乙二醛(glyoxal, GO),一种含有α-羰基结构的亲电化合物,通过糖类降解、脂质氧化和美拉德反应等途径形成并广泛存在于各种热加工食品中,是美拉德反应的中间产物及晚期糖基化终末产物羧甲基赖氨酸[Nε-(carboxyethyl)-lysine, CML]的前体物质。人体内葡萄糖异常代谢、葡萄糖修饰蛋白和核苷酸降解及脂质过氧化都会合成内源性GO。正常生理条件下血液中GO含量0.3 μmol/L[1],但患有慢性疾病人群(如1型和2型糖尿病及慢性肾病)的血清中二羰基化合物水平相对较高[2-4]。GO这类二羰基化合容易与蛋白质的亲核侧链或氨基酸的α-氨基发生反应,形成晚期糖基化终末产物(advanced glycation end products, AGEs)且在衰老过程中积累,尤其是在含有长寿蛋白质的组织中[5]。据估计,40%~50%的AGEs由源自GO的席夫碱加合物形成。文献证实,蛋白质中的AGEs与白内障、阿尔茨海默病和包括动脉粥样硬化在内的心血管疾病等慢性疾病有关[6-7]。同时,实验发现GO对三种细胞系表现出毒性,分别为MOLT-4(人早期T细胞白血病细胞系)、HeLaS3(人上皮癌细胞系)和HCT116(人结肠癌细胞系),并抑制DNA修复功能酶活性,如人类DNA聚合酶λ(ID50=56 μmol/L)和人类DNA聚合酶βλ(ID50=215 μmol/L)[8]。
为减少食品加工过程中产生的二羰基化合物及AGEs同时最大程度上不影响食品品质,开发更健康的产品,近些年对于其抑制剂的研究逐渐成为食品工作者的研究热点。目前被报道的抑制剂大多为具有抗氧化性的天然提取物,如多酚类化合物中的儿茶素、原花青素和没食子酸[9-12],黄酮类化合物中的芦丁和表儿茶素以及生物碱类等[13-15]。此外,还有益生菌类如Clostridium butyricum菌株和Lactobacillus brevis strain[16-17],氨基酸和蛋白质消化过程中的加合物[18-19]分别能在发酵条件下、食品加工过程中和胃肠消化后消除GO,降低GO的生物可及率,在不同环节减少食品中GO的含量,减轻人体对GO的消化吸收。
国内外学者对食品中二羰基化合物的研究大多聚焦于甲基乙二醛(methylglyoxal, MGO),对GO毒理和解毒机制的系统性综述较少,国内对于GO的关注也更多在于工业材料应用和生态环境影响而不是食源性GO对人体健康的风险和威胁。因此,本文聚焦于GO在食品加工过程中的产生和在各类食品中的分布,从GO毒理机制入手阐明其对人体健康的危害,综述目前研究中食源性GO毒性的缓解,天然抑制剂的作用机理及其在食品中的应用,有助于绿色健康食品的研发,同时也为新型二羰基化合物抑制剂的研究提供思路。
1 食源性GO
外源性GO的来源主要为食品加工,富含糖类、油脂和蛋白质的食物在加工过程中容易发生降解、过氧化和美拉德反应从而生成GO。焦糖化反应中,单糖、低聚糖和多糖都会在逆醛缩合和氧化过程中裂解形成二羰基化合物。乳果糖在热加工中降解为果糖和半乳糖,继续加热进一步降解生成MGO、GO和3-脱氧葡萄酮等二羰基化合物[20]。脂质氧化分解同样增加GO在食品中的积累,且产生的GO含量跟油脂的来源有关,同时也取决于脂质的不饱和程度[21],不饱和脂肪酸占比越高的油脂产生更多的GO,如橄榄油和鱼肝油。红花油在200 ℃条件下,GO[(155.2±29.6) ng/g]的生成明显高于MGO[(34.7±5) ng/g][22]。美拉德反应中还原糖羰基与蛋白质和氨基酸的游离氨基缩合形成席夫碱,再经Amadori重排后氧化脱水裂解生成GO。饼干在烘焙过程中发生美拉德反应从而形成对风味、香气和颜色至关重要的化合物,其中包括α-二羰基化合物、5-羟甲基糠醛和丙烯酰胺等有害化学物质。饼干配方中用果糖或葡萄糖会导致成品中GO含量比使用蔗糖制作时更高,且随着烘烤时间变长而增加[23]。
各类常见食品和饮料中均含有GO(表1),干果、蛋糕和糖果中的总二羰基浓度(>400 mg/kg)相对于茶、奶制品和软饮料中的总二羰基浓度(<10 mg/kg)更高一些[24]。油炸食品和含糖量较高的烘焙食品中GO含量相较饮料也更高,如油炸面团中GO含量为6.5~11.8 mg/kg,曲奇饼干中含量为4.8~26.0 mg/kg[25-26]。除了外界环境中的二羰基化合物,进入人体的外源性GO主要来自日常饮食,ARRIBAS-LORENZO[25]等研究发现在西班牙人群每天从饼干中摄入约213 μg的GO。因此不管是寻找能消除食品加工环节中产生二羰基化合物的抑制剂,还是改善加工条件来抑制GO生成都是有必要的。同时为预防和缓解食源性GO对健康的危害,减少GO和AGEs在食品和人体内的积累,对GO毒性和解毒机制的研究也必不可少。
表1 常见食品中GO的含量
Table 1 The content of glyoxal in common foods
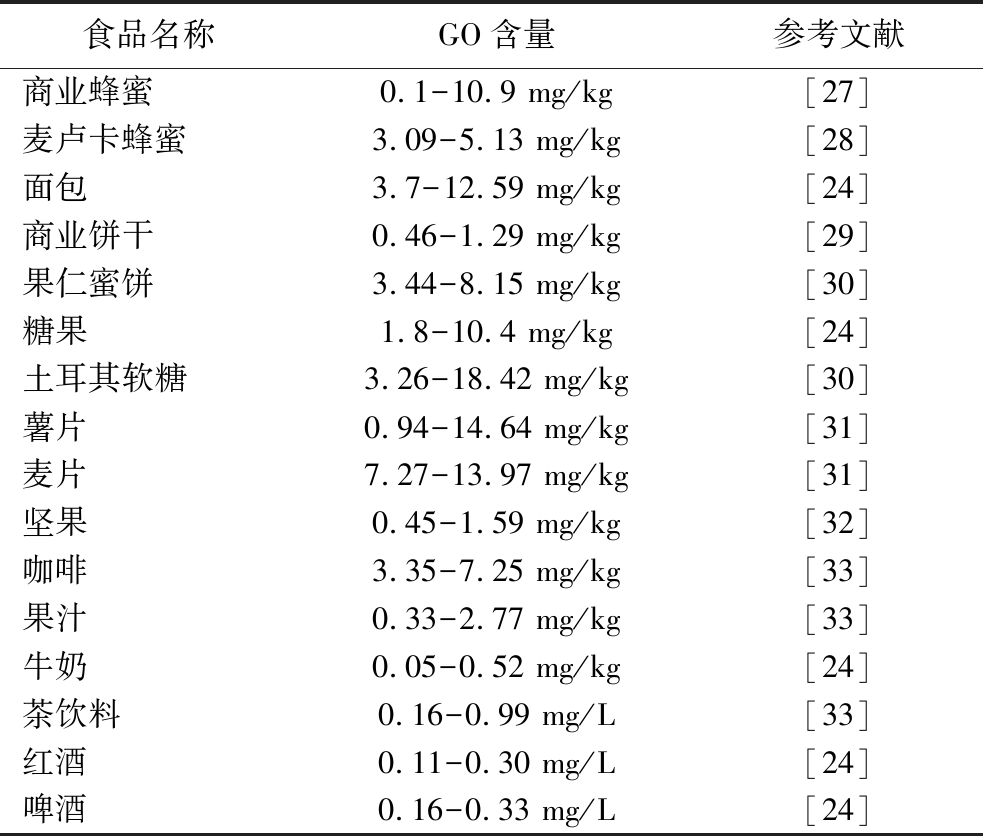
食品名称GO含量参考文献商业蜂蜜0.1-10.9 mg/kg[27]麦卢卡蜂蜜3.09-5.13 mg/kg[28]面包3.7-12.59 mg/kg[24]商业饼干0.46-1.29 mg/kg[29]果仁蜜饼3.44-8.15 mg/kg[30]糖果1.8-10.4 mg/kg[24]土耳其软糖3.26-18.42 mg/kg[30]薯片0.94-14.64 mg/kg[31]麦片7.27-13.97 mg/kg[31]坚果0.45-1.59 mg/kg[32]咖啡3.35-7.25 mg/kg[33]果汁0.33-2.77 mg/kg[33]牛奶0.05-0.52 mg/kg[24]茶饮料0.16-0.99 mg/L[33]红酒0.11-0.30 mg/L[24]啤酒0.16-0.33 mg/L[24]
