桔梗是临床上广泛应用的一味药食同源中药,生活中也常被做成泡菜食用。中国药典收载的桔梗是经净制、切制处理的饮片,而在古代比较常用的桔梗炮制方法还有蜜炙、米泔水制、百合制、醋炙、酒炙和姜炙等,米泔水炮制桔梗最早出现于宋代《小儿卫生总微论方》,载有“去芦,米泔水浸一宿,焙干用”,盛行于元代至明清两代,在《本草蒙筌》、《本草纲目》、《本草备要》等多部古籍中均载有此法[1],而目前鲜见使用此法炮制桔梗。为了探索其炮制目的,课题组前期通过高通量测序结果表明,米泔水炮制桔梗过程中存在微生物组成及丰度的变化,初期以欧文氏菌属、肠杆菌属、泛菌属等有害菌属为主,后期以乳球菌属、魏斯氏菌属、明串珠菌属等乳酸菌类为优势菌属[2],因此推测乳酸菌可能对桔梗中的成分有影响。已有学者利用乳酸菌发酵桔梗,如JUNG等[3]利用干酪乳杆菌(Lactobacillus casei)发酵桔梗,使桔梗总黄酮、总皂苷和桔梗皂苷D(Platycodon grandiflorum saponin D,PD)含量均显著增加,细胞毒性降低,细胞实验结果显示发酵桔梗提取物可通过丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)和核因子κB(nuclear factor kappa-B,NF-κB)信号通路发挥免疫刺激活性。WANG等[4]利用鼠李糖乳杆菌217-1发酵桔梗根,提高了桔梗皂苷 D、类黄酮和多酚的含量,桔梗发酵液抗氧化活性显著增强,并可降低葡聚糖硫酸钠诱导的小鼠溃疡性结肠炎的发生率。PD是桔梗的标志性成分,也是活性成分,具有抗炎、抗病毒、抗氧化、抗肿瘤等多种活性[5],尤其对于呼吸系统疾病方面,可通过抑制脂多糖诱导的炎症反应改善哮喘气道重塑[6]。LEE等[7]采用乳酸菌发酵桔梗,提高了PD的含量并显著减少咳嗽次数。因此,本研究采用MRS(DeMan-Rogosa- Sharpe)培养基分离纯化米泔水制桔梗炮制液中的优势菌,并采用纯种发酵的方法发酵桔梗,考察PD的含量变化,为探索米泔水炮制桔梗的原理提供依据。
1 材料与仪器
1.1 材料
桔梗生品饮片(批号:210501,产地:安徽亳州),北京同仁堂哈尔滨药店,由黑龙江省中医药科学院王伟明研究员鉴定为桔梗科植物桔梗Platycodon grandiflorum(Jacq.)A.DC.的干燥根;糯米,北京华联超市哈尔滨分店;桔梗皂苷D(纯度≥98%,批号:RFS-J01302004027),成都瑞芬思生物科技有限公司;3%过氧化氢酶试剂、革兰氏染色试剂盒、MRS琼脂、肉汤,青岛海博生物技术有限公司;Ezup柱式细菌基因组DNA抽提试剂盒、SanPrep柱式DNA胶回收试剂盒、引物,生工生物工程(上海)股份有限公司。
1.2 主要仪器
