多年来,用于工程化微生物细胞工厂的系统代谢工程、生物信息学和生物技术有了明显的发展,促进了生物基平台化学品的生产。由于谷氨酸棒杆菌(Corynebacterium glutamicum)具有公认安全(Generally Regarded As Safe, GRAS)的特性,已被广泛用于氨基酸、有机酸、醇类等化学品和燃料的工业生产[1],特别是它已成为氨基酸生产的主要菌种。据中国生物发酵产业协会统计,2021年中国的氨基酸发酵产品总产量约为639万t,氨基酸发酵在我国国民经济发展中扮演着重要角色。因此,构建高效谷氨酸棒杆菌微生物细胞工厂对于高产高效地生产氨基酸具有重要意义。
发酵过程需要将小分子质量的底物向胞内转运,与此同时,生成的产物向胞外转运。转运工程是细胞工厂开发的一个组成部分,可以解决底物的吸收、中间产物的转运、产物的再吸收和产物的分泌等。除了通过过量表达或敲除直接操纵转运蛋白基因外,还可以针对各自的转录抑制因子或激活因子来控制其表达。因此本文从谷氨酸棒杆菌的底物转运和氨基酸的分泌转运以及再吸收等方面进行综述。
1 底物的转运
对于高效的发酵过程来说,碳源的选择是生物工艺成功的第一个关键步骤。因此,底物的成本和可用性在工业生物制造中至关重要。代谢工程已经成功地用于扩大微生物细胞工厂的底物范围。谷氨酸棒杆菌底物转运系统如图1所示。
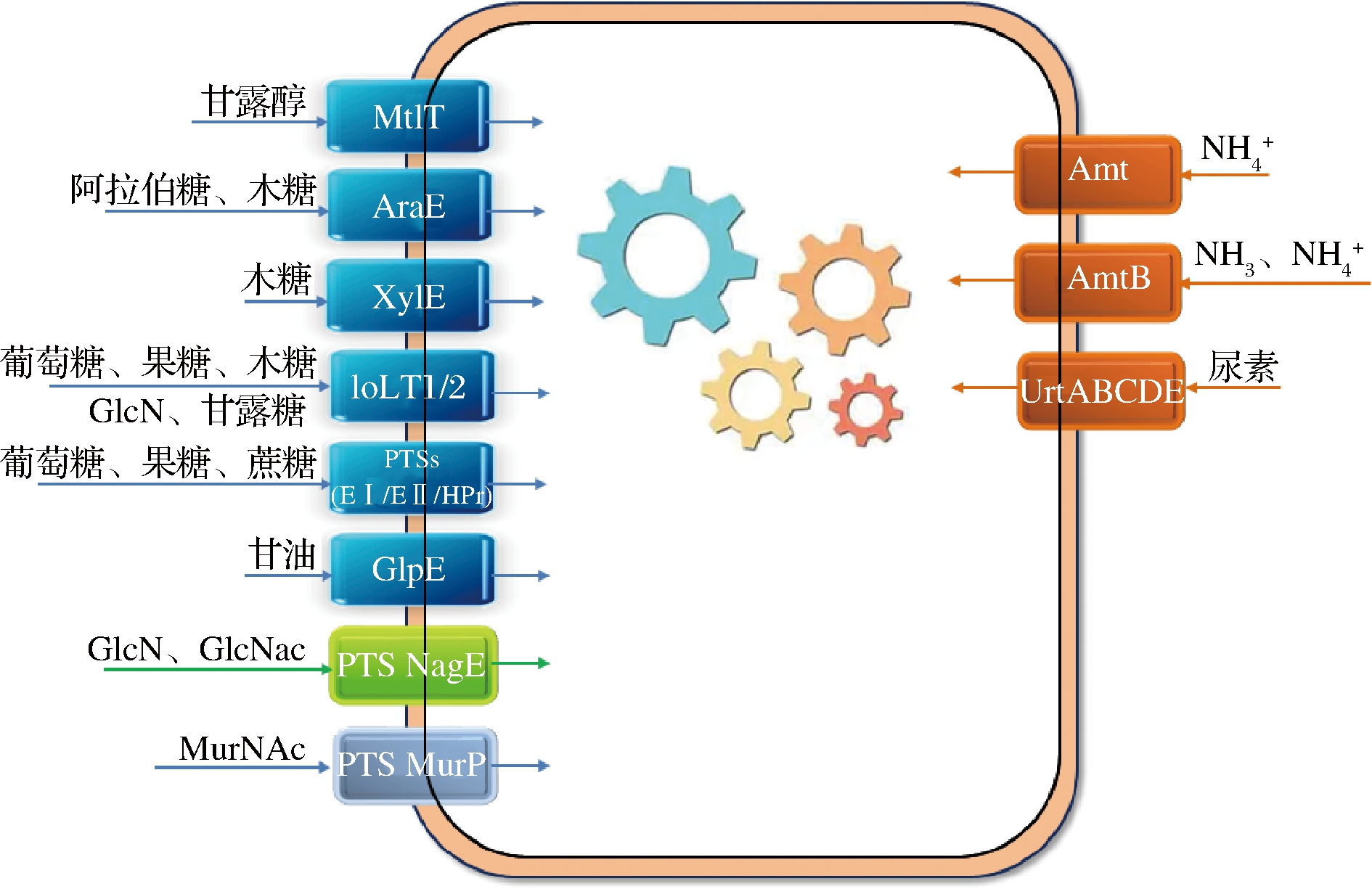
图1 谷氨酸棒杆菌细胞转运过程示意图
Fig.1 Schematic representation of transport processes in C.glutamicum cells
注:蓝色为碳源转运蛋白;橙色为氮源转运蛋白;灰色或绿色为外源转运蛋白
1.1 碳源转运
目前,氨基酸生产菌株严重依赖第一代原料、纯碳水化合物(如葡萄糖)作为唯一的碳源和能量来源。碳水化合物(如葡萄糖、果糖和蔗糖)主要通过磷酸烯醇-丙酮酸依赖性磷酸转移酶系统(phosphotransferase system,PTS)来转运和磷酸化。C.glutamicum ATCC 13032中含有4组PTS,包括葡萄糖PTS、果糖PTS、蔗糖PTS和一个未知PTS[2]。另外,葡萄糖、果糖和木糖可以被渗透酶IolT1和IolT2转运向胞内,并被激酶Glk和PpgK磷酸化[3]。与PTS介导的高亲和力转运相比,这种途径只在底物浓度高或PTSGlc不可用时运行,但不消耗磷酸烯醇丙酮酸,这有利于磷酸烯醇丙酮酸依赖的氨基酸(如芳香族氨基酸)的生物合成。在一个PTS缺陷的谷氨酸菌株中过量表达iolT1或iolT2与glk或ppgK结合,可以完全恢复葡萄糖的利用,并使L-赖氨酸产量提高20%[3]。当内源性iolT2基因和来自枯草杆菌的葡萄糖激酶基因glcK在谷氨酸棒杆菌野生型和L-赖氨酸生产菌GRLys1中过量表达时,葡萄糖消耗率可以增加28%[4]。敲除转录抑制基因sugR可以解除对包括PTS基因在内的糖酵解基因的抑制,从而增加葡萄糖消耗和生长速度,然而必须敲除ldhA以避免乳酸分泌造成的碳损失[4-6]。
目前,实现可持续性的战略倾向于使用来自工业废弃物流的碳原料,如戊糖、木糖、阿拉伯糖和多元醇甘油等。谷氨酸棒杆菌可以同时利用各种碳源。通过对碳代谢的详细了解,以及完善的遗传工具和工程策略,谷氨酸棒杆菌的底物谱得到进一步扩展。细胞工厂的构建需要解决一些替代碳源被吸收到谷氨酸棒杆菌细胞中的问题。
谷氨酸棒状杆菌不能代谢乳糖或半乳糖,因此不能利用乳清等乳制品基质来生产氨基酸。研究表明,以乳糖为唯一碳源的快速生长需要谷氨酸棒杆菌表达lac操纵子[7]。BARRETT等[8]建立了利用乳糖生产L-赖氨酸的方法。
甘油是生物柴油制造过程中的副产物,其供应量很大。谷氨酸棒杆菌不能使用甘油作为底物,但异源表达大肠杆菌(Escherichia coli)的甘油激酶(glycerol kinase,GlpK)和甘油-3-磷酸脱氢酶(GlpD)使谷氨酸棒杆菌在甘油上生长[9-10]。继续异源表达大肠杆菌的甘油通道蛋白GlpF加速了谷氨酸棒杆菌以甘油为唯一碳源的生长[9]。按照这一策略,甘油已被用作生产L-谷氨酸、L-赖氨酸、L-鸟氨酸、L-精氨酸和腐胺酸的碳源[9-10]。
