植物乳杆菌(Lactiplantibacillus plantarum)属于革兰氏阳性菌,是乳酸菌家族的主要成员之一,因其主要存在于传统、天然的食物而得到消费者的普遍认可,加上其合成的各类生物活性成分,所以被广泛应用于各类食品的制备中。
多糖广泛存在于植物、动物和微生物等多种生物中,并表现出与结构或来源相关的化学多样性、生理特性和生物活性。其中,胞外多糖(exopolysaccharide,EPS)是由乳酸菌在生长和代谢过程中合成并释放到细胞外的一种特殊的多糖。EPS通常具有分支程度不同、高分子质量、以单糖为单元结构、组成和理化性质各不相同的特性。根据单糖组成的不同,EPS可分为同多糖(homo-expolysaccharide, HoPS)和杂多糖(heteropolysaccharide,HePS),前者由单一的单糖组成,后者则由两种或两种以上单糖构成。
在过去的几十年里,嗜热链球菌[1]等乳酸菌EPS被大量研究、报道甚至应用,与之相比,针对植物乳杆菌EPS的研究还相对较少。本文从生物合成、分离、纯化、产量优化等方面综合论述了植物乳杆菌EPS的研究进展,并对植物乳杆菌EPS的结构、流变学特性、生物活性方面的最新研究进展进行了综述。
1 植物乳杆菌EPS的生物合成途径
EPS的生物合成是一个涉及多种基因和酶的复杂过程,其产物的复杂性与结构直接相关[2]。EPS的合成途径可归结为以下4种:①胞外合成途径(例如蔗糖酶);②ATP结合盒转运体依赖途径;③合成酶依赖途径;④Wzx/wzy依赖通路[3]。其中,胞外合成途径和Wzx/wzy依赖通路被认为是植物乳杆菌生物合成EPS的主要途径[4]。
L.plantarum HoPS的生物合成是一个相对简单的生化过程,该过程包括两个步骤:①在含有特定底物(蔗糖)的环境中利用胞外相关糖苷酶(蔗糖酶)的水解和聚合作用合成HoPS;②将形成的HoPS释放到培养体系中(图1)。
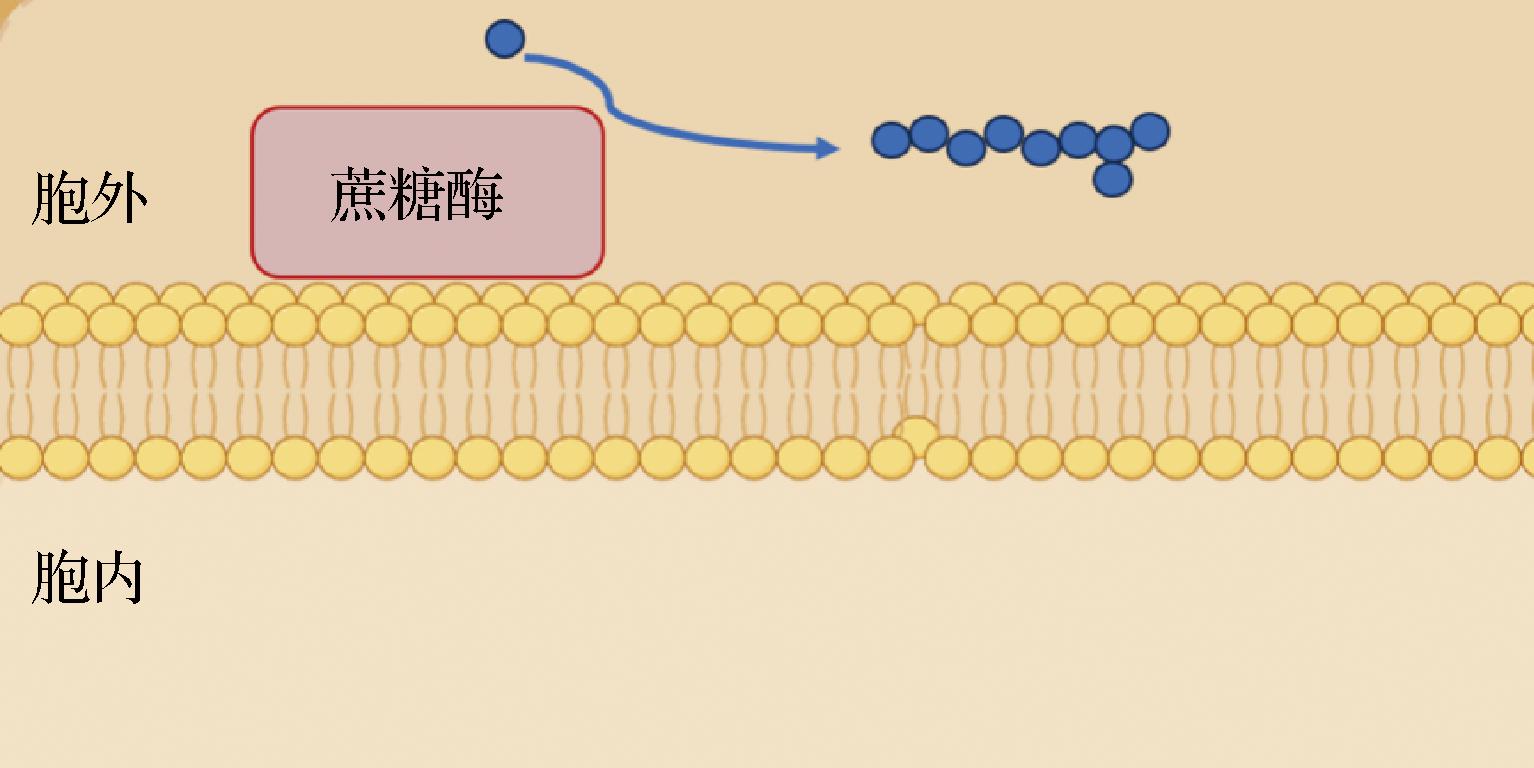
图1 植物乳杆菌HoPS生物合成途径
Fig.1 L.plantarum HoPS biosynthesis pathways
L.plantarum HePS的生物合成主要通过Wzx/wzy依赖通路,该通路包括4个步骤:①单糖和双糖运输和磷酸化(胞外→胞内);②糖核苷酸的形成(胞内);③EPS重复单元的集合和转移(胞内→胞外);④长链的聚合和释放(胞外)[3](图2)。
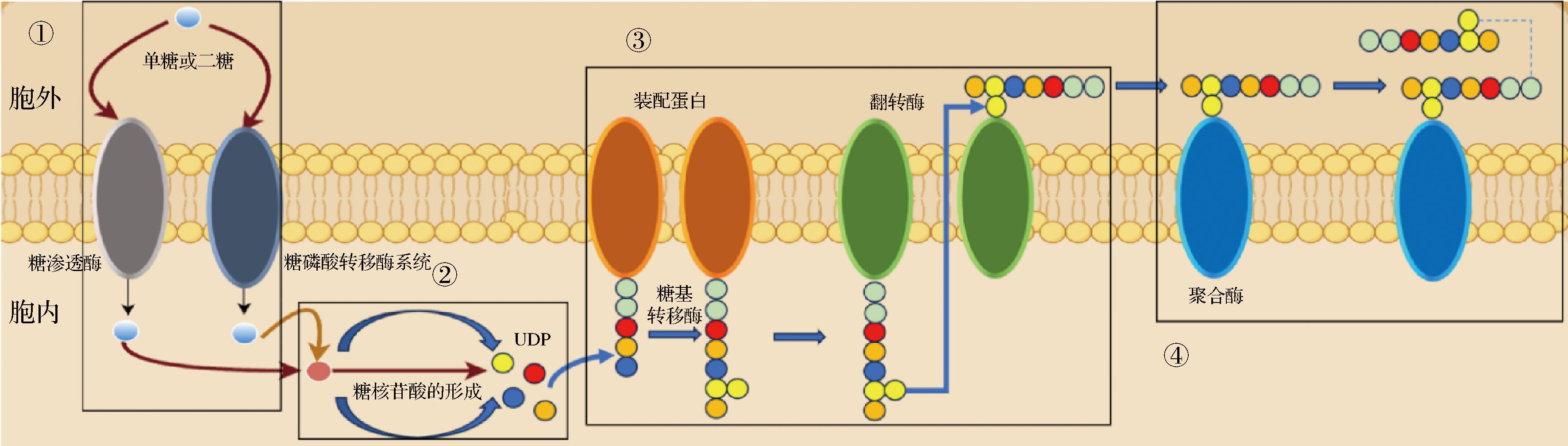
图2 植物乳杆菌HePS生物合成途径
Fig.2 L.plantarum HePS biosynthesis pathways
植物乳杆菌EPS合成基因簇通常位于一个11~22 kb的操纵子中,其涵盖13~23个特定功能模块[3]。根据功能类型的不同,这些模块可分为:调节基因(如epsB、epsC或epsD等)、重复单元合成基因(编码各种糖基转移酶GFT)、EPS组装基因(编码Wzx蛋白,Wzy蛋白和epsA)以及修饰基因(如乙酰化)等[5]。
尽管植物乳杆菌菌株间的遗传信息均不相同,但在EPS合成基因簇方面有的结构类似,有的功能雷同。例如,L.plantarum WCFS1的基因组显上4个与荚膜多糖合成有关的基因簇(cps1、cps2、cps3和cps4)[6-7]。L.plantarum IMC513、C904、LT52和K25编码EPS合成的基因簇均与WCFS1有一定的同源性[8]。L.plantarum ZJ316中鉴定出3个cps基因簇(cps3、cps4和部分cps2簇)与ST-III共享了大部分cps基因簇[9]。
2 植物乳杆菌EPS的产量优化
可能因为HoPS的生物合成相对简单,植物乳杆菌在非优化条件下便可合成大量EPS(2.18 g/L)[10],与之相比,合成过程更为复杂的HePS产率却往往不足1 g/L,因此,研究人员会利用单因素法、响应面法等实验模型来对培养基组成或发酵条件进行优化以提高目标菌株的EPS产率。IMRAN等[11]采用中心组合设计和响应面法对L.plantarum NTMI05和NTMI20发酵条件进行优化,发现在含有葡萄糖(20 g/L)、酵母浸膏(25 g/L)和(NH4)2SO4(2 g/L)的培养基中发酵72 h后,两者EPS最大产量分别为0.956和0.827 g/L,比优化前分别提高了385.28%和342.25%。