哺乳动物的奶中含有蛋白质、脂肪、乳糖、生物活性肽和微量元素等多种营养物质,人类驯化与利用乳用家畜具有悠久的历史,丰富了大众的食物图谱。不同物种奶的营养物质含量存在差异[1]。相比于牛奶,羊奶因其丰富的营养元素和细腻的口感逐渐引起人们的关注。绵羊奶的蛋白质、脂肪和乳糖等营养成分的单位含量均高于山羊奶[2-3]。但绵羊的产奶量相对于山羊较低,一个泌乳周期山羊产奶量在500~900 kg,而绵羊的产奶量则多为300~500 kg[4-5]。乳中除富含蛋白质、脂肪、乳糖等大分子营养成分外,还富含多种小分子代谢物,起到调控乳腺细胞生长、乳蛋白乳脂合成、抗菌抗病毒等多种功效。然而,乳畜泌乳性状调控机制复杂,造成了乳畜选育和饲养条件优化的困难。
代谢物是生命活动代谢过程产生或消耗的小分子物质,具有调控细胞生长、蛋白合成等多种生物学功能,也可作为家畜产奶、产肉性状和人类疾病等一些生物学过程的标志物。代谢物组(metabolome)是指一个生物样品中的全部小分子代谢物,而代谢组学(metabolomics)是使用先进的分析化学技术来测定细胞、组织和生物液体中的小分子代谢物,可全面检测生物样本中的小分子代谢物,从而进行一些生物学现象机制解析[6]。为探究不同物种乳间的代谢物差异,ZHAO等[7]研究了人与反刍动物乳中脂类的差异,寻找到了不同物种之间的标记物;SHI等[8]比较研究了不同品种水牛乳中的代谢变化,发现了相关生物标记物;WANG等[9]对人、牛与山羊乳汁进行了代谢组分析,找到了不同物种之间的差异代谢物,确定了部分生物标记物。一系列研究为泌乳机制的解析和不同物种甚至不同品种乳的鉴别提供了帮助。
代谢物表达随着生物学过程的变化而变化,是机体内在与外在环境共同作用的结果。为消除外在环境的影响,本研究在同一地点以相同的饲养条件喂养绵羊和山羊,在泌乳中期分别采集绵羊乳与山羊乳进行乳成分和非靶向代谢组比较分析,为两个物种的泌乳性状差异机制解析提供帮助,并为奶羊的选育和饲养提供参考。
1 材料和方法
1.1 动物和样品准备
本研究所选用的实验动物是关中山羊和戴寒杂交F1代(戴瑞奶绵羊♂×小尾寒羊♀)绵羊。分别选择了6只分娩后第90天的绵羊和山羊,均为1~2岁经产母羊。奶样在早晨饲喂前进行无菌采集,后置于液氮中,并立即运送到实验室进一步分析。
1.2 样品采集
样品采集时间均在分娩后第90天早上饲喂前。在泌乳第90天早上饲喂之前开始无菌手工挤乳,挤乳时先弃去前一小部分乳样,再开始采集试验样本。采集后奶样低温带回实验室用于乳成分检测以及代谢组学分析。
1.3 乳成分检测
使用乳成分分析仪(LactoScope FTIR Advanced)进行绵羊奶与山羊奶常规成分检测,检测指标包括脂肪、蛋白质、酪蛋白、乳糖、固形物和非乳脂固形物含量。
1.4 代谢物的分析
液相色谱-质谱联用仪(liquid chromatograph mass spectrometer,LC-MS/MS)分析由诺禾致源公司处理,并进行代谢物注释和鉴定分析。
1.4.1 代谢物的提取
首先,将样品和预冷的甲醇通过涡旋混合。混合后的样品在冰上孵化5 min,然后在15 000 r/min,4 ℃下离心5 min。用LC-MS级别的水将部分上清液稀释到含有60%甲醇的最终浓度。然后将样品转移到一个带有0.22 μm过滤器的新离心管中,在15 000×g、4 ℃下离心10 min。最后,将滤液注入LC-MS/MS系统处理。
1.4.2 LC-MS/MS分析
LC-MS/MS分析使用Vanquish UHPLC系统(ThermoFisher)和Orbitrap Q Exactive系列质谱仪(ThermoFishe)进行。样品被注入Hyperil Gold柱(100×2.1 mm, 1.9 μm),使用16 min的线性梯度,流速为0.2 mL/min。正极性模式的洗脱液为洗脱液A(水中0.1% FA)和洗脱液B(甲醇)。负极性模式的洗脱液为洗脱液A(5 mmol/L乙酸铵,pH 9.0)和洗脱液B(甲醇)。溶剂梯度设置如下。2% B,1.5 min;2%~100% B,12.0 min;100% B,14.0 min;100%~2% B,14.1 min;2% B,16 min。Q Exactive质谱仪在正/负极性模式下运行,喷雾电压3.2 kV,毛细管温度320 ℃,鞘内气体流速35 arb,辅助气体流速10 arb。
1.5 原始数据分析
1.5.1 乳成分数据分析
乳成分试验数据先用Excel 2016进行初步整理分析,利用SPSS 23进行单因素方差分析(ANOVA),以P<0.05为差异显著,结果以平均值±标准差表示。
1.5.2 乳成分数据分析
使用Compound Discoverer 3.1(CD3.1,Thermo Fisher)对LC-MS/MS产生的原始数据文件进行处理。而后将峰与mzCloud(https://www.mzcloud.org/)和ChemSpider(http://www.chemspider.com/)数据库进行匹配。使用统计软件R(R-3.4.3版本)、Python(Python 2.7.6版本)和CentOS(CentOS 6.6版本)进行统计分析。
1.5.3 代谢物数据分析
使用HMDB(人类代谢组数据库,http://www.hmdb.ca/)、KEGG(京都基因与基因组百科全书,http://www.genome.jp/kegg/)和Lipidmaps(脂质代谢途径研究计划,https://lipidmaps.org/)数据库对代谢物进行了注释。利用metaX进行主成分分析(principal component analysis,PCA)和偏最小二乘法判别分析(partial least squares discriminant analysis,PLS-DA),单变量分析(t检验)来计算统计学意义(P值)。根据一般变量权重值(variable important in projection,VIP)>1且P值<0.05,差异倍数(fold change,FC)>2或FC<0.5条件筛选出的代谢物被认为是差异代谢物。对差异代谢物进行代谢途径富集分析,当满足比值x/n>y/N时,认为代谢途径富集,当代谢途径P值<0.05时,认为代谢途径富集有统计学意义。
2 结果与分析
2.1 绵羊乳和山羊乳中乳常规成分差异
绵羊乳与山羊乳成分如表1所示,绵羊乳中脂肪、蛋白质、酪蛋白、乳糖、固形物和非乳脂固形物分别为(6.32±0.12)%、(5.32±0.02)%、(4.24±0.11)%、(5.06±0.09)%、(16.25±0.63)%和(9.93±0.23)%;绵羊乳的基本成分乳脂、蛋白质、酪蛋白、乳糖、固形物和非乳脂固形物均显著高于山羊乳(P<0.05)。