葡萄糖胺(glucosamine,GlcN)和N-乙酰葡萄糖胺(N-acetylglucosamine,Glc-NAc)是甲壳素的亚单位,甲壳素构成了甲壳类动物的外骨骼,是地球上最丰富的聚合物之一。而Glc-NAc和N-乙酰熊果苷酸(N-acetylrnuramic acid,MurNAc)是细菌细胞壁的亚单位,是发酵工业中的副产物。谷氨酸棒杆菌不能用甲壳素作为碳源[11],但是能够通过PtsG转运GlcN[12]。通常,Glc-NAc的代谢途径包括一个特定的转运系统和2个酶作用,由N-乙酰氨基葡糖-6-磷酸脱乙酰基酶(由nagA编码)和氨基葡糖苷-6-磷酸脱氨酶(由nagB编码)催化,将N-乙酰氨基葡萄糖转化为进入糖酵解途径的果糖-6-磷酸酯[11]。而在谷氨酸棒杆菌中,对Glc-NAc的转运则需要来自Corynebacterium glycinophilum的异源Glc-NAc特异性PTS NagE[13]。目前,过表达外源的nagA、nagB和nagE使谷氨酸棒杆菌能够利用GlcN产生L-瓜氨酸[14]、L-赖氨酸[15]、腐胺[12]和γ-氨基丁酸[16],产物产量或生物量形成和比生长速率与葡萄糖类似。表达E.coli的MurNAc特异性的PTS组分murP,则赋予了谷氨酸棒杆菌以MurNAc生长的能力[15]。
半纤维素是木质纤维素中第二丰富的成分(2%~30%),已经引起了人们极大的关注[17-18]。半纤维素生物质的糖化产生许多可发酵的糖,包括D-木糖、L-阿拉伯糖、D-甘露糖、D-半乳糖、L-鼠李糖、L-果糖、D-葡萄糖醛酸等以及醋酸。谷氨酸棒杆菌已被开发为能够利用半纤维素生物质生产增值产品的微生物细胞工厂。目前,谷氨酸棒杆菌中使用了3种转运木糖的跨膜蛋白:lolT1[19]、XylE[20]和AraE[21]。其中,AraE可以同时转运L-阿拉伯糖。在C.glutamicum ATCC 13032中表达C.glutamicum ATCC 31831的L-阿拉伯糖转运系统,增加了菌株以L-阿拉伯糖为碳源的生长速度[22-23]。在谷氨酸棒杆菌中异源表达大肠杆菌的阿拉伯糖代谢酶基因araBAD或同时表达C.glutamicum ATCC 31831中的L-阿拉伯糖转运蛋白基因araE,使其能够在以阿拉伯糖为唯一或辅助碳源时生长[22, 24]。谷氨酸棒杆菌以葡萄糖-阿拉伯糖混合物为碳源,能生产L-谷氨酸、L-赖氨酸、L-精氨酸和L-鸟氨酸[25]。利用稻草或麦麸水解物生产L-谷氨酸和L-赖氨酸的谷氨酸棒杆菌也已经被成功地设计[26]。
在谷氨酸棒杆菌中,甘露糖代谢途径由葡萄糖或果糖渗透酶(由ptsG或ptsF编码)以及磷酸甘露糖异构酶(由manA编码)组成。在厌氧条件下,manA和ptsF的共同过表达不仅加速了甘露糖的利用,而且还破坏了碳代谢物抑制,使谷氨酸棒杆菌中葡萄糖和甘露糖同时代谢[27]。目前,人们的注意力转移到了它的还原形式甘露醇上,因为甘露醇很容易从海洋植物的水解中获得。甘露醇的分解代谢途径包括2个跨膜转运系统和2个酶反应。首先,它通过跨膜蛋白MtlT从细胞外空间运输到细胞内空间,并通过甘露醇脱氢酶(由mtlD编码)转化为果糖[28]。随后,果糖通过由EI(由ptsIH编码)和EII(由ptsF编码)的膜蛋白组成的非特异性PTS转运系统,2次跨细胞膜转运,产生果糖-1-磷酸[29]。因此,有理由推测甘露醇转运体和甘露醇脱氢酶的充分表达是谷氨酸棒杆菌利用甘露醇的关键因素。
1.2 氮源转运
微生物已经发展出严格的氮素代谢调节机制,以满足不同环境条件的要求。铵、尿素、L-谷氨酰胺和其他含氮化合物都可以作为谷氨酸棒杆菌的氮源。其中,氨和尿素可以利用扩散作用穿过细胞膜,而铵[30]和尿素[31]、L-谷氨酰胺[32]的同化需要特定的转运系统。铵的转运系统主要依赖于2个特殊的载体,Amt(由amt编码)和AmtB(由amtB编码,原名amtP)[33]。与Amt渗透酶相反,AmtB渗透酶接受铵并将氨向胞内转运[30, 34]。尿素的吸收主要依靠被动扩散和尿素转运蛋白UrtABCDE,分别在尿素供给充足和不足的条件下进行[35]。
微生物对氮源的转运和同化对氨基酸的生产极为重要。通过敲除amtR以解除基因的反馈抑制来加速氮代谢,过量表达amtB以增强铵的转运,以及过量表达glnK以调节氮源代谢,被确定为提高L-精氨酸积累的有效修饰策略[36]。
2 氨基酸的转运
2.1 L-赖氨酸、L-精氨酸、L-鸟氨酸和L-瓜氨酸的转运
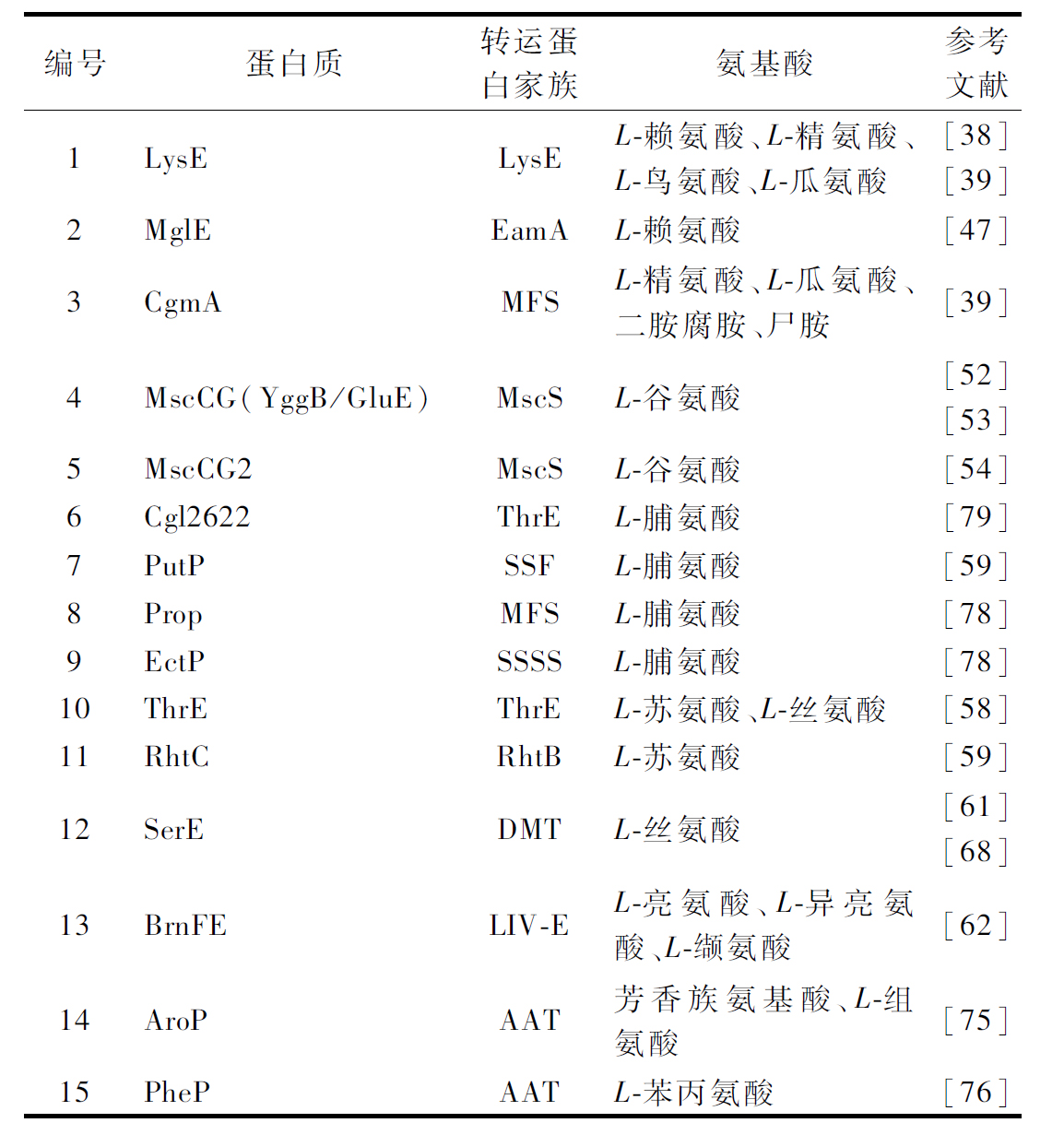