有学者比较了L.plantarum Q823在30 ℃和37 ℃下的EPS产量后发现,低温(30 ℃)比高温(37 ℃)更适合Q823产糖EPS产量(133.23 mg/L vs 13.18 mg/L) [12],上述结果与MIDIK等[13]的发现一致。还有研究将培养基组成和培养温度均纳入优化的范围,获得了更佳的EPS产量提升(2 064.69 mg/L)[14]。近年来,日益成熟的分子生物学方法成为EPS增产的新途径,尤其是对L.plantarum 中EPS合成蛋白相关基因过表达成为研究的热点[15],具体见表1。
表1 植物乳杆菌EPS产量的工艺优化
Table 1 Production optimization of L.plantarum EPS

菌株优化方法最高产量/(mg/L)参考文献SP8单因素法、响应面法280.015 [16]MF460单因素法515.48[13]KX014响应面法599.52[17]YM-2单因素法、响应面法257.362 [18]LPC-1单因素法、响应面法2 064.69[19]XN1904E单因素法、正交实验235.41[20]NTMI05响应面法956[11]MC5响应面法345.98[21]NCCP962单因素法、响应面法910[14]
3 植物乳杆菌EPS的分离纯化
植物乳杆菌EPS的分离方法如下:首先加热灭活内源性酶、微生物并使大分子蛋白发生变性,随后通过冷冻离心的方式将上述杂质去除,再向上清液中加入一定体积(通常为上清液体积的3倍)的冷乙醇进行沉淀,经离心后,取沉淀溶解于水中,加入三氯乙酸以去除残留的蛋白质,再次离心,取上清液进行透析(截留分子质量14 kDa)以去除三氯乙酸,最后冷冻干燥即得粗EPS(图3)。
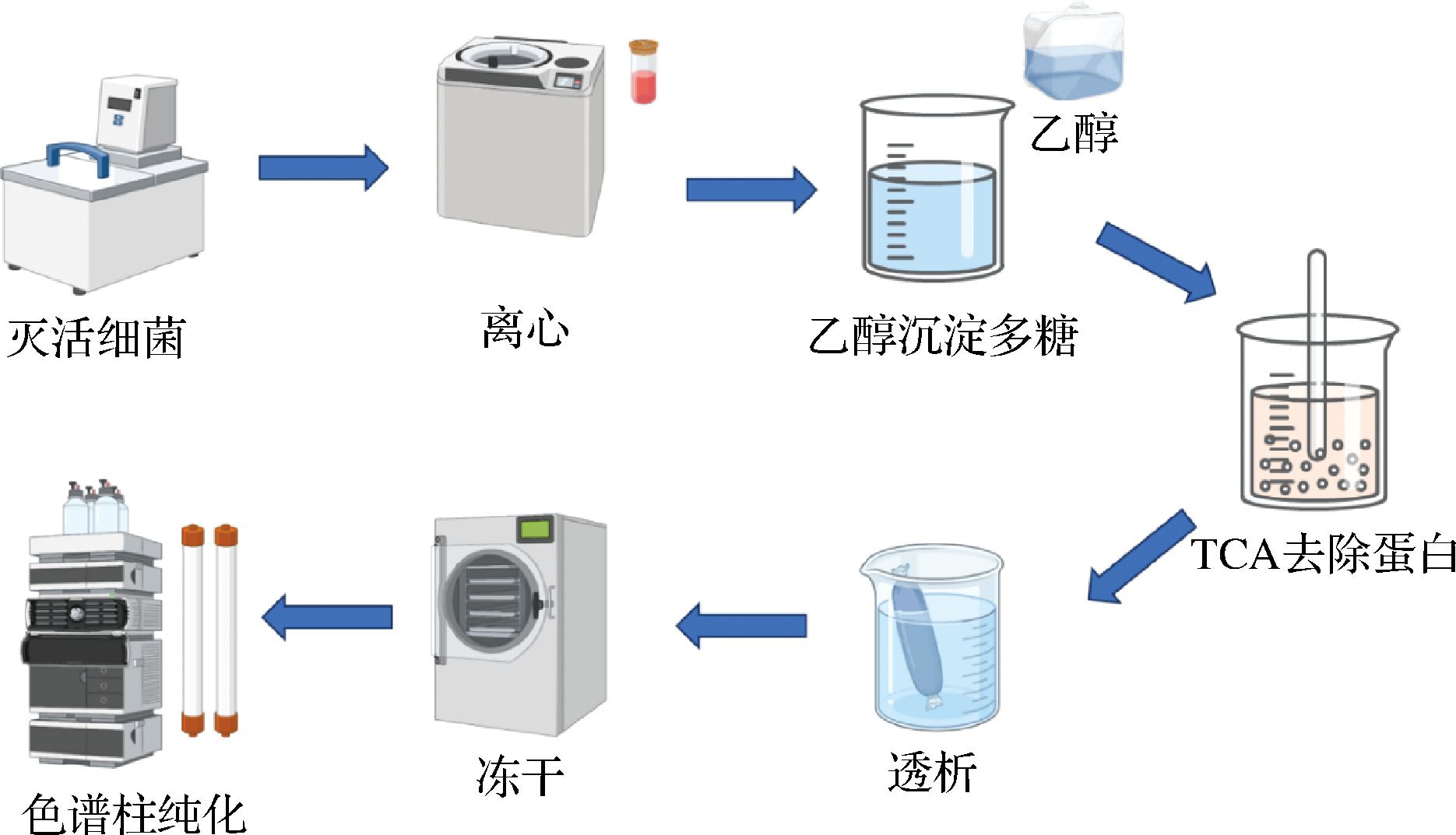
图3 植物乳杆菌EPS的分离方法
Fig.3 Isolation method of L.plantarum EPS
多糖的纯化是以获得单一组分为目的,对上述粗多糖进一步精细化分离的过程,目前本领域常用的EPS纯化方法是柱层析法,其中离子交换层析和凝胶过滤层析最受青睐(表2)。
表2 纯化植物乳杆菌EPS的层析方法
Table 2 Chromatography for the purification of L.plantarum EPS

层析类型常用填料分离原理优缺点分离结果参考文献离子交换层析DEAE-cellulose-52DEAE-Toyopearl 650MDEAE-Sepharose Fast FlowDEAE-Sepharose CL-6BQ Sepharose 离子交换机制价格低、比表面积大、流速慢流速快、耐高压化学稳定性佳、流速快耐高压,工作pH范围窄工作pH范围较宽,分离高效酸性多糖中性多糖碱性多糖[22][23][24][25][26]凝胶过滤层析Sepharose CL-6BSephacryl S系列Sephadex G系列分子筛机制分离范围广、选择性高流速低、不耐高压不同分子质量的多糖[27][28][29]
3.1 离子交换层析
离子交换层析是一种基于离子交换原理的分离纯化技术,通过离子交换剂(填料)上的平衡离子和流动相中的组分离子发生可逆交换时的结合力大小差异,对样品中的离子进行吸附、分离、洗脱等处理,从而获得高纯度的目标物质。当填料为阳离子交换剂时,适用于碱性多糖和中性多糖的分离纯化;而阴离子交换柱则更适用于各种酸性多糖、中性多糖和粘多糖的分离,因自然界中酸性EPS较多而应用较广。根据离子交换的强度不同,上述阳/阴离子交换剂又可进一步分为“强”和“弱”两类[3]。实际应用中,载有二乙基氨基乙基(dicthylaminoethyl,DEAE)基团的弱阴离子交换剂应用较为广泛,如DEAE-Sepharose FastFlow等。
3.2 凝胶过滤层析
凝胶过滤层析又叫排阻/分子筛层析,其原理是利用带孔凝胶将分子质量大小不同的EPS进行分离的技术,以此法分离可获得不同分子质量的EPS。为了避免多糖自身携带电荷发生聚集而影响最终的分离效果,通常在流动相和上样缓冲液中加入一定浓度的离子(如NaCl)来消除离子间的相互作用,Sepharose系列填料是首选填料。