表1 泌乳中期绵羊乳与山羊乳成分对比 单位:%
Table 1 Comparison of sheep milk and goat milk composition in mid-lactation
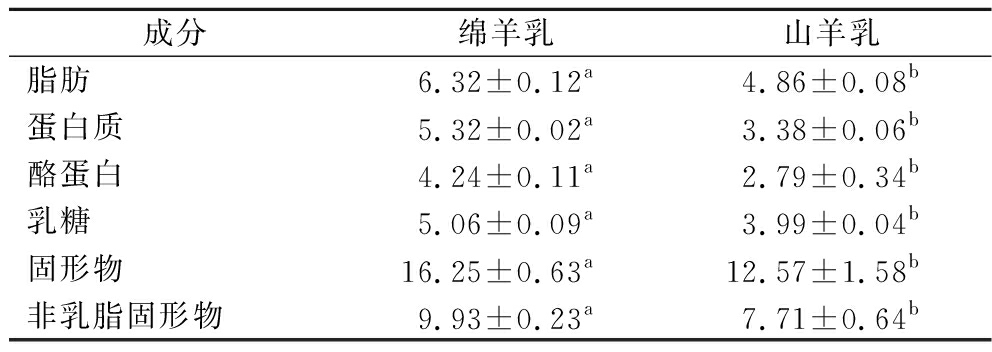
成分绵羊乳山羊乳脂肪6.32±0.12a4.86±0.08b蛋白质 5.32±0.02a3.38±0.06b酪蛋白4.24±0.11a2.79±0.34b乳糖5.06±0.09a3.99±0.04b固形物16.25±0.63a12.57±1.58b非乳脂固形物9.93±0.23a7.71±0.64b
注:同行肩注不同小写字母表示差异显著 (P<0.05), 相同字母表示差异不显著 (P>0.05)。
2.2 代谢物总览
利用LC-MS/MS技术,在绵羊乳和山羊乳中共发现1 513种代谢物,其中正离子模式下发现1 031种,负离子模式下482种[电子版增强出版附表1、附表2所示(https://doi.org/10.13995/j.cnki.11-1802/ts.036324)]。使用HMDB、KEGG和Lipidmaps对其进行注释分析,共有455种被注释,其中正离子模式下299种,负离子模式下156种。注释显示,共有15个类别,最大的类别是脂类和类脂分子(182个代谢物),其次是有机酸及其衍生物(69个代谢物)和有机杂环化合物(52个代谢物)。
表2 绵羊乳前10差异代谢物
Table 2 First 10 differential metabolites of sheep milk
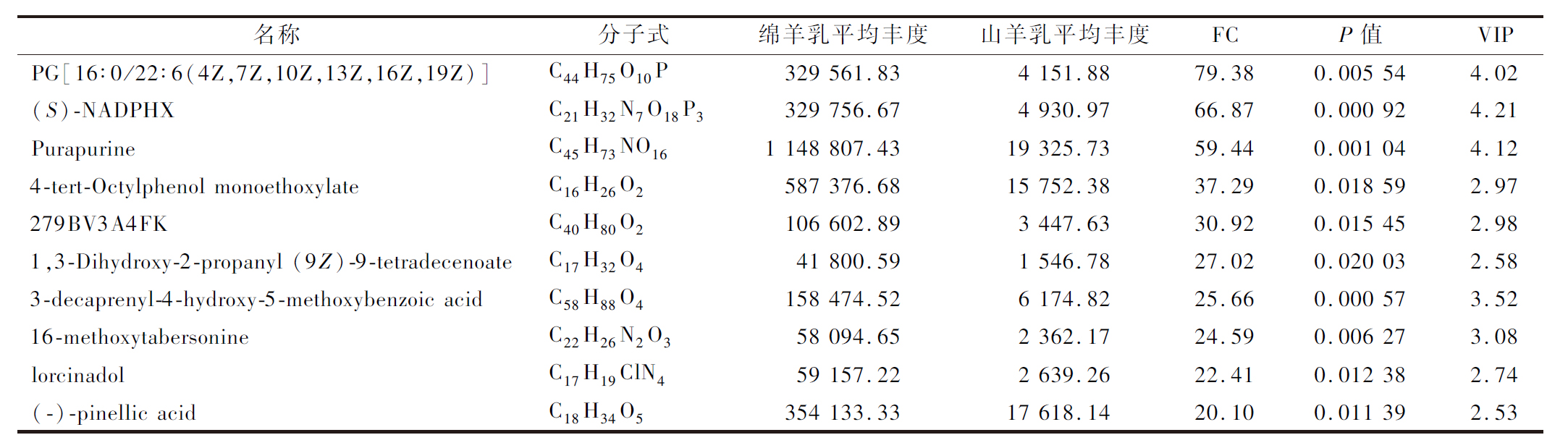
名称分子式绵羊乳平均丰度山羊乳平均丰度FCP值VIPPG[16∶0/22∶6(4Z,7Z,10Z,13Z,16Z,19Z)]C44H75O10P329 561.834 151.8879.380.005 544.02(S)-NADPHXC21H32N7O18P3329 756.674 930.9766.870.000 924.21PurapurineC45H73NO161 148 807.4319 325.7359.440.001 044.124-tert-Octylphenol monoethoxylateC16H26O2587 376.6815 752.3837.290.018 592.97279BV3A4FKC40H80O2106 602.893 447.6330.920.015 452.981,3-Dihydroxy-2-propanyl (9Z)-9-tetradeceno-ateC17H32O441 800.591 546.7827.020.020 032.583-decaprenyl-4-hydroxy-5-methoxybenzoic acidC58H88O4158 474.526 174.8225.660.000 573.5216-methoxytabersonineC22H26N2O358 094.652 362.1724.590.006 273.08lorcinadolC17H19ClN459 157.222 639.2622.410.012 382.74(-)-pinellic acidC18H34O5354 133.3317 618.1420.100.011 392.53
2.3 多元统计分析
本研究应用PCA来确定绵羊乳与山羊乳代谢物之间的样品分离和聚集程度。聚集的点表示观察到的变量高度相似,离散的点代表观察到的变量的显著差异。结果显示,在正离子模式下,PC1、PC2分别为34.60%和21.10%(图2-a);在负离子模式下,PC1、PC2分别为35.30%和23.96%(图2-b)。结果表明,绵羊乳和山羊乳代谢物有明显的分离,具有不同的代谢物特征。
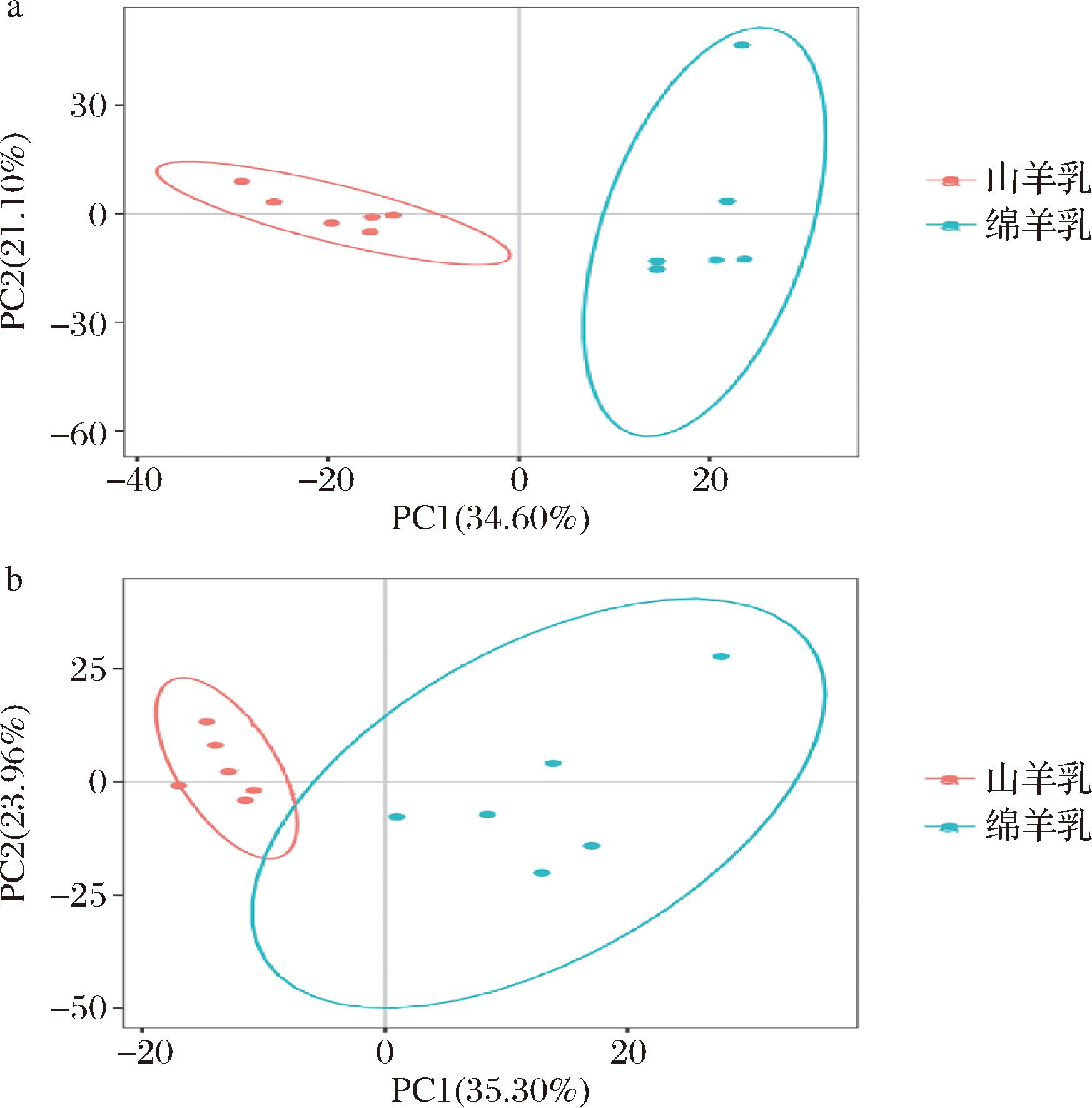
a-正离子模式;b-负离子模式
图1 绵羊乳和山羊乳代谢物PCA得分
Fig.1 PCA scores of milk metabolites in sheep and goats
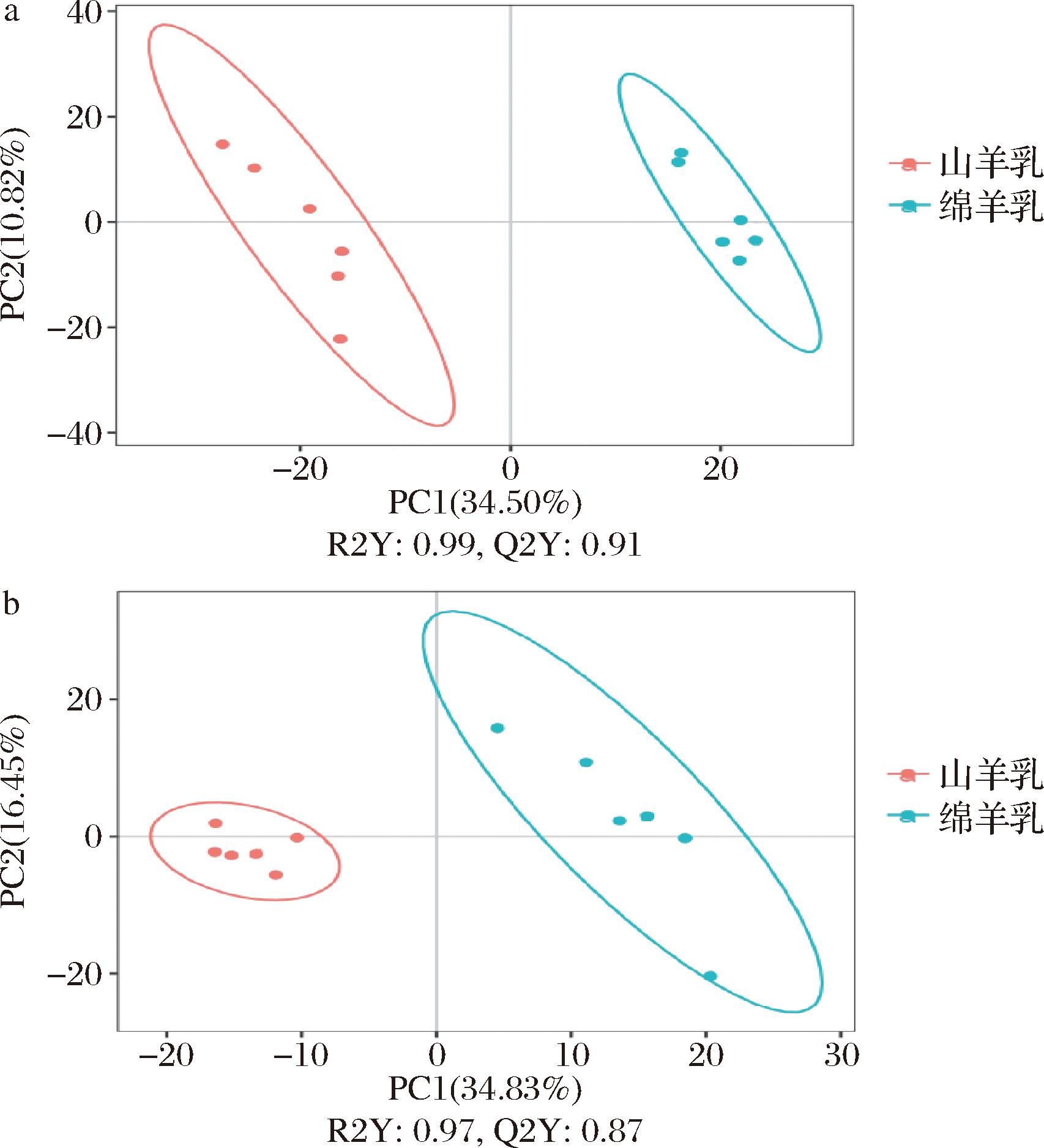
a-正离子模式;b-负离子模式
图2 绵羊乳和山羊乳代谢物PLS-DA得分
Fig.2 PLS-DA score plots of milk metabolites in sheep and goat
研究采用了PLS-DA来确定各组之间的具体差异。在绵羊乳与山羊乳代谢物的比较中,正离子模式下PLS-DA模型的R2为0.99,Q2为0.91(图3-a);负离子模式下PLS-DA模型的R2为0.97,Q2为0.87(图3-b)。这些结果表明,后续分析可信。
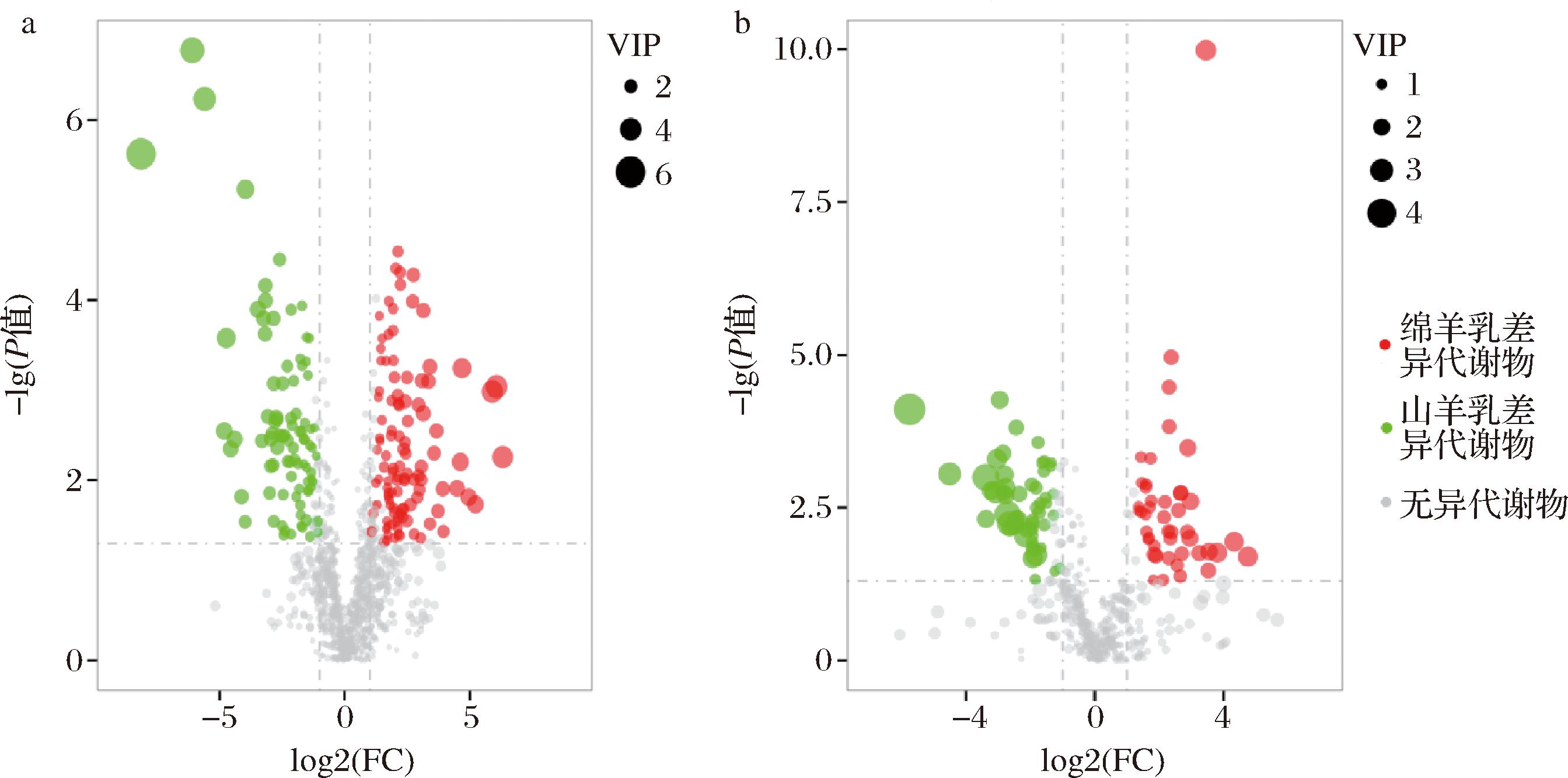
a-正离子模式;b-负离子模式
图3 绵羊乳和山羊乳差异代谢物火山图
Fig.3 Volcano map of milk differential metabolites in sheep and goats
2.4 差异代谢物分析
根据VIP>1;FC>2或FC<0.5和P<0.05筛选,绵羊乳和山羊乳代谢物中有292种为差异代谢物。绵羊乳优势代谢物共153种,其中正离子模式108种,负离子模式45种;山羊乳优势代谢物共139种,其中正离子模式88种,负离子模式51种(图3)。绵羊乳优势代谢物中含量最高的10种代谢物是PG[16∶0/22∶6(4Z,7Z,10Z,13Z,16Z,19Z)]、(S)-NADPHX Purapurine、4-tert-Octylphenol monoethoxylate、279BV3A4FK、1,3-Dihydroxy-2-propanyl (9Z)-9- tetradecenoate、3-decaprenyl-4-hydroxy-5- methoxybenzoic acid、16-methoxytabersonine、lorcinadol与(-)-pinellic acid;山羊乳优势代谢物中含量最高的10种代谢物是APC、Biocytin、Adenosine 3-phosphate 5-phosphosulfate、Fluocinolone、β-Syringin、Astragaloside IV、aloesin、Adenosine diphosphate (ADP)、Myricyl palmitate与4-hydroxy-3-octaprenylbenzoic acid(表2、表3)。
