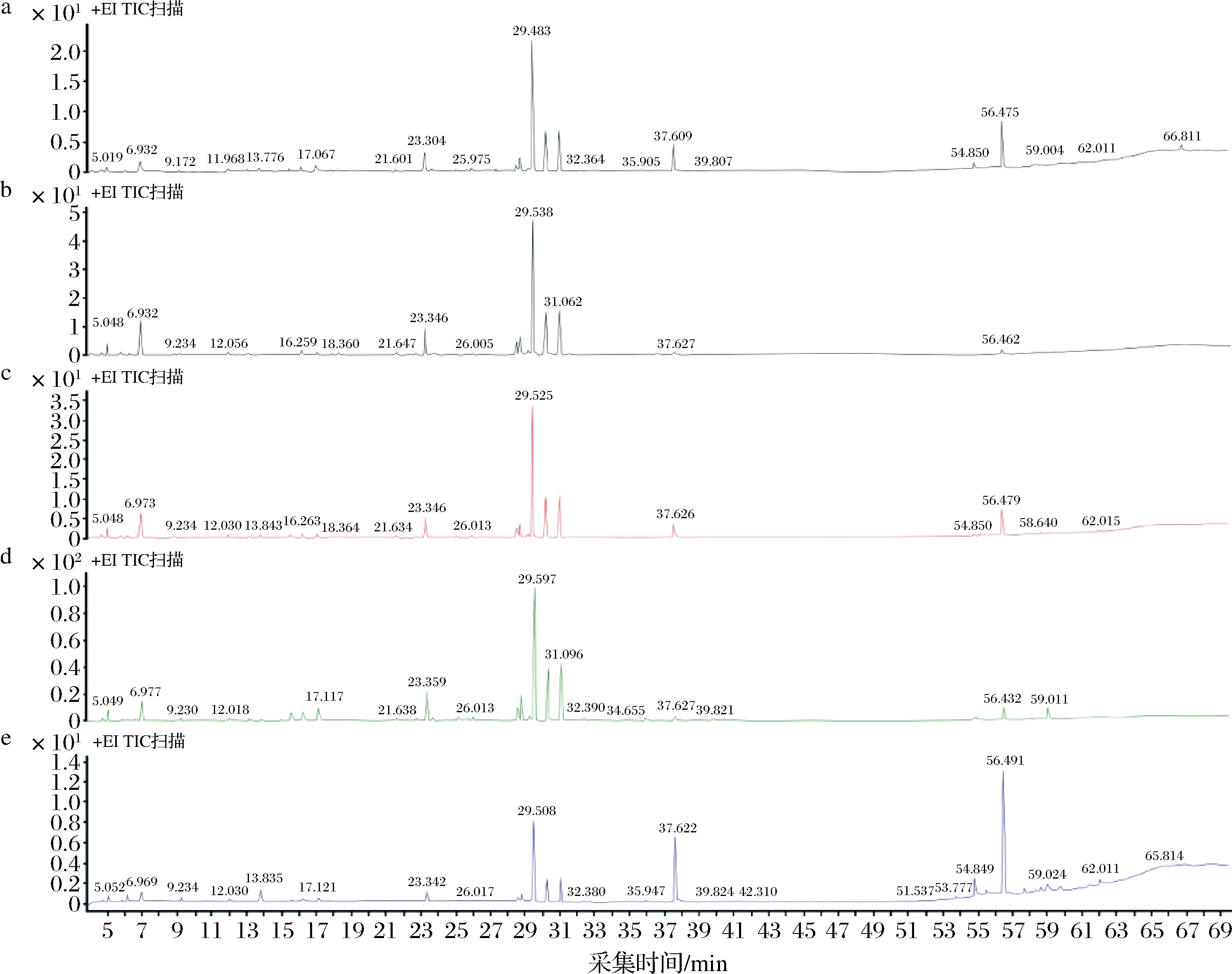
a-超临界CO2姜油;b-水蒸气蒸馏法姜精油;c-乙醇溶剂姜油;d-超临界CO2姜精油;e-超临界CO2姜油树脂(表1同)
图1 不同提取工艺提取物的GC-MS总离子流色谱图
Fig.1 GC-MS total ion chromatograms of products under different extraction processes
表1 不同提取工艺提取物的GC-MS化学组成分析结果
Table 1 GC-MS chemical composition analysis results of extracts from different extraction processes
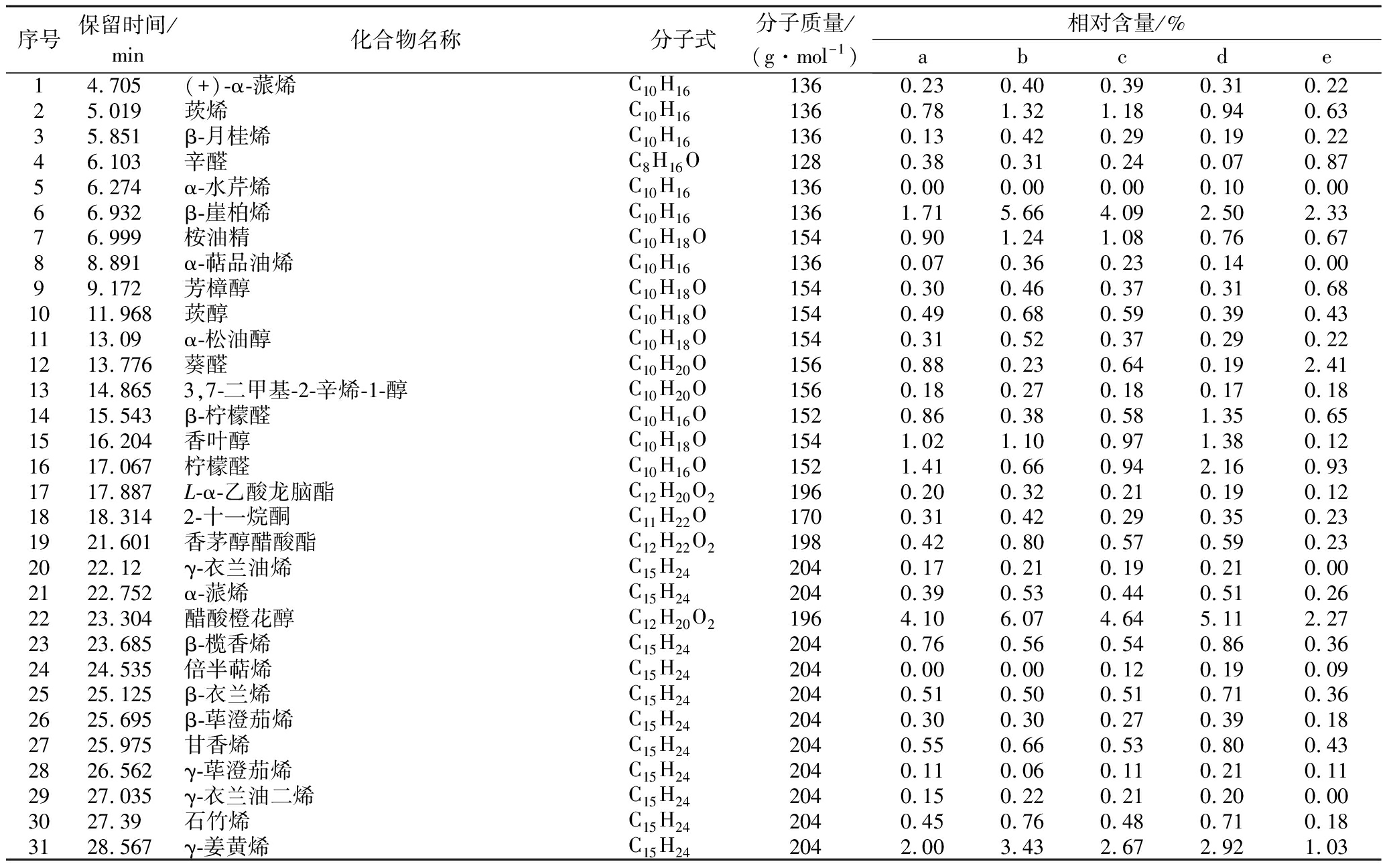
序号保留时间/min化合物名称分子式分子质量/(g·mol-1)相对含量/%abcde14.705(+)-α-蒎烯C10H161360.230.400.390.310.2225.019莰烯C10H161360.781.321.180.940.6335.851β-月桂烯C10H161360.130.420.290.190.2246.103辛醛C8H16O1280.380.310.240.070.8756.274α-水芹烯C10H161360.000.000.000.100.0066.932β-崖柏烯C10H161361.715.664.092.502.3376.999桉油精C10H18O1540.901.241.080.760.6788.891α-萜品油烯C10H161360.070.360.230.140.0099.172芳樟醇C10H18O1540.300.460.370.310.681011.968莰醇C10H18O1540.490.680.590.390