对于组分复杂的粗多糖而言,需要通过上述两种层析法结合使用才能到各组分的完全分离目的。一般会先用离子交换层析对粗多糖中的酸/碱多糖进行分离和收集,而未挂柱直接被洗脱下来的中性多糖部分,需要后续的凝胶过滤层析进一步根据分子质量的差异进行分离和纯化。
4 植物乳杆菌EPS的结构特征
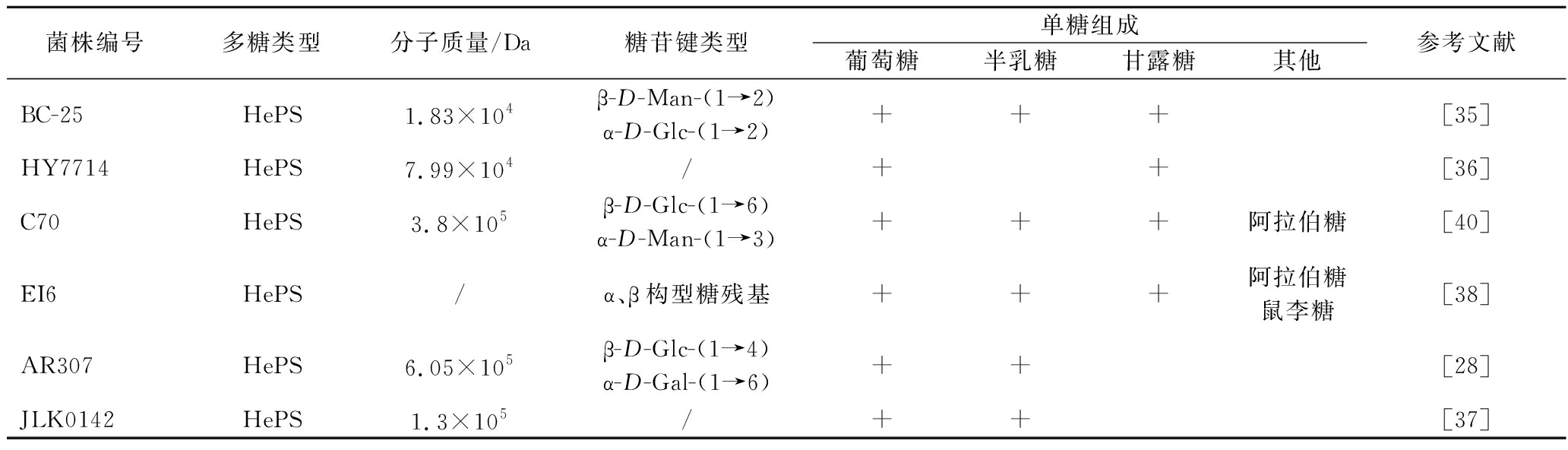
如表3所示,L.plantarum 合成的EPS多为HePS,只有少数是HoPS(葡聚糖)[30],而其HePS通常以葡萄糖、半乳糖、甘露糖为主要的单糖组成[31-38],只有少数L.plantarum合成的HePS还含有阿拉伯糖、木糖等单糖[28, 39-40]。
表3 植物乳杆菌EPS的结构特征
Table 3 Structure feature of L.plantarum EPS
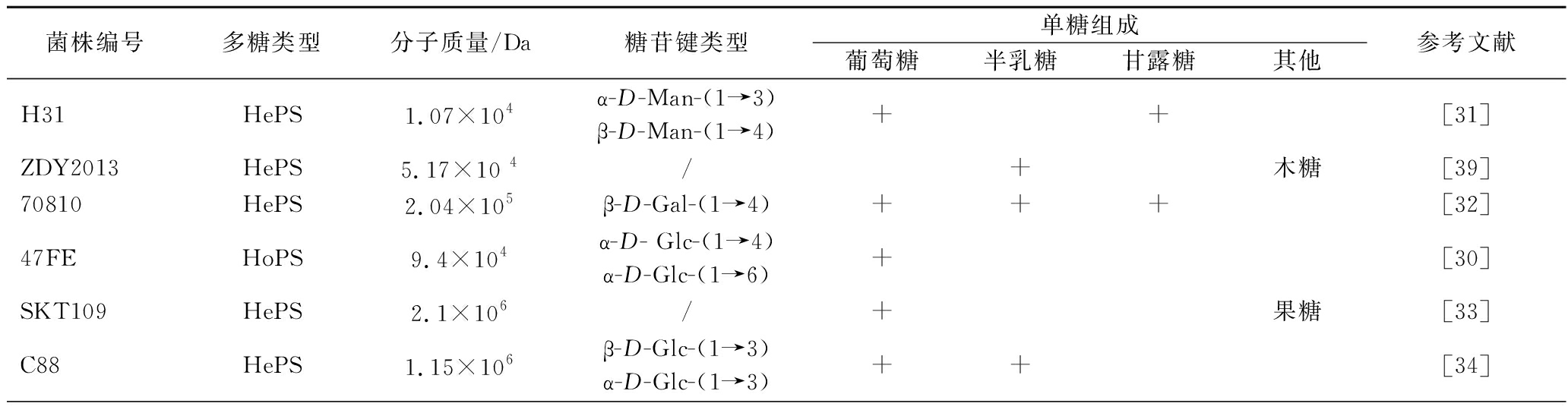
菌株编号多糖类型分子质量/Da糖苷键类型单糖组成葡萄糖半乳糖甘露糖其他参考文献H31HePS1.07×104α-D-Man-(1→3)β-D-Man-(1→4)++[31]ZDY2013HePS5.17×10 4/+木糖[39]70810HePS2.04×105β-D-Gal-(1→4)+++[32]47FEHoPS9.4×104α-D- Glc-(1→4)α-D-Glc-(1→6)+[30]SKT109HePS2.1×106/+果糖[33]C88HePS1.15×106β-D-Glc-(1→3)α-D-Glc-(1→3)++[34]
续表3
菌株编号多糖类型分子质量/Da糖苷键类型单糖组成葡萄糖半乳糖甘露糖其他参考文献BC-25HePS1.83×104β-D-Man-(1→2)α-D-Glc-(1→2) +++[35]HY7714HePS7.99×104/++[36]C70HePS3.8×105β-D-Glc-(1→6)α-D-Man-(1→3)+++阿拉伯糖[40]EI6HePS/α、β构型糖残基+++阿拉伯糖鼠李糖[38]AR307HePS6.05×105β-D-Glc-(1→4)α-D-Gal-(1→6)++[28]JLK0142HePS1.3×105/++[37]
表4 植物乳杆菌EPS的生物活性
Table 4 Biological activity of L.plantarum EPS
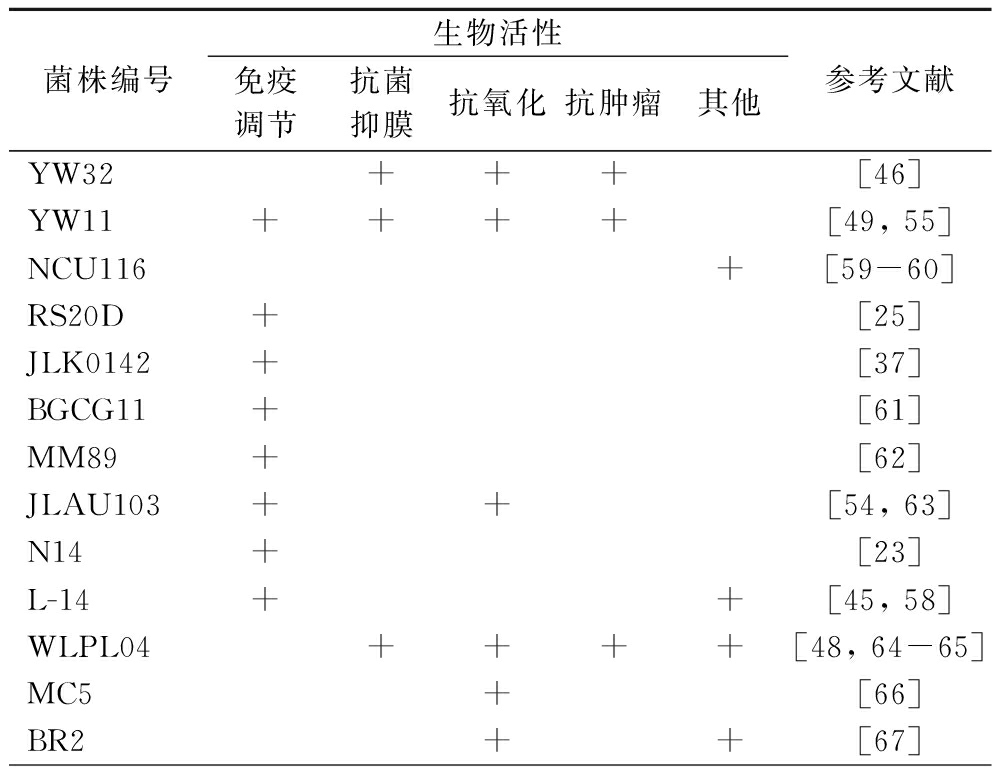
菌株编号生物活性免疫调节抗菌抑膜抗氧化抗肿瘤其他参考文献YW32+++[46]YW11++++[49, 55]NCU116+[59-60]RS20D+[25]JLK0142+[37]BGCG11+[61]MM89+[62]JLAU103++[54, 63]N14+[23]L-14++[45, 58]WLPL04++++[48, 64-65]MC5+[66]BR2++[67]
续表4
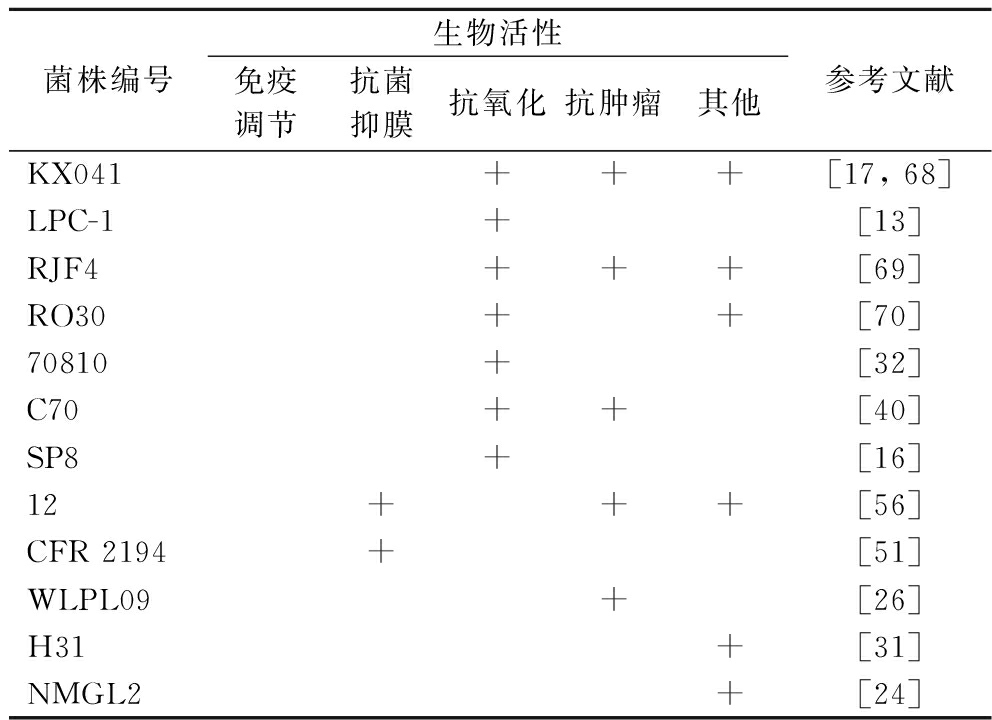
菌株编号生物活性免疫调节抗菌抑膜抗氧化抗肿瘤其他参考文献KX041+++[17, 68]LPC-1+[13]RJF4+++[69]RO30++[70]70810+[32]C70++[40]SP8+[16]12+++[56]CFR 2194+[51]WLPL09+[26]H31+[31]NMGL2+[24]
在糖苷键的类型方面,植物乳杆菌EPS显示出很大的相似性。其主链上的葡萄糖主要是α/β-D-(1→3)和α/β-D-(1→4)糖苷键[30, 32, 34],α-D-(1→2)和β-(1→6)键的出现概率较低[35, 40],侧链和分支点上α/β-D-(1→3)、α/β-(1→6)和β-(1→4)键的比例不同[4]。这种共性可将新发现的EPS分子定向到特定的单糖键上,并有助于核磁共振分析[41]。
有报道指出,乳酸菌HePS分子质量范围为4×104~6×106Da,HoPS分子质量在106Da以上[41],除了L.plantarum 47FE合成的HoPS外(9.4×104 Da)[30],表3中其他植物乳杆菌菌株的EPS平均分子质量分布基本与上述报道一致(1.83×104~2.1×106 Da)。
5 植物乳杆菌EPS的流变学特性