对谷氨酸棒杆菌转运过程的研究开创了所有细菌中小分子质量化合物的转运领域[37-38]。谷氨酸棒杆菌的LysE系统是细菌中第1个具有遗传和生化特性的氨基酸转运系统。LysE是一种跨膜蛋白,在L-赖氨酸、L-精氨酸、L-鸟氨酸和L-瓜氨酸的转运中发挥主要作用[39-40]。LysE是谷氨酸棒杆菌中唯一活跃的L-赖氨酸分泌转运蛋白[39],而L-精氨酸可以通过LysE和CgmA转运[40](表1)。通过增强LysE的表达,L-赖氨酸的产量都获得了提高[4, 41-42],而L-精氨酸、L-瓜氨酸和L-鸟氨酸的产量分别提高了13.6%[43]、45%[40]和21.8%[44]。基因lysE的敲除可以提高L-赖氨酸衍生化合物的产量,如尸胺[45]和5-氨基戊酸乙酯(5-aminovalerate,5AVA)[46]。XIAO等[47]通过基因敲除C.glutamicum23604菌株中广泛存在的3种氨基酸跨膜转运蛋白,即 MscCG(GluE)、BrnE/BrnF和赖氨酸胞内转运蛋白LysP后,L-赖氨酸的产量分别增加了9.0%、12.3%和10.0%,而当gluE和brnE/brnF基因缺失时,副产物氨基酸的量显著减少。近期,研究者在牛肠道微生物组文库中鉴定了一个新型高效的L-赖氨酸转运蛋白MglE,并在1株工业谷氨酸棒杆菌菌株中表达,使其赖氨酸转化率和产量分别提高了7.8%和9.5%[48]。
CgmA是一种渗透酶,负责转运L-精氨酸、L-瓜氨酸、二胺腐胺和尸胺(表1)。基因cgmA的过量表达弥补了LysE在L-精氨酸转运方面的不足[40]。敲除基因cgmA避免腐胺和尸胺的转运以增加γ-氨基丁酸(γ-aminobutyric acid,GABA)和5AVA的前体,被用作生产衍生化合物GABA和5AVA的方法[16]。基因cgmA受转录因子CgmR的调节[49],敲除抑制基因cgmR可提高L-精氨酸、尸胺和腐胺的产量[40, 50-51]。编码GABA转运系统的gabP的缺失也可以增加GABA的产生[52]。
2.2 L-谷氨酸和L-脯氨酸的转运
在利用谷氨酸棒杆菌建立的工业L-谷氨酸生产中,细胞内合成的L-谷氨酸通过机械敏感通道蛋白(MscCG和MscCG2)转运(表1)。MscCG(由ncgl1221编码)是谷氨酸棒杆菌的机械敏感通道,MscCG的过表达增加了L-谷氨酸的产生[53],MscCG的缺失则减少了L-谷氨酸的产生[54]。除了MscCG,机械敏感通道MscCG2支持C.glutamicum Z188菌株的L-谷氨酸转运;然而,它不存在于C.glutamicum ATCC 13032中[55]。L-谷氨酸是许多氨基酸生物合成的直接前体。因此,为了将L-谷氨酸转化为其他氨基酸,而不是将其分泌到发酵培养基中,改造其转运系统成为提高氨基酸产量的有效策略。谷氨酸转运蛋白MscCG的缺失,导致L-谷氨酸的分泌减少,增加了L-精氨酸[56]、L-鸟氨酸[44]、谷氨酰胺[57]和GABA[16]的生物合成。由于谷氨酸转运蛋白MscCG2存在于C.glutamicum S9114中,同时敲除MscCG2和MscCG可以完全阻断L-谷氨酸的转运,用于生产L-精氨酸、L-脯氨酸、L-鸟氨酸和L-瓜氨酸的细胞工厂的构建。
早在1998年就出现了关于谷氨酸棒杆菌的ProP、EctP等L-脯氨酸吸收系统的报道[58],但直到2022年才第一次报道了将细胞内合成的L-脯氨酸分子运输到培养基的L-脯氨酸转运蛋白Cgl2622[59]。PutP是脯氨酸-钠离子协同转运蛋白,扮演了协助脯氨酸进出细胞的角色[60]。L-脯氨酸的生物合成途径是其他谷氨酸族氨基酸的竞争性代谢途径。敲除putP以阻止L-脯氨酸的分泌,从而减少L-脯氨酸的生物合成已被用作提高L-精氨酸[61]和L-鸟氨酸[62]产量的修饰方法,它可以在不影响生长的情况下尽可能地减少L-脯氨酸的积累。
2.3 L-苏氨酸和L-丝氨酸的转运
ThrE能转运L-苏氨酸和L-丝氨酸[63] (表1)。SIMIC等[64]在L-苏氨酸生产菌中过表达thrE,培养72 h后,L-苏氨酸的产量增加了40%。在谷氨酸棒杆菌中表达大肠杆菌L-苏氨酸转运蛋白RhtC也可以有效促进L-苏氨酸的分泌及合成[65]。近期有研究表明,ThrE可能不是主要的L-丝氨酸转运蛋白[66],NCgl0580 编码蛋白参与了L-丝氨酸的分泌转运[67-68]。
表1 氨基酸的转运蛋白
Table 1 Transporters of amino acids
2.4 支链氨基酸和L-甲硫氨酸的转运
BrnFE 是转运支链氨基酸L-亮氨酸、L-异亮氨酸和L-缬氨酸所必需的渗透酶,BrnFE的过表达提高了它们的转运率[69] (表1)。尽管在谷氨酸棒杆菌中BrnFE不是转运L-甲硫氨酸的关键[70],但是过表达BrnFE也增加了L-甲硫氨酸的产量[71]。BrnFE 的表达受转录因子Lrp 的激活,在产生L-异亮氨酸的C.glutamicum JHI3-156中共表达lrp和brnFE,使L-异亮氨酸的产量从24.3 g/L增加到26.9 g/L[72]。过表达Lrp 及BrnFE 亦能显著提高谷氨酸棒杆菌L-缬氨酸的产量[73-75]。而brnQ基因编码的转运蛋白是谷氨酸棒杆菌中3种支链氨基酸(brancled-chain amino acid,BCAA)的共同和唯一的吸收转运系统[76],为了进一步提高BCAA的产量,敲除brnQ基因也是重要的方法[77]。而在过表达BrnFE的同时,敲除brnQ基因,使L-异亮氨酸的产量达到29.0 g/L[78]。在L-鸟氨酸或L-精氨酸发酵过程中,BCAA被当作副产物处理,因此BCAA转运蛋白常被确定为开发L-鸟氨酸生产菌株[62, 79]和L-精氨酸生产菌株[61]的敲除位点。