DHP9272恒温培养箱,上海一恒科学仪器有限公司;BSA224S电子天平,德国赛多利斯科学仪器有限公司;LC-2030C 3D Plus高效液相色谱仪,日本岛津公司;3730XL测序仪、2720 cycler PCR仪,美国应用生物系统公司;DYY-5电泳仪,北京六一仪器厂;HC-2518R高速冷冻离心机,BBI。
2 实验方法
2.1 米泔水制桔梗炮制液中乳酸菌的分离鉴定
2.1.1 米泔水制桔梗炮制液的制备
取糯米适量,粉碎,过五号筛,每1 g糯米粉加水50 mL,搅拌均匀,得米泔水混悬液[8],每10 g桔梗生品饮片加米泔水混悬液60 mL,于28 ℃浸泡24 h,摇匀,无菌吸取炮制液适量,用无菌生理盐水梯度稀释。
2.1.2 菌株鉴定
2.1.2.1 初步鉴定
挑取MRS固体培养基的单一菌落,采用革兰氏染色试剂盒和过氧化氢酶实验进行初步鉴定。
2.1.2.2 DNA提取、PCR扩增及高通量测序
菌株活化:将筛选得到的菌株接种至MRS液体培养基中于37 ℃振荡培养12 h。
DNA提取:按Ezup柱式细菌基因组DNA抽提试剂盒使用说明提取DNA。测得OD260/OD280的比值为1.82,表明提取的DNA较纯。
PCR扩增:采用细菌特异性引物,引物序列为27F:AGTTTGATCMTGGCTCAG;1492R:GGTTACCTTGTTACGACTT。扩增体系见表1,扩增程序如下:95 ℃预变性5 min,94 ℃变性30 s,57 ℃退火30 s,72 ℃延伸90 s,共进行30个循环,最后于72 ℃延伸10 min。将PCR扩增后产物进行凝胶电泳,电压为150 V,电泳结束后观察条带,切取含目的DNA片段的琼脂糖凝胶块,采用SanPrep柱式DNA胶回收试剂盒进行纯化回收。委托生工生物工程(上海)股份有限公司对筛选得到的菌株进行16S rDNA测序。
表1 PCR反应体系
Table 1 Reaction system of PCR

试剂体积/μL10 X PCR BufferdNTP (各 10 mmol/L)Taq Plus DNA Polymerase(5 U/μL)50 mmol/L MgSO4共12.5引物F (10 μmol/L)1引物R (10 μmol/L)1模板 (DNA)1ddH2O9.5总计25
2.1.2.3 系统发育树的构建
将测序所得16S rDNA序列上传至GenBank数据库进行BLAST分析,选取相似度较高的序列,采用MEGA11软件的Neighbor-Joining法构建系统发育树,bootstrap值设为1 000。
2.2 纯种发酵桔梗对PD含量的影响
2.2.1 发酵桔梗的制备[9]
精密称取桔梗粉末(过二号筛)20 g于锥形瓶中,加入200 mL蒸馏水,于121 ℃灭菌20 min,按2%质量比接入各菌液(菌液浓度为105 CFU/mL),于37 ℃、150 r/min振荡培养48 h,未发酵组加入等量蒸馏水作为空白对照。
2.2.2 PD含量测定[10]
2.2.2.1 对照品溶液制备