表3 山羊乳前10差异代谢物
Table 3 First 10 differential metabolites of goat milk

名称分子式绵羊乳平均丰度山羊乳平均丰度FCP值VIPAPCC33H38N4O88 615.142 430 960.67282.172.39E-066.10BiocytinC16H28N4O4S9 478.78649 020.5768.471.70E-074.81Adenosine 3-phosphate 5-phosphosulfateC10H15N5O13P2S92 266.524 962 627.3153.797.78E-054.52FluocinoloneC21H26F2O65 691.04276 271.8748.565.88E-074.40β-SyringinC17H24O96 385.48180 485.3728.260.002 842.96Astragaloside IVC41H68O1410 185.96271 393.4126.640.000 263.51aloesinC19H22O93 152.8674 517.3923.630.004 492.72Adenosine diphosphate (ADP)C10H15N5O10P213 974.27318 071.0022.760.000 893.09Myricyl palmitateC46H92O27 748.58163 797.8721.140.003 512.874-hydroxy-3-octaprenylbenzoic acidC47H70O33 593.0862 710.8217.450.015 242.44
2.5 差异代谢物功能富集分析
KEGG途径富集显示,绵羊乳和山羊乳的优势代谢物分别在正负离子模式下富集到32和19条功能通路(表4、表5)。在负离子模式下富集到苯丙氨酸、酪氨酸和色氨酸的生物合成、磷酸盐和次磷酸盐代谢两条通路存在差异。其相应代谢物Biliverdin、L-Pyroglutamic acid、bilirubin等与抗炎和抗氧化有关;Acetyl-CoA、D-Fructose 1-phosphate、D-Sedoheptulose 7-phosphate、Phosphoenolpyruvic acid等与能量代谢相关。
表4 正离子模式下KEGG富集通路
Table 4 KEGG enrichment pathway in positive ion mode
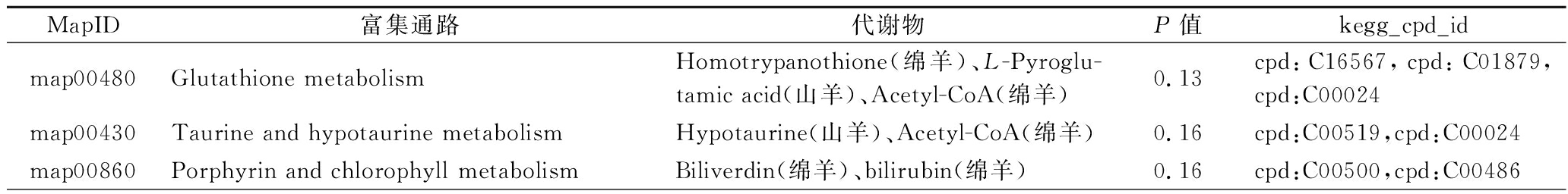
MapID富集通路代谢物P值kegg_cpd_idmap00480Glutathione metabolismHomotrypanothione(绵羊)、L-Pyroglu-tamic acid(山羊)、Acetyl-CoA(绵羊)0.13cpd:C16567,cpd:C01879,cpd:C00024map00430Taurine and hypotaurine metabolismHypotaurine(山羊)、Acetyl-CoA(绵羊)0.16cpd:C00519,cpd:C00024map00860Porphyrin and chlorophyll metabolismBiliverdin(绵羊)、bilirubin(绵羊)0.16cpd:C00500,cpd:C00486
续表4
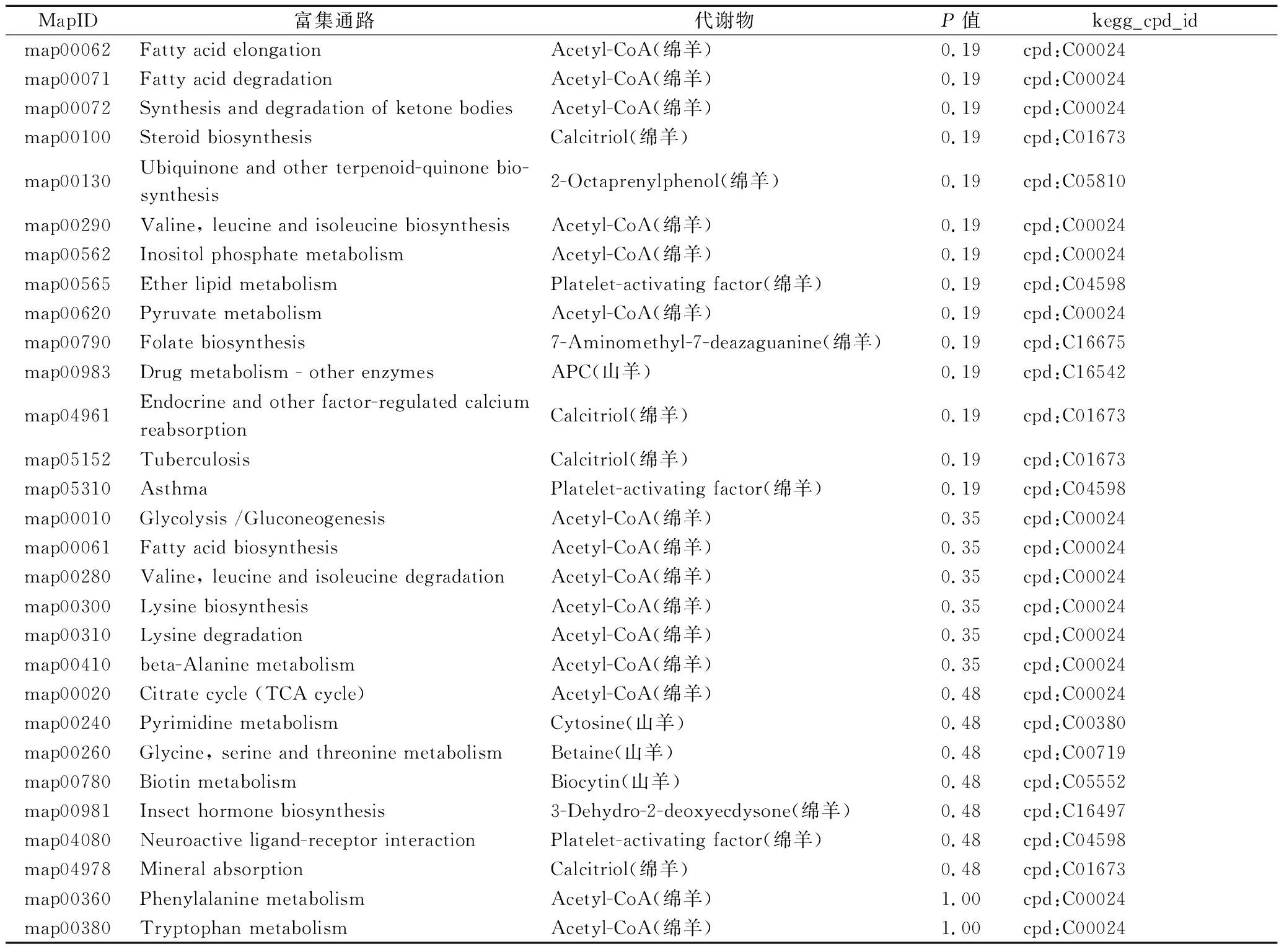
MapID富集通路代谢物P值kegg_cpd_idmap00062Fatty acid elongationAcetyl-CoA(绵羊)0.19cpd:C00024map00071Fatty acid degradationAcetyl-CoA(绵羊)0.19cpd:C00024map00072Synthesis and degradation of ketone bodiesAcetyl-CoA(绵羊)0.19cpd:C00024map00100Steroid biosynthesisCalcitriol(绵羊)0.19cpd:C01673map00130Ubiquinone and other terpenoid-quinone bio-synthesis2-Octaprenylphenol(绵羊)0.19cpd:C05810map00290Valine, leucine and isoleucine biosynthesisAcetyl-CoA(绵羊)0.19cpd:C00024map00562Inositol phosphate metabolismAcetyl-CoA(绵羊)0.19cpd:C00024map00565Ether lipid metabolismPlatelet-activating factor(绵羊)0.19cpd:C04598map00620Pyruvate metabolismAcetyl-CoA(绵羊)0.19cpd:C00024map00790Folate biosynthesis7-Aminomethyl-7-deazaguanine(绵羊)0.19cpd:C16675map00983Drug metabolism - other enzymesAPC(山羊)0.19cpd:C16542map04961Endocrine and other factor-regulated calcium reabsorptionCalcitriol(绵羊)0.19cpd:C01673map05152TuberculosisCalcitriol(绵羊)0.19cpd:C01673map05310AsthmaPlatelet-activating factor(绵羊)0.19cpd:C04598map00010Glycolysis /GluconeogenesisAcetyl-CoA(绵羊)0.35cpd:C00024map00061Fatty acid biosynthesisAcetyl-CoA(绵羊)0.35cpd:C00024map00280Valine, leucine and isoleucine degradationAcetyl-CoA(绵羊)0.35cpd:C00024map00300Lysine