2.5 芳香族氨基酸和L-组氨酸的转运
AroP和PheP参与谷氨酸棒杆菌对芳香族氨基酸的吸收转运(表1)。PheP是L-苯丙氨酸的特异性转运蛋白[80]。AroP是一个通用的芳香族氨基酸转运蛋白,也能吸收转运L-组氨酸[81]。破坏aroP减少了对L-组氨酸、L-色氨酸、L-酪氨酸和L-苯丙氨酸作为唯一氮源的利用,这表明存在一个迄今为止未知的这些氨基酸的替代转运系统[82]。
3 展望
在过去几十年里,谷氨酸棒杆菌在工业生物技术方面取得了显著进展,尽管如此,葡萄糖和蔗糖仍然是氨基酸工业生产的主要碳源。考虑到全球资源和粮食短缺的挑战,高效利用替代碳源是发展谷氨酸棒杆菌微生物细胞工厂的关键因素之一。因此,未来的研究应着重于设计和开发谷氨酸棒杆菌吸收转运系统,通过工程合成基因表达对各种替代碳源途径的组合优化,使其能够直接利用农业废弃物、食品加工残留物和工业废弃物进行发酵生产氨基酸。
转运蛋白对氨基酸生产的重要性已经得到了令人信服的证明。在发酵生产某种氨基酸时,必须将细胞中的氨基酸分子运输到细胞外,才能使该种氨基酸的产量提高;而在发酵生产某种氨基酸的衍生物时,需要将这种氨基酸的分泌转运蛋白失活,从而使该氨基酸在胞内大量积累,为其衍生物的合成提供更多的前体。转运蛋白对于氨基酸的转运往往不具有特异性的,因此需要:(1)进一步鉴定转运蛋白及其特异性,并验证其对氨基酸转运的重要性;(2) 根据所生产氨基酸品种的需求对已知转运蛋白进行改造。
目前,对谷氨酸棒杆菌基因组学、转录组学、系统生物学和生理学方面的研究结果已被用于代谢工程工具和策略的开发。新的代谢工程工具和策略的应用将使谷氨酸棒杆菌成为一个更有前途的微生物细胞工厂,从而促进氨基酸行业的可持续发展。
[1] HERMANN T.Industrial production of amino acids by coryneform bacteria[J].Journal of Biotechnology, 2003, 104(1-3):155-172.
[2] 韩武洋, 刘金雷, 杜红燕, 等.谷氨酸棒状杆菌葡萄糖代谢阻断工程菌的构建[J].食品科学, 2017, 38(2):65-74.
HAN W Y, LIU J L, DU H Y, et al.Construction of engineered strain blocking glucose metabolism in Corynebacterium glutamicum[J].Food Science, 2017, 38(2):65-74.
[3] LINDNER S N, SEIBOLD G M, HENRICH A, et al.Phosphotransferase system-independent glucose utilization in Corynebacterium glutamicum by inositol permeases and glucokinases[J].Applied and Environmental Microbiology, 2011, 77(11):3 571-3 581.
[4] PÉREZ-GARC A F, PETERS-WENDISCH P, WENDISCH V F.Engineering Corynebacterium glutamicum for fast production of L-lysine and L-pipecolic acid[J].Applied Microbiology and Biotechnology, 2016, 100(18):8 075-8 090.
A F, PETERS-WENDISCH P, WENDISCH V F.Engineering Corynebacterium glutamicum for fast production of L-lysine and L-pipecolic acid[J].Applied Microbiology and Biotechnology, 2016, 100(18):8 075-8 090.
[5] ENGELS V, WENDISCH V F.The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum[J].Journal of Bacteriology, 2007, 189(8):2 955-2 966.