2 GO毒性机制
包括GO在内的α-二羰基化合物在体内浓度增加可能导致组织和细胞功能失调,这种代谢状态也称为二羰基应激,造成活性氧(reactive oxygen species, ROS)和促炎细胞因子释放增加以及DNA结构损伤等,产生致突变性和致癌性相关的潜在健康风险。活性二羰基化合物对人体危害极大,即使体内有应对这些化合物毒性作用的有效机制,也不能完全排除饮食中α-二羰基化合物对健康产生的负面影响,例如GO已被证明会引起细胞毒性(浓度为1~10 mmol/L)、内皮细胞形态改变、线粒体功能障碍、细胞骨架重排、屏障功能障碍以及抑制DNA合成、细胞复制和呼吸链功能[34]。GO在体内毒性机制的阐明是预防和缓解二羰基应激的关键,下文将从GO通过与蛋白质等大分子交联形成晚期糖基化终末产物,在细胞内引起氧化应激,诱发炎症三个方面介绍GO的毒性机制(图1)。
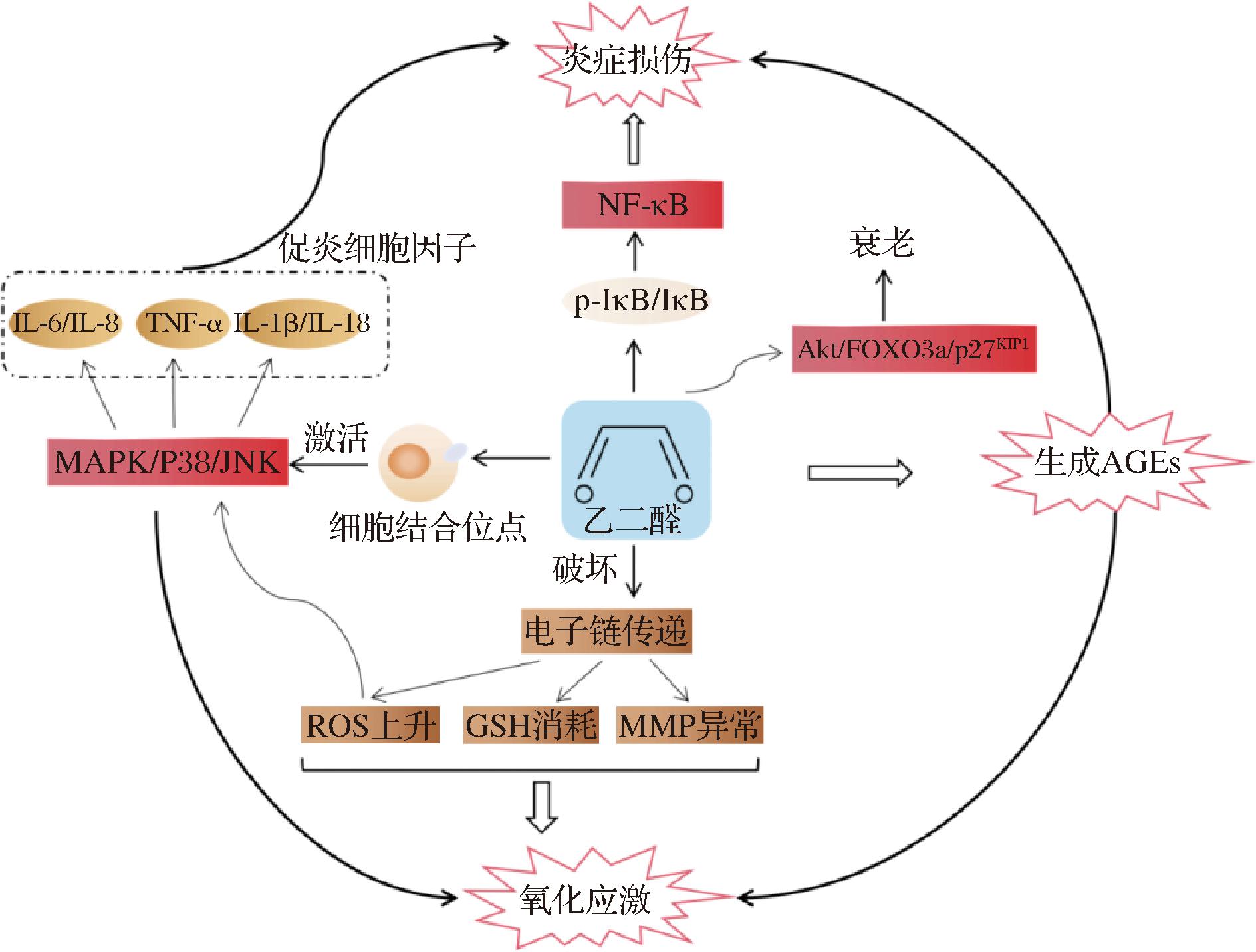
图1 GO的毒性作用机制
Fig.1 Mechanism of toxicity of glyoxal
2.1 形成晚期糖基化终末端产物
GO是一种相对不稳定的缺电子分子,属于亲电子化合物,可以与亲核试剂(具有活性电子对的分子)反应,生成共价键。在生理条件下GO易与氨基酸和蛋白质上的游离氨基以及核苷酸发生交联形成多类稳定加合物,即晚期糖基化终末产物,含有AGEs的交联蛋白能抵抗蛋白质水解从而在组织和体液中积累[35]。而AGEs本身在体内会造成自由基增加,促进促炎细胞分子释放,引起氧化应激和炎症反应水平上升,导致糖尿病并发症如胰岛素抵抗、动脉粥样硬化、糖尿病肾病和视网膜疾病的产生[36-37]。同时,AGEs在脑部积累与神经退行性疾病的早发和加速进展也存在一定关联。GO与DNA双链体可以形成GO-鸟嘌呤从而使核酸内部产生交联损伤[38-39],NEVIN等[39]研究发现GO能引起精子中CML的水平显著升高,而AGEs位于精子细胞的部位包括其最主要的头部,对DNA完整性产生有害影响。和MGO的性质相似,GO具有较强糖基化活性,能与多种蛋白质相互作用,如牛血清白蛋白、β-突触核蛋白和血红蛋白,修饰蛋白质的赖氨酸和精氨酸残基,形成多种产物如CML、羧甲基精氨酸(carboxymethylarginine, CMA)和氢咪唑酮(glyoxal-derived hydroimidazolone, G-H1、G-H2和G-H3),进入红细胞与细胞蛋白相互作用降低其变形能力。GO能诱导人血清白蛋白(human serum albumin, HSA)糖基化,促进HSA中AGEs交联形成[40],修饰HSA上与药物等外源性化合物结合的位点,存在影响药物-HSA在体内的吸收、分布和代谢的可能[41]。BANERJEE等[42]实验发现GO与血红蛋白和肌红蛋白在25 ℃下孵育七天后,血红蛋白的吸光度、固有荧光和表面疏水性均降低(280 nm),热稳定性增加,MALDI-TOF结果表明与血红蛋白上的Arg-31α、Arg-40β和Arg-104β位点结合生成氢咪唑酮加合物,修饰肌红蛋白上的Lsy-133和Lsy-145位点形成CML。G-H1和特定的AGEs与长期亚临床动脉粥样硬化的严重程度相关,并可能在长期2型糖尿病患者血管并发症的“负代谢记忆”中发挥重要作用[43]。肥胖但其他方面健康人群能通过增加G-H1等物质的排泄分数和肾脏清除率来防止代谢应激期间的氧化损伤和AGEs的积累[44]。
与蛋白质、核酸等大分子发生加合和交联反应形成AGEs是GO在体内产生毒性的主要途径,且AGEs会导致糖尿病、肾衰竭、神经退行性疾病和与衰老相关的慢性并发症[45]。因此,在食品工业生产和体内消化吸收后抑制GO及其衍生AGEs的形成是缓解二羰基应激的有效手段,同时减少食源性AGEs的摄入,减轻人体免疫负担。
2.2 引起氧化应激
GO本身具有的细胞毒性与氧化应激有关,毒性通过损害细胞抗氧化酶系统而显著增加。作为含有亲电基团的活性物质,GO由于其高活性可与大分子相互作用,并通过谷胱甘肽(glutathione, GSH)和NADP(H)等氧化还原辅助因子影响细胞的氧化还原,导致细胞损伤。谷胱甘肽是一种含有三肽的活性半胱氨酸残基,是细胞中主要的可溶性抗氧化分子,在细胞质、细胞核和线粒体中含量丰富[46-48]。参与糖酵解的酶能被GO大量修饰,显著抑制GAPDH的活性,从而通过削弱糖酵解促进多种疾病的病理过程[49]。GOUDARZI等人通过实验研究GO对离体大鼠肝线粒体的线粒体膜电位(mitochondrial membrane potential, MMP)、丙二醛(malondialdehyde, MDA)、ROS形成、GSH含量和蛋白质羰基化的影响,结果表明GO作用于肝线粒体,破坏电子传递链,增加线粒体中ROS形成,造成脂质过氧化、线粒体膜损伤、GSH氧化和蛋白质羰基化,导致ROS和MDA水平上升,GSH耗尽以及MMP破坏,毒性作用呈剂量依赖性且涉及糖尿病及其并发症的病理机制[49]。XIE等[50]通过比色分析、荧光定量和Western Blotting检测人内皮细胞暴露于GO后的氧化应激,结果同样显示在经GO处理后细胞中的谷胱甘肽活性异常、线粒体膜电位崩溃,此外细胞毒性还表现为切割氧化蛋白的二硫键从而影响细胞内氧化应激清除的硫氧还蛋白酶-1。