EPS的流变学特性关系到它在各个领域的应用,因此对EPS流变学特性影响因素(如温度、pH、分子质量等)的深入研究至关重要。AFREEN等[14]从温度、浓度和pH三个角度对L.plantarum NCCP 962 EPS进行了评估,发现该EPS溶液的持水性和持油性与黏度呈正相关性,表明该聚合物具有氢键持水的能力,与其他市售的水胶体相比,其发泡能力更强也更稳定,分离自L.plantarum K25和MTCC 9510的EPS也被证明有类似的持水性[42-43]。L.plantarum DM5合成的葡聚糖含有86.5%的α-(1→6)和13.5%的支链α-(1→3),因其无毒的生物相容性和独特的流变学特性而有望在食品工业中作为胶凝剂使用[44]。
6 植物乳杆菌EPS生物活性
6.1 免疫调节活性
人体免疫系统具有识别和清除病原微生物、肿瘤细胞和其他抗原的功能,在人体防御中发挥着至关重要的作用。大量研究在细胞因子水平等多方面对L.plantarum EPS抗炎或抑炎现象的原理进行了分析,例如促炎机制有促进NO释放[37]、促炎因子[白细胞介素-6(interleukin-6, IL-6)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)等]表达上调[25],抑炎机制有下调促炎因子表达[23]、阻断脂多糖与Toll样受体4(Toll like receptor 4,TLR4)的相互作用抑制促炎介质[环氧合酶-2(cyclooxygenase-2,COX-2)、IL-6、TNF-α和IL-1β][45]。
6.2 抗菌抑膜活性
生物膜是细菌在生长过程中为提高自身的稳定性和耐药性而分泌的一种物理保护屏障,尤其对病原菌而言,生物膜的抑制或消除可直接影响其存活能力。L.plantarum YW32合成的EPS(32 kDa)对多种致病菌(金黄色葡萄球菌、单增李斯特菌、铜绿假单胞菌和伤寒沙门氏菌)的生物膜形成均具有剂量依赖性的抑制作用[46],类似的抑膜作用同样被发现于L.plantarum 12[47]、WLPL04[48]和YW11[49],其机制可能是EPS通过修饰细菌细胞表面,阻碍病原菌菌体的初始附着,或通过信号分子下调参与生物膜形成的基因表达来干扰生物膜活性[50]。除了抑膜活性外,L.plantarum EPS还有抗菌作用,例如L. plantarum CFR 2194制备而来的蛋白质多糖聚合物溶液对多种食源性致病菌具有显著的抗菌活性[51],有学说认为这种抗菌活性来自于EPS对细菌细胞包膜(特别是肽聚糖层)的结构破坏[52]。
6.3 抗氧化活性
大量实验数据表明L. plantarum EPS具有抗氧化能力[53],一方面,L. plantarum EPS在体外可清除羟基和超氧阴离子等自由基,如L. plantarum KX041 和JLAU103 EPS在体外对ABTS阳离子、羟基、DPPH和超氧阴离子自由基均有显著的清除效果[17, 54];另一方面,L. plantarum EPS在体内可刺激各类抗氧化酶合成水平的增加,如衰老小鼠摄入L.plantarum YW11 EPS[50 mg/(kg·d)]后可有效缓解氧化应激,提高其血清谷胱甘肽过氧化物酶、超氧化物歧化酶、过氧化氢酶水平和总抗氧化能力[55]。
6.4 抗肿瘤活性
L.plantarum EPS(YW32、WLPL09、70810)对各类癌细胞模型(Caco-2、BGC-823、HT-29、HCT8和HepG2细胞)均有一定的抗肿瘤效果[26, 32, 46],其机制可能与增殖细胞核抗原、促凋亡蛋白(Bax、Cyt C、caspase-3、caspase-8和caspase-9)表达的上调以及抗凋亡蛋白(Bcl-2)表达的下调有关[56]。更进一步的动物实验通过调节肠道菌群和代谢物、增强肠道屏障、抑制NF-κB通路和激活caspase级联方面来解释了L. plantarum EPS缓解C57BL/6小鼠结肠癌症状[57]。上述层层递进的研究结果表明,L. plantarum EPS具有强大的抗癌潜力。
6.5 其他活性作用
L. plantarum H31的EPS H31-2可降低胰岛素抵抗HepG2细胞上清液的糖浓度,并上调HepG2细胞中AMPK、AKT-2和GLUT-4基因(葡萄糖代谢关键基因)的表达,揭示了EPS H31-2在预防和缓解糖尿病方面的潜在价值[31]。L. plantarum L-14 EPS利用TLR2和AMPK信号通路抑制脂肪生成,从而改善肥胖和肥胖相关疾病,可用于肥胖和代谢紊乱的预防和治疗[58]。L.plantarum BR2、RJF4和AAS3 EPS的体外降解胆固醇效率分别为47.5%、42.24%和41.6%[21]。此外,L. plantarum EPS还具有调节肠道菌群[24, 55]、改善肠屏障、促进肠稳态[59]、修复皮肤[38]等益生作用。