[6] XU J Z, ZHANG J L, LIU D D, et al.Increased glucose utilization and cell growth of Corynebacterium glutamicum by modifying the glucose-specific phosphotransferase system (PTSGlc) genes[J].Canadian Journal of Microbiology, 2016, 62(12):983-992.
[7] BRABETZ W, LIEBL W, -H SCHLEIFER K.Studies on the utilization of lactose by Corynebacterium glutamicum, bearing the lactose operon of Escherichia coli[J].Archives of Microbiology, 1991, 155(6):607-612.
[8] BARRETT E, STANTON C, ZELDER O, et al.Heterologous expression of lactose- and galactose-utilizing pathways from lactic acid bacteria in Corynebacterium glutamicum for production of lysine in whey[J].Applied and Environmental Microbiology, 2004, 70(5):2 861-2 866.
[9] RITTMANN D, LINDNER S N, WENDISCH V F.Engineering of a glycerol utilization pathway for amino acid production by Corynebacterium glutamicum[J].Applied and Environmental Microbiology, 2008, 74(20):6 216-6 222.
[10] MEISWINKEL T M, RITTMANN D, LINDNER S N, et al.Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum[J].Bioresource Technology, 2013, 145:254-258.
[11] MATANO C, KOLKENBROCK S, HAMER S N, et al.Corynebacterium glutamicum possesses β-N-acetylglucosaminidase[J].BMC Microbiology, 2016, 16(1):177.
[12] UHDE A, YOUN J W, MAEDA T, et al.Glucosamine as carbon source for amino acid-producing Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2013, 97(4):1 679-1 687.
[13] MATANO C, UHDE A, YOUN J W, et al.Engineering of Corynebacterium glutamicum for growth and L-lysine and lycopene production from N-acetyl-glucosamine[J].Applied Microbiology and Biotechnology, 2014, 98(12):5 633-5 643.
[14] EBERHARDT D, JENSEN J V, WENDISCH V F.L-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources[J].AMB Express, 2014, 4(1):85.
[15] SGOBBA E, BLÖBAUM L, WENDISCH V F.Production of food and feed additives from non-food-competing feedstocks:Valorizing N-acetylmuramic acid for amino acid and carotenoid fermentation with Corynebacterium glutamicum[J].Frontiers in Microbiology, 2018, 9:2046.
