含氯苯酚类化合物 (chlorinated phenols,CPs) 是一类重要的杀菌剂、杀虫剂、除草剂、防腐剂和黏合剂,被广泛运用于农药、医药、染料等领域。常见的CPs有19种,根据氯原子取代数分为一氯苯酚(monochlorophenol,MoCP)、二氯苯酚(dichlorophenol,DiCP)、三氯苯酚 (trichlorophenol,TriCP)、四氯苯酚 (tetrachlorophenol,TeCP) 和五氯苯酚(pentachlorophenol,PCP),因氯原子取代位置不同存在多对同分异构体。CPs对人体具有强生物毒性和遗传毒性,对肝肾功能、生殖系统、血液系统、神经系统等具有极大损伤,摄入过量会引发急性中毒,多种CPs共存时会表现出毒性增强的协同作用[1-4]。在水产养殖中,CPs作为杀菌剂和杀螺剂被大量使用,极易通过养殖水传播并污染水产品。同时,CPs化学性质稳定,难以降解,易发生蓄积污染,可通过食物链进入人体并威胁生命健康。因此,美国环境保护署将2-MoCP、2, 4-DiCP、2, 4, 6-TriCP、PCP列入水环境中129种优先控制污染物黑名单[5]。我国将PCP列为食品安全监管抽检项目,在GB 31650—2019《食品安全国家标准 食品中兽药最大残留限量》中规定动物源性食品禁止使用PCP及其钠盐。尽管我国尚未将除PCP之外的其他18种CPs纳入食品安全监管范围,但鉴于CPs的强毒性和协同毒性,很有必要建立一种操作简便、重现性好并适用于水产品中19种CPs残留的高通量确证分析方法。
针对CPs测定已报道的分析方法有表面增强拉曼光谱法[6]、电化学分析法[7]、免疫分析法[8]、GC[9-10]、液相色谱法 (liquid chromatography, LC)[11-12]、GC-MS[13-16]和液相色谱-串联质谱法 (LC-MS)[17]等。WANG等[13]运用GC-MS建立了测定水中17种CPs的方法;胡苹等[14]运用GC-MS建立了测定皮革制品中19种CPs的方法,检出限为0.006~0.015 mg/kg,其中2, 4-DiCP与2, 5-DiCP未分离;吕明旭等[15]运用GC-MS建立了测定纺织品中19种CPs的方法,定量下限为0.020~0.082 mg/kg,其中2, 4-DiCP与2, 5-DiCP未分离;穆应花等[16]运用十八烷基键合硅胶(C18)净化建立了测定鱼肉中19种CPs的GC-MS方法,回收率为70.6%~115%,其中2, 6-DiCP与3, 5-DiCP未分离。相较于GC-MS需柱前衍生化、操作繁琐,LC-MS前处理简单、选择性强、灵敏度高,已广泛应用于农、兽药残留检测;张书芬等[17]运用十八烷基键合硅胶 (C18) 净化建立了测定鱼肉中19种CPs的超高效液相色谱-飞行时间质谱方法,定量限为16~30 μg/kg,回收率为65.7%~98.2%。目前,针对CPs测定方法的研究主要集中在水、皮革、纺织品等样品类型[3,6-15],对动物源性食品中水产品类型的研究较少或不全,多数方法仅针对单一或少量CPs进行测定[3,6-13]。多壁碳纳米管 (multi-walled carbon nanotubes,MWCNTs) 材料的化学性质稳定、比表面积大、吸附性能优良、易于改性和修饰,近年来在复杂基质样品的前处理技术领域引起广泛关注[18-22]。本研究基于MWCNTs吸附材料,建立了一种操作简便、重现性好、可同时测定水产品中19种CPs残留的多壁碳纳米管净化-液相色谱-串联质谱法,可为水产品中氯酚类化合物残留的安全监管提供技术支撑。
1 材料与方法
1.1 材料与试剂
19种CPs标准品:2-氯苯酚、4-氯苯酚、2, 6-二氯苯酚、2, 3, 6-三氯苯酚、2, 4, 5-三氯苯酚、2, 3, 4, 5-四氯苯酚、2, 3, 4, 6-四氯苯酚、2, 3, 5, 6-四氯苯酚,美国o2si公司;2, 4-二氯苯酚、2, 5-二氯苯酚、3, 4-二氯苯酚、3, 5-二氯苯酚、2, 3, 4-三氯苯酚、2, 3, 5-三氯苯酚、2, 4, 6-三氯苯酚、3, 4, 5-三氯苯酚、五氯酚酸钠,美国Dr.Ehrenstorfer公司;3-氯苯酚,环境保护部标准样品研究所;2, 3-二氯苯酚,中国天津阿尔塔科技有限公司。
