全球人口老龄化速度正在逐渐加快。2000年我国进入人口老龄化社会,且近年来人口老龄化程度逐步加深。据WHO报道,预计2015—2050年期间,世界60岁以上人口的比例将从12%升至22%;2024年国家统计局发布的国民经济和社会发展统计公报显示:我国60周岁及以上人口占总人口的21.1%,并将于2035年之前进入高度老龄化社会。在老年健康管理方面,《“健康中国2030”规划纲要》强调要加强重点人群的健康服务,对老年常见病、慢性病进行健康指导和综合干预,强化老年人健康管理,促进健康老龄化。《“十四五”国民健康规划》也将“预防为主”列入基本原则之一,同时指出要强化健康教育,提高老年人主动健康能力[1]。
研究表明,随着年龄增长,肠道菌群逐渐失衡。而肠道微生物菌群及其代谢产物在调节肠道健康和疾病方面发挥着核心作用。肠道微生物菌群组成的变化与炎症和代谢紊乱有关,包括炎症性肠病(inflammatory bowel disease,IBD)、高血压、糖尿病、心血管疾病、癌症和神经衰退疾病[2-3]。本文首先总结老年人肠道菌群变化特征及相关疾病、改善老年人肠道菌群失衡相关疾病的途径,重点阐述益生菌在改善老年人代谢综合征、炎症性肠病、肿瘤和癌症、神经退行性疾病中的作用以及研究进展,以期为老年人健康管理和疾病治疗提供理论依据。
1 肠道菌群组成随年龄增长的变化
人类肠道微生物群含有约100万亿个肠道细菌[4],其组成在一生中经历了巨大变化(图1)。刚出生时婴儿的胃肠道是无菌的。随着婴儿的生长,胃肠道逐渐被微生物定植,且受喂养方式影响。一般来说,母乳喂养的婴儿中乳酸杆菌属和双歧杆菌属的丰度较高,而未母乳喂养的婴儿肠道微生物中拟杆菌属含量更高[5]。随着年龄增长,成人肠道微生物多样性增加,虽随种族、饮食和生活方式的不同稍有差异[6],但都以厚壁菌门、拟杆菌门和放线菌门为优势菌群[7]。随着年龄持续增长,进入衰老后肠道内菌群多样性下降,厚壁菌门和双歧杆菌减少,而变形菌门和拟杆菌门增加;特别是有抗炎作用和产生丁酸(盐)以及短链脂肪酸(short chain fatty acids, SCFAs)的梭菌簇XIVa和粪杆菌显著降低,还有产关键代谢产物的嗜黏蛋白阿克曼氏菌(可降解黏蛋白)和布氏瘤胃球菌(可分解膳食抗性淀粉)也随年龄增长降低[8],此外,老年人往往会积累更多与衰老疾病相关的代谢物,如胆碱、三甲胺、N8-乙酰亚精胺[9]。