[16] JORGE J M, NGUYEN A Q, PÉREZ-GARC A F, et al.Improved fermentative production of gamma-aminobutyric acid via the putrescine route:Systems metabolic engineering for production from glucose, amino sugars, and xylose[J].Biotechnology and Bioengineering, 2017, 114(4):862-873.
A F, et al.Improved fermentative production of gamma-aminobutyric acid via the putrescine route:Systems metabolic engineering for production from glucose, amino sugars, and xylose[J].Biotechnology and Bioengineering, 2017, 114(4):862-873.
[17] AVANTHI A, KUMAR S, SHERPA K, et al.Bioconversion of hemicelluloses of lignocellulosic biomass to ethanol:An attempt to utilize pentose sugars [J].Biofuels-UK, 2017, 8(4):431-44.
[18] CHANDEL A K, GARLAPATI V K, SINGH A K, et al.The path forward for lignocellulose biorefineries:Bottlenecks, solutions, and perspective on commercialization[J].Bioresource Technology, 2018, 264:370-381.
[19] BRÜSSELER C, RADEK A, TENHAEF N, et al.The myo-inositol/proton symporter IolT1 contributes to D-xylose uptake in Corynebacterium glutamicum[J].Bioresource Technology, 2018, 249:953-961.
[20] YIM S S, CHOI J W, LEE S H, et al.Modular optimization of a hemicellulose-utilizing pathway in Corynebacterium glutamicum for consolidated bioprocessing of hemicellulosic biomass[J].ACS Synthetic Biology, 2016, 5(4):334-343.
[21] CHEN Z, HUANG J H, WU Y, et al.Metabolic engineering of Corynebacterium glutamicum for the production of 3-hydroxypropionic acid from glucose and xylose[J].Metabolic Engineering, 2017, 39:151-158.
[22] KAWAGUCHI H, SASAKI M, VERT S A A, et al.Engineering of an L-arabinose metabolic pathway in Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2008, 77(5):1 053-1 062.
S A A, et al.Engineering of an L-arabinose metabolic pathway in Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2008, 77(5):1 053-1 062.
[23] ZHANG Y, SHANG X L, LAI S J, et al.Development and application of an Arabinose-inducible expression system by facilitating inducer uptake in Corynebacterium glutamicum[J].Applied and Environmental Microbiology, 2012, 78(16):5 831-5 838.
[24] GOPINATH V, MURALI A, DHAR K S, et al.Corynebacterium glutamicum as a potent biocatalyst for the bioconversion of pentose sugars to value-added products [J].Applied Microbiology and Biotechnology, 2012, 93(1):95-106.
[25] SCHNEIDER J, NIERMANN K, WENDISCH V F.Production of the amino acids L-glutamate, L-lysine, L-ornithine and L-arginine from Arabinose by recombinant Corynebacterium glutamicum[J].Journal of Biotechnology, 2011, 154(2-3):191-198.
[26] GOPINATH V, MEISWINKEL T M, WENDISCH V F, et al.Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2011, 92(5):985-996.
[27] SASAKI M, TERAMOTO H, INUI M, et al.Identification of mannose uptake and catabolism genes in Corynebacterium glutamicum and genetic engineering for simultaneous utilization of mannose and glucose[J].Applied Microbiology and Biotechnology, 2011, 89(6):1 905-1 916.
[28] PENG X, OKAI N, VERT S A A, et al.Characterization of the mannitol catabolic operon of Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2011, 91(5):1 375-1 387.
S A A, et al.Characterization of the mannitol catabolic operon of Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2011, 91(5):1 375-1 387.
[29] LASLO T, VON ZALUSKOWSKI P, GABRIS C, et al.Arabitol metabolism of Corynebacterium glutamicum and its regulation by AtlR[J].Journal of Bacteriology, 2012, 194(5):941-955.
[30] SIEWE R M, WEIL B, BURKOVSKI A, et al.Functional and genetic characterization of the (methyl)ammonium uptake carrier of Corynebacterium glutamicum[J].Journal of Biological Chemistry, 1996, 271(10):5 398-5 403.
[31] BECKERS G, BENDT A K, KR MER R, et al.Molecular identification of the urea uptake system and transcriptional analysis of urea transporter- and urease-encoding genes in Corynebacterium glutamicum[J].Journal of Bacteriology, 2004, 186(22):7 645-7 652.
MER R, et al.Molecular identification of the urea uptake system and transcriptional analysis of urea transporter- and urease-encoding genes in Corynebacterium glutamicum[J].Journal of Bacteriology, 2004, 186(22):7 645-7 652.
[32] SIEWE R M, WEIL B, KR MER R.Glutamine uptake by a sodium-dependent secondary transport system inCorynebacterium glutamicum[J].Archives of Microbiology, 1995, 164(2):98-103.
MER R.Glutamine uptake by a sodium-dependent secondary transport system inCorynebacterium glutamicum[J].Archives of Microbiology, 1995, 164(2):98-103.
[33] JAKOBY M, KR MER R, BURKOVSKI A.Nitrogen regulation in Corynebacterium glutamicum:Isolation of genes involved and biochemical characterization of corresponding proteins[J].FEMS Microbiology Letters, 1999, 173(2):303-310.
MER R, BURKOVSKI A.Nitrogen regulation in Corynebacterium glutamicum:Isolation of genes involved and biochemical characterization of corresponding proteins[J].FEMS Microbiology Letters, 1999, 173(2):303-310.
[34] JAKOBY M, NOLDEN L, MEIER-WAGNER J, et al.AmtR, a global repressor in the nitrogen regulation system of Corynebacterium glutamicum[J].Molecular Microbiology, 2000, 37(4):964-977.
[35] SIEWE R M, WEIL B, BURKOVSKI A, et al.Urea uptake and urease activity in Corynebacterium glutamicum[J].Archives of Microbiology, 1998, 169(5):411-416.
[36] XU M J, LI J, SHU Q F, et al.Enhancement of l-arginine production by increasing ammonium uptake in an AmtR-deficient Corynebacterium crenatum mutant[J].Journal of Industrial Microbiology &Biotechnology, 2019, 46(8):1 155-1 166.