.431113.09α-松油醇C10H18O1540.310.520.370.290.221213.776葵醛C10H20O1560.880.230.640.192.411314.8653,7-二甲基-2-辛烯-1-醇C10H20O1560.180.270.180.170.181415.543β-柠檬醛C10H16O1520.860.380.581.350.651516.204香叶醇C10H18O1541.021.100.971.380.121617.067柠檬醛C10H16O1521.410.660.942.160.931717.887L-α-乙酸龙脑酯C12H20O21960.200.320.210.190.121818.3142-十一烷酮C11H22O1700.310.420.290.350.231921.601香茅醇醋酸酯C12H22O21980.420.800.570.590.232022.12γ-衣兰油烯C15H242040.170.210.190.210.002122.752α-蒎烯C15H242040.390.530.440.510.262223.304醋酸橙花醇C12H20O21964.106.074.645.112.272323.685β-榄香烯C15H242040.760.560.540.860.362424.535倍半萜烯C15H242040.000.000.120.190.092525.125β-衣兰烯C15H242040.510.500.510.710.362625.695β-荜澄茄烯C15H242040.300.300.270.390.182725.975甘香烯C15H242040.550.660.530.800.432826.562γ-荜澄茄烯C15H242040.110.060.110.210.112927.035γ-衣兰油二烯C15H242040.150.220.210.200.003027.39石竹烯C15H242040.450.760.480.710.183128.567γ-姜黄烯C15H242042.003.432.672.921.03