biosynthesisAcetyl-CoA(绵羊)0.35cpd:C00024map00310Lysine degradationAcetyl-CoA(绵羊)0.35cpd:C00024map00410beta-Alanine metabolismAcetyl-CoA(绵羊)0.35cpd:C00024map00020Citrate cycle (TCA cycle)Acetyl-CoA(绵羊)0.48cpd:C00024map00240Pyrimidine metabolismCytosine(山羊)0.48cpd:C00380map00260Glycine, serine and threonine metabolismBetaine(山羊)0.48cpd:C00719map00780Biotin metabolismBiocytin(山羊)0.48cpd:C05552map00981Insect hormone biosynthesis3-Dehydro-2-deoxyecdysone(绵羊)0.48cpd:C16497map04080Neuroactive ligand-receptor interactionPlatelet-activating factor(绵羊)0.48cpd:C04598map04978Mineral absorptionCalcitriol(绵羊)0.48cpd:C01673map00360Phenylalanine metabolismAcetyl-CoA(绵羊)1.00cpd:C00024map00380Tryptophan metabolismAcetyl-CoA(绵羊)1.00cpd:C00024
表5 负离子模式下KEGG富集通路
Table 5 KEGG enrichment pathway in negative ion mode

MapID富集通路代谢物P值kegg_cpd_idmap00400Phenylalanine, tyrosine and tryptophan biosynthesisD-Fructose 1-phosphate(山羊)、Phos-phoenolpyruvic acid(山羊)0.03cpd:C01094,cpd:C00074map00440Phosphonate and phosphinate metabolismPhosphoenolpyruvic acid(山羊)、Rhizocticin D(绵羊)0.03cpd:C00074,cpd:C17961map01200Carbon metabolismPhosphoenolpyruvic acid(山羊)、D-Sedohep-tulose 7-phosphate(山羊)0.09cpd:C00074,cpd:C05382map01230Biosynthesis of amino acidsPhosphoenolpyruvic acid(山羊)、D-Sedohep-tulose 7-phosphate(山羊)0.09cpd:C00074,cpd:C05382map00010Glycolysis /GluconeogenesisPhosphoenolpyruvic acid(山羊)0.19cpd:C00074map00030Pentose phosphate pathwayD-Sedoheptulose 7-phosphate(山羊)0.19cpd:C05382map00051Fructose and mannose metabolismD-Fructose 1-phosphate(山羊)0.19cpd:C01094map00562Inositol phosphate metabolismD-myo-Inositol 1,4-bisphosphate(山羊)0.19cpd:C01220map00750Vitamin B6 metabolism4-Hydroxy-L-threonine(山羊)0.19cpd:C06056map04070Phosphatidylinositol signaling systemD-myo-Inositol 1,4-bisphosphate(山羊)0.19cpd:C01220map00020Citrate cycle (TCA cycle)Phosphoenolpyruvic acid(山羊)0.35cpd:C00074map00330Arginine and proline metabolismPhosphocreatine(山羊)0.35cpd:C02305map00620Pyruvate metabolismPhosphoenolpyruvic acid(山羊)0.35cpd:C00074map04979Cholesterol metabolismTaurochenodeoxycholic acid(山羊)0.35cpd:C05465map00120Primary bile acid biosynthesisTaurochenodeoxycholic acid(山羊)0.48cpd:C05465map00240Pyrimidine metabolismdUDP(山羊)0.48cpd:C01346map00520Amino sugar and nucleotide sugar metabo-lismN-Acetylneuraminic acid(山羊)0.48cpd:C00270
续表5
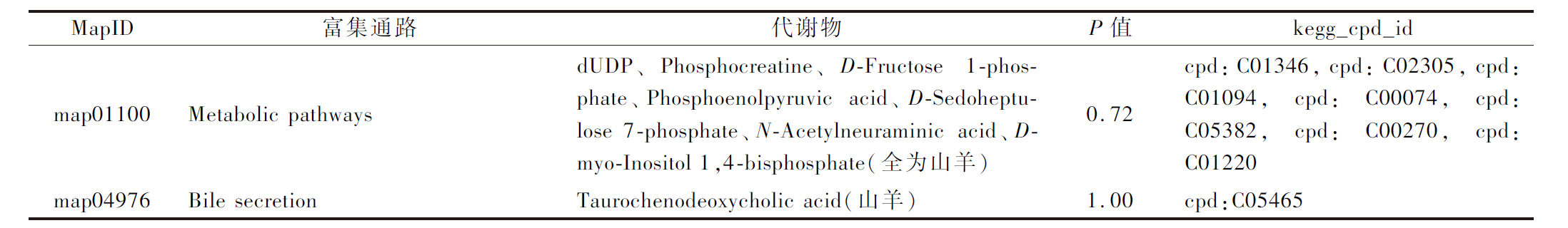
MapID富集通路代谢物P值kegg_cpd_idmap01100Metabolic pathwaysdUDP、Phosphocreatine、D-Fructose 1-phos-phate、Phosphoenolpyruvic acid、D-Sedohep-tulose 7-phosphate、N-Acetylneuraminic acid、D-myo-Inositol 1,4-bisphosphate(全为山羊)0.72cpd:C01346,cpd:C02305,cpd:C01094,cpd:C00074,cpd:C05382,cpd:C00270,cpd:C01220map04976Bile secretionTaurochenodeoxycholic acid(山羊)1.00cpd:C05465
3 讨论