精密称取PD对照品适量,加甲醇制成每毫升含0.5 mg的溶液,即得。
2.2.2.2 供试品溶液制备
将2.2.1节制备的样品抽滤,滤液加蒸馏水定容至200 mL,精密量取20 mL,用水饱和正丁醇振摇提取3次,每次20 mL,合并正丁醇液,用氨试液50 mL洗涤,弃去氨液,再用正丁醇饱和的水50 mL洗涤,弃去水液,正丁醇液回收溶剂至干,残渣加甲醇溶解并定容到10 mL量瓶中,摇匀,0.22 μm滤膜过滤,备用。
2.2.2.3 色谱条件与系统适用性试验
采用ZORBAX Eclipse XDB-C18(4.6 mm×150 mm,5 μm)色谱柱,以乙腈-水(28∶72,体积比)为流动相,蒸发光散射检测器检测;理论塔板数数按PD峰计算不低于3 000,进样量10 μL。
分别精密吸取对照品溶液10、20 μL,供试品溶液10 μL,进样测定,以外标两点法对数方程计算。
2.2.3 数据处理
采用SPSS 24.0软件对数据进行统计学分析,用GraphPad Prism 9作图。数据结果以平均值±标准差表示,组间差异比较采用t检验和ANOVA单因素方差分析。P<0.05表示差异有统计学意义,P<0.01表示差异具有显著性。
3 结果与分析
3.1 优势菌株的分离及初步鉴定结果
分离获得3株优势菌株,分别为MZJ01、MZJ02、MZJ03,革兰氏染色均为阳性,过氧化氢酶试验为阴性,初步判定3株细菌均为乳酸菌。各菌株培养菌落的形态学如图1所示。MZJ01:菌落圆形,乳白色,中央凸起湿润,边缘光滑,菌体椭球状,单个或成对排列;MZJ02:菌落圆形,白色,湿润,边缘光滑,菌体椭球状,单个或成对排列;MZJ03:菌落圆形,白色,半透明,干燥,菌体椭球状,成对或链状排列。
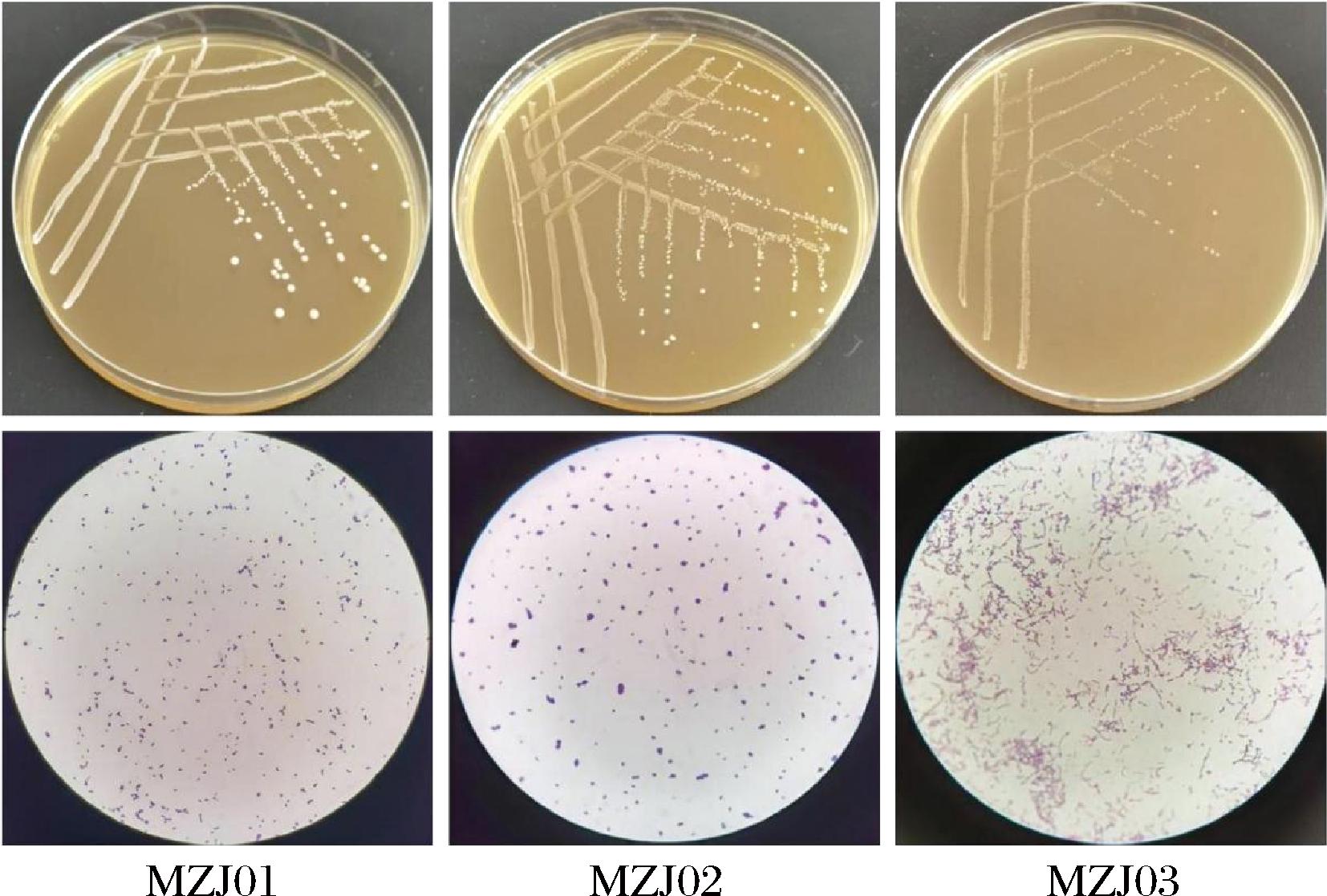
图1 不同菌的菌落形态及革兰氏染色图(1 000×)
Fig.1 Photograph of colony morphology and Gram staining of different strains (1 000×)
3.2 乳酸菌16S rDNA序列同源性比对结果
通常情况下,16S rDNA序列同源性超过98.7%的菌株被认为是同一物种[11]。将筛选所得菌株的16S rDNA序列上传至NCBI数据库,进行同源性比对,结果见表2。MZJ01和食窦魏斯氏菌(Weissellacibaria)同源性最高,MZJ02和肠球菌属(Enterococcus)多株细菌同源性均较高,MZJ03和假肠膜明串珠菌(Leuconostoc pseudomesenteroides)同源性最高。所有菌株同源性均大于98.7%,和标准菌株序列覆盖率均达到100%,E值都为0.0。
表2 分离菌株的16S rDNA序列同源性比对结果