[37] MARIN K, KR MER R.Amino acid transport systems in biotechnologically relevant bacteria[M]//Amino Acid Biosynthesis-Pathways, Regulation and Metabolic Engineering.Berlin, Heidelberg:Springer Berlin Heidelberg, 2007:289-325.
MER R.Amino acid transport systems in biotechnologically relevant bacteria[M]//Amino Acid Biosynthesis-Pathways, Regulation and Metabolic Engineering.Berlin, Heidelberg:Springer Berlin Heidelberg, 2007:289-325.
[38] EGGELING L.Exporters for production of amino acids and other small molecules[M]//Amino Acid Fermentation.Tokyo:Springer Japan, 2016:199-225.
[39] VRLJIC M, SAHM H, EGGELING L.A new type of transporter with a new type of cellular function:L-lysine export from Corynebacterium glutamicum[J].Molecular Microbiology, 1996, 22(5):815-826.
[40] LUBITZ D, JORGE J M P, PÉREZ-GARC A F, et al.Roles of export genes cgmA and lysE for the production of L-arginine and L-citrulline by Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2016, 100(19):8 465-8 474.
A F, et al.Roles of export genes cgmA and lysE for the production of L-arginine and L-citrulline by Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2016, 100(19):8 465-8 474.
[41] BLOMBACH B, HANS S, BATHE B, et al.Acetohydroxyacid synthase, a novel target for improvement of L-lysine production by Corynebacterium glutamicum[J].Applied and Environmental Microbiology, 2009, 75(2):419-427.
[42] UNTHAN S, BAUMGART M, RADEK A, et al.Chassis organism from Corynebacterium glutamicum-a top-down approach to identify and delete irrelevant gene clusters[J].Biotechnology Journal, 2015, 10(2):290-301.
[43] XU M J, RAO Z M, YANG J, et al.The effect of a LYSE exporter overexpression on L-arginine production in Corynebacterium crenatum[J].Current Microbiology, 2013, 67(3):271-278.
[44] ZHANG B, YU M, ZHOU Y, et al.Systematic pathway engineering of Corynebacterium glutamicum S9114 for L-ornithine production[J].Microbial Cell Factories, 2017, 16(1):158.
[45] KIND S, NEUBAUER S, BECKER J, et al.From zero to hero - production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum[J].Metabolic Engineering, 2014, 25:113-123.
[46] ROHLES C M, GIEßELMANN G, KOHLSTEDT M, et al.Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate[J].Microbial Cell Factories, 2016, 15(1):1-13.
[47] XIAO J, WANG D T, WANG L, et al.Increasing L-lysine production in Corynebacterium glutamicum by engineering amino acid transporters[J].Amino Acids, 2020, 52(10):1 363-1 374.
[48] MALLA S, VAN DER HELM E, DARBANI B, et al.A novel efficient L-lysine exporter identified by functional metagenomics[J].Frontiers in Microbiology, 2022, 13:855736.
[49] ITOU H, WATANABE N, YAO M, et al.Crystal structures of the multidrug binding repressor Corynebacterium glutamicum CgmR in complex with inducers and with an operator[J].Journal of Molecular Biology, 2010, 403(2):174-184.
[50] KIND S, WITTMANN C.Bio-based production of the platform chemical 1, 5-diaminopentane[J].Applied Microbiology and Biotechnology, 2011, 91(5):1 287-1 296.
[51] NGUYEN A Q D, SCHNEIDER J, WENDISCH V F.Elimination of polyamine N-acetylation and regulatory engineering improved putrescine production by Corynebacterium glutamicum[J].Journal of Biotechnology, 2015, 201:75-85.
[52] ZHAO Z, DING J Y, MA W H, et al.Identification and characterization of γ-aminobutyric acid uptake system GabPCg (NCgl0464) in Corynebacterium glutamicum[J].Applied and Environmental Microbiology, 2012, 78(8):2 596-2 601.
[53] KRUMBACH K, SONNTAG C K, EGGELING L, et al.CRISPR/Cas12a mediated genome editing to introduce amino acid substitutions into the mechanosensitive channel MscCG of Corynebacterium glutamicum[J].ACS Synthetic Biology, 2019, 8(12):2 726-2 734.
[54] NAKAMURA J, HIRANO S, ITO H, et al.Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce L-glutamic acid production[J].Applied and Environmental Microbiology, 2007, 73(14):4 491-4 498.
[55] WANG Y, CAO G Q, XU D Y, et al.A novel Corynebacterium glutamicum L-glutamate exporter[J].Applied and Environmental Microbiology, 2018, 84(6):e02691-e02617.
[56] CHEN M L, CHEN X L, WAN F, et al.Effect of tween 40 and DtsR1 on L-arginine overproduction in Corynebacterium crenatum[J].Microbial Cell Factories, 2015, 14(1):119.
[57] LV Q L, HU M K, TIAN L Z, et al.Enhancing L-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy[J].Bioresource Technology, 2021, 341:125799.
[58] PETER H, WEIL B, BURKOVSKI A, et al.Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes:Identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP[J].Journal of Bacteriology, 1998, 180(22):6 005-6 012.
[59] LIU J, LIU M S, SHI T, et al.CRISPR-assisted rational flux-tuning and arrayed CRISPRi screening of an L-proline exporter for L-proline hyperproduction[J].Nature Communications, 2022, 13:891.
[60] PETER H, BADER A, BURKOVSKI A, et al.Isolation of the putP gene of Corynebacterium glutamicum and characterization of a low-affinity uptake system for compatible solutes[J].Archives of Microbiology, 1997, 168(2):143-151.
[61] HUANG M Z, ZHAO Y, LI R, et al.Improvement of L-arginine production by in silico genome-scale metabolic network model guided genetic engineering[J].3 Biotech, 2020, 10(3):126.
[62] ZHANG B, REN L Q, YU M, et al.Enhanced L-ornithine production by systematic manipulation of L-ornithine metabolism in engineered Corynebacterium glutamicum S9114[J].Bioresource Technology, 2018, 250:60-68.