多壁碳纳米管 (外径<8 nm、8~15 nm、20~30 nm、30~50 nm、>50 nm,长度0.5~2 μm),中国Aladdin公司;十八烷基键合硅胶 (C18),中国CNW公司;磷酸氢二钠、一水合柠檬酸、无水MgSO4、NaCl,中国广州化学试剂厂;乙腈、甲醇 (色谱纯),中国CNW公司;甲酸、氨水、乙酸铵 (分析纯),中国广州化学试剂厂。
实际样品购自本地市场,空白样品经方法确认。
1.2 仪器与设备
Triple Quad TM 6500+三重四级杆质谱仪,美国AB Sciex公司;UPLC-30A超高效液相色谱仪、UW6200H分析天平,日本Shimadzu公司;Synergy超纯水系统,德国Merck公司;Allegra X-30R离心机,美国Beckman公司;TurboVap® LV氮吹仪,瑞典Biotage公司。
1.3 方法
1.3.1 标准溶液的配制
称取经折算的CPs标准品,甲醇定容,配制100 μg/mL相应单标标准储备液。分别吸取适量体积19种CPs单标标准储备液于10 mL容量瓶,甲醇定容,配制19种CPs混合标准中间液。使用空白基质溶液配制19种CPs混合标准系列工作液。
1.3.2 样品制备
分别均取鲫鱼、鲩鱼、罗氏虾、带子等肌肉部分,充分匀浆,置于-18 ℃冷冻保存,备用。
1.3.3 样品前处理
称取2 g (精确至0.01 g) 试样于50 mL离心管中,加入5 mL磷酸氢二钠-柠檬酸缓冲液,涡旋振荡5 min;加入10 mL乙腈,涡旋振荡5 min,超声提取10 min;加入5 g无水MgSO4和1.5 g NaCl,涡旋振荡2 min,以8 000 r/min离心5 min;吸取上清液5 mL于内含500 mg无水MgSO4、5 mg MWCNTs (外径8~15 nm)、100 mg C18的15 mL离心管中,涡旋振荡2 min,以8 000 r/min离心5 min;吸取上清液4 mL于40 ℃下氮吹至近干,用甲醇-水(1∶1,mL∶mL) 溶液复溶至2 mL,经0.22 μm有机相微孔滤膜过滤,上机测定。
1.3.4 色谱条件
色谱柱为Phenomenex Kinetex® C18柱 (100 mm×3.0 mm,2.6 μm);流速0.3 mL/min;柱温40 ℃;进样量为5.0 μL;流动相为10 mmol/L乙酸铵溶液(A)、甲醇(B);梯度洗脱程序:0.0~1.0 min,40% B;1.0~6.0 min,40%~50% B;6.0~13.0 min,50%~90% B;13.0~16.0 min,90% B;16.0~16.1 min,90%~40% B;16.1~18.0 min,40% B。
1.3.5 质谱条件
离子化模式为电喷雾电离负离子模式 (ESI-);扫描方式为多反应监测模式 (MRM);气帘气 (CUR) 为206.8 kPa;碰撞气(CAD)为62.1 kPa;电喷雾电压(IS)为-4 500 V;离子源温度(TEM)为500 ℃;雾化气 (GS1) 为344.7 kPa;辅助气 (GS2) 为379.2 kPa。
1.3.6 数据处理
使用MultiQuant 3.0.3、WPS Office和Origin 2022进行数据处理。
2 结果与分析
2.1 色谱条件的优化
19种CPs存在多对同分异构体,色谱分离具有一定难度。通过比较甲醇-水、乙腈-水体系,以及水相中添加不同体积分数甲酸(0.05%、0.1%、0.2%、0.3%)、氨水(0.05%、0.1%、0.2%、0.3%)和乙酸铵(5、10、15、20 mmol/L) 时的洗脱效果,考察不同流动相组合方式对19种CPs峰形、灵敏度、保留效果、分离效果等的影响。结果表明,流动相为甲醇-水体系时目标峰的响应强度、保留效果和分离效果均优于乙腈-水体系;加入甲酸或氨水均会抑制目标峰的响应强度,存在多对目标峰重合,分离效果较差;水相中添加一定浓度的乙酸铵可改善19种CPs峰形和分离效果。进一步考察乙酸铵添加量,发现目标峰响应强度会随乙酸铵浓度的提高而降低,但较低浓度的乙酸铵会出现基线漂移现象。因此,最终选择甲醇和10 mmol/L乙酸铵溶液为流动相。