Table 2 Results of homology comparison of 16S rDNA sequence of isolated strains
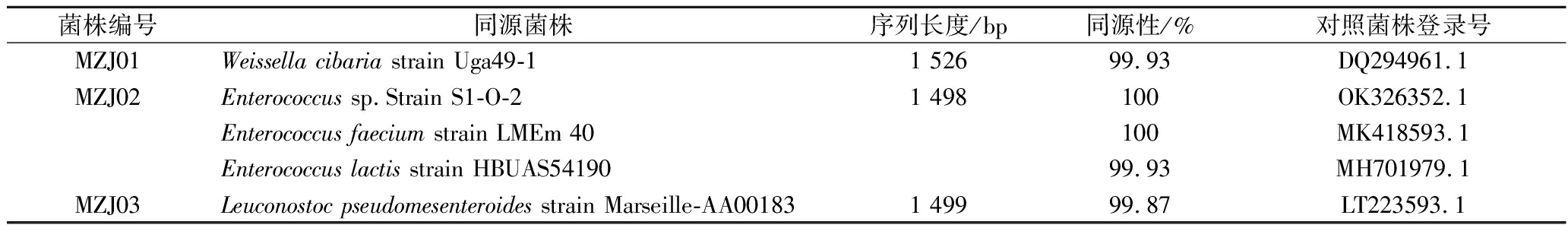
菌株编号同源菌株序列长度/bp同源性/%对照菌株登录号MZJ01Weissella cibaria strain Uga49-11 52699.93DQ294961.1MZJ02Enterococcus sp.Strain S1-O-21 498100OK326352.1Enterococcus faecium strain LMEm 40100MK418593.1Enterococcus lactis strain HBUAS5419099.93MH701979.1MZJ03Leuconostoc pseudomesenteroides strain Marseille-AA001831 49999.87LT223593.1
3.3 系统发育树的构建
以3株乳酸菌测序所得序列,与其高同源性菌株序列以及Coxiella burnetii外群序列为对象,构建系统发育树[12],见图2。系统发育树各分支自展值均高于50,分支结构与同源性比对结果一致。综合所有实验数据,可以判定:MZJ01为食窦魏斯氏菌(Weissella cibaria);MZJ02为肠球菌(Enterococcus);MZJ03为假肠膜明串珠菌(Leuconostoc pseudomesenteroides)。

图2 基于16S rDNA序列的乳酸菌系统发育树
Fig.2 Phylogenetic tree of lactic acid bacteria based on 16S rDNA sequence
3.4 PD含量测定结果
图3为PD对照品、桔梗生品及发酵桔梗的HPLC图。
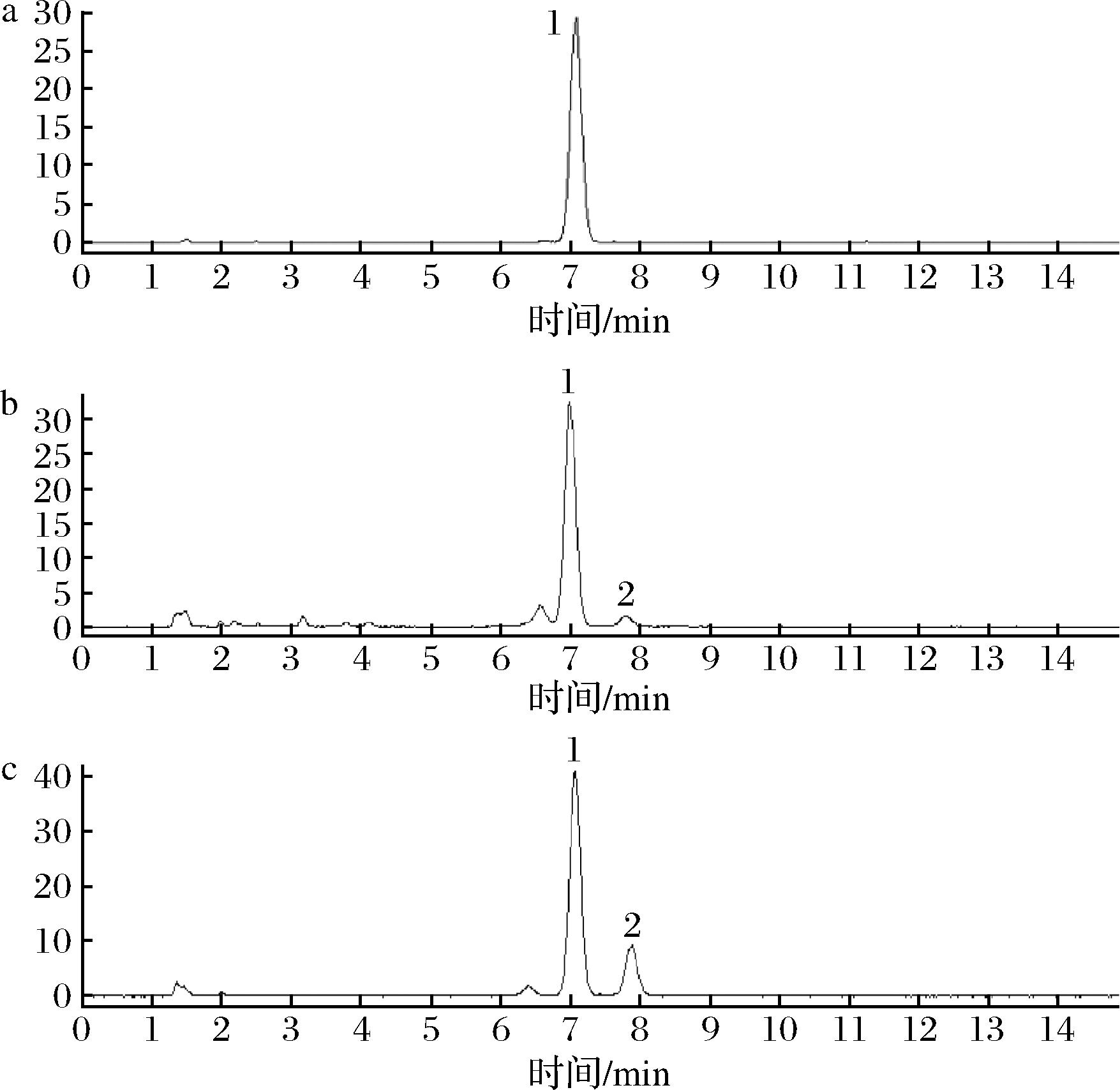
a-PD;b-桔梗生品;c-发酵桔梗
图3 PD对照品及样品的HPLC图
Fig.3 HPLC diagram of platycodon saponin D reference substance and the samples
各组PD的含量如图4,MZJ01和MZJ02发酵桔梗提取液中PD的含量显著高于未发酵组(P<0.01),PD的含量分别提高8.19%和10.72%,而MZJ03发酵桔梗提取液中PD的含量高于未发酵组,但不显著(P>0.05)。另外,从图3可知,与生品相比,发酵桔梗在保留时间近8.0 min处的峰2的峰面积也明显升高,具体是何成分有待于进一步分析。
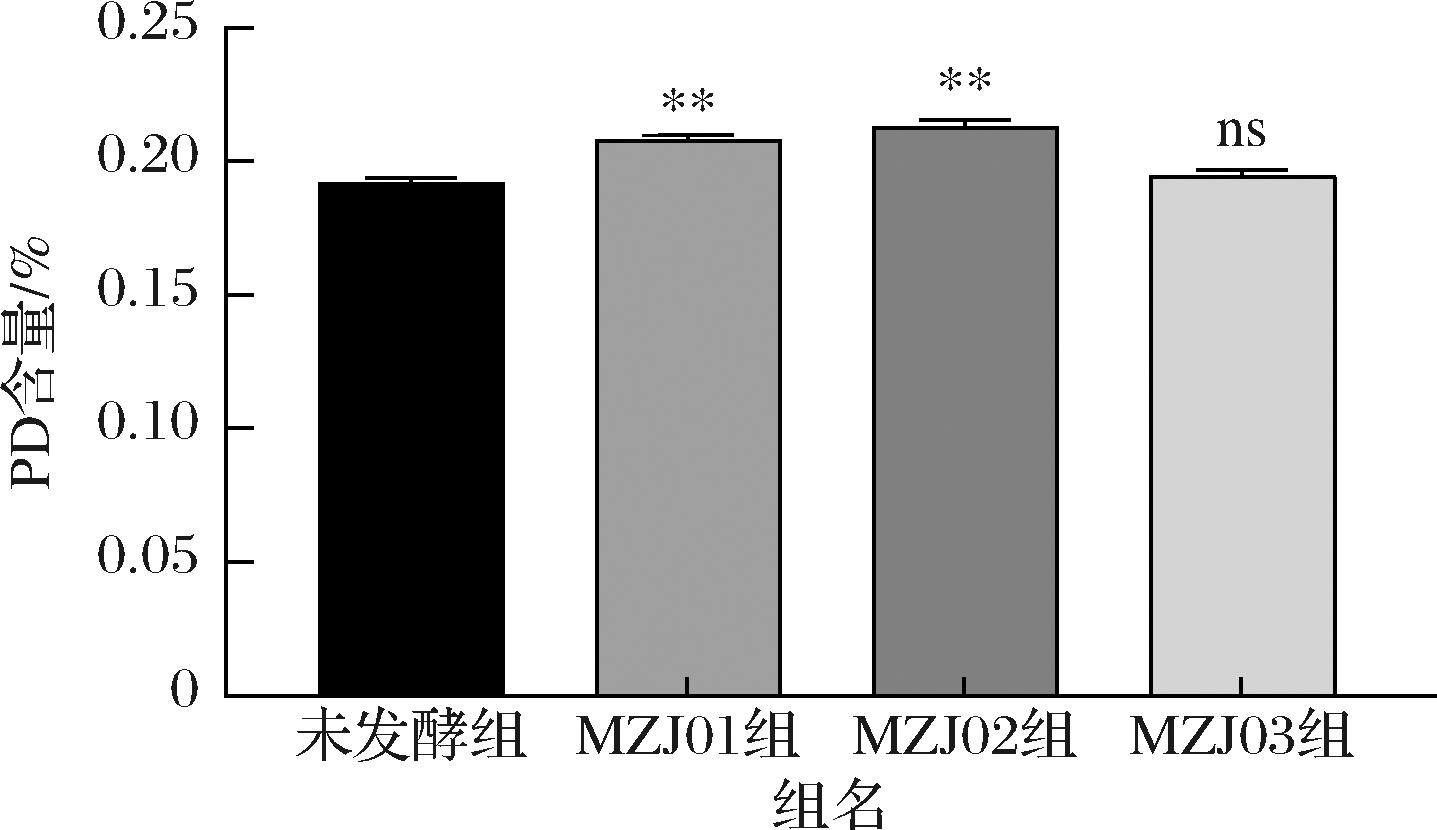
图4 不同菌种发酵桔梗样品中PD的含量
Fig.4 Content of PD in PG fermented by different strains
注:与未发酵组相比,**P<0.01,*P<0.05。