续表1

序号保留时间/min化合物名称分子式分子质量/(g·mol-1)相对含量/%abcde3228.768α-姜黄烯C15H222024.045.264.124.922.133329.228β-己烯C15H242040.851.290.891.320.533429.483α-姜烯C15H2420433.0437.0336.5134.5819.753530.25α-法尼烯C15H2420412.1613.9113.3514.316.613630.551α-芹子烯C15H242040.250.270.230.360.243731.028β-倍半水芹烯C15H242049.3811.3010.5711.115.383831.476反式-γ-没药烯C15H242040.270.400.290.350.173932.364揽香醇C15H26O2220.490.090.000.600.624034.629反式水合倍半香桧烯C15H26O2220.350.000.000.360.244135.9054-(1,5-二甲基-4-己烯)-1-甲基-2-环己烯-1-醇C15H26O2220.360.000.000.490.324237.609姜酮C11H14O31946.830.983.971.5716.904339.8077-纤维醇-反-水合倍半香桧烯C15H26O2220.320.000.000.460.414454.856-姜醇C17H24O32761.400.000.520.353.494555.5036-姜酮C17H24O42920.440.000.120.111.214656.4756-姜酚C17H26O42947.220.644.411.6319.954757.6728-姜酚C19H30O43220.590.000.430.251.484858.646-姜二醇二乙酸酯C21H32O63800.250.000.250.240.814959.004邻苯二甲酸二酯C24H38O43901.220.000.211.691.605062.01110-姜二酮C21H32O43480.460.000.230.091.77
由5种提取物GC-MS总离子流色谱图(图1)和21个主要化合物百分含量比较图(图2)可知,水蒸气蒸馏法姜精油和超临界CO2姜精油、超临界CO2姜油与乙醇溶剂姜油分别较为相似,超临界CO2姜油树脂则与前4种差异较大,这主要是由于各提取工艺的原理及参数有所不同。由表1还可看出,分子质量大于156小于222的化合物中除姜酮外,超临界CO2姜精油中含量均高于姜油树脂,而分子质量大于222的化合物中,超临界CO2姜精油中含量均小于姜油树脂,显示通过改变超临界CO2萃取压力可以实现一定程度选择性分离,分别得到分子质量较小沸点较低与水蒸气蒸馏法姜精油较为接近的超临界CO2姜精油和富含姜酚类化合物的姜油树脂,这种明显的选择性分离效果也可从图2-c看出。
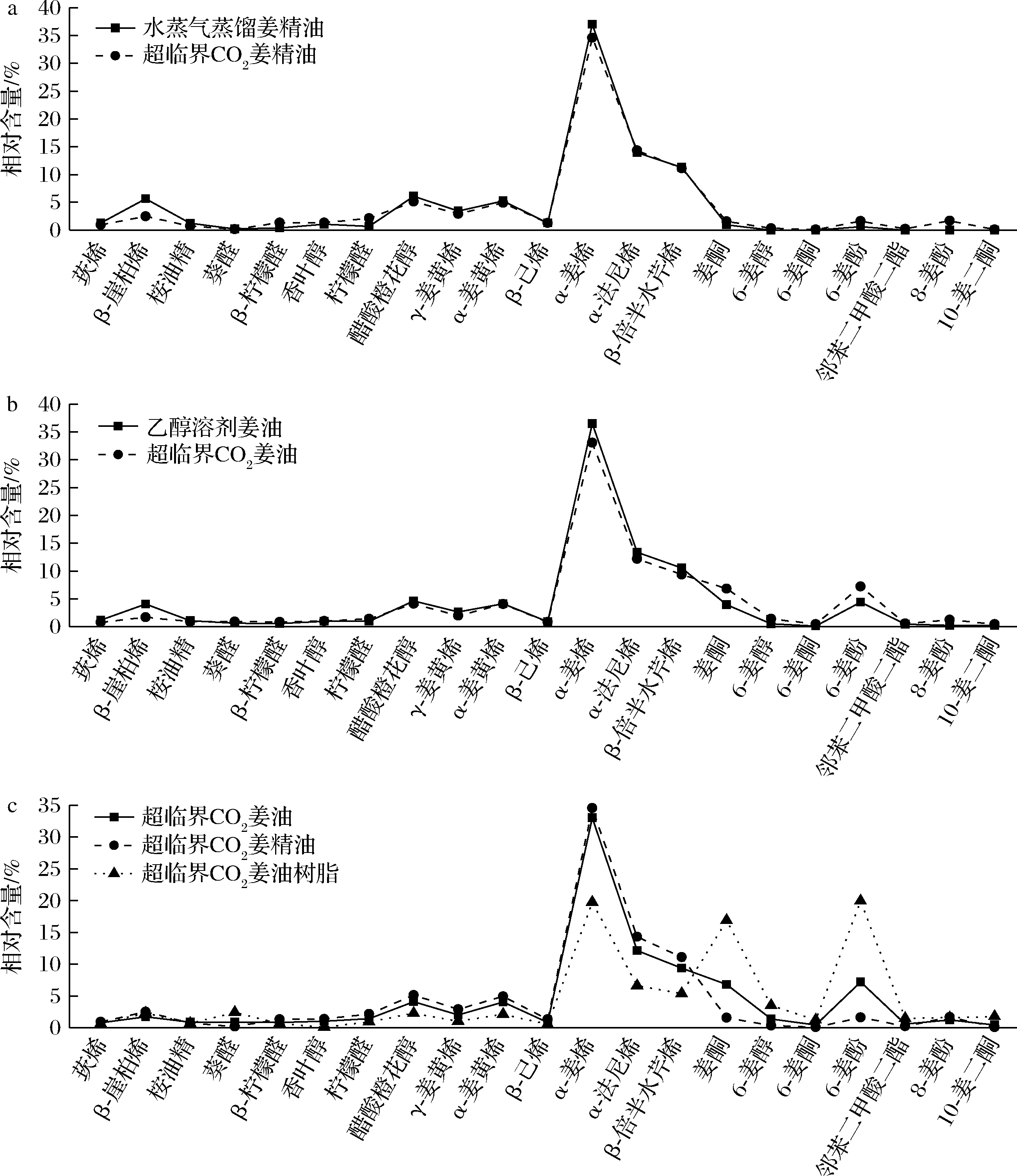
a-水蒸气蒸馏姜精油和超临界CO2姜精油的比较;b-乙醇溶剂姜油和超临界CO2姜油的比较;c-超临界CO2姜油、 姜精油和姜油树脂的比较
图2 不同提取工艺5种提取物中21个主要化合物百分含量比较
Fig.2 Comparison of the percentages of 21 main compounds in 5 extracts of different extraction processes
2.2 HPLC色谱图及定量分析结果
标准品及不同提取方法所得提取物样品姜酚类化合物的HPLC分析色谱图及定量检测结果分别如图3和表2所示。