通过比较Phenomenex Kinetex® C18柱、Phenomenex Kinetex® F5柱和Thermo Accucore aQ柱对19种CPs峰形、灵敏度、保留效果、分离效果等的影响。结果发现,F5柱虽为五氟苯基丙基固定相,适用于卤代、共轭、异构体或强极性化合物的分离分析,但验证后发现19种CPs的响应强度普遍很弱,尤其是2-MoCP和2, 6-DiCP,难以满足检测灵敏度要求;在Kinetex® C18柱上,19种CPs峰形尖锐、保留时间适中、响应强度最优,除2, 3, 5-TriCP与2, 4, 5-TriCP分离度小于1.5之外,最大程度地实现了同分异构体之间的分离,获得了更好的灵敏度和色谱分离度 (见图1)。因此,最终选用Phenomenex Kinetex® C18色谱柱。
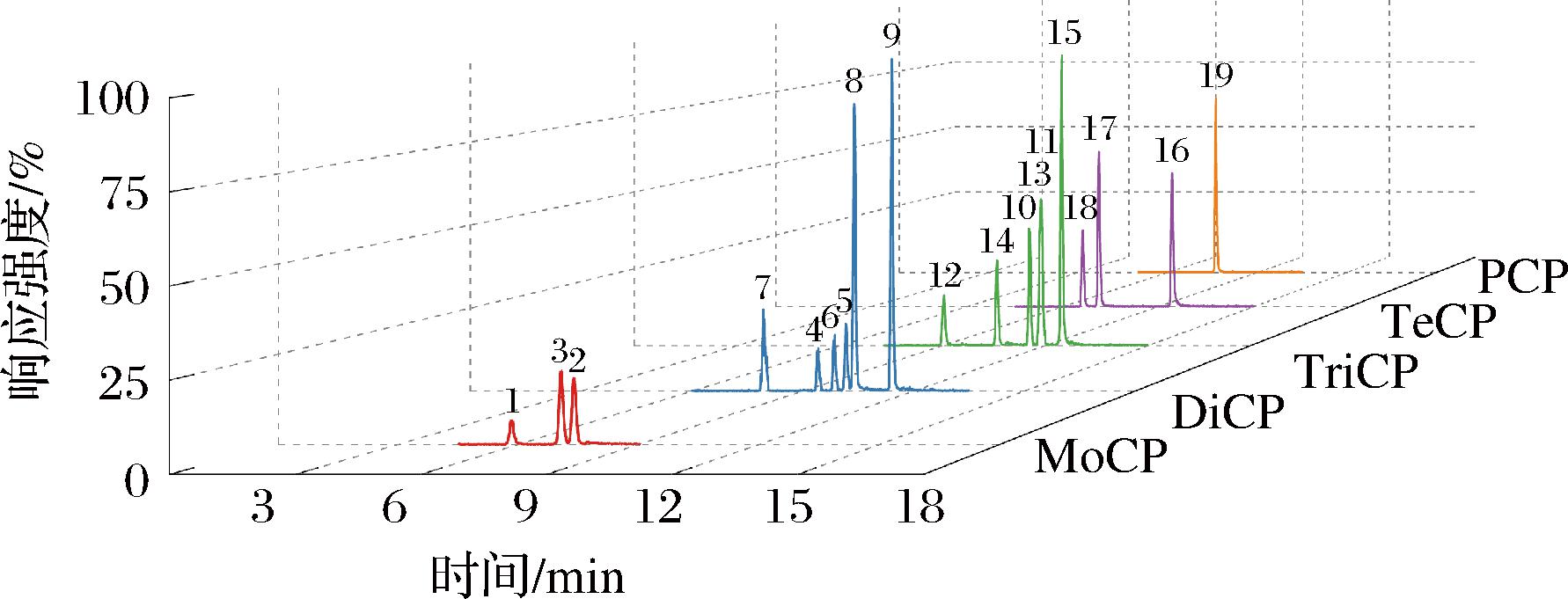
图1 19种CPs色谱图
Fig.1 Chromatogram of 19 CPs
注:2-MoCP为250 μg/L;2, 6-DiCP为125 μg/L;3-MoCP、4-MoCP、2, 3-DiCP、2, 4-DiCP、2, 5-DiCP、2, 3, 6-TriCP为50 μg/L;其余11种CPs为5 μg/L。
2.2 质谱条件的优化
19种CPs均含苯环结构,因苯环共轭双键的键长、键能均等化而具有稳定的化学结构,使其难以碎裂而获得合适特征离子。因此,主要采用氯的同位素35Cl和37Cl来分别组成子离子与母离子相同的不同离子对进行定性、定量分析 (见表1)。
表1 19种CPs保留时间和质谱参数
Table 1 Retention time and mass spectral parameters of 19 CPs

编号化合物缩写CAS号保留时间/min离子对(m/z)去簇电压(DP)/V碰撞能量(CE)/V12-氯苯酚2-MoCP95-57-85.8123-氯苯酚3-MoCP108-43-07.3834-氯苯酚4-MoCP106-48-97.05126.9/126.9*128.9/128.9126.9/35.1-70-10-10-3442, 3-二氯苯酚2, 3-DiCP576-24-99.4152, 4-二氯苯酚2, 4-DiCP120-83-210.1862, 5-二氯苯酚2, 5-DiCP583-78-89.8672, 6-二氯苯酚2, 6-DiCP87-65-07.9683, 4-二氯苯酚3, 4-DiCP95-77-210.4093, 5-二氯苯酚3, 5-DiCP591-35-511.41162.9/162.9*160.9/160.9160.9/124.9-80-10-10-24102, 3, 4-三氯苯酚2, 3, 4-TriCP15950-66-011.54112, 3, 5-三氯苯酚2, 3, 5-TriCP933-78-811.86122, 3, 6-三氯苯酚2, 3, 6-TriCP933-75-59.04132, 4, 5-三氯苯酚2, 4, 5-TriCP95-95-411.92142, 4, 6-三氯苯酚2, 4, 6-TriCP88-06-210.60153, 4, 5-三氯苯酚3, 4, 5-TriCP609-19-812.49194.9/194.9*196.9/196.9194.9/35.1-90-10-10-55162, 3, 4, 5-四氯苯酚2, 3, 4, 5-TeCP4901-51-312.42172, 3, 4, 6-四氯苯酚2, 3, 4, 6-TeCP58-90-210.13182, 3, 5, 6-四氯苯酚2, 3, 5, 6-TeCP935-95-59.62228.9/228.9*230.9/230.9228.9/35.1-95-10-10-5519五氯酚PCP87-86-510.60262.8/262.8*264.8/264.8262.8/35.1-90-8-8-55
注:*为定量离子。
2.3 提取条件的优化