因此,随着老年人肠道菌群的变化,肠道功能下降,黏蛋白和淀粉降解减少,SCFAs产生量减少,必需氨基酸和维生素合成减少[10],使得机会致病菌生长和繁殖进而促进炎症,同时疾病相关代谢物的增多也会引起或加剧各种老年人相关疾病。
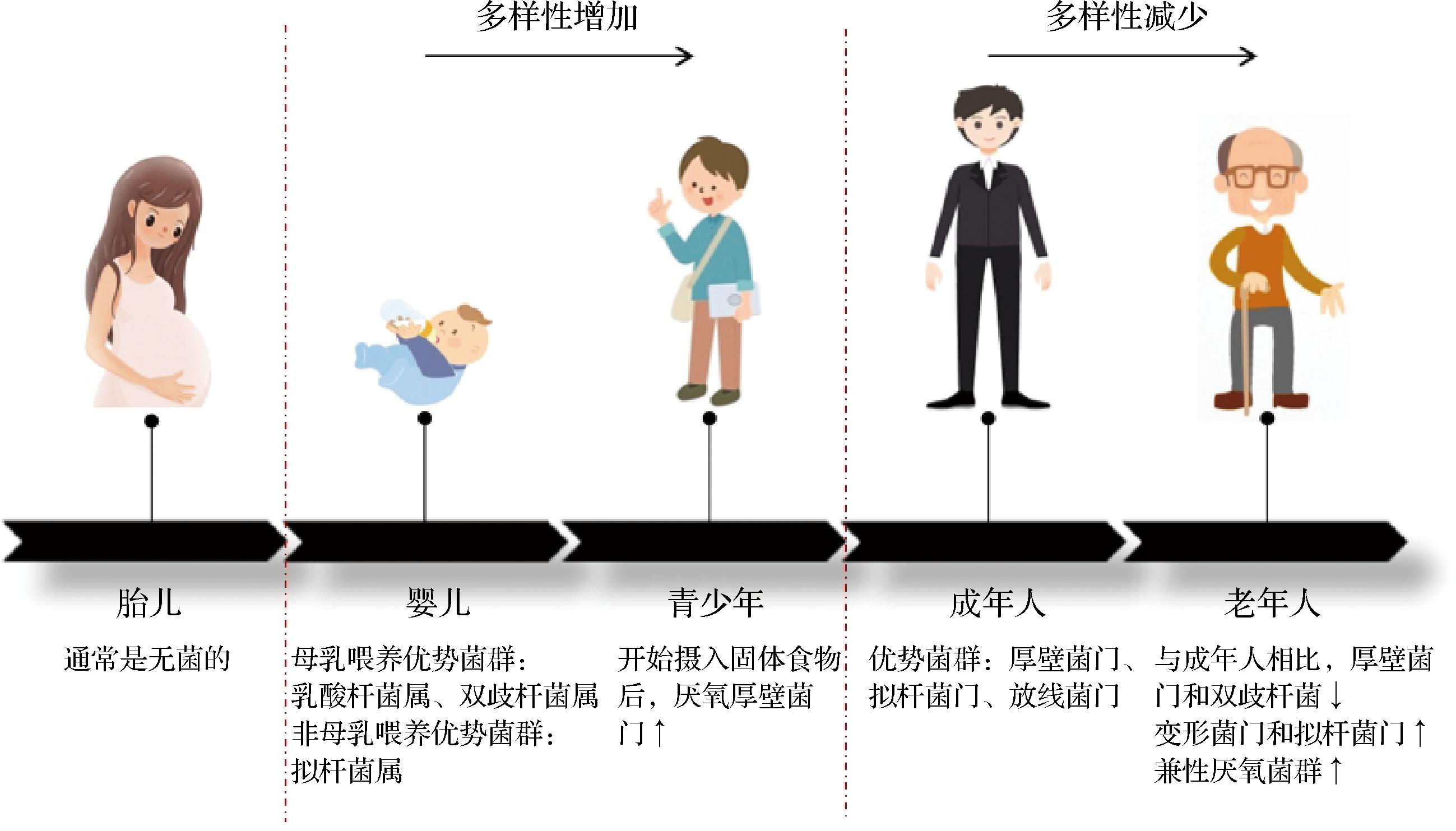
图1 不同生命阶段肠道菌群组成的变化
Fig.1 Changes of gut microbiota composition in different life stages
注:↑表丰度增加,↓表丰度降低。
2 老年人肠道菌群失衡相关疾病及改善途径
稳定的肠道菌群以共生方式保护宿主肠道健康,抵抗外界干扰、维持正常结构功能和代谢功能[8]。老年人肠道菌群组成的改变会引起肠道生理变化(胃动力障碍、肠神经系统退化等),较高的炎症反应和免疫衰老[11]。免疫衰老导致的免疫细胞的数量和功能下降使得老年人对疾病的易感性增加,更易引发感染和免疫系统性疾病,包括胃肠道疾病(例如IBD)、高血压与心血管疾病、糖尿病、癌症、神经退行性疾病等[12]。JACKSON等[13]发现老年人中普氏粪杆菌丰度减少而肠杆菌科丰度增加,进而导致老年人虚弱指数升高;在阿尔茨海默病(Alzheimer’s disease,AD)老年患者的微生物组中缺乏瘤胃球菌科和毛螺科的相关菌属,而阿利斯特属和拟杆菌属丰度更高[14];产SCFAs的粪杆菌属、罗斯氏菌属和普氏菌属的相对丰度与帕金森病(Parkinson’s disease,PD)呈负相关,并伴有肠道神经系统改变[15]。
目前改善老年人肠道菌群失衡相关疾病的主要途径是药物治疗、粪便微生物群移植(fecal microbial transplantation, FMT)和益生菌。常见的西药包括左旋多巴(针对神经退行性疾病)、血管紧张素转化酶抑制剂(高血压)、β受体拮抗剂(心脑血管疾病),双胍类、糖苷酶抑制剂等(糖尿病)。