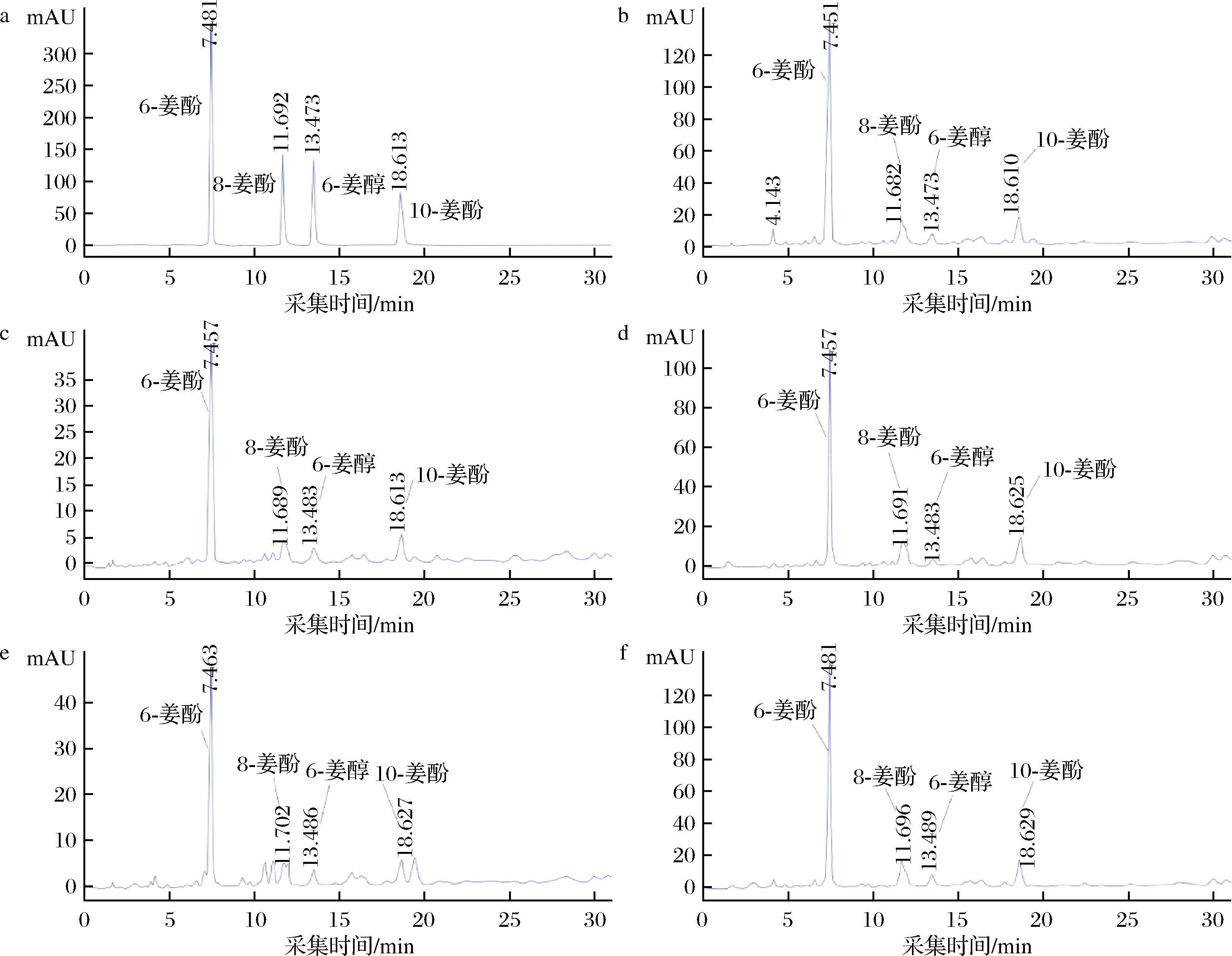
a-标准品;b-超临界CO2姜油;c-水蒸气蒸馏姜精油;d-乙醇溶剂姜油;e-超临界CO2姜精油;f-超临界CO2
图3 标准品及不同提取工艺提取物HPLC图
Fig.3 HPLC chromatograms of standard products and extracts from different extraction processes
表2 不同提取方法所得提取物样品姜酚类物质测定结果 单位:mg/g
Table 2 Determination results of gingerols in extract samples obtained by different extraction methods
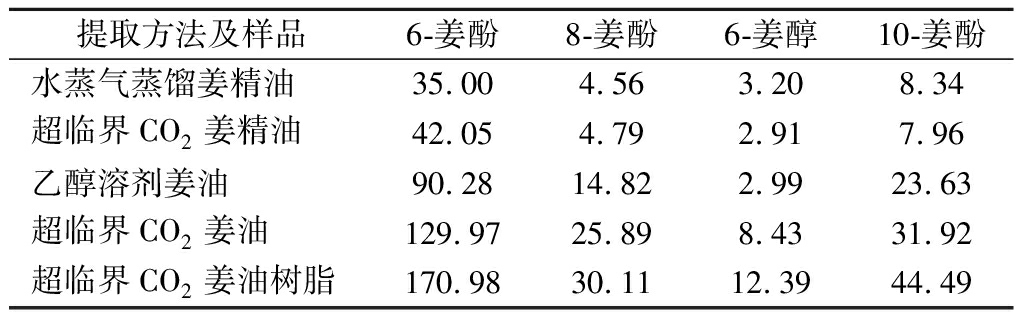
提取方法及样品6-姜酚8-姜酚6-姜醇10-姜酚水蒸气蒸馏姜精油35.004.563.208.34超临界CO2姜精油42.054.792.917.96乙醇溶剂姜油90.2814.822.9923.63超临界CO2姜油129.9725.898.4331.92超临界CO2姜油树脂170.9830.1112.3944.49
由表2可知不同工艺得到的5种提取物中姜酚类物质含量水蒸气蒸馏姜精油和超临界CO2姜精油、超临界CO2姜油与乙醇溶剂姜油较接近,超临界CO2姜油树脂中含量最高,尤其与同时得到的超临界CO2精油相比,显示通过改变超临界CO2提取工艺条件参数可以进行姜酚类物质的选择性分离富集。超临界CO2提取是根据在不同提取压力、温度条件时超临界CO2流体密度不同,对被提取物的溶解度能力不同来进行提取分离的,其中压力影响最大[22],随着压力增大,超临界CO2密度增大,溶解能力增强,通过改变提取过程的压力可以对提取物进行一定程度选择性提取[23-24]。