羊奶含有的生物活性物质,有着人体健康。BITTANTE等[10]对绵羊乳和山羊乳成分分析发现,固形物含量分别为17.22%与11.57%,脂肪的含量分别为6.36%与3.07%,蛋白质、酪蛋白、乳糖、灰分等物质含量分别为5.12%、4.01%、4.81%、0.85%与3.28%、2.59%、4.12%、0.79%。本研究结果与其相符,绵羊乳相比山羊乳具有更高单位含量的脂肪、乳糖、蛋白质、酪蛋白、固形物和非乳脂固形物。乳脂作为婴儿发育和生长所需的基本物质,绵羊奶能为婴儿提供更多的乳脂,并且绵羊奶中所提供的脂肪球半径较小,更有助于消化与吸收,促进生长发育[11]。乳蛋白含有人体生长发育所需的氨基酸,是人类摄取蛋白的重要来源之一。生物活性肽的主要来源之一是乳蛋白,其对多种慢性疾病有辅助治疗的作用[12]。同时绵羊奶不易引起乳糖不耐受症,具有更广阔的适用人群,近年来逐渐成为乳制品行业新的增长点[13]。
乳中除富含蛋白质、脂肪、乳糖等大分子营养成分外,还富含多种小分子代谢物,起到调控乳腺细胞生长、乳蛋白乳脂合成、抗菌抗病毒等多种功效。为全面分析乳代谢物的特征,科学家们进行了多项研究。CABONI等[14]通过GC-MS代谢组学鉴定了绵羊奶和山羊奶中38种代谢物;另一项通过GC-MS的研究,从绵羊奶中鉴定出112种代谢物;通过UHPLC-QTOF技术,在牛奶中鉴定了1 686种代谢物[15];通过LC-MS/MS代谢组学分析,ZWIERZCHOWSKI等[16]鉴定出正常奶牛与跛脚奶牛奶中168种差异代谢物;YUAN等[17]利用LC-MS /MS代谢组学鉴定出荷斯坦奶牛与地中海水牛的奶中差异代谢物23种。本研究采用LC-MS/MS鉴定了绵羊乳与山羊乳中的代谢物,共检测到1 513种,是乳代谢组研究的良好补充。
山羊乳腺组织单位面积腺泡数量高于绵羊,而腺泡数量是决定产奶量的关键因素之一。本研究发现,山羊乳中Cytosine、dUDP、Phosphocreatine、Adenosine diphosphate (ADP)、cytidine 5′-monophosphate等优势代谢物均与能量代谢相关,可能是乳腺腺泡数量高的原因之一。同时,aloesin、Astragaloside IV和Platelet-activating factor与血管生成相关,丰富的血管为腺泡供给充足的能量,有助于提高产奶量[18-19]。DL-Glutamine能够促进生长激素的释放,从而调节血流分配,增加乳腺的血流量,间接地促进乳腺发育。同时,生长激素能够协同多种激素促进乳腺的发育以及导管和腺泡的形成,间接的影响产奶量的高低[20]。Oxogestone phenpropionate、Nandrolone cyclotate、Velloquercetin和Fluocinolone等多种类固醇皮质类物质能够促进乳腺的发育。BOMFIM[21]指出皮质醇在体内能够促进PRLR和GHR基因表达增加,上皮细胞的反应性可能提高。并且观察到皮质醇对泌乳中期山羊的产奶量起到正相关作用,能够在不改变乳腺上皮细胞增殖和凋亡率的情况下提高产奶量。皮质醇还能提高瓜氨酸的合成,瓜氨酸进一步转化的产物能够提高奶牛的产奶量[22]。
乳成分形成方面,在绵羊乳中关于氨基酸和脂质相关的优势代谢物较多,这可能与高乳脂与乳蛋白相关。7-Aminomethyl-7-deazaguanine、(6R)-L-erythro-6,7-dihydrobiopterin等参与叶酸的生物合成。研究表明叶酸不仅能够提高机体免疫力,还能提高乳品质(如乳脂、乳蛋白等)[23]。Canthaxanthin能够参与胡萝卜素的合成。胡萝卜素能够降低牛乳中的体细胞数和提高产奶量[24-25],并且胡萝卜素的提高对乳脂、乳蛋白和干物质有积极的影响,对乳糖无影响[26]。磷脂不仅是生物膜的重要组成部分,更是组成甘油三酯的材料。甘油三酯经过分解在乳腺中进一步合成乳脂[27]。PG[16∶0/22∶6(4Z,7Z,10Z,13Z,16Z,19Z)]、TG(18∶2(9Z,12Z)/20∶1(11Z)/20∶1(11Z))[iso3]、Lysophosphatidylinositol和L-serine phosphoethanolamine等磷脂类物质的存在,也可能是绵羊奶中高乳脂存在的原因。
在羊乳中含有多种与抗肿瘤、抗炎、抗菌、抗氧化以及免疫调节等功能相关的代谢物,与FLIS[28]的研究相符。在绵羊乳中,Osthol、Phenylisocyanate、Desethylamiodarone、Calcitriol、Glycyrrhizin、Anisomycin、Sulbutiamine、streptonigrin等优势代谢物在抗肿瘤(癌)、调控细胞周期与凋亡等方面具有一定的作用[29-35]。Osthol通过抑制癌细胞生长和诱导细胞凋亡来发挥显著的抗癌特性,在卵巢癌、宫颈癌、结肠癌和前列腺癌等均有应用[29]。Desethylamiodarone阻止细胞周期的G0 /G1期,并通过反向调节Bcl-2和Bax水平以及减少Akt和ERK1/2激活来诱导半胱天冬酶介导的细胞凋亡[31]。在山羊乳中,Succinobucol、Cucurbitacin D、APC、Avasimibe、Pristimerin、Betulin、Scoparone、Ixabepilone、Borrelidin等优势代谢物表现出抗肿瘤(癌)以及细胞凋亡等相关作用[36-43];如Succinobucol有效抑制VCAM-1表达和细胞-细胞黏附,有利于抑制乳腺癌的转移[36];Cucurbitacin D通过诱导细胞凋亡和抑制NF-κB和STAT3的组成性活化而具有抗癌作用[37]。可能在泌乳过程中乳腺细胞会呈现一些癌细胞的生物学特征,此类物质的存在使得乳腺细胞能维持正常细胞状态,不至于癌化。
绵羊乳中Biliverdin、bilirubin、Canthaxanthin、Sanggenon D、Phloridzin等优势代谢物可能与抗炎、抗氧化以及免疫相关[44-48]。Biliverdin是血红素分解代谢的副产物,具有强大的内源性抗氧化和抗炎特性[44]。bilirubin是一种强大的抗氧化剂,具有与维生素C和维生素E的抗氧化作用类似的生理特性。特别是具有保护磷脂免受过氧化物酶产生的自由基而氧化损伤的主要能力[45]。同时,山羊乳中的Tofacitinib、flumizole、Urolithin B、(+)-Riboflavin、aloesin等优势代谢物主要表现出抗炎、抗氧化以及免疫相关等作用[49-53];Tofacitinib抑制JAK的磷酸化和激活,阻止STAT的磷酸化和激活以及随后的基因转录,减少细胞因子的产生,先天性和适应性免疫反应都被调控[49]。Urolithin相关物质可能是通过增强肝脏和肾脏的抗氧化能力、减弱炎症反应和细胞凋亡,从而改善了衰老小鼠的肝肾功能障碍和组织病理学变化[51]。此类代谢物可能对泌乳期间乳房起保护作用,降低炎症发生率。