除了通过损害抗氧化性系统,GO还能通过胞内信号转导,激活AGEs-RAGE信号通路,增加细胞内ROS水平,激活MAPK和JNK信号通路,引起细胞氧化应激。此外,AGEs与受体RAGE结合会导致ROS和GO水平增加,GO的增加进一步导致AGEs含量上升,并加重机体的氧化应激状态。除了与RAGE受体识别反应外,HALKOUM等[51]还研究发现,人角质形成细胞经GO刺激后,不仅ROS和晚期糖基化终产物水平上升从而引起氧化应激,还激活蛋白激酶B/FOXO3a /p27KIP1途径介导细胞周期停滞进入早期衰老以及p16INK4/pRb途径维持的晚期衰老。此外,红细胞能吸收外源性GO,并将其大部分转化为乙醇酸,其中约1%转化为草酸,而这种草酸盐形成途径可能会增强糖尿病和其他与氧化应激相关的疾病[52]。二羰基化合物通过破坏胞内抗氧化系统和激活信号转导通路引起氧化应激,造成组织和细胞功能障碍,各类疾病诱因与风险增加。
2.3 诱发炎症反应
GO可以与许多细胞结合位点相互作用,如AGEs受体,RAGE。RAGE是一种多配体模式识别受体,能够放大炎症反应,持续激活其介导的氧化应激和炎症反应。SUBRAMANIAN等[53]发现,被注射GO的大鼠体内RAGE的表达增强。有研究表明RAGE的过度表达可加重小鼠肾小球内皮细胞功能障碍和炎症反应,加重疾病的进展[54]。AGEs-RAGE相互作用介导的炎症效应机制的激活涉及多种细胞内途径,包括MAPK信号途径。人内皮细胞暴露于GO后,细胞内丝裂原活化蛋白激酶(MAP)途径被激活,磷酸化的MAPK途径的表达水平立即增加,包括P-ERK(105%)、P-JNK(314%)和P-P38(159%),从而加重细胞的炎症损伤[50]。GOLD是GO衍生的晚期糖基化终产物,LEE等研究发现GOLD通过MAPK途径诱导鼠细胞炎症机制相关的促炎细胞因子释放,如肿瘤坏死因子α(TNF-α)、白细胞介素IL-6、IL-1β和IL-18[55],GO本身也同样通过MAPK途径引起人肠道细胞(Caco-2和HT-29)中IL-8的产生[56],经GO处理的人角质细胞中IL-8 mMRA表达上升,均作用呈剂量依赖性[51]。糖尿病性肾病也是一种慢性炎症疾病,而NF-κB通路的激活已被证明是肾脏损伤中炎症的主要触发因素。ZHU等[57]和LIU等[58]实验通过检测p-IκB和IκB蛋白的表达,研究GO对人胚胎肾细胞中NF-κB通路的影响,GO浓度为0.5、1和2 mmol/L时,p-IκB蛋白的表达上调并且p-IκB/IκB的比率显著增加,而有研究报道IκB蛋白在缺氧缺血性脑损伤后磷酸化激活NF-κB信号通路,表明GO可能通过激活NF-κB信号通路诱导人胚胎肾细胞炎症损伤。
二羰基应激诱导的细胞毒性危害并不独立,氧化应激与炎症反应就有着密切的关系。机体在免疫调节的过程中产生ROS以抵抗某些疾病和外来抗原,但ROS的过量积累与抗氧化系统的损伤则导致异常的炎症反应,引发促炎基因转录和促炎细胞因子释放同时放大氧化应激,进一步促进炎症细胞激活,形成恶性循环。因此,抗氧化系统稳态的维持和相应信号通路的调节是缓解和抑制GO毒性值得关注的2个途径。
3 GO抑制剂的作用机制及应用
3.1 捕获和结合
为缓解二羰基化合物及其衍生AGEs诱导的细胞毒性,最主要的办法是对活性二羰基化合物进行捕获或清除,从而抑制AGEs的形成,减轻二羰基应激。抑制剂大多是从植物中提取具有抗氧化性的天然化学产物,具有原料易获取、营养价值高和副作用少等特点。一些研究也表明,增加饮食中天然抗氧化剂的摄入量,如大多数植物中的类黄酮和其他酚类化合物,能有效预防与氧化应激相关疾病[59-60]。HUANG等[61]使用玉米糠中的阿魏酰化低聚糖来抑制AGEs在葡萄糖-氨基酸和葡萄糖-牛血清白蛋白模型中形成,结果表明阿魏酰化低聚糖在高温碱性环境下水解释放阿魏酸,阿魏酸酚羟基对位的氢具有足够的活性,可与二羰基化合物缩合,减少AGEs的形成。同时,阿魏酸上的甲氧基激活了苯环上的π电子,使得苯环上的氢更容易被羰基化合物取代而对其进行捕获。而ZHANG等[62]利用玉米须提取物来清除酪蛋白葡萄糖-脂肪酸模型体系中GO和MGO,进而抑制CML的形成,结果显示玉米丝提取物中含有多种黄酮类化合物,如山柰酚、槲皮素和原花青素等,具有较强的抗氧化和清除自由基的能力,与GO形成单价加合物或三加合物来捕获二羰基化合物,从而抑制CML的形成,抑制率可达76.57%。
除了通过与二羰基化合物加合,抑制剂还能通过自身的抗氧化活性抑制糖自氧化,与氨基酸或蛋白质上的结合位点竞争来缓解GO诱导的毒性作用[40,63]。SARMAH等人利用柑橘果实中的柚皮苷和柚皮素进行实验,测试这2种类黄酮植物化学物质对GO诱导的糖化作用、晚期糖化终产物和HSA原纤维形成的影响。结果表明,柚皮苷和柚皮素对GO介导的HSA糖化作用具有抑制,对AGEs的形成也有类似的抑制作用,增加了被GO修饰HSA的游离氨基的百分比,对蛋白质的伯氨基显示出一定的屏蔽作用,并保护它们免受GO介导的糖化。同时,赖氨酸和精氨酸残基与类黄酮的相互作用也可以防止残基在糖化过程中被修饰。此外,柚皮苷和柚皮素能与HSA的ⅡA亚结构域结合,且柚皮素还能和ⅢA子结构域结合,保护更多的残基,形成氢键和其他非共价键为HSA的结构提供稳定性,在一定程度上抑制了HSA中GO诱导的纤维状聚集体形成[40]。槲皮素也被发现能通过与结合位点中的赖氨酸残基相互作用来抑制葡萄糖诱导的蛋白质糖化[64]。
3.2 调节解毒系统