本研究在较低超临界CO2萃取压力下提取得到生姜中化学组成通常为无极性或极性较小、挥发性较强的超临界CO2姜精油,然后通过升高萃取压力,也即增加超临界CO2的溶解能力,将有一定极性且挥发性弱分子质量较大化学组成的姜油树脂进一步提取出来,也即通过改变萃取压力进行选择性一次提取分离,分别得到超临界CO2姜精油和富集6-姜酚的超临界CO2姜油树脂2种提取物。
3 结论
分别采用水蒸气蒸馏法、有机溶剂法以及3种超临界CO2提取工艺对同一种植产地同品系生姜中的挥发性及脂溶性成分进行了提取分离,得到不同提取工艺下5种提取物,对这些提取物中的化学组成以及重要生物活性成分姜酚类化合物分别进行了GC-MS定性、归一化法及用标准品的HPLC定量分析表征,结果表明:
(1)水蒸气蒸馏法得到的挥发性姜精油收率最低,超临界CO2提取法得到的姜油收率最高,其次为乙醇溶剂法所得姜油,在超临界CO2选择性提取中可以一次性分别得到姜精油和姜油树脂,姜油树脂的收率高于姜精油。
(2)提取物的GC-MS定性、归一化法以及HPLC对重要生物活性成分姜酚类化合物的定量分析表明:超临界CO2姜油和乙醇溶剂姜油组成较为接近,水蒸气蒸馏法姜精油和超临界CO2姜精油化学组成较为接近,富含6-姜酚等姜酚类化合物的超临界CO2姜油树脂化学组成与前述4种提取物化学组成差异较大。
(3)超临界CO2提取工艺可以通过改变或调整工艺参数得到3种不同的生姜提取物,即超临界CO2姜精油、姜油和姜油树脂,从收率、化学组成以及姜酚类物质含量分析检测结果的比较表明,超临界CO2提取作为一种绿色高效提取工艺是生姜中挥发性及脂溶性成分提取的优选提取分离技术,可得到不同提取物以分别用作香精香料、食品添加剂及健康医药产品等的优质原料。
[1] 于梓芃,黄泽天,崔文甲,等.生姜的营养成分及其加工产品研究进展[J].中国果菜,2021,41(1):15-20.