但长期服用某一种药物会导致肠道微生物群失衡和细菌耐药性增强[16]。FMT可将健康供体的粪便物质移植到受体的肠道中来恢复和重建受体肠道微生态平衡和多样性,通过促进SCFAs的产生来降低肠道通透性,从而降低艰难梭菌感染、IBD等疾病的严重程度,保持肠上皮屏障完整性[17]。但是FMT可能会造成腹泻、低热、感染和脓毒症等潜在不良反应[18];同时,该技术在健康供体的选择,治疗持续时间等方面还有待优化。益生菌以其促进肠道健康和免疫系统的能力而闻名,不仅可帮助调节人体肠道菌群中与年龄相关的失衡,促进健康菌株生长,还可以调节免疫系统,通过调节表达和细胞分化,产生抗衰老作用,因此益生菌是改善老年人肠道菌群失衡相关疾病风险最低、效果显著的最佳选择。
3 益生菌改善老年人肠道菌群失衡相关疾病的作用及作用机制
由于老年人肠道菌群失衡导致的免疫衰老和抗炎能力减弱,老年人更易引发炎症和免疫系统性疾病,包括代谢综合征、IBD、肿瘤和癌症以及神经退行性疾病等。目前已经有许多临床研究发现益生菌可以用于治疗肠道菌群失衡所引起的疾病(表1),其中,乳酸杆菌属和双歧杆菌属是构成益生菌的重要菌属,服用形式包括菌粉、菌剂、胶囊和发酵乳,但益生菌的功效具有高度的菌株特异性,因此有些菌株干预可以产生显著影响,有些菌株干预效果甚微。但总体来说益生菌可以通过增加对黏膜表面的黏附来抑制病原体的黏附,竞争性地消除病原微生物,产生抗菌物质,调节免疫功能,保护肠黏膜并重建肠道菌群结构[19]。下面具体综述益生菌改善老年人肠道菌群失衡相关疾病的作用及作用机制。
表1 不同益生菌干预对中老年人疾病的影响
Table 1 Effect of different probiotics intervention on intestinal flora imbalance diseases
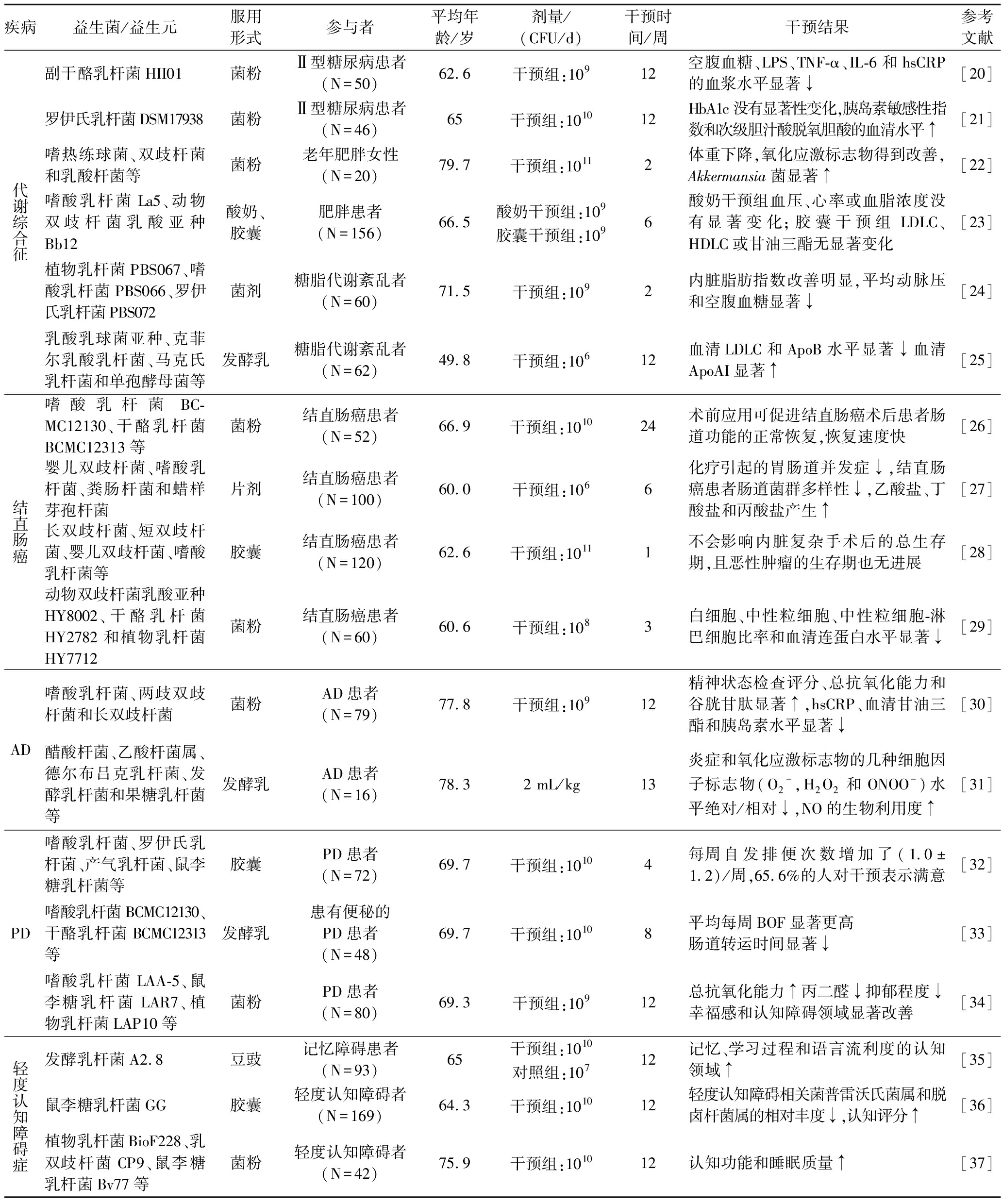