生物体内能采用各种方法对活性二羰基化合物进行解毒,主要依赖于乙二醛酶系统和NADPH依赖性解毒酶。乙二醛酶系统由乙二醛酶Ⅰ和Ⅱ(Glyoxalase, Glo1和Glo2)组成,是参与二羰基应激解毒的主要酶,能将细胞质中的活性二羰基化合物代谢为D-乳酸,Glo1的表达水平和活性降低会导致MGO和GO的积累。乙二醛酶系统根据其辅因子可进一步分类分为GSH依赖性和GSH非依赖性。细胞中GSH依赖性乙二醛酶系统是GO解毒的主要途径。GSH与GO非酶促结合形成单硫缩醛,在Glo1的作用下转化为S-羟乙酰谷胱甘肽,之后Glo2将S-羟乙酰谷胱甘肽水解为乙醇酸盐。CIANFRUGLIA等[65]研究发现苹果多酚能增加受慢性高葡萄糖诱导氧化应激和二羰基应激的细胞中的GSH水平,调控Glo1和Glo2的活性,提高细胞的总抗氧化能力。苹果中含有多种类黄酮物质,有研究提出经类黄酮处理的细胞中GSH水平的增加可能与γ-谷氨酰-半胱氨酸合成酶催化亚基启动子的反式激活有关[66]。黄酮类儿茶素、桑黄素和槲皮素可增强神经元细胞中的乙二醛酶通路,导致Glo1和Glo2过表达,增加GSH水平并降低ROS浓度[67]。2型糖尿病病人进行维生素D补充后,体内Glo1表达增加、RAGE受体转录下调和血清中AGEs减少[68]。
3.3 细胞免疫通路调控
天然抑制剂除了通过捕获GO来清除二羰基化合物,调控GO解毒途径,还能通过调节细胞信号通路来缓解二羰基应激。类黄酮能够调节多种信号通路涉及细胞免疫和代谢途径,包括NF-κB、MAPK、ERK和Nrf2[69-70],调节和减少基因的促凋亡和促炎产物表达,抑制导致细胞凋亡的激酶和磷酸酶激活。此外,类黄酮增加细胞内GSH水平还能防止谷氨酸介导的Ca2+内流,调节与细胞存活相关的信号通路。类黄酮被自由基氧化后,生成的醌参与细胞抗氧化和修复活动的信号通路。ROS在免疫反应中被用作信号分子,抗氧化剂的存在可以阻止ROS介导的分子和通路靶标的磷酸化,从而阻止它们的激活和转录,如桑黄素能够阻断ROS和炎症细胞因子对NF-κB通路的激活,防止诱导细胞死亡的信号级联反应[71]。同时,除了调控上述提到的细胞信号通路,抑制剂还能通过调节信号通路影响乙二醛酶解毒系统。十字花科蔬菜中富集的硫代葡萄糖苷经酶促转化为异硫氰酸酯,其中的萝卜硫素可通过降低MAPK信号通路(ERK1/2、JNK和p38)磷酸化来抵消糖基化引起的损伤,从而增强原代新生大鼠心肌细胞和SH-SY5Y神经母细胞瘤细胞中的乙二醛酶系统,且不影响从健康个体分离的外周血单核细胞中Glo1的活性[72-74]。在葡萄、浆果和花生中发现的白藜芦醇能通过上调ERK通路和Nrf2的核转移来上调乙二醛酶系统表达[75],另一种天然酚类化合物芒果苷可通过上调Nrf2信号,提高乙二醛酶活性,防止AGEs的形成[76]。
各类GO抑制剂的类型、来源和抑制剂机理归纳见图2和表2,除了天然化学产物,益生菌也表现出缓解食源性二羰基化合物诱导细胞毒性的特性。LI等[16]用Caco-2细胞模型检测Clostridium butyricum菌株缓解食源性醛类诱导的氧化应激和炎症损伤,实验表明C.butyricum能提高受到GO胁迫的Caco-2细胞活性,降低GO、MGO和4-羟基己烯醛等食源性醛诱导的ROS水平和自噬程度,上调Nrf2信号途径的表达,如HO-1、SOD-1和CAT,并维持在适当的水平,同时调控NLRP3和NLRP6蛋白来减轻氧化应激和炎症,且C.butyricum本身对Caco-2细胞的活性没有影响[16]。
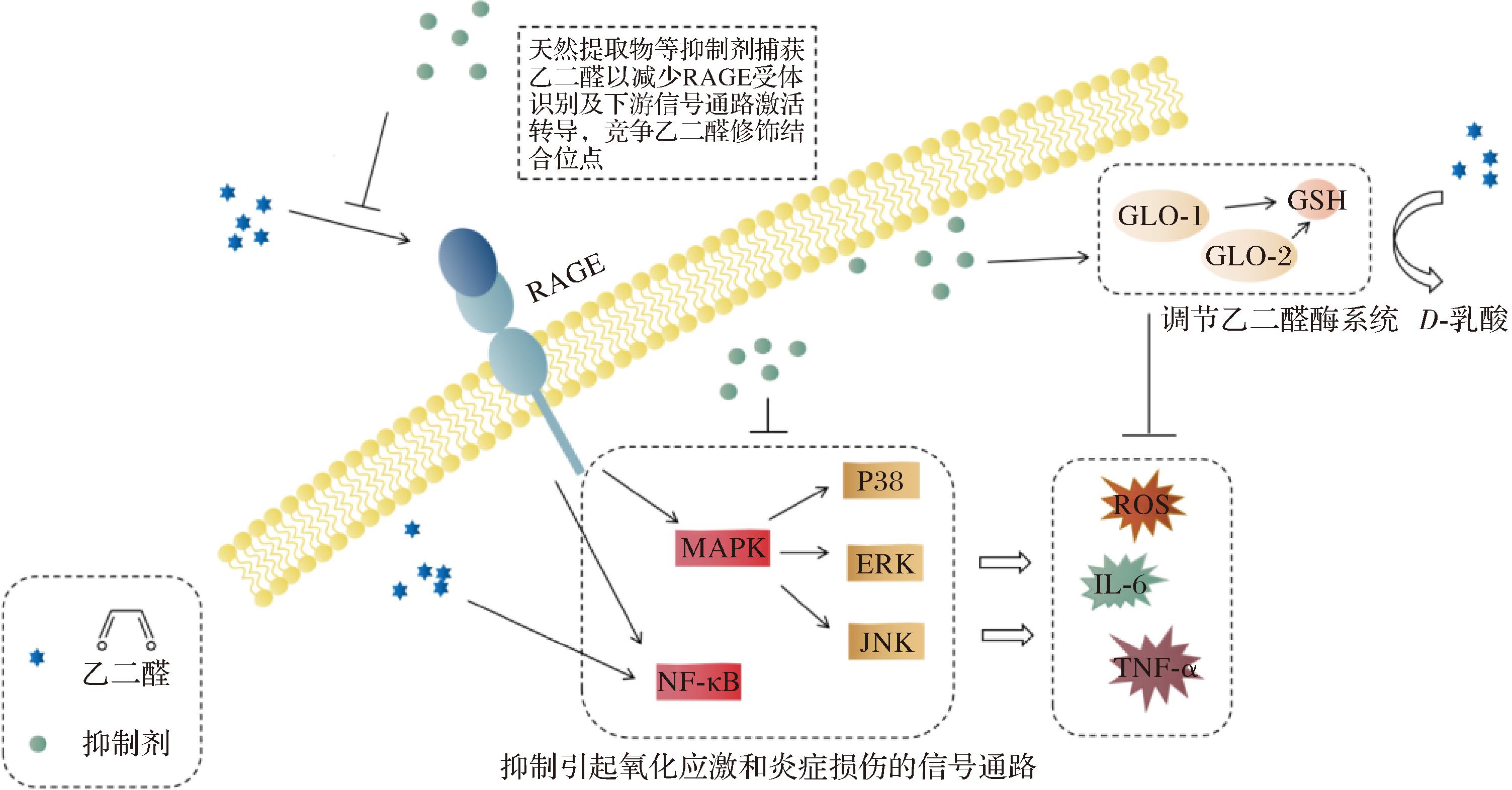
图2 抑制剂缓解GO毒性机理
Fig.2 Mechanism of inhibition of glyoxal toxicity
表2 GO抑制剂类型、来源和抑制机理
Table 2 Types, sources, and inhibitory mechanisms of glyoxal inhibitors
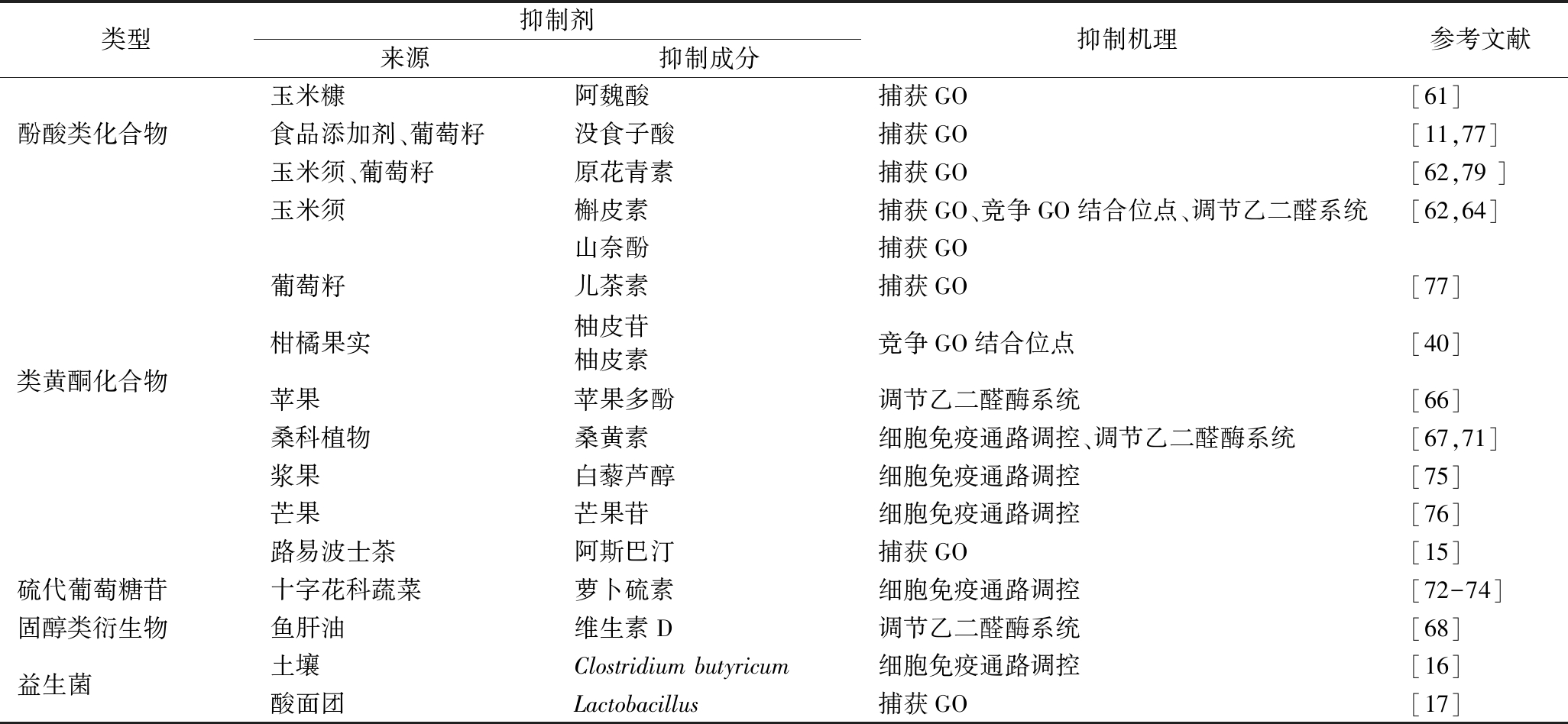
类型抑制剂来源抑制成分抑制机理参考文献酚酸类化合物玉米糠阿魏酸捕获GO[61]食品添加剂、葡萄籽没食子酸捕获GO[11,77]玉米须、葡萄籽原花青素捕获GO[62,79 ]类黄酮化合物玉米须槲皮素捕获GO、竞争GO结合位点、调节乙二醛系统[62,64]山奈酚捕获GO葡萄籽儿茶素捕获GO[77]柑橘果实柚皮苷柚皮素竞争GO结合位点[40]苹果苹果多酚调节乙二醛酶系统[66]桑科植物桑黄素细胞免疫通路调控、调节乙二醛酶系统[67,71]浆果白藜芦醇细胞免疫通路调控[75]芒果芒果苷细胞免疫通路调控[76]路易波士茶阿斯巴汀捕获GO[15]硫代葡萄糖苷十字花科蔬菜萝卜硫素细胞免疫通路调控[72-74]固醇类衍生物鱼肝油维生素D调节乙二醛酶系统[68]益生菌土壤Clostridium butyricum细胞免疫通路调控[16]酸面团Lactobacillus捕获GO[17]
3.4 抑制剂在食品中的应用
越来越多的文献表明,二羰基化合物和AGEs积累会影响组织中的细胞功能在分子和细胞衰老的进程中发挥致病作用。为预防食品中的二羰基化合物对人体的潜在的危害风险,消除GO及其在食品生产过程中与糖类、油脂和蛋白质进一步反应生成的AGEs,可行的方法之一是在食品加工环节添加能对GO进行捕获和清除的抑制剂,阻断AGEs形成,从而减少食源性GO和AGEs摄入。从食物和饮料中摄入的外源性抗氧化剂在细胞防御和生存中发挥重要作用,并被证明有助于身体对抗氧化应激和炎症[78]。XUE等[79]实验发现山奈中的活性成分能还原烤牛肉饼中的二羰基化合物,从而抑制杂环胺和AGEs形成。ZHU等[77]通过实验得出,0.5 g/kg的葡萄籽提取物能有效的在油炸和空气油炸条件下能减少鸡胸肉中GO和MGO的产生,从而抑制成品中CML和羧乙基赖氨酸[Nε-(carboxyethyl)-lysine, CEL]的水平。经乳酸菌发酵的酸面团在烘烤后GO的生物可及性指数降低,体外消化后GO增加的程度减少,能减轻对AGEs在体内的积累[17]。褐藻苷苔提取物具有良好的抗氧化作用,能降低豆浆中GO含量,与105 ℃高压灭菌处理的豆浆相比,0.5%的褐藻苷苔提取物联合紫外热超声处理能减少豆浆中78%的GO[80]。具有天然抗氧化性的茶叶提取物是一种常见的食品添加成分,对食品中的二羰基化合物及AGEs具有一定的清除能力。BEDNARSKA等[15]实验发现未发酵的路易波士茶提取物中含有阿司巴汀等类黄酮物质,抑制蛋白质糖基化的同时与GO加合来捕获二羰基化合物。新鲜马铃薯片在50 g/L绿茶提取物预浸泡处理后炸成的薯片成品中GO含量下降20.3%,MGO含量下降69.7%,荧光AGEs含量下降42.9%[81]。这不仅为了生产更健康的薯片提供了思路,还发掘了茶叶行业中的边角料绿茶粉尘的再利用。