除上述作用外,还有一些代谢物可抑制或缓解一些其他疾病。在绵羊乳中stearoylcarnitine、3-[(3-Hydroxyundecanoyl)oxy]-4-(trimethylammonio)butanoate、Hydroxyhexanoycarnitine、Propionylcarnitine、cis-5-Tetradecenoylcarnitine、Hexanoylcarnitine、3-hydroxyoctanoylcarnitine等酰基肉碱相关的优势代谢物种类较多,这些代谢物可抑制或缓解一些疾病,例如代谢紊乱、心血管疾病、糖尿病、抑郁症、神经系统疾病等[54]。山羊乳中的Pivagabine、Avagacestat、gosogliptin、Ulimorelin、Prunin等优势代谢物,可抑制或缓解抑郁症、阿尔茨海默症以及糖尿病等疾病[55]。Caroxazone在研究中表现出抗抑郁的作用[56]。一系列研究表明羊乳具有优秀的抗病特征,可作为病患群体的潜在功能饮品。
4 结论
研究发现绵羊乳中乳脂、乳糖、蛋白质、固形物和非脂肪固形物等物质均显著高于山羊乳;通过LC-MS/MS非靶向代谢组技术对绵羊乳与山羊乳代谢物进行了全面对比分析,共鉴定出1 513种代谢物,其中差异代谢物292种,明确了各自的代谢物特征;对差异代谢物进行生物学功能分析,发现绵羊乳和山羊乳中多种代谢物与乳成分、泌乳量、抗炎和抗氧化等作用相关。本研究为家畜泌乳性状调控机制解析提供了一定帮助,为奶羊的饲养提供了一定参考。
[1] SUN Y X, WANG C N, SUN X M, et al.Proteomic analysis of differentially expressed whey proteins in Guanzhong goat milk and Holstein cow milk by iTRAQ coupled with liquid chromatography-tandem mass spectrometry[J].Journal of Dairy Science, 2020, 103(10):8732-8740.
[2] FERRO M M, TEDESCHI L O, ATZORI A S.The comparison of the lactation and milk yield and composition of selected breeds of sheep and goats[J].Translational Animal Science, 2017, 1(4):498-506.
[3] PIETRZAK-FIE KO R, KAMELSKA-SADOWSKA A M.The comparison of nutritional value of human milk with other mammals’ milk[J].Nutrients, 2020, 12(5):1404.
KO R, KAMELSKA-SADOWSKA A M.The comparison of nutritional value of human milk with other mammals’ milk[J].Nutrients, 2020, 12(5):1404.
[4] 李景芳, 陆东林.我国乳用羊品种及其生产性能[J].新疆畜牧业, 2013(7):4-6.LI J F, LU D L.Chinese dairy sheep breeds and their production performance[J].Xinjiang Journal of Animal Husbandry, 2013(7):4-6.
[5] 宋宇轩, 安小鹏, 张磊, 等.奶绵羊产业概况及中国奶绵羊产业的前景分析[J].中国乳业, 2019(8):16-21.SONG Y X, AN X P, ZHANG L, et al.General situation of dairy sheep industry and prospect analysis of dairy sheep industry in China[J].China Dairy, 2019(8):16-21.
[6] GOLDANSAZ S A, GUO A C, SAJED T, et al.Livestock metabolomics and the livestock metabolome:A systematic review[J].PLoS One, 2017, 12(5):e0177675.
[7] ZHAO L L, ZHANG J X, GE W P, et al.Comparative lipidomics analysis of human and ruminant milk reveals variation in composition and structural characteristics[J].Journal of Agricultural and Food Chemistry, 2022, 70(29):8994-9006.
[8] SHI W, YUAN X, CUI K Q, et al.LC-MS/MS based metabolomics reveal candidate biomarkers and metabolic changes in different buffalo species[J].Animals:an Open Access Journal from MDPI, 2021, 11(2):560.
[9] WANG L N, LI X D, LIU L, et al.Comparative lipidomics analysis of human, bovine and caprine milk by UHPLC-Q-TOF-MS[J].Food Chemistry, 2020, 310:125865.[10] BITTANTE G, AMALFITANO N, BERGAMASCHI M, et al.Composition and aptitude for cheese-making of milk from cows, buffaloes, goats, sheep, dromedary camels, and donkeys[J].Journal of Dairy Science, 2022, 105(3):2132-2152.