疾病益生菌/益生元服用形式参与者平均年龄/岁剂量/(CFU/d)干预时间/周干预结果参考文献代谢综合征副干酪乳杆菌HII01菌粉Ⅱ型糖尿病患者(N=50)62.6干预组:10912空腹血糖、LPS、TNF-α、IL-6和hsCRP的血浆水平显著↓[20]罗伊氏乳杆菌DSM17938菌粉Ⅱ型糖尿病患者(N=46)65干预组:101012HbA1c没有显著性变化,胰岛素敏感性指数和次级胆汁酸脱氧胆酸的血清水平↑[21]嗜热练球菌、双歧杆菌和乳酸杆菌等菌粉老年肥胖女性(N=20)79.7干预组:10112体重下降,氧化应激标志物得到改善,Akkermansia菌显著↑[22]嗜酸乳杆菌La5、动物双歧杆菌乳酸亚种Bb12酸奶、胶囊肥胖患者(N=156)66.5酸奶干预组:109胶囊干预组:1096酸奶干预组血压、心率或血脂浓度没有显著变化;胶囊干预组LDLC、HDLC或甘油三酯无显著变化[23]植物乳杆菌PBS067、嗜酸乳杆菌PBS066、罗伊氏乳杆菌PBS072菌剂糖脂代谢紊乱者(N=60)71.5干预组:1092内脏脂肪指数改善明显,平均动脉压和空腹血糖显著↓[24]乳酸乳球菌亚种、克菲尔乳酸乳杆菌、马克氏乳杆菌和单孢酵母菌等发酵乳糖脂代谢紊乱者(N=62)49.8干预组:10612血清LDLC和ApoB水平显著↓血清ApoAI显著↑[25]结直肠癌嗜酸乳杆菌BC-MC12130、干酪乳杆菌BCMC12313等菌粉结直肠癌患者(N=52)66.9干预组:101024术前应用可促进结直肠癌术后患者肠道功能的正常恢复,恢复速度快[26]婴儿双歧杆菌、嗜酸乳杆菌、粪肠杆菌和蜡样芽孢杆菌片剂结直肠癌患者(N=100)60.0干预组:1066化疗引起的胃肠道并发症↓,结直肠癌患者肠道菌群多样性↓,乙酸盐、丁酸盐和丙酸盐产生↑[27]长双歧杆菌、短双歧杆菌、婴儿双歧杆菌、嗜酸乳杆菌等胶囊结直肠癌患者(N=120)62.6干预组:10111不会影响内脏复杂手术后的总生存期,且恶性肿瘤的生存期也无进展[28]动物双歧杆菌乳酸亚种HY8002、干酪乳杆菌HY2782和植物乳杆菌HY7712菌粉结直肠癌患者(N=60)60.6干预组:1083白细胞、中性粒细胞、中性粒细胞-淋巴细胞比率和血清连蛋白水平显著↓[29]AD嗜酸乳杆菌、两歧双歧杆菌和长双歧杆菌菌粉AD患者(N=79)77.8干预组:10912精神状态检查评分、总抗氧化能力和谷胱甘肽显著↑,hsCRP、血清甘油三酯和胰岛素水平显著↓[30]醋酸杆菌、乙酸杆菌属、德尔布吕克乳杆菌、发酵乳杆菌和果糖乳杆菌等发酵乳AD患者(N=16)78.32 