7 结语
L.plantarum EPS是一种天然、安全、可再生、可生物降解的生物大分子,因其独特的理化特性和加工性能而具有作为乳化剂、稳定剂和增稠剂广泛应用于食品工业中的潜在价值。然而,与其他乳酸菌类似,L.plantarum EPS的产量和得率普遍不高。尽管针对培养基组成、发酵条件的优化一定程度上提高了EPS的相对产量,却依然无法满足商业化应用的需求,寻找新的产糖策略变得尤为重要。近年来,随着分子生物学技术的日益成熟,对EPS合成酶(GFT等)相关基因的积极调控或过表达成为优化EPS产量的新技术[15],此外,合成生物学也是未来调节EPS产量一种跨学科技术,例如敲除其他副产物的基因为EPS合成保留更多的能量和基础物质,再如调整EPS合成通路上的基因表达及对应酶活力等。
除了产量有待提高外,L.plantarum EPS在生物活性方面的研究也有必要进一步地深入开展。从目前所公开的研究来看,虽然大量体外实验的数据支持L.plantarum EPS的各种潜在益生价值(调节免疫、抗肿瘤、抗氧化等),但还有几点不足有待改进:①生物学活性背后更深层次的机制及其与EPS精细结构(分子质量、单糖组成、糖苷键类型和分支大小等)的关联性需要明确;②体外活性需要临床研究来进一步的临床研究以考察其在复杂的人体内的实际作用;③利用修饰的方法对EPS结构进行改造以增强其现有的生物学活性或定向打造特定功能的L.plantarum EPS。
[1] XIA W, HAN J, ZHU S M, et al.Structural elucidation of the exopolysaccharide from Streptococcus thermophilus XJ53 and the effect of its molecular weight on immune activity[J].International Journal of Biological Macromolecules, 2023, 230:123177.
[2] WELMAN A D, MADDOX I S.Exopolysaccharides from lactic acid bacteria:Perspectives and challenges[J].Trends in Biotechnology, 2003, 21(6):269-274.
[3] ZEIDAN A A, POULSEN V K, JANZEN T, et al.Polysaccharide production by lactic acid bacteria:From genes to industrial applications[J].FEMS Microbiology Reviews, 2017, 41(Supp_1):S168-S200.
[4] ZHOU Y, CUI Y H, QU X J.Exopolysaccharides of lactic acid bacteria:Structure, bioactivity and associations:A review[J].Carbohydrate Polymers, 2019, 207:317-332.
[5] LAWS A, GU Y C, MARSHALL V.Biosynthesis, characterisation, and design of bacterial exopolysaccharides from lactic acid bacteria[J].Biotechnology Advances, 2001, 19(8):597-625.
[6] REMUS D M, VAN KRANENBURG R, VAN SWAM I I, et al.Impact of 4 Lactobacillus plantarum capsular polysaccharide clusters on surface glycan composition and host cell signaling[J].Microbial Cell Factories, 2012, 11:149.