CPs常用提取溶剂有乙腈、甲醇、乙酸乙酯等。乙腈因其可适用的目标物极性范围较宽、组织渗透力较强、对脂类等杂质溶解度较小、蛋白质沉淀作用较强等优势,是目前使用最为广泛的提取溶剂。19种CPs的pKa值为4.75~9.18,多数pKa值在6~8.5,以中等极性为主。实验使用磷酸氢二钠-柠檬酸缓冲液体系,考察不同pH条件下目标物的形态和性质变化,以及对提取效果的影响。结果如图2所示,2, 3, 4, 5-TeCP和PCP对pH值敏感,其他CPs受pH值影响相对不明显;pH值在5~5.8时,两者为分子形态,存在MWCNTs吸附现象,回收率较低;pH值在5.8~7.4时,回收率逐步提高,可能是因为两者逐步由分子形态向离子形态转变,为分子形态和离子形态共存,与MWCNTs表面孔隙吸附形态存在差异而得以改善;pH>7.4之后,回收率呈下降趋势,可能是因为两者逐步完全转化为离子形态,影响了目标物在水相与有机相之间的分配比,抑制了目标物由水相转移至有机相的效率。因此,最终选用缓冲液pH值为7.4。
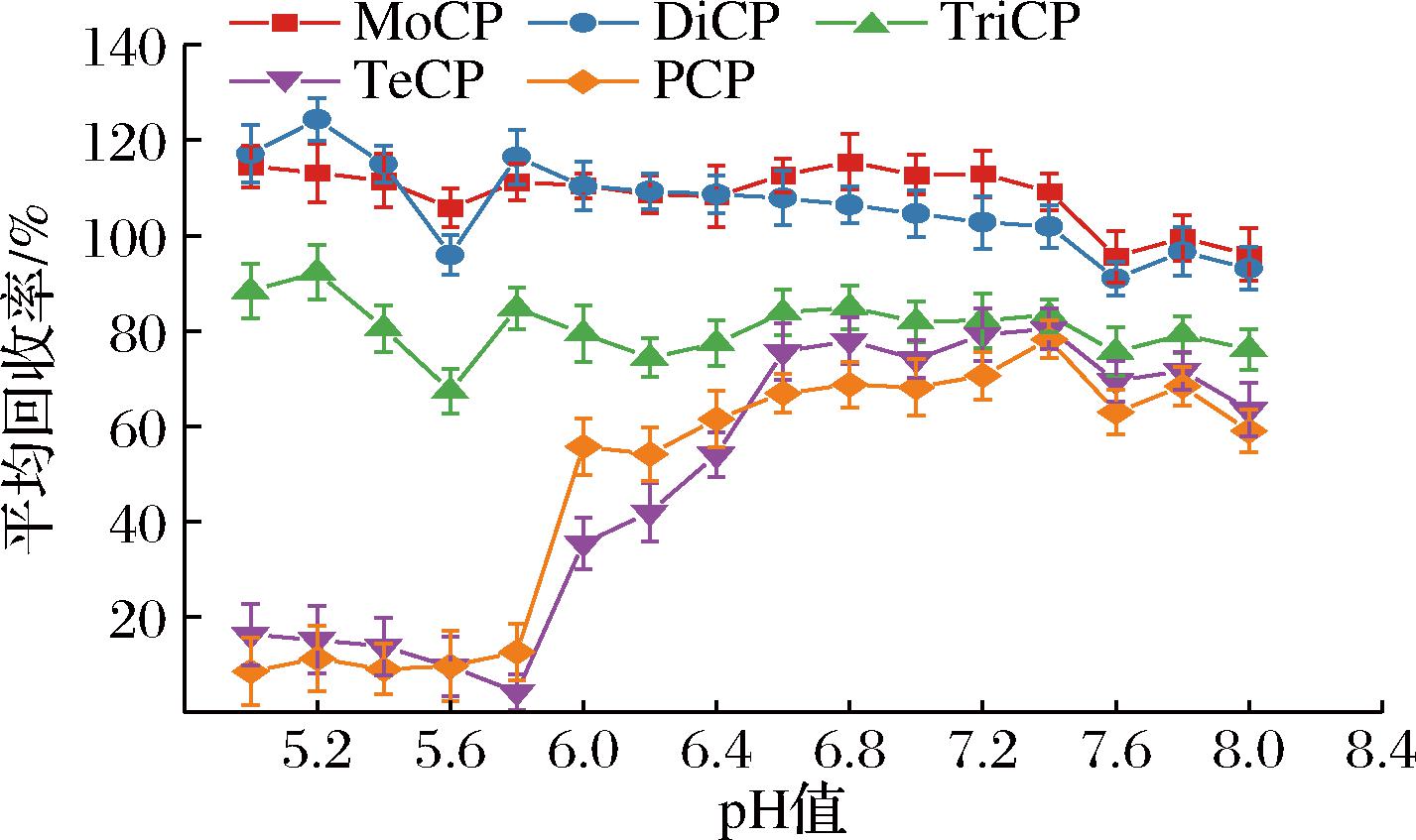
图2 缓冲液pH值对提取效果的影响
Fig.2 Influence of buffer pH on extraction effect
2.4 净化方式的优化
2.4.1 多壁碳纳米管管径的选择
MWCNTs是由碳六元环结构组成的类石墨平面卷曲而成的纳米级中空管。考察相同用量、不同管径下MWCNTs的净化效果,结果如图3,MWCNTs外径为8~15 nm时,平均回收率最佳,19种CPs回收率均在60%~120%;MWCNTs外径≥8 nm时,回收率随外径尺寸增大而逐渐降低,这是因为MWCNTs外径越小,比表面积越大,吸附能力越强[18];MWCNTs外径<8 nm时,回收率开始下降,此时吸附能力过强,出现吸附部分目标物现象,尤其对2, 3, 4, 5-TeCP和PCP的吸附。因此,最终选用外径8~15 nm MWCNTs进行净化。
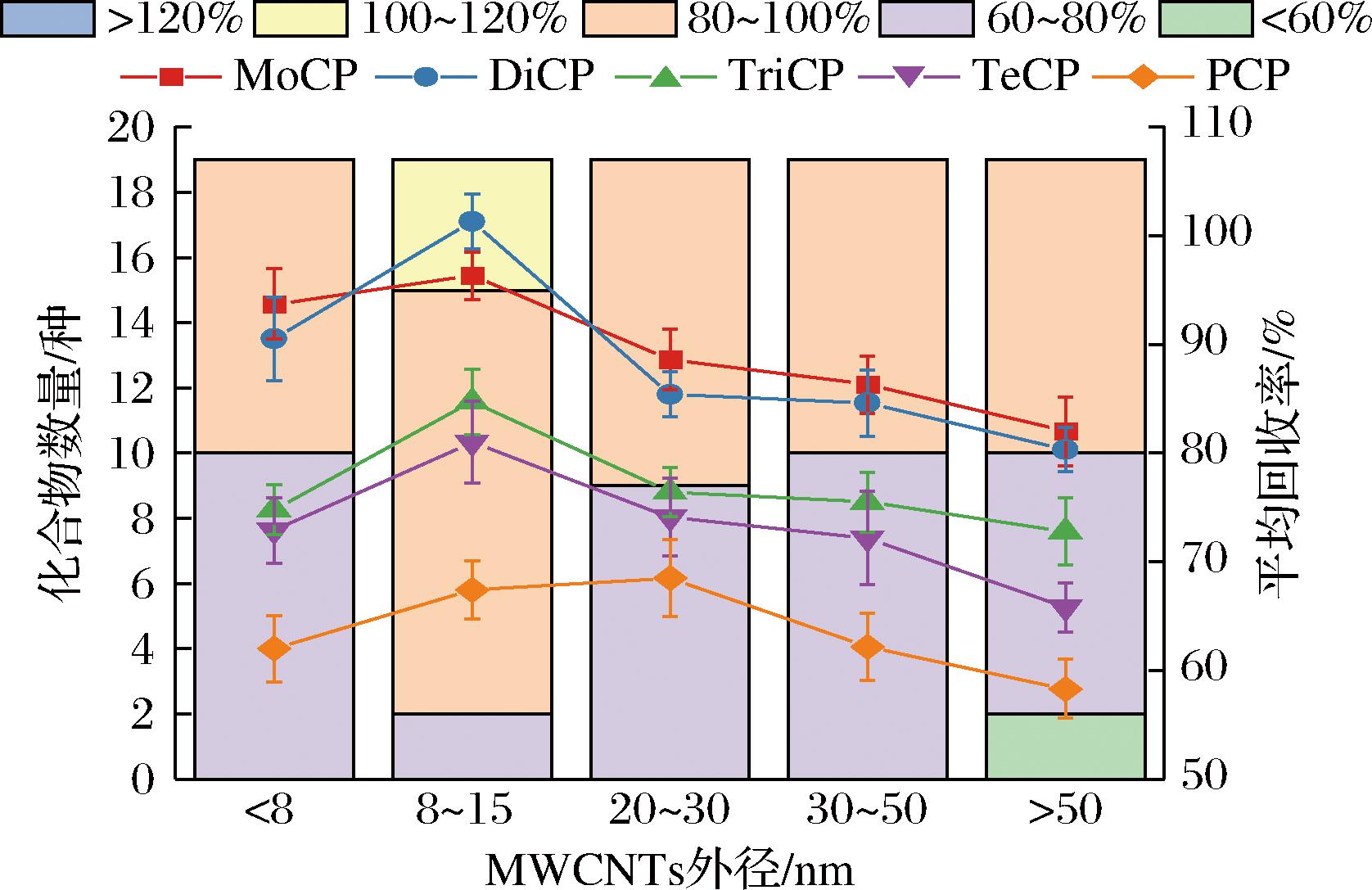
图3 MWCNTs外径对净化效果的影响
Fig.3 Influence of MWCNTs outer diameter on purification effect
2.4.2 多壁碳纳米管用量的选择
MWCNTs可吸附样品基质中色素、有机酸、重金属离子等杂质[19]。考察相同管径、不同用量下MWCNTs的净化效果,结果如图4,MWCNTs用量在3~100 mg时,回收率先升高、后下降,这是因为用量过多的MWCNTs在吸附杂质的同时吸附了部分目标物,甚至在较大用量下2, 3, 4, 5-TeCP和PCP被近乎完全吸附;用量过少的MWCNTs可能存在净化不完全,3 mg用量时出现回收率大于120%。因此,最终选用5 mg MWCNTs进行净化。

图4 MWCNTs用量对净化效果的影响
Fig.4 Influence of MWCNTs dosage on purification effect
2.4.3 多壁碳纳米管吸附时间的选择
采用涡旋振荡方式,考察MWCNTs吸附时间分别为1、2、5、10、15、20、30、60 min时的净化效果。由图5可知,1~2 min时,净化效果随吸附时间延长而提高;2 min之后,回收率呈下降趋势,出现吸附部分目标物现象。因此,最终选用涡旋振荡2 min吸附净化。
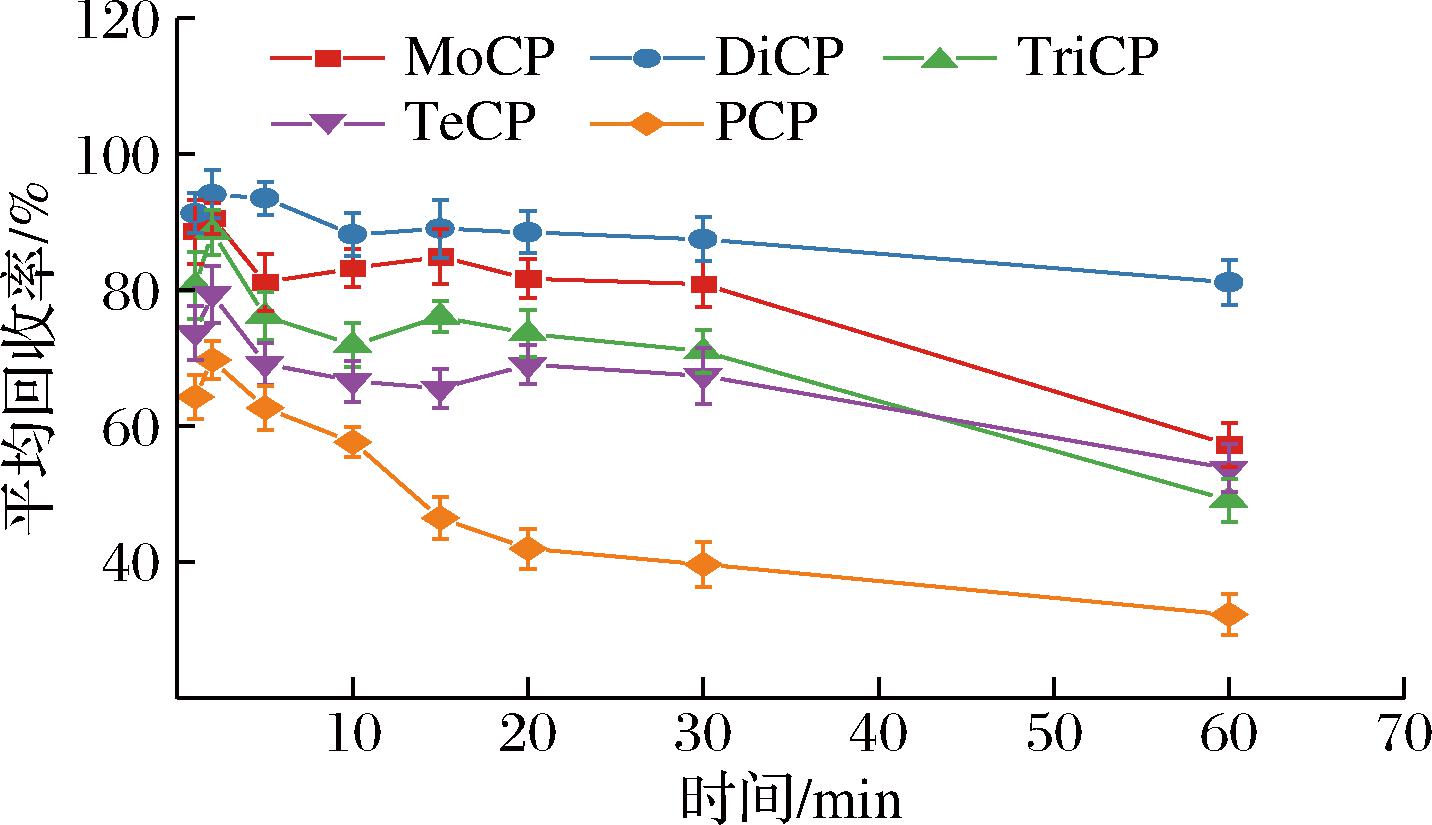
图5 吸附时间对净化效果的影响
Fig.5 Influence of adsorption time on purification effect
2.5 基质效应
因与样品基质带入的共流出物之间存在电离竞争作用,目标物在基质效应的影响下会出现响应强度不同程度的增大或减小现象,这将影响测定结果的准确性。为了评估基质效应的影响程度,可通过计算基质因子 (MF=K1/K2,其中K1为基质匹配标准曲线斜率、K2为溶剂标准曲线斜率) 进行评价:若MF=1,表明无基质效应;若MF<1,表明为基质抑制效应;若MF>1,表明为基质增强效应[23]。一般认为,当0.8
表2 不同水产品中19种CPs基质因子
Table 2 Matrix factors of 19 CPs in different aquatic products
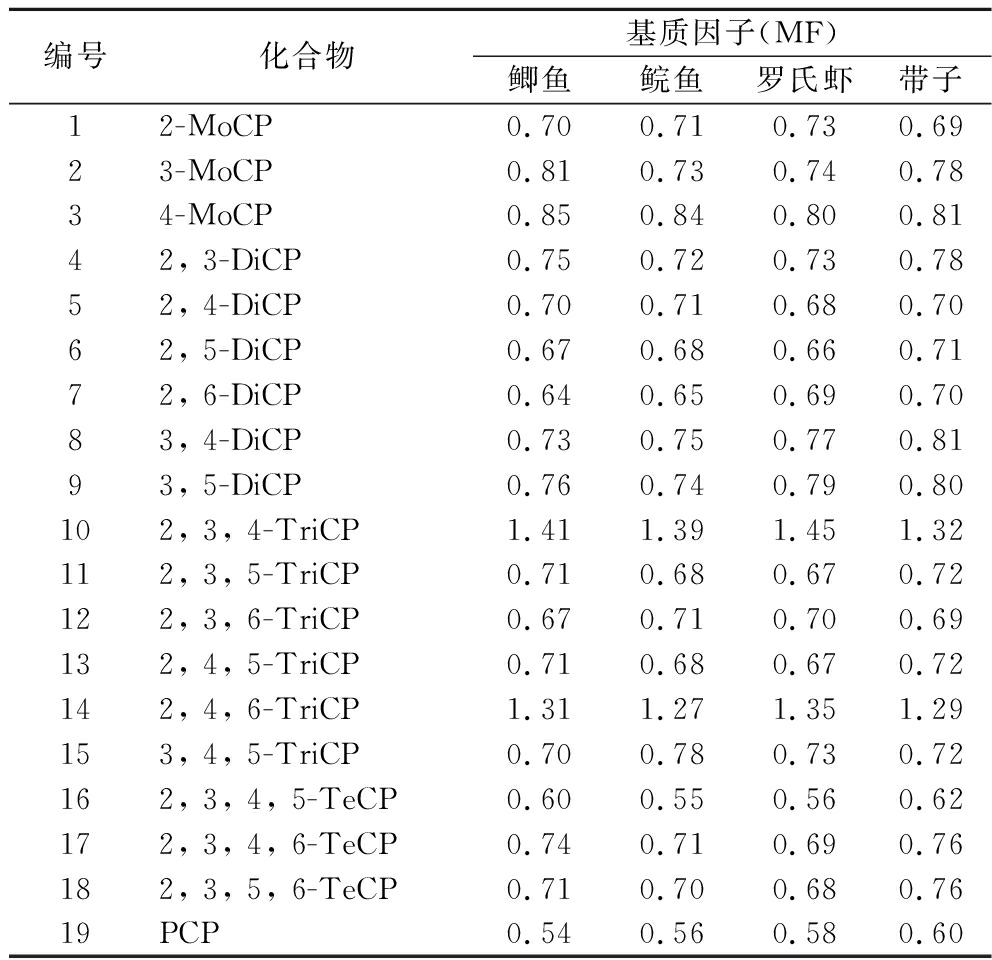