4 讨论与结论
在前期高通量测序基础上,本研究采用MRS培养基分离纯化米泔水制桔梗溶液中的优势菌,共分离获得3株菌,经鉴定均为乳酸菌,分别为食窦魏斯氏菌、肠球菌和假肠膜明串珠菌,与高通量测序结果基本一致[2]。3株菌均可用于食品发酵,魏斯氏菌属可通过代谢及转化合成反应生成酮类、醛类、醇类、酯类、萜类等物质而改善食品风味[13-14],该菌能产胞外多糖,具有抑菌及抗氧化活性[15-16];明串珠菌属常和魏斯氏菌共存于发酵食品中[14],在泡菜、芒果汁、黄豆酱和酒中均分离得到假肠膜明串珠菌,也能够产生胞外多糖,具有抑菌活性,有较好的水溶性和持水能力等[17-19];肠球菌可用于奶酪的制作,既能促进奶酪的发酵过程,还能水解蛋白和脂类而产生特殊口感和香味[20],肠球菌也具有一定的益生作用及安全性[21]。
本研究利用MZJ01和MZJ02两株乳酸菌发酵桔梗显著提高了PD的含量,同样也有学者利用其他菌发酵桔梗提高了PD的含量,JUNG等[22]利用香菇菌丝对PG提取物进行发酵,PD的含量提高了18.3倍;PARK等[23]利用啤酒酵母菌(Saccharomyces cerevisiae)对PG提取物进行发酵,PD含量提高了4倍。分析PD含量提高的原因,可能与这些菌能够产生较高活性的β-D-葡萄糖苷酶相关。桔梗提取物中含有两种主要的PD前体物质,分别为桔梗皂苷E(PE)和桔梗皂苷D3(PD3),它们可以通过β-D-葡萄糖苷酶水解转化为PD[24]。据报道,桔梗提取物中含有约2%的桔梗皂苷,其中PE、PD3和PD的比例分别约占8.3%、15.4%和20.9%,3种桔梗皂苷的结构区别在于PD的C-3位置的葡萄糖单元,而β-D -葡萄糖苷酶可通过切断PD3和PE的C-3处的1个和2个葡萄糖分子而转化为PD,即PE→PD3→PD[25-26]。本研究中3株乳酸菌发酵桔梗对PD的转化能力不同,可能与其所产生的β-D-葡萄糖苷酶活性相关,MZJ01和MZJ02产β-D-葡萄糖苷酶的能力可能优于MZJ03,后续可进一步通过实验进行验证。另外,还可以PE和PD3为前体物质,通过3株菌在一定条件下发酵,考察PD的含量变化以验证其内在机制。也可通过筛选高产β-D-葡萄糖苷酶的食用菌发酵桔梗以提高PD的含量,从而增强其营养保健和药用价值。
通过以上转化机制可推测米泔水炮制桔梗的机制:米泔水为乳酸菌的生长繁殖提供了充足的碳源,促进了乳酸菌的生长而产生大量的β- D -葡萄糖苷酶,促进了桔梗中PE和PD3向PD的转化,提高了PD的含量,更有利于增强其生物活性。PD是桔梗的重要皂苷,具有抗氧化、抗炎等多种药理作用[27],后续可通过药理学实验进行验证。
本研究结果显示,MZJ02和肠球菌属多种细菌相似度均大于98.7%,这主要是由于已报道的肠球菌属细菌序列具有高度相似性(99%),16S rDNA序列分析技术在种水上平难以准确鉴定,后续可利用MALDI-TOF MS等技术对肠球菌进行种水平鉴定[28-29]。