4 总结与展望
GO等二羰基化合物广泛分布于各类食品原料和成品,并在食品加工和贮存过程中积累以及形成AGEs等有害化合物,对人体健康产生威胁。除了内源性合成的GO,随着日常饮食摄入的外源性GO与体内的氨基酸、核酸和蛋白质等分子反应形成AGEs,破坏细胞的形态和功能并在组织中沉积从而导致多种疾病发生。同时,GO破坏细胞内的电子传递链引起ROS水平上升、GSH消耗增加和线粒体膜电位异常等氧化应激,与细胞上的结合位点相互作用,如RAGE受体,激活MAPK和NF-κB途径及其下游信号转导,诱导ROS产生和促炎细胞因子释放导致炎症损伤和进一步的氧化应激。此外,肿瘤生长、皮肤细胞衰老、糖尿病和肾病等慢性疾病进展都与GO暴露有关。目前研究的抑制剂主要通过对GO进行捕获或者竞争GO修饰和结合的位点,调节体内乙二醛酶等解毒系统的活性和表达,调控与氧化应激和炎症损伤相关的信号通路以应对二羰基应激诱导的毒性作用。抑制剂的种类大多是从植物中分离的天然化合物,如葡萄籽和莲房中提取的原花青素、柑橘果实中分离的柚皮苷和柚皮素等。除此之外,乳酸菌和丁酸菌等肠道益生菌也被研究用于应对GO等二羰基化合物及其衍生物毒性应激。
二羰基化合物在食品加工过程中的产生是不可避免的,但研究开发有效消除GO形成,捕获食品中积累的GO以及减轻GO诱导细胞毒性的抑制物质仍是值得关注的焦点。益生菌类抑制剂与天然化合物相比便利性和安全性更高,应用性也更为广泛,但其具体作用机制和清除效果仍有待研究。而胃肠道的消化吸收是食源性GO进入人体的必由之路,未来的研究也可以关注GO及益生菌类抑制剂在消化道中的变化规律,了解其生理效应的关键,确定GO在胃肠道中的分子靶点和反应产物,以及影响GO从食物基质中释放的因素等,有助于了解其他食物成分如何影响体内GO和AGEs的形成,有益于抑制剂的开发和应用。
[1] HAN Y C, RANDELL E, VASDEV S, et al.Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes[J].Molecular and Cellular Biochemistry, 2007, 305(1-2):123-131.
[2] HANSSEN N M J, WESTERINK J, SCHEIJEN J L J M, et al.Higher plasma methylglyoxal levels are associated with incident cardiovascular disease and mortality in individuals with type 2 diabetes[J].Diabetes Care, 2018, 41(8):1689-1695.
[3] ODANI H, SHINZATO T, MATSUMOTO Y, et al.Increase in three alpha,beta-dicarbonyl compound levels in human uremic plasma:Specific in vivo determination of intermediates in advanced Maillard reaction[J].Biochemical and Biophysical Research Communications, 1999, 256(1):89-93.
[4] SCHEIJEN J L J M, SCHALKWIJK C G.Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry:Evaluation of blood specimen[J].Clinical Chemistry and Laboratory Medicine, 2014, 52(1):85-91.
[5] FU M X, REQUENA J R, JENKINS A J, et al.The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions[J].The Journal of Biological Chemistry, 1996, 271(17):9982-9986.
[6] AVILA F, FRIGUET B, SILVA E.Photosensitizing activity of endogenous eye lens chromophores:An attempt to unravel their contributions to photo-aging and cataract disease[J].Photochemistry Photobiology, 2015, 91(4):767-779.