[7] KLEEREBEZEM M, BOEKHORST J, VAN KRANENBURG R, et al.Complete genome sequence of Lactobacillus plantarum WCFS1[J].Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(4):1990-1995.
[8] GARCIA-GONZALEZ N, BOTTACINI F, VAN SINDEREN D, et al.Comparative genomics of Lactiplantibacillus plantarum:Insights into probiotic markers in strains isolated from the human gastrointestinal tract and fermented foods[J].Frontiers in Microbiology, 2022, 13:854266.
[9] LI P, LI X, GU Q, et al.Comparative genomic analysis of Lactobacillus plantarum ZJ316 reveals its genetic adaptation and potential probiotic profiles[J].Journal of Zhejiang University.Science.B, 2016, 17(8):569-579.[10] ADESULU-DAHUNSI A T, JEYARAM K, SANNI A I, et al.Production of exopolysaccharide by strains of Lactobacillus plantarum YO175 and OF101 isolated from traditional fermented cereal beverage[J].PeerJ, 2018, 6:e5326.
[11] IMRAN M Y M, REEHANA N, JAYARAJ K A, et al.Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20[J].International Journal of Biological Macromolecules, 2016, 93:731-745.
[12] VERA PINGITORE E, PESSIONE A, FONTANA C, et al.Comparative proteomic analyses for elucidating metabolic changes during EPS production under different fermentation temperatures by Lactobacillus plantarum Q823[J].International Journal of Food Microbiology, 2016, 238:96-102.
[13] MIDIK F, TOKATL M, BA
M, BA DER ELMAC
DER ELMAC S, et al.Influence of different culture conditions on exopolysaccharide production by indigenous lactic acid bacteria isolated from pickles[J].Archives of Microbiology, 2020, 202(4):875-885.
S, et al.Influence of different culture conditions on exopolysaccharide production by indigenous lactic acid bacteria isolated from pickles[J].Archives of Microbiology, 2020, 202(4):875-885.