[1] 邓亚羚, 任洪民, 叶先文, 等.桔梗的炮制历史沿革、化学成分及药理作用研究进展[J].中国实验方剂学杂志, 2020, 26(2):190-202.DENG Y L, REN H M, YE X W, et al.Progress of historical evolution of processing, chemical composition and pharmacological effect of platycodonis radix[J].Chinese Journal of Experimental Traditional Medical Formulae, 2020, 26(2):190-202.
[2] 于鑫鑫, 丁纯洁, 陈丽艳, 等.米泔水炮制桔梗过程中微生物变化规律研究[J].中国现代中药, 2022, 24(11):2093-2100.YU X X, DING C J, CHEN L Y, et al.Microbial changes in Platycodon grandiflorum processing with rice-washed water[J].Modern Chinese Medicine, 2022, 24(11):2093-2100.
[3] JUNG J I, LEE H S, KIM S M, et al.Immunostimulatory activity of hydrolyzed and fermented Platycodon grandiflorum extract occurs via the MAPK and NF-κB signaling pathway in RAW 264.7 cells[J].Nutrition Research and Practice, 2022, 16(6):685-699.
[4] WANG Z, LI C H, HE X, et al.Platycodon grandiflorum root fermentation broth reduces inflammation in a mouse IBD model through the AMPK/NF-κB/NLRP3 pathway[J].Food &Function, 2022, 13(7):3946-3956.
[5] XIE L, ZHAO Y X, ZHENG Y, et al.The pharmacology and mechanisms of platycodin D, an active triterpenoid saponin from Platycodon grandiflorus[J].Frontiers in Pharmacology, 2023, 14:1148853.