[7] DRENTH H, ZUIDEMA S U, KRIJNEN W P, et al.Advanced Glycation End-Products Are Associated With the Presence and Severity of Paratonia in Early Stage Alzheimer Disease[J].Journal of the American Meadical Directors Association, 2017, 18(7):636.e7-636.e12.
[8] AMOROSO A, MAGA G, DAGLIA M.Cytotoxicity of α-dicarbonyl compounds submitted to in vitro simulated digestion process[J].Food Chemistry, 2013, 140(4):654-659.
[9] 吴夏青. 儿茶素对糖苷酶和蛋白质非酶糖基化的抑制作用及机制研究[D].南昌:南昌大学,2019.WU X Q.Inhibitory effect and mechanism of catechins on glycosidase and protein non-enzymatic glycation[D]. Nanchang:Nanchang University, 2019.
[10] 赵扩权, 张芬, 罗庆等.莲房原花青素对AGEs诱导的小鼠肠道组织损伤和微生物紊乱的改善作用[J].中国食品学报, 2022, 22(5):70-83.ZHAO K Q, ZHANG F, LUO Q, et al.Effect of lotus seedpod oligomeric procyanidins on tissue injury and intestinal microbial disturbance of mice induced by AGEs[J].Journal of Chinese Institute of Food Science and Technology, 2022, 22(5):70-83.
[11] HOU Y, XIE Z J, CUI H Q, et al.Trapping of glyoxal by propyl, octyl and dodecyl gallates and their mono-glyoxal adducts[J].Food Chemistry, 2018, 269:396-403.
[12] 杨志军. EGCG抑制甲基乙二醛及晚期糖基化终末产物对PC12细胞损伤作用研究[D]. 沈阳:辽宁大学,2020.YANG Z J.The inhibitory effect of EGCG on PC12 cells damage induced by methylglyoxal and advanced glycation end products[D]. Shenyang:Liaoning University, 2020.
[13] 蒋楠, 王富静, 封亮等.基于非酶糖基化反应的槐花抑制AGEs形成的活性组分筛选[J].中国中药杂志, 2019, 44(14):3100-3106.JIANG N, WANG F J, FENG L, et al.Screening for active components of Sophorae Flos on inhibiting AGEs formation based on non-enzymatic glycation reaction[J].China Journal of Traditional Chinese Materia Medica, 2019, 44 (14):3100-3106.
[14] 刘荟萃, 李巨秀.几种抑制剂抗晚期糖基化/脂质过氧化终产物(AGEs/ALEs)作用的比较[J].中国食品学报, 2015, 15(10):11-18.LIU M J, LI J X.Inhibitory effect of different inhibitor formation of advanced glycation endproducts (AGEs) and advanced lipoxidation endproducts(ALEs) in vitro[J].Journal of Chinese Institute Food Science and Technology, 2015, 15(10):11-18.
[15] BEDNARSKA K, FECKA I. Aspalathin and other rooibos flavonoids trapped α-dicarbonyls and inhibited formation of advanced glycation end products in vitro[J]. International Journal of Molecular Sciences, 2022, 23(23):14738.
[16] LI J Y, SHEN H K, ZHAO Z J, et al.Protective effects of Clostridium butyricum against oxidative stress induced by food processing and lipid-derived aldehydes in Caco-2 cells[J].Applied Microbiology and Biotechnology, 2020, 104:9343-9361.