[14] AFREEN A, AHMED Z, KHALID N, et al.Optimization and cholesterol-lowering activity of exopolysaccharide from Lactiplantibacillus paraplantarum NCCP 962[J].Applied Microbiology and Biotechnology, 2023, 107(4):1189-1204.
[15] SOUMYA M P, PARAMESWARAN R, MADHAVAN NAMPOOTHIRI K.Nisin controlled homologous over-expression of an exopolysaccharide biosynthetic glycosyltransferase gene for enhanced EPS production in Lactobacillus plantarum BR2[J].Bioresource Technology, 2023, 385:129387.
[16] ZHANG L, ZHAO B, LIU C J, et al.Optimization of biosynthesis conditions for the production of exopolysaccharides by Lactobacillus plantarum SP8 and the exopolysaccharides antioxidant activity test[J].Indian Journal of Microbiology, 2020, 60(3):334-345.
[17] WANG X, SHAO C G, LIU L, et al.Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041[J].International Journal of Biological Macromolecules, 2017, 103:1173-1184.
[18] 司天昭, 柳陈坚, 秦晓萌, 等.植物乳杆菌YM-2菌株胞外多糖生物合成工艺优化[J].食品科学, 2017, 38(10):24-30.SI T Z, LIU C J, QIN X M, et al.Optimization of biosynthesis conditions for the production of exopolysaccharides by Lactobacillus plantarum YM-2[J].Food Science, 2017, 38(10):24-30.
[19] 张文平, 赵英杰, 罗晟, 等. 高产胞外多糖植物乳杆菌筛选及其发酵工艺优化[J]. 食品与发酵工业, 2019, 45(21): 38-45.ZHANG W P, ZHAO Y J, LUO S, et al. Screening of Lactobacillus plantarum with higher yield of exopolysaccharides andoptimization of fermentation conditions[J]. Food and Fermentation Industries, 2019, 45(21): 38-45.
[20] 陈绮, 雷文平, 肖茜, 等.植物乳杆菌XN1904E产胞外多糖发酵条件优化[J].乳业科学与技术, 2020, 43(3):1-5.CHEN Q, LEI W P, XIAO Q, et al.Optimization of fermentation conditions for exopolysaccharide production by Lactobacillus plantarum XN1904E[J].Journal of Dairy Science and Technology, 2020, 43(3):1-5.
[21] ZHAO X F, LIANG Q.Optimization, probiotic characteristics, and rheological properties of exopolysaccharides from Lactiplantibacillus plantarum MC5[J].Molecules, 2023, 28(6):2463.
[22] ZHANG X L, LIU Y, ZHAO X G, et al.A novel viscous hydrophilic colloidal polysaccharide produced by Lactiplantibacillus plantarum T1:Structural characterization, rheological behavior and biological activity[J].Process Biochemistry, 2023, 131:101-113.
[23] MUROFUSHI Y, VILLENA J, MORIE K, et al.The Toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14[J].Molecular Immunology, 2015, 64(1):63-75.
[24] YAO M K, ZHANG M, LAI T T, et al.Characterization and in vitro fecal microbiota regulatory activity of a low-molecular-weight exopolysaccharide produced by Lactiplantibacillus plantarum NMGL2[J].Foods, 2022, 11(3):393.
[25] ZHU Y T, WANG X J, PAN W S, et al.Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity[J].International Journal of Biological Macromolecules, 2019, 121:342-349.
[26] JIANG B S, TIAN L L, HUANG X L, et al.Characterization and antitumor activity of novel exopolysaccharide APS of Lactobacillus plantarum WLPL09 from human breast milk[J].International Journal of Biological Macromolecules, 2020, 163:985-995.
[27] LIU T, ZHOU K, YIN S, et al.Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan pickle[J].International Journal of Biological Macromolecules, 2019, 134:516-526.
[28] FENG X W, ZHANG H, LAI P F H, et al.Structure characterization of a pyruvated exopolysaccharide from Lactobacillus plantarum AR307[J].International Journal of Biological Macromolecules, 2021, 178:113-120.
[29] HUANG Y Y, WU J M, WU W T, et al.Structural, antioxidant, and immunomodulatory activities of an acidic exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010[J].Frontiers in Nutrition, 2022, 9:1073071.
[30] ABO SAIF F A A, SAKR E A E.Characterization and bioactivities of exopolysaccharide produced from probiotic Lactobacillus plantarum 47FE and Lactobacillus pentosus 68FE[J].Bioactive Carbohydrates and Dietary Fibre, 2020, 24:100231.
[31] HUANG Z H, LIN F X, ZHU X Y, et al.An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway[J].International Journal of Biological Macromolecules, 2020, 143:775-784.
[32] WANG K, LI W, RUI X, et al.Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810[J].International Journal of Biological Macromolecules, 2014, 67:71-78.
[33] WANG J, ZHAO X, TIAN Z, et al.Isolation and characterization of exopolysaccharide-producing Lactobacillus plantarum SKT109 from Tibet kefir[J].Polish Journal of Food and Nutrition Sciences, 2015, 65(4):269-279.
[34] ZHANG L, LIU C H, LI D, et al.Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88[J].International Journal of Biological Macromolecules, 2013, 54:270-275.
[35] ZHOU K, ZENG Y T, YANG M L, et al.Production, purification and structural study of an exopolysaccharide from Lactobacillus plantarum BC-25[J].Carbohydrate Polymers, 2016, 144:205-214.
[36] LEE K, KIM H J, KIM S A, et al.Exopolysaccharide from Lactobacillus plantarum HY7714 protects against skin aging through skin-gut axis communication[J].Molecules, 2021, 26(6):1651.
[37] WANG J, WU T, FANG X B, et al.Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu[J].International Journal of Biological Macromolecules, 2018, 115:985-993.
[38] ZAGHLOUL E H, IBRAHIM M I A.Production and characterization of exopolysaccharide from newly isolated marine probiotic Lactiplantibacillus plantarum ei6 with in vitro wound healing activity[J].Frontiers in Microbiology, 2022, 13:903363.
[39] ZHANG Z H, LIU Z Q, TAO X Y, et al.Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities[J].Carbohydrate Polymers, 2016, 153:25-33.
[40] AYYASH M, ABU-JDAYIL B, ITSARANUWAT P, et al.Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk[J].International Journal of Biological Macromolecules, 2020, 144:938-946.
[41] LYNCH K M, ZANNINI E, COFFEY A, et al.Lactic acid bacteria exopolysaccharides in foods and beverages:Isolation, properties, characterization, and health benefits[J].Annual Review of Food Science and Technology, 2018, 9:155-176.
[42] YUNYUN J, MIAO C, SHUJUAN S, et al.Factors affecting of Lactobacillus plantarum K25 fermentation to produce exopolysaccharide and its application [J].Journal of Food Science and Technology, China, 2018, 36(1):25-34.