[11] MOATSOU G, SAKKAS L.Sheep milk components:Focus on nutritional advantages and biofunctional potential[J].Small Ruminant Research, 2019, 180:86-99.
[12] MOHANTY D P, MOHAPATRA S, MISRA S, et al.Milk derived bioactive peptides and their impact on human health: A review[J].Saudi Journal of Biological Sciences, 2016, 23(5):577-583.
[13] NGUYEN H T H, AFSAR S, DAY L.Differences in the microstructure and rheological properties of low-fat yoghurts from goat, sheep and cow milk[J].Food Research International, 2018, 108:423-429.
[14] CABONI P, MURGIA A, PORCU A, et al.A metabolomics comparison between sheep’s and goat’s milk[J].Food Research International, 2019, 119:869-875.
[15] ROCCHETTI G, GALLO A, NOCETTI M, et al.Milk metabolomics based on ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to discriminate different cows feeding regimens[J].Food Research International, 2020, 134:109279.
[16] ZWIERZCHOWSKI G, ZHANG G S, MANDAL R, et al.Milk metabotyping identifies metabolite alterations in the whole raw milk of dairy cows with lameness[J].Journal of Agricultural and Food Chemistry, 2020, 68(15):4507-4514.
[17] YUAN X, SHI W, JIANG J P, et al.Comparative metabolomics analysis of milk components between Italian Mediterranean buffaloes and Chinese Holstein cows based on LC-MS/MS technology[J].PLoS One, 2022, 17(1):e0262878.
[18] WAHEDI H M, JEONG M, CHAE J K, et al.Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo[J].Phytomedicine, 2017, 28:19-26.
[19] SHENG S, XU J L, LIANG Q Y, et al.Astragaloside IV inhibits bleomycin-induced ferroptosis in human umbilical vein endothelial cells by mediating LPC[J].Oxidative Medicine and Cellular Longevity, 2021, 2021:6241242.
[20] 李真, 李庆章.奶山羊乳腺发育过程中生长激素、胰岛素及其受体的变化规律研究[J].中国农业科学, 2010, 43(8):1730-1737.LI Z, LI Q Z.Development change of growth hormone, insulin and their receptors in mammary gland of dairy goats[J].Scientia Agricultura Sinica, 2010, 43(8):1730-1737.
[21] BOMFIM G F, MERIGHE G K F, DE OLIVEIRA S A, et al.Acute and chronic effects of cortisol on milk yield, the expression of key receptors, and apoptosis of mammary epithelial cells in Saanen goats[J].Journal of Dairy Science, 2022, 105(1):818-830.
[22] AMIN A B, ZHANG L, ZHANG J Y, et al.Metagenomic and metabolomic insights into the mechanism underlying the disparity in milk yield of Holstein cows[J].Frontiers in Microbiology, 2022, 13:844968.
[23] 刘雪琴, 王迪, 米思远, 等.叶酸补饲对隐性乳房炎奶牛免疫力及产奶性状相关基因表达的影响[J].畜牧兽医学报, 2020, 51(11):2731-2742.LIU X Q, WANG D, MI S Y, et al.The effect of folic acid supplementation on the expression of genes related to immunity and milking traits in subclinical mastitis cows[J].Chinese Journal of Animal and Veterinary Sciences, 2020, 51(11):2731-2742.