[17] OZGOLET M, YAMAN M, ZEKI DURAK M, et al.The effect of five different sourdough on the formation of glyoxal and methylglyoxal in bread and influence of in vitro digestion[J].Food Chemistry, 2022, 371:131141.
[18] 林佳钰, 黄才欢, 郑洁,等.氨基酸同时消除甲醛和乙二醛的机理及消减产物的细胞毒性[J].食品科学, 2023, 44(4):278-285.LIN J Y, HUANG C H, ZHENG J, et al.Mechanisms for simultaneous removal of formaldehyde and glyoxal by amino acids and cytotoxicity of their products[J].Food Science, 2023, 44(4):278-285.
[19] JIANG K Y, HUANG C H, JIAO R, et al.Adducts formed during protein digestion decreased the toxicity of five carbonyl compounds against Caco-2 cells[J].Journal of Hazardous Materials, 2019, 363:26-33.
[20] DONG L, YU Z T, ZHAO R M, et al.The effect of lactulose thermal degradation products on β-lactoglobulin:Linear-, loop-, and cross-link structural modifications and reduced digestibility[J].Food Chemistry, 2023, 403:134333.
[21] SHIBAMOTO T.Analytical methods for trace levels of reactive carbonyl compounds formed in lipid peroxidation systems[J].Journal of Pharmaceutical and Biomedical Analysis, 2006, 41(1):12-25.
[22] JIANG Y P, HENGEL M, PAN C P, et al.Determination of toxic α-dicarbonyl compounds, glyoxal, methylglyoxal, and diacetyl, released to the headspace of lipid commodities upon heat treatment.[J].Journal of Agricultural and Food Chemistry, 2013, 61(5):1067-1071.
[23] FALLICO B, GRASSO A, ARENA E.Hazardous chemical compounds in cookies:The role of sugars and the kinetics of their formation during baking[J].Foods, 2022, 11(24):4066.
[24] MAASEN K, SCHEIJEN J L J M, OPPERHUIZEN A, et al.Quantification of dicarbonyl compounds in commonly consumed foods and drinks;presentation of a food composition database for dicarbonyls[J].Food Chemistry, 2021, 339:128063.
[25] ARRIBAS-LORENZO G, MORALES F J.Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants[J].Journal of Agricultural and Food Chemistry, 2010, 58(5):2966-2972.
[26] LIU H C, LI J X.Changes in glyoxal and methylglyoxal content in the fried dough twist during frying and storage[J].European Food Research and Technology, 2014, 238(2):323-331.
[27] ARENA E, BALLISTRERI G, TOMASELLI F, et al.Survey of 1,2-dicarbonyl compounds in commercial honey of different floral origin[J].Journal of food science and technology, 2011, 76(8):C1203-C1210.
[28] MARSHALL S M, SCHNEIDER K R, CISNEROS K V, et al.Determination of antioxidant capacities, α-dicarbonyls, and phenolic phytochemicals in Florida varietal honeys using HPLC-DAD-ESI-MS(n.)[J].Journal of Agricultural and Food Chemistry, 2014, 62(34):8623-8631.
[29] YAMAN M, DEMIRCI M, EDE-CINTESUN E, et al.Investigation of formation of well-known AGEs precursors in cookies using an in vitro simulated gastrointestinal digestive system[J].Food Chemistry.2022, 373(Pt A):131451.
[30] UGUR HALIME, GORUNMEK MIHRAC, CATAK, JALE, et al.Determination and assessment of the most potent precursors of advanced glycation end products in baklava and Turkish delight by HPLC[J].Food Science and Technology, 2020, 42:e08522.
[31] CENGIZ S, KI
![]() C, ÇEBI N, et al.Determination of the most potent precursors of advanced glycation end products (AGEs) in chips, crackers, and breakfast cereals by high performance liquid chromatography (HPLC) using precolumn derivatization with 4-nitro-1, 2-phenlenediamine[J].Microchemical Journal, 2020, 158:105170.
C, ÇEBI N, et al.Determination of the most potent precursors of advanced glycation end products (AGEs) in chips, crackers, and breakfast cereals by high performance liquid chromatography (HPLC) using precolumn derivatization with 4-nitro-1, 2-phenlenediamine[J].Microchemical Journal, 2020, 158:105170.
[32] LIU W T, WANG Y T, XU D C, et al.Investigation on the contents of heat-induced hazards in commercial nuts[J].Food Research International, 2023, 163:112041.
[33] WANG C, LU Y L, HUANG Q J, et al.Levels and formation of α-dicarbonyl compounds in beverages and the preventive effects of flavonoids[J].Journal of Food Science and Technology, 2017, 54(7):2030-2040.
[34] SLIMAN S M, EUBANK T D, KOTHA S R, et al.Hyperglycemic oxoaldehyde, glyoxal, causes barrier dysfunction, cytoskeletal alterations, and inhibition of angiogenesis in vascular endothelial cells:Aminoguanidine protection[J].Molecular and cellular biochemistry, 2010, 333(1-2):9-26.
[35] LÜTH H J, OGUNLADE V, KUHLA B, et al.Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer′s disease brains[J].Cerebral Cortex, 2005, 15(2):211-220.
[36] COHEN M P, ZIYADEH F N, CHEN S. Amadori-modified glycated serum proteins and accelerated atherosclerosis in diabetes: Pathogenic and therapeutic implications[J]. Journal of Laboratory and Clinical Medicine, 2006, 147(5):211-219.
[37] FOSMARK D S, TORJESEN P A, KILHOVD B K, et al. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated withretinopathy in patients with type 2 diabetes mellitus[J]. Metabolism, 2006,55:232-236.
[38] VILANOVA B, FERN NDEZ D, CASASNOVAS R, et al.Formation mechanism of glyoxal-DNA adduct, a DNA cross-link precursor[J].International Journal of Biological Macromolecules, 2017, 98:664-675.
NDEZ D, CASASNOVAS R, et al.Formation mechanism of glyoxal-DNA adduct, a DNA cross-link precursor[J].International Journal of Biological Macromolecules, 2017, 98:664-675.
[39] NEVIN C, MCNEIL L, AHMED N, et al.Investigating the glycating effects of glucose, glyoxal and methylglyoxal on human sperm[J].Scientific Reports, 2018, 8(1):9 002.
[40] SARMAH S, GOSWAMI A, KUMAR B V, et al.Mitigation of ribose and glyoxal induced glycation, AGEs formation and aggregation of human serum albumin by citrus fruit phytochemicals naringin and naringenin:An insight into their mechanism of action[J].Food Research International, 2022, 157:111358.
[41] IFTEKHAR S, LI Z, TAO P Y, et al.Analysis of the binding of warfarin to glyoxal- and methylglyoxal-modified human serum albumin by ultrafast affinity extraction[J].Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 2022, 1211:123500.
[42] BANERJEE S, CHAKRABORTI A S.Structural alterations of hemoglobin and myoglobin by glyoxal:A comparative study[J].International Journal of Biological Macromolecules, 2014, 66:311-318.
[43] SAREMI A, HOWELL S, SCHWENKE D C, et al.Advanced glycation end products, oxidation products, and the extent of atherosclerosis during the VA diabetes trial and follow-up study[J].Diabetes care, 2017, 40(4):591-598.
[44] PERKINS R K, MIRANDA E R, KARSTOFT K, et al.Experimental hyperglycemia alters circulating concentrations and renal clearance of oxidative and advanced glycation end products in healthy obese humans[J].Nutrients, 2019, 11(3):532.
[45] KOUKA P, PRIFTIS A, STAGOS D, et al.Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts[J].International journal of molecular medicine, 2017, 40(3):703-712.
[46] KOUKA P, CHATZIEFFRAIMIDI G A, RAFTIS G, et al.Antioxidant Effects of an olive oil total polyphenolic fraction from a Greek Olea europaea variety in different Cell cultures[J].Phytomedicine:International Journal of Phytotherapy and Phytopharmacology, 2018, 47:135-142.