[6] 赵院院, 赵一颖, 贺红娟, 等.基于网络药理学和实验验证探讨桔梗改善哮喘的机制研究[J].中药药理与临床, 2021, 37(6):82-89.ZHAO Y Y, ZHAO Y Y, HE H J, et al.Mechanism of Jiegeng in improving asthma:An exploration based on network pharmacology and experimental verification[J].Pharmacology and Clinics of Chinese Materia Medica, 2021, 37(6):82-89.
[7] LEE S, HAN E H, LIM M K, et al.Fermented Platycodon grandiflorum extracts relieve airway inflammation and cough reflex sensitivity in vivo[J].Journal of Medicinal Food, 2020, 23(10):1060-1069.
[8] 赵玉霞. 樟帮特色米泔漂苍术工艺及药效学研究[D].南昌:江西中医药大学, 2019.ZHAO Y X.Study on the technology and pharmacodynamics of bleaching Atractylodes lancea with Zhangbang special rice bran[D].Nanchang:Jiangxi University of Traditional Chinese Medicine, 2019.
[9] LEE K S, SEONG B J, KIM S I, et al.Changes in platycoside components and antimicrobial activities of Bronchus disease-inducing bacteria of fermented Platycodon grandiflorum root by lactic acid bacteria[J].Journal of the Korean Society of Food Science and Nutrition, 2016, 45(7):1017-1025.[10] 国家药典委员会. 中华人民共和国药典:一部[M].北京:中国医药科技出版社, 2020:289.Chinese Pharmacopoeia Commission.Pharmacopoeia of the People’s Republic of China:Part One[M].Beijing:China Medical Science and Technology Press, 2020:289.