编号化合物基质因子(MF)鲫鱼鲩鱼罗氏虾带子12-MoCP0.700.710.730.6923-MoCP0.810.730.740.7834-MoCP0.850.840.800.8142, 3-DiCP0.750.720.730.7852, 4-DiCP0.700.710.680.7062, 5-DiCP0.670.680.660.7172, 6-DiCP0.640.650.690.7083, 4-DiCP0.730.750.770.8193, 5-DiCP0.760.740.790.80102, 3, 4-TriCP1.411.391.451.32112, 3, 5-TriCP0.710.680.670.72122, 3, 6-TriCP0.670.710.700.69132, 4, 5-TriCP0.710.680.670.72142, 4, 6-TriCP1.311.271.351.29153, 4, 5-TriCP0.700.780.730.72162, 3, 4, 5-TeCP0.600.550.560.62172, 3, 4, 6-TeCP0.740.710.690.76182, 3, 5, 6-TeCP0.710.700.680.7619PCP0.540.560.580.60
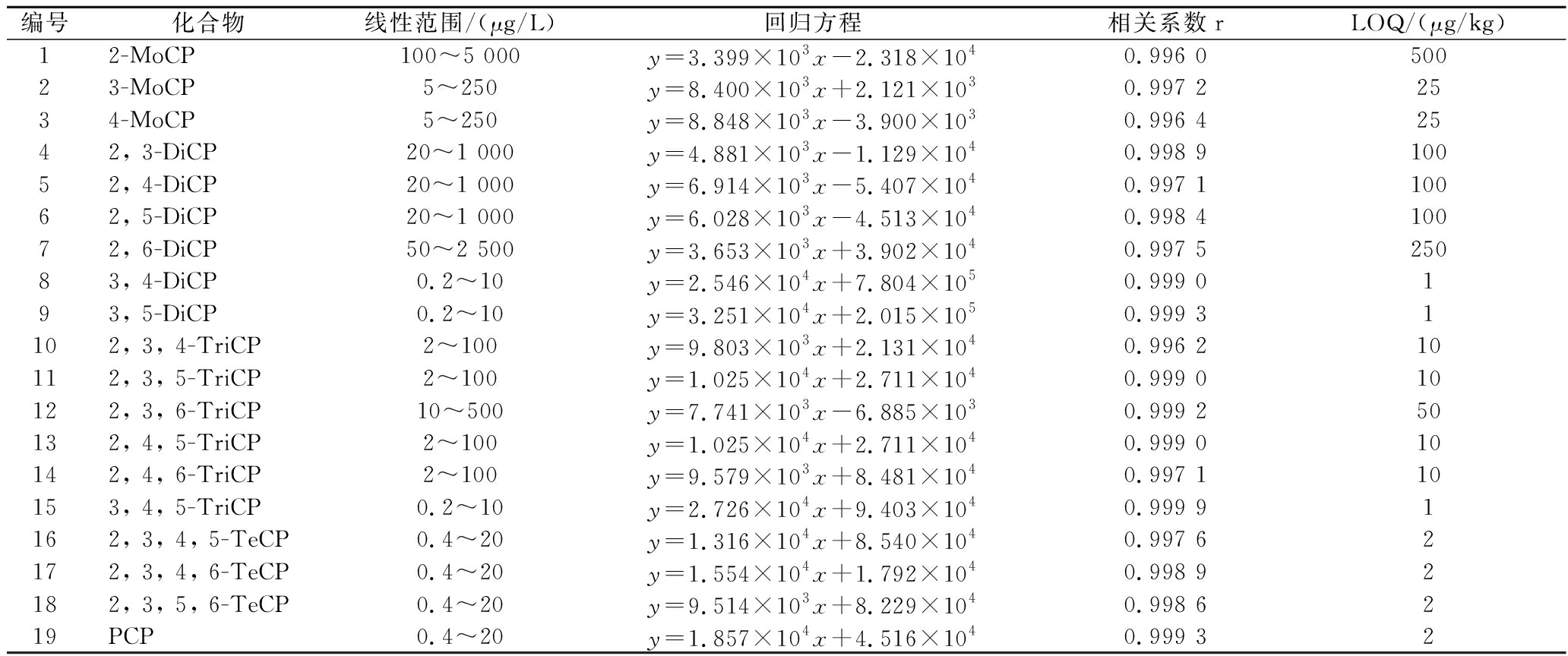
2.6 线性范围与方法定量限
使用空白基质配制浓度为0.2~5 000 μg/L系列标准工作液,以浓度为横坐标、峰面积为纵坐标进行拟合,绘制标准曲线。以10倍信噪比(S/N),计算方法定量限(limit of quantitation, LOQ)。由表3可知,在线性范围内19种CPs均具有良好线性关系,相关系数r≥0.996,方法定量限为1~500 μg/kg。
表3 19种CPs标准曲线回归方程、相关系数和定量限
Table 3 Standard curve regression equations, correlation coefficients and limits of quantification for 19 CPs
编号化合物线性范围/(μg/L)回归方程相关系数rLOQ/(μg/kg)12-MoCP100~5 000y=3.399×103x-2.318×1040.996 050023-MoCP5~250y=8.400×103x+2.121×1030.997 22534-MoCP5~250y=8.848×103x-3.900×1030.996 42542, 3-DiCP20~1 000y=4.881×103x-1.129×1040.998 910052, 4-DiCP20~1 000y=6.914×103x-5.407×1040.997 110062, 5-DiCP20~1 000y=6.028×103x-4.513×1040.998 410072, 6-DiCP50~2 500y=3.653×103x+3.902×1040.997 525083, 4-DiCP0.2~10y=2.546×104x+7.804×1050.999 0193, 5-DiCP0.2~10y=3.251×104x+2.015×1050.999 31102, 3, 4-TriCP2~100y=9.803×103x+2.131×1040.996 210112, 3, 5-TriCP2~100y=1.025×104x+2.711×1040.999 010122, 3, 6-TriCP10~500y=7.741×103x-6.885×1030.999 250132, 4, 5-TriCP2~100y=1.025×104x+2.711×1040.999 010142, 4, 6-TriCP2~100y=9.579×103x+8.481×1040.997 110153, 4, 5-TriCP0.2~10y=2.726×104x+9.403×1040.999 91162, 3, 4, 5-TeCP0.4~20y=1.316×104x+8.540×1040.997 62172, 3, 4, 6-TeCP0.4~20y=1.554×104x+1.792×1040.998 92182, 3, 5, 6-TeCP0.4~20y=9.514×103x+8.229×1040.998 6219PCP0.4~20y=1.857×104x+4.516×1040.999 32
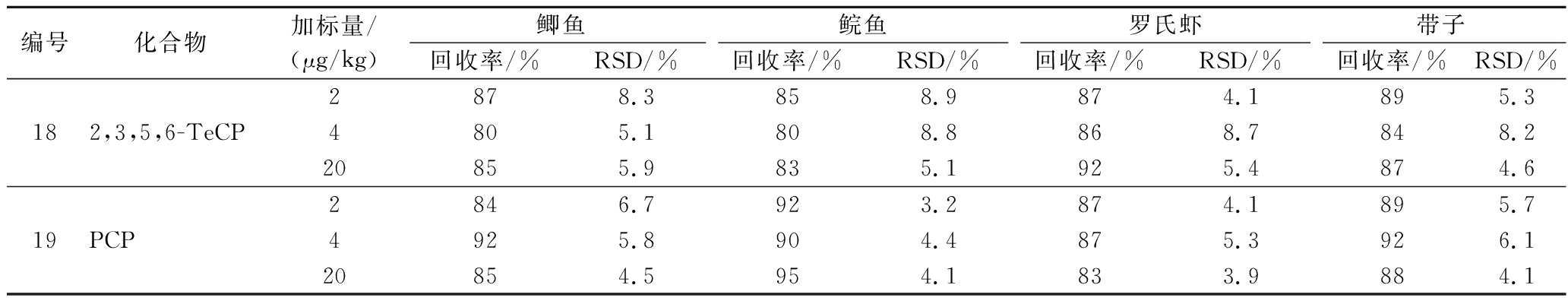
2.7 准确度与精密度