mL/kg13炎症和氧化应激标志物的几种细胞因子标志物(O2-,H2O2和ONOO-)水平绝对/相对↓,NO的生物利用度↑[31]PD嗜酸乳杆菌、罗伊氏乳杆菌、产气乳杆菌、鼠李糖乳杆菌等胶囊PD患者(N=72)69.7干预组:10104每周自发排便次数增加了(1.0±1.2)/周,65.6%的人对干预表示满意[32]嗜酸乳杆菌BCMC12130、干酪乳杆菌BCMC12313等发酵乳患有便秘的PD患者(N=48)69.7干预组:10108平均每周BOF显著更高肠道转运时间显著↓[33]嗜酸乳杆菌LAA-5、鼠李糖乳杆菌LAR7、植物乳杆菌LAP10等菌粉PD患者(N=80)69.3干预组:10912总抗氧化能力↑丙二醛↓抑郁程度↓幸福感和认知障碍领域显著改善[34]轻度认知障碍症发酵乳杆菌A2.8豆豉记忆障碍患者(N=93)65干预组:1010对照组:10712记忆、学习过程和语言流利度的认知领域↑[35]鼠李糖乳杆菌GG胶囊轻度认知障碍者(N=169)64.3干预组:101012轻度认知障碍相关菌普雷沃氏菌属和脱卤杆菌属的相对丰度↓,认知评分↑[36]植物乳杆菌BioF228、乳双歧杆菌CP9、鼠李糖乳杆菌Bv77等菌粉轻度认知障碍者(N=42)75.9干预组:101012认知功能和睡眠质量↑[37]
注:表中↑表增加,↓表降低;LPS(lipopolysaccharide),脂多糖;TNF-α(tumor necrosis factor-α),肿瘤坏死因子α;IL-6(interleukin-6),白细胞介素-6;hsCRP(high sensitivity C reactive protein),血清高敏C反应蛋白;HbA1c(glycosylated hemoglobin, type A1C),血红蛋白A1c;AST(aspartate aminotransferase),谷草转氨酶;LDLC(low density lipoprotein cholesterol),低密度脂蛋白胆固醇;HDLC(high