YU Z P,HUANG Z T,CUI W J,et al.Research progress on nutritional components and processing products of ginger[J].China Fruit & Vegetable, 2021,41(1):15-20.
[2] NOGUEIRA DE MELO G A, GRESPAN R, FONSECA J P, et al.Inhibitory effects of ginger (Zingiber offcinale Roscoe) essential oil on leukocyte migration in vivo and in vitro[J].Journal of Natural Medicines, 2011, 65(1):241-246.
[3] 李辉.水蒸气蒸馏法提取姜精油[J].粮油加工,2010(11):16-20.
LI H.Study on the essential oil from ginger by steam distillation[J].Cereals and Oils Processing,2010(11):16-20.
[4] 刘红霞,丁荣良,仝锦豪,等.姜精油提取工艺优化及对比成分分析[J].中国调味品,2021,46(8):101-104.
LIU H X,DING R L,TONG J H,et al.Optimization of extraction process of ginger essential oil and comparative analysis of its components[J].China Condiment,2021,46(8):101-104.
[5] 高珊.姜精油的提取及抗氧化作用研究[J].食品安全导刊,2016(21):155-156.
GAO S.Research on extraction and antioxidant effect of ginger essential oil [J].China Food Safety Magazine,2016(21):155-156.
[6] 欧阳辉,田启建,陈功锡,等.超临界萃取姜精油工艺的优化[J].食品与发酵工业,2010,36(1):171-174.
OUYANG,TIAN Q J,CHEN G X,et al.Optimization of supercritical carbon dioxide extraction of ginger essential oil with response surface methodology[J].Food and Fermentation Industries,2010,36(1):171-174.
[7] LEI H,ZHOU R J,WEI Q N,et al.Optimization of supercritical carbon dioxide extraction of ginger essential oil by response surface method[J].Agricultural Science & Technology,2016,17(9):2 178-2 182.
[8] 骆海林,陆宁.同时蒸馏-萃取法(SDE)提取/GC-MS分析姜精油的组分[J].中国调味品,2010,35(10):104-106;109.
LUO H L,LU N.Analysis of ginger essential oil constituents by simultaneous distillation and solvent extraction combined with GC-MS[J].China Condiment,2010,35(10):104-106;109.
[9] 宋丽丽,李科娜,杨旭,等.小黄姜精油提取工艺优化、成分分析及其生物活性研究[J].轻工学报,2020,35(5):7-15.
SONG L L,LI K N,YANG X,et al.Study on the optimization of extraction process,composition analysis and biological activity of essential oil from Zingiber offcinale Roscoe[J].Journal of Light Industry,2020,35(5):7-15.
[10] 王颖.GC/MS法测定不同产地鲜姜精油化学成分[J].广东化工,2016,43(7):176;191.
WANG Y.Chemical compositions analysis of essential oil for fresh gingers from different districts by GC/MS[J].Guangdong Chemical Industry,2016,43(7):176;191.