以空白鲫鱼、鲩鱼、罗氏虾、带子为基质,在低、中、高水平 (1、2、10倍定量限) 进行加标回收试验,平行测定6次,计算回收率和相对标准偏差 (relative standard deviation, RSD),考察方法的准确度和精密度。结果见表4,19种CPs平均回收率在80%~96%,RSD在2.4%~8.9%,满足GB/T 27404—2008《实验室质量控制规范 食品理化检测》中准确度和精密度的相关要求。
表4 19种CPs平均回收率和相对标准偏差
Table 4 Average recovery and relative standard deviation of 19 CPs

编号化合物加标量/(μg/kg)鲫鱼鲩鱼罗氏虾带子回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%12-MoCP500834.6812.8845.5833.81 000804.3825.6813.2833.85 000823.6827.5833.2853.323-MoCP25855.5882.5884.5857.350907.4887.6882.9897.2250914.0854.2914.3904.434-MoCP25883.6896.1837.5888.750857.8927.3826.0847.8250836.7888.2822.6927.742, 3-DiCP100853.5867.2855.9865.6200887.3916.9896.3806.11 000844.2876.4843.5834.452, 4-DiCP100905.3896.3947.8883.2200878.0842.4926.1947.21 000895.1863.9897.1884.262, 5-DiCP100826.6885.2805.8815.4200867.0866.2844.7866.51 000876.7826.3843.0805.572, 6-DiCP250803.9814.7817.8823.1500823.6834.9853.3852.82 500807.2838.8833.8832.683, 4-DiCP1872.7905.0885.4875.62883.7957.3873.4883.110884.8958.8907.2844.393, 5-DiCP1857.4816.6847.6836.92815.0813.9838.2815.510873.6864.1812.4834.0102, 3, 4-TriCP10895.9873.4895.7883.620865.8825.0922.5856.1100913.1878.3956.3878.3112, 3, 5-TriCP10953.1878.0943.4895.520883.6878.5885.5873.3100966.2883.5917.9824.9122, 3, 6-TriCP50947.1924.2887.6893.9100908.3923.2945.4905.0500943.8957.0946.0946.6132, 4, 5-TriCP10953.1878.0943.4895.520883.6878.5885.5873.3100966.2883.5917.9824.9142, 4, 6-TriCP10874.6857.7844.1896.720896.2853.6848.7874.0100928.3833.1802.7877.2153, 4, 5-TriCP1896.5847.9905.9863.02847.5853.1932.6872.510853.7914.7874.2884.7162, 3, 4, 5-TeCP2886.7833.2897.7893.14816.2898.3885.7844.920906.9916.7832.7858.4172, 3, 4, 6-TeCP2957.6865.3933.2963.24906.2934.7835.8947.120873.2934.4863.5898.8
续表4