density lipoprotein cholesterol),高密度脂蛋白胆固醇;ApoB(apolipoprotein B),载脂蛋白B;ApoAI(apolipoprotein A-I),载脂蛋白AI;IPA(indole-3-Propionic acid),吲哚-3-丙酸;BDNF(brain-derived neurotrophic factor),血清脑源性神经营养因子;BOF(bowel opening frequency),开肠频率。
3.1 代谢综合征
代谢综合征是指人体的蛋白质、脂肪、碳水化合物等物质发生代谢紊乱的病理状态,是一组复杂的代谢紊乱症候群,是导致糖尿病、心脑血管疾病的危险因素。由于肠道菌群失衡导致的免疫衰老使得老年人抵御感染和炎症的能力降低,老年人更容易产生葡萄糖代谢和脂质代谢异常的症状进而引发代谢综合征[38]。部分益生菌菌株可改变肠道菌群,降低血糖水平与胰岛素抵抗、降低总胆固醇和HDLC的浓度,延缓体重增加[39]。FORD等[40]发现补充两歧双歧杆菌HA-132、短双歧杆菌HA-129和长双歧杆菌HA-135的老年女性肠道微生物群稳定性更高,体重更轻;此外CICERO等[24]发现服用植物乳杆菌PBS067、嗜酸乳杆菌PBS066和罗伊氏乳杆菌PBS072的代谢综合征患者内脏脂肪指数得到明显改善且显著降低了平均动脉压和空腹血糖水平;然而,在BELLIKCI-KOYU等[41]的研究中,连续饮用12周开菲尔的代谢综合征患者后空腹胰岛素、胰岛素抵抗指数、TNF-α、γ干扰素(interferon γ,IFN-γ)以及收缩压和舒张压虽显著降低,但与对照组无显著差异,这可能是因为代谢综合征是一种复杂的疾病,涉及早发性糖尿病、胰岛素抵抗和肥胖,单独使用益生菌并不能有效地控制血糖指数,可将益生菌作为一种预防糖尿病的补充或辅助疗法,更能有效地调节糖尿病患者的肠道菌群,控制血糖水平进而更有效地缓解老年人的糖尿病。朱建丰等[42]用盐酸二甲双胍和双歧杆菌乳杆菌三联活菌片联合治疗2型糖尿病,发现三联活菌片的加入可显著降低老年患者血清各项检测指标水平,治疗效果显著高于单独使用盐酸二甲双胍,证实了益生菌对代谢综合征的辅助治疗效果。