[47] VESKOUKIS A, KERASIOTI E, PRIFTIS A, et al.A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds:The biomarker issue[J].Current Opinion in Toxicology, 2019, 13:99-109.
[48] CHEN Y, QIN W, LI Z H, et al.Site-specific chemoproteomic profiling of targets of glyoxal[J].Future Medicinal Chemistry, 2019, 11(23):2979-2987.
[49] GOUDARZI M, KALANTARI H, REZAEI M.Glyoxal toxicity in isolated rat liver mitochondria[J].Human &Experimental Toxicology, 2018, 37(5):532-539.
[50] XIE M Z, GUO C, DONG J Q, et al.Glyoxal damages human aortic endothelial cells by perturbing the glutathione, mitochondrial membrane potential, and mitogen-activated protein kinase pathways[J].BMC Cardiovascular Disorders, 2021, 21(1):603.
[51] HALKOUM R, SALNOT V, CAPALLERE C, et al. Glyoxal induces senescence in human keratinocytes through oxidative stress and activation of the protein kinase B/FOXO3a/p27KIP1 pathway[J]. The Journal of Investigative Dermatology, 2022, 142(8):2068-2078.e7.
[52] KNIGHT J, WOOD K D, LANGE J N, et al.Oxalate formation from glyoxal in erythrocytes[J].Urology, 2016, 88:226.e11-5.
[53] SUBRAMANIAN U, SHARMA A, VENKATACHALAM G, et al.Induction of renal damage and modulation of redox potential in rats infused with glyoxal[J].Biomedicine &Preventive Nutrition, 2012, 2(2):119-124.
[54] SHU A M, DU Q, CHEN J, et al.Catalpol ameliorates endothelial dysfunction and inflammation in diabetic nephropathy via suppression of RAGE/RhoA/ROCK signaling pathway[J].Chemico-biological interactions, 2021, 348:109625.
[55] LEE H W, GU M J, KIM Y, et al.Glyoxal-lysine dimer, an advanced glycation end product, induces oxidative damage and inflammatory response by interacting with RAGE[J].Antioxidants, 2021, 10(9):1486.
[56] KUNTZ S, KUNZ C, RUDLOFF S.Carbonyl compounds methylglyoxal and glyoxal affect interleukin-8 secretion in intestinal cells by superoxide anion generation and activation of MAPK p38[J].Molecular Nutrition &Food Research, 2010, 54(10):1458-1467.
[57] ZHU J J, YU B Y, FU C C, et al.LXA4 protects against hypoxic-ischemic damage in neonatal rats by reducing the inflammatory response via the IκB/NF-κB pathway[J].International Immunopharmacology.2020, 89:107095.
[58] LIU D, CHEN J L, XIE Y Z, et al.Investigating the molecular mechanisms of glyoxal-induced cytotoxicity in human embryonic kidney cells:Insights from network toxicology and cell biology experiments[J].Environmental toxicology, 2022, 37(9):2269-2280.
[59] HALLIWELL B.Dietary polyphenols:good, bad, or indifferent for your health?[J].Cardiovascular Research, 2007, 73(2):341-347.
[60] DE O RIOS A, ANTUNES L M, DE LP BIANCHI M.Bixin and lycopene modulation of free radical generation induced by cisplatin-DNA interaction[J].Food Chemistry, 2008, 113(4):1113-1118.
[61] HUANG J Q, REN J Y, TAO G, et al.Maize bran feruloylated oligosaccharides inhibited AGEs formation in glucose/amino acids and glucose/BSA models[J].Food Research International, 2019, 122:443-449.
[62] ZHANG D W, WANG Y Z, LIU H L.Corn silk extract inhibit the formation of Nε -carboxymethyllysine by scavenging glyoxal/methyl glyoxal in a casein glucose-fatty acid model system[J].Food Chemistry, 2020, 309:125 708.
[63] PENG X F, MA J Y, CHENG K W, et al.The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread[J].Food Chemistry, 2010, 119(1):49-53.
[64] ALAM M M, AHMAD I, NASEEM I.Inhibitory effect of quercetin in the formation of advance glycation end products of human serum albumin:An in vitro and molecular interaction study[J].International Journal of Biological Macromolecules, 2015, 79:336-343.
[65] CIANFRUGLIA L, MORRESI C, BACCHETTI T, et al.Protection of Polyphenols against Glyco-Oxidative Stress:Involvement of Glyoxalase Pathway[J].Antioxidants, 2020, 9(10):1006.
[66] MYHRSTAD M C W, CARLSEN H, NORDSTRÖM O, et al.Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter[J].Free Radical Biology &Medicine, 2002, 32(5):386-393.
[67] FRANDSEN J, NARAYANASAMY P.Flavonoid Enhances the Glyoxalase Pathway in Cerebellar Neurons to Retain Cellular Functions[J].Scientific Reports, 2017, 7(1):5126.
[68] OMIDIAN M, DJALALI M, JAVANBAKHT M H, et al.Effects of vitamin D supplementation on advanced glycation end products signaling pathway in T2DM patients:a randomized, placebo-controlled, double blind clinical trial[J].Diabetology &metabolic syndrome, 2019, 11(1):86.
[69] JIN H N, LEE W S, EUN S Y, et al.Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway[J].International Journal of Oncology, 2014, 45(4):1629-1637.
[70] JIANG W, LUO T, LI S, et al.Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons[J].PLoS ONE, 2016, 11(4):e0152371.
[71] KIM J M, LEE E K, PARK G L, et al.Morin modulates the oxidative stress-induced NF-kappaB pathway through its anti-oxidant activity[J].Free Radical Research, 2010, 44(4):454-461.
[72] ALFARANO M, PASTORE D, FOGLIANO V, et al.The Effect of Sulforaphane on Glyoxalase I Expression and Activity in Peripheral Blood Mononuclear Cells[J].Nutrients, 2018, 10(11):1773.
[73] LIU Y W, LIU X L, KONG L, et al.Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation[J].Biomedicine &Pharmacotherapy=Biomedecine &Pharmacotherapie, 2019, 109:2145-2154.
[74] ANGELONI C, MALAGUTI M, RIZZO B, et al.Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity[J].Chemical research in toxicology, 2015, 28(6):1234-1245.
[75] FARKHONDEH T, FOLGADO S L, POURBAGHER-SHAHRI A M, et al.The therapeutic effect of resveratrol:Focusing on the Nrf2 signaling pathway[J].Biomedicine &Pharmacotherapy=Biomedecine &Pharmacotherapie, 2020, 127:110 234.
[76] LIU Y W, CHENG Y Q, LIU X L, et al.Mangiferin upregulates glyoxalase 1 through activation of Nrf2/ARE signaling in central neurons cultured with high Glucose[J].Molecular Neurobiology, 2017, 54(6):4060-4070.
[77] ZHU Z S, FANG R, YANG J, et al.Air frying combined with grape seed extract inhibits Nε-carboxymethyllysine and Nε-carboxyethyllysine by controlling oxidation and glycosylation[J].Poultry Science, 2021, 100(2):1308-1318.
[78] RAHMAN I.Dietary polyphenols mediated regulation of oxidative stress and chromatin remodeling in inflammation[J].Nutrition reviews, 2008, 66 Suppl 1(Suppl.1):S42-S45.
[79] XUE C Y, QUAN W, LI Y, et al.Mitigative capacity of Kaempferia galanga L.and kaempferol on heterocyclic amines and advanced glycation end products in roasted beef patties and related mechanistic analysis by density functional theory[J].Food chemistry, 2022, 385:132660.
[80] PARK J J, OLAWUYI I F, LEE W Y.Effect of combined UV-thermosonication and Ecklonia cava extract on advanced glycation end-products in soymilk[J].Journal of Food Process Engineering, 2023, 46(1):e14208.
[81] WANG W T, WANG H X, WU Z J, et al. Reduction in five harmful substances in fried potato chips by pre-soaking treatment with different tea extracts[J]. Foods, 2023, 12(2):321.