编号化合物加标量/(μg/kg)鲫鱼鲩鱼罗氏虾带子回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%回收率/%RSD/%182,3,5,6-TeCP2878.3858.9874.1895.34805.1808.8868.7848.220855.9835.1925.4874.619PCP2846.7923.2874.1895.74925.8904.4875.3926.120854.5954.1833.9884.1
2.8 实际样品测定
运用上述方法,对市售20份水产品进行19种CPs测定,在1份鲩鱼样品中测出PCP含量2.3 μg/kg,其他水产品中均未检出CPs。
3 结论
本研究基于MWCNTs吸附材料,对提取条件、MWCNTs管径、MWCNTs用量、吸附净化时间等进行了研究和优化,建立了一种操作简便、重现性好、可同时测定水产品中19种含氯苯酚类残留的多壁碳纳米管净化-液相色谱-串联质谱法。经验证,该方法在低、中、高不同加标水平下的准确度和精密度良好,能满足相关技术要求,可适用于水产品中19种含氯苯酚类化合物残留的高通量确证分析。
[1] World Health Organization.IPCS international programme on chemical safety:Health and safety guide N0.19, pentachlorophenol [EB/OL].(2014-05-25) [2024-02-27].http://www.inchem.org/documents/hsg/hsg/hsg019.htm.
[2] CHEN C Y, DU Y, ZHOU Y J, et al.Formation of nitro(so) and chlorinated products and toxicity alteration during the UV/monochloramine treatment of phenol[J].Water Research, 2021, 194:116914.
[3] 林仰锋. 固相萃取-液相色谱法测定水中2, 4-二氯酚、2, 4, 6-三氯酚、五氯酚实验的优化方案[J].城镇供水, 2021(1):69-73.LIN Y F.Optimized experiment for determination of 2, 4-dichlorophenol, 2, 4, 6-trichlorophenol and pentachlorophenol in water by solid phase extraction and liquid chromatography[J].City and Town Water Supply, 2021(1):69-73.
[4] 杨霓云, 刘征涛, 王宏, 等.五氯苯酚与邻氯苯酚和2, 4-二氯苯酚对斑马鱼的联合毒性[J].环境科学研究, 2006, 19(6):145-148.YANG N Y, LIU Z T, WANG H, et al.Joint toxicity effects of PCP, 2-CP and 2, 4-DCP to zebrafish[J].Research of Environmental Sciences, 2006, 19(6):145-148.
[5] United States Environmental Protection Agency.Sediment toxicity identification evaluation (TIE), phases I, II, and III guidance document.[EB/OL].(2007-09) [2024-02-27].https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1003GR1.txt.
[6] 张泸文, 余婉松, 夏苏捷, 等.基于表面增强拉曼光谱的养殖水中五氯酚残留检测[J].食品与机械, 2019, 35(12):82-86;157.ZHANG L W, YU W S, XIA S J, et al.Detection of pentachlorophenol in fishery water using surface-enhanced Raman spectroscopy[J].Food &Machinery, 2019, 35(12):82-86;157.