[24] 袁博, 卢金河, 赵晓静, 等.β-胡萝卜素对奶牛生产性能及血液指标的影响[J].中国饲料, 2022(23):81-86.YUAN B, LU J H, ZHAO X J, et al.Effects of β-carotene on production performance and blood parameter of dairy cows[J].China Feed, 2022(23):81-86.
[25] 马吉锋, 常国新, 王建东, 等.β-胡萝卜素对奶牛泌乳及繁殖性能影响的研究[J].黑龙江畜牧兽医, 2015(1):86-88.MA J F, CHANG G X, WANG J D, et al.Effect of β-carotene on lactation and reproductive performance of dairy cows[J].Heilongjiang Animal Science and Veterinary Medicine, 2015(1):86-88.
[26] 孙胜祥. 不同β-胡萝卜素水平对奶牛产奶性能的影响[J].青海畜牧兽医杂志, 2010, 40(6):9-11.SUN S X.Effect of different level of β-carotene on milking performance of dairy cow[J].Chinese Qinghai Journal of Animal and Veterinary Sciences, 2010, 40(6):9-11.
[27] 赵萌. 中国荷斯坦牛不同泌乳阶段乳腺基因差异表达研究[D].泰安:山东农业大学, 2017.ZHAO M.The internal self-regulatory rules inside the mammary gland of chinese holstein cow during lactation cycle[D].Tai’an:Shandong Agricultural University, 2017.
[28] FLIS Z, MOLIK E.Importance of bioactive substances in sheep’s milk in human health[J].International Journal of Molecular Sciences, 2021, 22(9):4364.
[29] SHOKOOHINIA Y, JAFARI F, MOHAMMADI Z, et al.Potential anticancer properties of osthol:A comprehensive mechanistic review[J].Nutrients, 2018, 10(1):36.
[30] YE B, YANG J L, CHEN L J, et al.Induction of apoptosis by phenylisocyanate derivative of quercetin:Involvement of heat shock protein[J].Anti-Cancer Drugs, 2007, 18(10):1165-1171.
[31] BOGNAR Z, CSEH A M, FEKETE K, et al.Amiodarone’s major metabolite, desethylamiodarone inhibits proliferation of B16-F10 melanoma cells and limits lung metastasis formation in an in vivo experimental model[J].PLoS One, 2020, 15(9):e0239088.
[32] MA J G, MA Z H, LI W, et al.The mechanism of calcitriol in cancer prevention and treatment[J].Current Medicinal Chemistry, 2013, 20(33):4121-4130.
[33] JAIN R, ALI HUSSEIN M, PIERCE S, et al.Oncopreventive and oncotherapeutic potential of licorice triterpenoid compound glycyrrhizin and its derivatives:Molecular insights[J].Pharmacological Research, 2022, 178:106138.
[34] USHIJIMA H, MONZAKI R, ONODERA A.Suppressive effects of anisomycin on the proliferation of B16 mouse melanoma cells in vitro[J].Anticancer Research, 2021, 41(12):6113-6121.
[35] STARLING-SOARES B, CARRERA-BASTOS P, BETTENDORFF L.Role of the synthetic B1 vitamin sulbutiamine on health[J].Journal of Nutrition and Metabolism, 2020, 2020:9349063.
[36] CAO H Q, ZHANG Z W, ZHAO S, et al.Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression[J].Journal of Controlled Release, 2015, 205:162-171.
[37] SIKANDER M, HAFEEZ B B, MALIK S, et al.Cucurbitacin D exhibits potent anti-cancer activity in cervical cancer[J].Scientific Reports, 2016, 6:36594.
[38] PHELPS R A, BROADBENT T J, STAFFORINI D M, et al.New perspectives on APC control of cell fate and proliferation in colorectal cancer[J].Cell Cycle, 2009, 8(16):2549-2556.
[39] LIU J Y, FU W Q, ZHENG X J, et al.Avasimibe exerts anticancer effects on human glioblastoma cells via inducing cell apoptosis and cell cycle arrest[J].Acta Pharmacologica Sinica, 2021, 42(1):97-107.
[40] CHEN R Z, YANG F, ZHANG M, et al.Cellular and molecular mechanisms of pristimerin in cancer therapy:Recent advances[J].Frontiers in Oncology, 2021, 11:671548.
[41] HAN Y H, MUN J G, JEON H D, et al.Betulin inhibits lung metastasis by inducing cell cycle arrest, autophagy, and apoptosis of metastatic colorectal cancer cells[J].Nutrients, 2019, 12(1):66.
[42] LI N, YANG F, LIU D Y, et al.Scoparone inhibits pancreatic cancer through PI3K/Akt signaling pathway[J].World Journal of Gastrointestinal Oncology, 2021, 13(9):1164-1183.
[43] IBRAHIM N K.Ixabepilone:Overview of effectiveness, safety, and tolerability in metastatic breast cancer[J].Frontiers in Oncology, 2021, 11:617874.
[44] SHIELS R G, HEWAGE W, PENNELL E N, et al.Biliverdin and bilirubin sulfonate inhibit monosodium urate induced sterile inflammation in the rat[J].European Journal of Pharmaceutical Sciences, 2020, 155:105546.
[45] JANGI S, OTTERBEIN L, ROBSON S.The molecular basis for the immunomodulatory activities of unconjugated bilirubin[J].The International Journal of Biochemistry &Cell Biology, 2013, 45(12):2843-2851.
[46] ESATBEYOGLU T, RIMBACH G.Canthaxanthin:From molecule to function[J].Molecular Nutrition &Food Research, 2017, 61(6):1600469.
[47] WU Y X, KIM Y J, KWON T H, et al.Anti-inflammatory effects of mulberry (Morus alba L.) root bark and its active compounds[J].Natural Product Research, 2020, 34(12):1786-1790.
[48] BALDISSEROTTO A, MALISARDI G, SCALAMBRA E, et al.Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications[J].Molecules, 2012, 17(11):13275-13289.
[49] L PEZ-SANROM
PEZ-SANROM N A, ESPLUGUES J V, DOM
N A, ESPLUGUES J V, DOM NECH E.Pharmacology and safety of tofacitinib in ulcerative colitis[J].Gastroenterología y Hepatología (English Edition), 2021, 44(1):39-48.
NECH E.Pharmacology and safety of tofacitinib in ulcerative colitis[J].Gastroenterología y Hepatología (English Edition), 2021, 44(1):39-48.
[50] WISEMAN E H, MCILHENNY H M, BETTIS J W.Flumizole, a new nonsteroidal anti-inflammatory agent[J].Journal of Pharmaceutical Sciences, 1975, 64(9):1469-1475.
[51] CHEN P, CHEN F C, LEI J X, et al.Gut microbial metabolite urolithin B attenuates intestinal immunity function in vivo in aging mice and in vitro in HT29 cells by regulating oxidative stress and inflammatory signalling[J].Food &Function, 2021, 12(23):11938-11955.
[52] SUWANNASOM N, KAO I, PRUß A, et al.Riboflavin:The health benefits of a forgotten natural vitamin[J].International Journal of Molecular Sciences, 2020, 21(3):950.
[53] ZAID A N, AL RAMAHI R.Depigmentation and anti-aging treatment by natural molecules[J].Current Pharmaceutical Design, 2019, 25(20):2292-2312.
[54] DAMBROVA M, MAKRECKA-KUKA M, KUKA J, et al.Acylcarnitines:Nomenclature, biomarkers, therapeutic potential, drug targets, and clinical trials[J].Pharmacological Reviews, 2022, 74(3):506-551.
[55] ESPOSITO G, LUPARINI M R.Pivagabine:A novel psychoactive drug[J].Arzneimittel-Forschung, 1997, 47(11A):1306-1309.
[56] MORETTI A, CACCIA C, MARTINI A, et al.Effect of caroxazone, a new antidepressant drug, on monoamine oxidases in healthy volunteers[J].British Journal of Clinical Pharmacology, 1981, 11(5):511-515.