[11] 裴亚萍.山东大(黄)姜精油香气成分的SFE/SD-GC-MS分析[J].食品研究与开发,2020,41(3):188-195.
PEI Y P.The analysis on volatile compositions of Shandong ginger essential oil by SFE/SD-GC-MS[J].Food Research and Development,2020,41(3):188-195.
[12] 于大胜,崔秀伟,张福鑫,等.生姜风味物质不同提取方法对比分析[J].安徽农业科学,2008,36(21):8 878-8 870.
YU D S,CUI X W,ZHANG F X,et al.Contrasting analysis on ginger flavor substances by using different extraction ways[J].Journal of Anhui Agricultural Sciences,2008,36(21):8 878-8 880.
[13] SEMWAL R B,SEMWAL D K,COMBRINCK S, et al.Gingerols and shogaols:Important nutraceutical principles from ginger[J].Phytochemistry,2015,117:554-568.
[14] MA R H,NI Z J,ZHU Y Y,et al.A recent update on the multifaceted health benefits associated with ginger and its bioactive components[J].Food & Function,2021,12(2):519-542.
[15] SCHWERTNER H A,RIOS D C.High-performance liquid chromatographic analysis of 6-gingerol,8-gingerol,10-gingerol,and 6-shogaol in ginger-containing dietary supplements,spices,teas,and beverages[J].Journal of Chromatography B,2007,856(1-2):41-47.
[16] 李鹏辉,严辉,朱邵晴,等.干姜HPLC指纹图谱建立及5种成分测定[J].中成药,2019,41(9):2 246-2 251.
LI P H,YAN H,ZHU S Q, et al.Establishment of HPLC fingerprint of dried ginger and determination of five components [J].Chinese Traditional Patent Medicine,2019,41(9):2 246-2 251.
[17] 凌育赵.水蒸气蒸馏提取沙姜中特性组分-沙姜油的研究[J].中国调味品,2005, 30(8):28-31.
LING Y Z.Study on steam distillation law draw of characteristic component-Kaempferia galanga L.oil[J].Chinese Condiment,2005, 30(8):28-31.
[18] 张鲁明.生姜姜油的提取、成分分析及及其抑菌活性研究[D].长沙:湖南农业大学,2010.
ZHANG L M.Studies on ginger oil extraction and chemical components and its antibacterial activity from Zingiber offcinale Roscoe[D].Changsha:Hunan Agricultural University,2010.
[19] 余德顺,黄炯,夏文,等.一种从生姜中萃取分离姜精油及富含姜酚姜油树脂的方法:CN201110257527.9[P].2011-09-02.
YU D S,HUANG J,XIA W,et al.Method for extracting and separating ginger essential oil and ginger oleoresin rich in gingerol from ginger:CN102965197A[P].2011-09-02.
[20] 陈可可,滕桂平,余德顺,等.姜及其相关产品中主要姜辣素成分的HPLC检测分析[J].食品科技,2019,44(2):324-328.
CHEN K K,TENG G P,YU D S,et al.Analysis of main gingerols in ginger and its related products by HPLC[J].Food Science and Technology,2019,44(2):324-328.
[21] ZHANG X,IWAOKA W T,HUANG A S,et al.Gingerol decreases after processing and storage of ginger[J].Journal of Food Science,1994,59(6):1 338-1 340.
[22] AHANGARI H,KING J W,EHSANI A,et al.Supercritical fluid extraction of seed oils-A short review of current trends[J].Trends in Food Science & Technology,2021, 111:249-260.
[23] 周玉凤,张海东,熊昆,等.超临界CO2萃取植物功能性油脂的研究进展[J].食品工业科技,2019,40(20):334-339.
ZHOU Y F,ZHANG H D,XIONG K,et al.Research progress in supercritical CO2 extraction of functional oils of plant[J].Science and Technology of Food Industry,2019,40(20):334-339.
[24] 廖传华, 黄振仁.超临界CO2流体萃取技术:工艺开发及其应用[M].北京:化学工业出版社, 2004:18-26.
LIAO C H, HUANG Z R.Supercritical CO2 Fluid Extraction Technology-Process Development and Application[M].Beijing:Chemical Industry Press, 2004:18-26.