[7] 梁凤. 功能材料ARPOP-1在电化学分析氯酚类有机污染物中的应用[D].武汉:中南民族大学, 2020.LIANG F.Application of functional material ARPOP-1 in the electrochemical analysis of chlorophenol organic pollutants[D].Wuhan:South-central University for Nationalities, 2020.
[8] 龚蕾, 周陶鸿, 彭青枝, 等.免疫层析法快速检测动物源性食品中五氯酚酸钠含量[J].食品安全质量检测学报, 2022, 13(3):888-893.GONG L, ZHOU T H, PENG Q Z, et al.Rapid determination of sodium pentachlorophenolate in animal-derived foods by immunochromatography[J].Journal of Food Safety &Quality, 2022, 13(3):888-893.
[9] BEN HASSINE S, HAMMAMI B, TOUIL S, et al.Determination of chlorophenols in water samples using solid-phase extraction enrichment procedure and gas chromatography analysis[J].Bulletin of Environmental Contamination and Toxicology, 2015, 95(5):654-660.[10] 曾家源, 彭虹, 刘小珍.固相萃取-气相色谱法测定水中13种酚类化合物[J].广东化工, 2020, 47(2):140-141;146.ZENG J Y, PENG H, LIU X Z.Determinating 13 phenolic compounds in water by solid phase extraction-gas chromatography[J].Guangdong Chemical Industry, 2020, 47(2):140-141;146.
[11] FIROUZY M, HASHEMI P.Ionic liquid-based magnetic needle headspace single-drop microextraction combined with HPLC/UV for the determination of chlorophenols in wastewater[J].Journal of Chromatographic Science, 2023, 61(8):743-749.
[12] LU W H, FU S C, SUN X Z, et al.Magnetic solid-phase extraction using polydopamine-coated magnetic multiwalled carbon nanotube composites coupled with high performance liquid chromatography for the determination of chlorophenols[J].The Analyst, 2021, 146(20):6252-6261.
[13] WANG X W, CHEN R H, LUAN T G, et al.Full automatic determination of chlorophenols in water using solid-phase microextraction/on-fiber derivatization and gas chromatography-mass spectrometry[J].Journal of Separation Science, 2012, 35(8):1017-1026.
[14] 胡苹, 李大刚, 陈崇城, 等.水蒸气蒸馏-气相色谱-质谱法测定皮革制品中19种含氯苯酚[J].理化检验-化学分册, 2020, 56(11):1205-1211.HU P, LI D G, CHEN C C, et al.Determination of nineteen chlorophenols in leather products by water vapor distillation-GC-MS[J].Physical Testing and Chemical Analysis (Part B (Chemical Analysis)), 2020, 56(11):1205-1211.
[15] 吕明旭, 胡苹, 廖耀祖.KOH超声萃取/气相色谱-质谱法同时检测纺织品中19种含氯苯酚[J].东华大学学报(自然科学版), 2022, 48(6):71-76;111.LYU M X, HU P, LIAO Y Z.Simultaneous determination of nineteen chlorophenols in textiles by gas chromatography-mass spectrometry combined with KOH ultrasonic extraction[J].Journal of Donghua University (Natural Science), 2022, 48(6):71-76;111.
[16] 穆应花, 邢家溧, 沈坚, 等.QuEChERS-气相色谱-质谱法检测鱼肉中19种氯酚类化合物[J].色谱, 2022, 40(5):477-487.MU Y H, XING J L, SHEN J, et al.Determination of 19 chlorophenols in fish by QuEChERS-gas chromatography-mass spectrometry[J].Chinese Journal of Chromatography, 2022, 40(5):477-487.
[17] 张书芬, 徐晓蓉, 穆应花, 等.超高效液相色谱-四极杆-飞行时间质谱法同时测定鱼肉样品中19种氯酚类化合物[J].分析化学, 2023, 51(7):1179-1195.ZHANG S F, XU X R, MU Y H, et al.Simultaneous determination of 19 kinds of chlorophenols in fish samples by ultra-performance liquid chromatography-quadrupole-time of flight-mass spectrometry[J].Chinese Journal of Analytical Chemistry, 2023, 51(7):1179-1195.
[18] 崔丽丽, 朴向民, 冯志伟, 等.多壁碳纳米管QuEChERS/气相色谱-质谱联用法快速检测黄芪中16种农药[J].分析测试学报, 2020, 39(8):1034-1039.CUI L L, PIAO X M, FENG Z W, et al.Determination of 16 pesticides in Astragalus membranaceus by multi-walled carbon nanotubes QuEChERS/gas chromatography-mass spectrometry[J].Journal of Instrumental Analysis, 2020, 39(8):1034-1039.
[19] 黄华, 谢文东, 谷雨, 等.多壁碳纳米管分散固相萃取净化超高效液相色谱串联质谱测定果蔬中50种农药残留量[J].食品与发酵工业, 2022, 48(17):282-290.HUANG H, XIE W D, GU Y, et al.Determination of 50 pesticide residues in fruits and vegetables by QuEChERS extraction with multi-walled carbon nanotubes (MWCNTs) coupled to UPLC-MS/MS[J].Food and Fermentation Industries, 2022, 48(17):282-290.
[20] BIZINA E V, EFROSININA A V, FARAFONOVA O V, et al.Nanocomposites based on multi-walled carbon nanotubes, magnetite nanoparticles, and core-shell molecularly imprinted polymers in piezoelectric sensors for the determination of macrolide antibiotics[J].Journal of Analytical Chemistry, 2023, 78(11):1566-1574.
[21] LI J, DONG C, AN W J, et al.Simultaneous enantioselective determination of two new isopropanol-triazole fungicides in plant-origin foods using multiwalled carbon nanotubes in reversed-dispersive solid-phase extraction and ultrahigh-performance liquid chromatography-tandem mass spectrometry[J].Journal of Agricultural and Food Chemistry, 2020, 68(21):5969-5979.
[22] 杨志敏, 常巧英, 李坚, 等.羟基多壁碳纳米管分散固相萃取/气相色谱-串联质谱法测定党参中75种农药残留[J].分析测试学报, 2022, 41(6):827-834.YANG Z M, CHANG Q Y, LI J, et al.Determination of 75 pesticide residues in Codonopsis Radix by gas chromatography-tandem mass spectrometry with dispersive solid phase extraction based on hydroxylated multi-walled carbon nanotubes[J].Journal of Instrumental Analysis, 2022, 41(6):827-834.
[23] 夏宝林, 严秋钫, 杨娜, 等.增强型除脂固相萃取技术结合超高效液相色谱-串联质谱法测定动物源性食品中五氯酚残留量[J].食品安全质量检测学报, 2019, 10(14):4698-4705.XIA B L, YAN Q F, YANG N, et al.Determination of pentachlorophenol residue in animal-origin foods by EMR-Lipid-ultra performance liquid chromatography-tandem mass spectrometry[J].Journal of Food Safety &Quality, 2019, 10(14):4698-4705.