[11] ERKO S, EBERS J.Taxonomic parameters revisited:Tarnished gold standards[J].Microbiology Today, 2006, 33:152-155.
[12] BEN BRAÏEK O, GHOMRASSI H, CREMONESI P, et al.Isolation and characterisation of an enterocin P-producing Enterococcus lactis strain from a fresh shrimp (Penaeus vannamei)[J].Antonie Van Leeuwenhoek, 2017, 110(6):771-786.
[13] 刘长蕾, 文宇萍, 李冠洋, 等.魏斯氏菌的研究进展[J].食品与机械, 2022, 38(9):227-233.LIU C L, WEN Y P, LI G Y, et al.Recent progress of Weissella[J].Food &Machinery, 2022, 38(9):227-233.
[14] 李巧玉, 方芳, 堵国成, 等.魏斯氏菌在发酵食品中的应用[J].食品与发酵工业, 2017, 43(10):241-247.LI Q Y, FANG F, DU G C, et al.The application of Weissella strains in fermented food[J].Food and Fermentation Industries, 2017, 43(10):241-247.
[15] YU H S, JANG H J, LEE N K, et al.Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi[J].LWT, 2019, 112:108229.
[16] KIBAR H, ARSLAN Y E, CEYLAN A, et al.Weissella cibaria EIR/P2-derived exopolysaccharide:A novel alternative to conventional biomaterials targeting periodontal regeneration[J].International Journal of Biological Macromolecules, 2020, 165:2900-2908.
[17] YE G B, LI G L, WANG C L, et al.Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice[J].Carbohydrate Polymers, 2019, 207:218-223.
[18] YANG Y F, FENG F, ZHOU Q Q, et al.Isolation, purification and characterization of exopolysaccharide produced by Leuconostoc pseudomesenteroides YF32 from soybean paste[J].International Journal of Biological Macromolecules, 2018, 114:529-535.
[19] ZHOU Q Q, FENG F, YANG Y F, et al.Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine[J].International Journal of Biological Macromolecules, 2018, 107:2234-2241.
[20] MORANDI S, CREMONESI P, POVOLO M, et al.Enterococcus lactis sp.nov., from Italian raw milk cheeses[J].International Journal of Systematic and Evolutionary Microbiology, 2012, 62(Pt_8):1992-1996.
[21] FU X M, LYU L, WANG Y, et al.Safety assessment and probiotic characteristics of Enterococcus lactis JDM1[J].Microbial Pathogenesis, 2022, 163:105380.
[22] JUNG J A, NOH J H, JANG M S, et al.Safety evaluation of fermented Platycodon grandiflorus (Jacq.) A.DC.extract:Genotoxicity, acute toxicity, and 13-week subchronic toxicity study in rats[J].Journal of Ethnopharmacology, 2021, 275:114138.
[23] PARK E J, LEE H J.Immunomodulatory effects of fermented Platycodon grandiflorum extract through NF-κB signaling in RAW 264.7 cells[J].Nutrition Research and Practice, 2020, 14(5):453-462.
[24] AHN H J, YOU H J, PARK M S, et al.Biocatalysis of platycoside E and platycodin D3 using fungal extracellular β-glucosidase responsible for rapid platycodin D production[J].International Journal of Molecular Sciences, 2018, 19(9):2671.
[25] HA I J, HA Y W, KANG M, et al.Enzymatic transformation of platycosides and one-step separation of platycodin D by high-speed countercurrent chromatography[J].Journal of Separation Science, 2010, 33(13):1916-1922.
[26] HA Y W, NA Y C, SEO J J, et al.Qualitative and quantitative determination of ten major saponins in Platycodi Radix by high performance liquid chromatography with evaporative light scattering detection and mass spectrometry[J].Journal of Chromatography A, 2006, 1135(1):27-35.
[27] 李明泽. 桔梗皂苷D的微生物转化和抗氧化性分析[D].延吉:延边大学, 2018.LI M Z.Microbial transformation and antioxidant analysis of Platycodon grandiflorum saponin D[D].Yanji:Yanbian University, 2018.
[28] BELLOSO DAZA M V, CORTIMIGLIA C, BASSI D, et al.Genome-based studies indicate that the Enterococcus faecium Clade B strains belong to Enterococcus lactis species and lack of the hospital infection associated markers[J].International Journal of Systematic and Evolutionary Microbiology, 2021, 71(8):004948.
[29] KIM E, YANG S M, KIM H J, et al.Differentiating between Enterococcus faecium and Enterococcus lactis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry[J].Foods, 2022, 11(7):1046.