[43] ISMAIL B, NAMPOOTHIRI K M.Molecular characterization of an exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510 and its efficacy to improve the texture of starchy food[J].Journal of Food Science and Technology, 2014, 51(12):4012-4018.
[44] DAS D, GOYAL A.Characterization and biocompatibility of glucan:A safe food additive from probiotic Lactobacillus plantarum DM5[J].Journal of the Science of Food and Agriculture, 2014, 94(4):683-690.
[45] KWON M, LEE J, PARK S, et al.Exopolysaccharide isolated from Lactobacillus plantarum L-14 has anti-inflammatory effects via the toll-like receptor 4 pathway in LPS-induced RAW 264.7 cells[J].International Journal of Molecular Sciences, 2020, 21(23):9283.
[46] MAHDHI A, LEBAN N, CHAKROUN I, et al.Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation[J].Microbial Pathogenesis, 2017, 109:214-220.
[47] 宋莹龙, 梁雪, 宋杏, 等.Lactobacillus plantarum-12胞外多糖抑制志贺氏菌生物膜形成的研究[J].食品研究与开发, 2018, 39(8):144-151.SONG Y L, LIANG X, SONG X, et al.The study on the inhibition of Shigella biofilm formation by the exopolysaccharides of Lactobacillus plantarum-12[J].Food Research and Development, 2018, 39(8):144-151.
[48] LIU Z Q, ZHANG Z H, QIU L, et al.Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04[J].Journal of Dairy Science, 2017, 100(9):6895-6905.
[49] ZHANG M, LAI T T, YAO M K, et al.Interaction of the exopolysaccharide from Lactobacillus plantarum YW11 with casein and bioactivities of the polymer complex[J].Foods, 2021, 10(6):1153.
[50] SALIMI F, FARROKH P.Recent advances in the biological activities of microbial exopolysaccharides[J].World Journal of Microbiology &Biotechnology, 2023, 39(8):213.
[51] MADHU A N, PRAPULLA S G.Evaluation and functional characterization of a biosurfactant produced by Lactobacillus plantarum CFR 2194[J].Applied Biochemistry and Biotechnology, 2014, 172(4):1777-1789.
[52] ABDALLA A K, AYYASH M M, OLAIMAT A N, et al.Exopolysaccharides as antimicrobial agents:Mechanism and spectrum of activity[J].Frontiers in Microbiology, 2021, 12:664395.
[53] LIU C F, TSENG K C, CHIANG S S, et al.Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides[J].Journal of the Science of Food and Agriculture, 2011, 91(12):2284-2291.
[54] MIN W H, FANG X B, WU T, et al.Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103[J].Journal of Bioscience and Bioengineering, 2019, 127(6):758-766.
[55] ZHANG J, ZHAO X, JIANG Y Y, et al.Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir[J].Journal of Dairy Science, 2017, 100(8):6025-6041.
[56] SUN M Y, LIU W W, SONG Y L, et al.The effects of Lactobacillus plantarum-12 crude exopolysaccharides on the cell proliferation and apoptosis of human colon cancer (HT-29) cells[J].Probiotics and Antimicrobial Proteins, 2021, 13(2):413-421.
[57] MA F L, SONG Y L, SUN M Y, et al.Exopolysaccharide produced by Lactiplantibacillus plantarum-12 alleviates intestinal inflammation and colon cancer symptoms by modulating the gut microbiome and metabolites of C57BL/6 mice treated by azoxymethane/dextran sulfate sodium salt[J].Foods, 2021, 10(12):3060.
[58] LEE J, PARK S, OH N, et al.Oral intake of Lactobacillus plantarum L-14 extract alleviates TLR2- and AMPK-mediated obesity-associated disorders in high-fat-diet-induced obese C57BL/6J mice[J].Cell Proliferation, 2021, 54(6):e13039.
[59] ZHOU X T, ZHANG D D, QI W C, et al.Exopolysaccharides from Lactobacillus plantarum NCU116 facilitate intestinal homeostasis by modulating intestinal epithelial regeneration and microbiota[J].Journal of Agricultural and Food Chemistry, 2021, 69(28):7863-7873.
[60] ZHOU X T, QI W C, HONG T, et al.Exopolysaccharides from Lactobacillus plantarum NCU116 regulate intestinal barrier function via STAT3 signaling pathway[J].Journal of Agricultural and Food Chemistry, 2018, 66(37):9719-9727.
[61] DINI M, PECIKOZA U, DJOKI
M, PECIKOZA U, DJOKI J, et al.Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats[J].Frontiers in Pharmacology, 2018, 9:1.
J, et al.Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats[J].Frontiers in Pharmacology, 2018, 9:1.
[62] RAJOKA M S R, MEHWISH H M, KITAZAWA H, et al.Techno-functional properties and immunomodulatory potential of exopolysaccharide from Lactiplantibacillus plantarum MM89 isolated from human breast milk[J].Food Chemistry, 2022, 377:131954.
[63] WANG J, FANG X B, WU T, et al.In vitro immunomodulatory effects of acidic exopolysaccharide produced by Lactobacillus planetarium JLAU103 on RAW264.7 macrophages[J].International Journal of Biological Macromolecules, 2020, 156:1308-1315.
[64] LIU Z Q, DONG L Y, JIA K Y, et al.Sulfonation of Lactobacillus plantarum WLPL04 exopolysaccharide amplifies its antioxidant activities in vitro and in a Caco-2 cell model[J].Journal of Dairy Science, 2019, 102(7):5922-5932.
[65] SUN X, ZHANG H F, MA C L, et al.Alleviation of anxiety/depressive-like behaviors and improvement of cognitive functions by Lactobacillus plantarum WLPL04 in chronically stressed mice[J].The Canadian Journal of Infectious Diseases &Medical Microbiology = Journal Canadien Des Maladies Infectieuses et De La Microbiologie Medicale, 2021, 2021:6613903.
[66] ZHAO X F, LIANG Q.EPS-producing Lactobacillus plantarum MC5 as a compound starter improves rheology, texture, and antioxidant activity of yogurt during storage[J].Foods, 2022, 11(11):1660.
[67] SASIKUMAR K, KOZHUMMAL VAIKKATH D, DEVENDRA L, et al.An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods[J].Bioresource Technology, 2017, 241:1152-1156.
[68] WANG T, WANG P P, YIN L, et al.Dietary Lactiplantibacillus plantarum KX041 attenuates colitis-associated tumorigenesis and modulates gut microbiota[J].Food Science and Human Wellness, 2023, 12(5):1626-1636.
[69] DILNA S V, SURYA H, ASWATHY R G, et al.Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4[J].LWT- Food Science and Technology, 2015, 64(2):1179-1186.
[70] ELMANSY E A, ELKADY E M, ASKER M S, et al.Exopolysaccharide produced by Lactiplantibacillus plantarum RO30 isolated from Romi cheese:Characterization, antioxidant and burn healing activity[J].World Journal of Microbiology &Biotechnology, 2022, 38(12):245.