[63] SIMIC P, SAHM H, EGGELING L.L-threonine export:Use of peptides to identify a new translocator from Corynebacterium glutamicum[J].Journal of Bacteriology, 2001, 183(18):5 317-5 324.
[64] SIMIC P, WILLUHN J, SAHM H, et al.Identification of glyA (encoding serine hydroxymethyltransferase) and its use together with the exporter ThrE to increase L-threonine accumulation by Corynebacterium glutamicum[J].Applied and Environmental Microbiology, 2002, 68(7):3 321-3 327.
[65] DIESVELD R, TIETZE N, FÜRST O, et al.Activity of exporters of Escherichia coli in Corynebacterium glutamicum, and their use to increase L-threonine production[J].Journal of Molecular Microbiology and Biotechnology, 2009, 16(3-4):198-207.
[66] 张晓梅, 高宇洁, 杨玲, 等.谷氨酸棒杆菌中氨基酸分泌转运蛋白及其代谢改造研究进展[J].生物工程学报, 2020, 36(11):2 250-2 259.
ZHANG X M, GAO Y J, YANG L, et al.Amino acid exporters and metabolic modification of Corynebacterium glutamicum:A review[J].Chinese Journal of Biotechnology, 2020, 36(11):2 250-2 259.
[67] 陈紫薇. 谷氨酸棒杆菌中L-丝氨酸转运蛋白的研究[D].无锡:江南大学, 2018.
CHEN Z W.Investigation of L-serine exporter in Corynebacterium glutamicum[D].Wuxi:Jiangnan University, 2018.
[68] ZHANG X M, GAO Y J, CHEN Z W, et al.High-yield production of L-serine through a novel identified exporter combined with synthetic pathway in Corynebacterium glutamicum[J].Microbial Cell Factories, 2020, 19(1):115.
[69] KENNERKNECHT N, SAHM H, YEN M R, et al.Export of L-isoleucine from Corynebacterium glutamicum:A two-gene-encoded member of a new translocator family[J].Journal of Bacteriology, 2002, 184(14):3 947-3 956.
[70] TRÖTSCHEL C, DEUTENBERG D, BATHE B, et al.Characterization of methionine export in Corynebacterium glutamicum[J].Journal of Bacteriology, 2005, 187(11):3 786-3 794.
[71] QIN T Y, HU X Q, HU J Y, et al.Metabolic engineering of Corynebacterium glutamicum strain ATCC 13032 to produce L-methionine[J].Biotechnology and Applied Biochemistry, 2015, 62(4):563-573.
[72] YIN L, SHI F, HU X, et al.Increasing L-isoleucine production in Corynebacterium glutamicum by overexpressing global regulator Lrp and two-component export system BrnFE[J].Journal of Applied Microbiology, 2013, 114(5):1 369-1 377.
[73] CHEN C, LI Y Y, HU J Y, et al.Metabolic engineering of Corynebacterium glutamicum ATCC 13869 for L[J].Metabolic Engineering, 2015, 29:66-75.
[74] 陈诚, 李颜颜, 尹良鸿, 等.全局调控因子Lrp的表达强化谷氨酸棒状杆菌发酵生产L-缬氨酸[J].食品与生物技术学报, 2016, 35(9):920-927.
CHEN C, LI Y Y, YIN L H, et al.Expression of global regulator lrp facilitates L-valine production in Corynebacterium glutamicum[J].Journal of Food Science and Biotechnology, 2016, 35(9):920-927.
[75] 张海灵, 李颜颜, 王小元.代谢工程改造谷氨酸棒状杆菌合成及分泌途径生产L-缬氨酸[J].生物工程学报, 2018, 34(10):1 606-1 619.
ZHANG H L, LI Y Y, WANG X Y.Metabolic engineering of L-valine synthesis and secretory pathways in Corynebacterium glutamicum for higher production[J].Chinese Journal of Biotechnology, 2018, 34(10):1 606-1 619.
[76] TAUCH A, HERMANN T, BURKOVSKI A, et al.Isoleucine uptake in Corynebacterium glutamicum ATCC 13032 is directed by the brnQ gene product[J].Archives of Microbiology, 1998, 169(4):303-312.
[77] ZHANG Y C, LIU Y D, ZHANG S Y, et al.Metabolic engineering of Corynebacterium glutamicum WM001 to improve L-isoleucine production[J].Biotechnology and Applied Biochemistry, 2021, 68(3):568-584.
[78] XIE X X, XU L L, SHI J M, et al.Effect of transport proteins on L-isoleucine production with the L-isoleucine-producing strain Corynebacterium glutamicum YILW[J].Journal of Industrial Microbiology &Biotechnology, 2012, 39(10):1 549-1 556.
[79] DONG J J, KAN B J, LIU H, et al.CRISPR-Cpf1-assisted engineering of Corynebacterium glutamicum SNK118 for enhanced L-ornithine production by NADP-dependent glyceraldehyde-3-phosphate dehydrogenase and NADH-dependent glutamate dehydrogenase[J].Applied Biochemistry and Biotechnology, 2020, 191(3):955-967.
[80] ZHAO Z, DING J Y, LI T, et al.The ncgl1108 (PheP (Cg)) gene encodes a new L-Phe transporter in Corynebacterium glutamicum[J].Applied Microbiology and Biotechnology, 2011, 90(6):2 005-2 013.
[81] WEHRMANN A, MORAKKABATI S, KR MER R, et al.Functional analysis of sequences adjacent to dapE of Corynebacterium glutamicum reveals the presence of aroP, which encodes the aromatic amino acid transporter[J].Journal of Bacteriology, 1995, 177(20):5 991-5 993.
MER R, et al.Functional analysis of sequences adjacent to dapE of Corynebacterium glutamicum reveals the presence of aroP, which encodes the aromatic amino acid transporter[J].Journal of Bacteriology, 1995, 177(20):5 991-5 993.
[82] SHANG X L, ZHANG Y, ZHANG G Q, et al.Characterization and molecular mechanism of AroP as an aromatic amino acid and histidine transporter in Corynebacterium glutamicum[J].Journal of Bacteriology, 2013, 195(23):5 334-5 342.