益生菌改善糖尿病和肥胖等代谢综合征的可能机制如图2-a所示。一方面益生菌通过抑制LPS水平来减少促炎细胞因子的分泌,TNF-α介导的细胞凋亡[41],增加抗炎细胞因子IL-10以及与IL-10相关的胰岛素抵抗,最终达到预防和稳定糖尿病的目的。另一方面,益生菌刺激肠道中SCFAs产生,进而促进脂肪细胞分泌脂联素,达到控制血糖的目的;SCFAs还可以通过激活GPR41和GPR43使EEC和肠L细胞分泌PYY和GLP-1、GIP[40],降低食欲来改善肥胖;同时GLP-1可促进胰岛素分泌而达到降血糖的作用[43]。
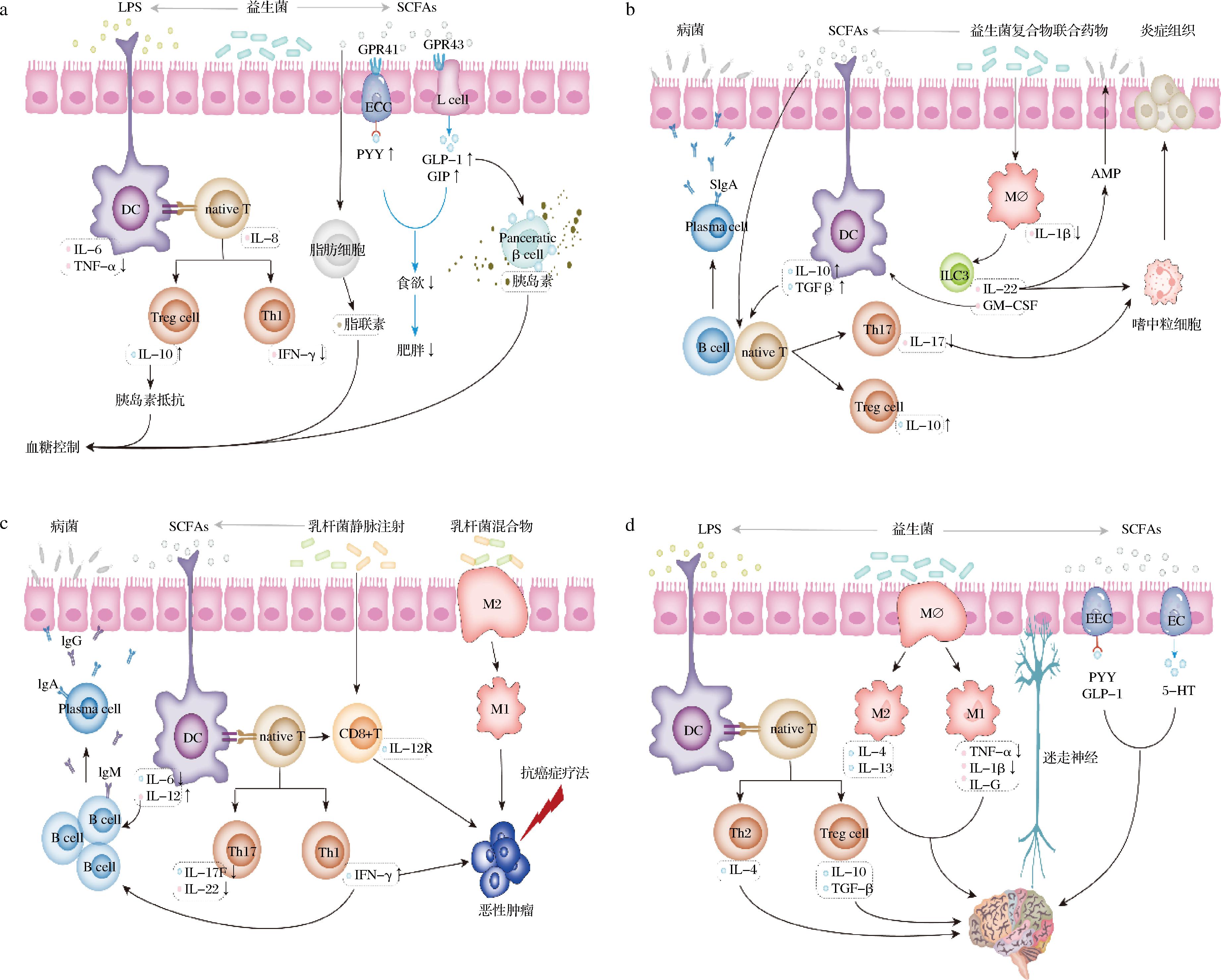
a-糖尿病;b-IBD;c-癌症;d 神经退行性疾病
图2 益生菌缓解老年人肠道菌群失衡相关疾病机理图
Fig.2 Mechanism of probiotics in the treatment of diseases related to gut microbiota imbalance in the elderly
注:图中↑表增加细胞因子的分泌,↓表降低细胞因子的分泌;EEC(enteroendocrine cell),肠内分泌细胞;ECC(enterochromaffin cells),肠嗜铬细胞;L cell,肠L细胞;GPR41(G-protein coupled receptor 41),G蛋白偶联受体41;GPR43(G-protein coupled receptor 43),G蛋白偶联受体43;PYY(peptide YY),多肽YY;GLP-1(glucagon-like peptide-1),胰高血糖素样肽-1;GIP(glucose indolence insulin-promoting polypeptide),葡萄糖依赖性促胰岛素多肽;AMP(antimicrobial peptide),抗菌肽;TGF-b(transforming growth factor-b),转化生长因子b;ILC3(group 3 innate lymphoid cell),3型天然淋巴细胞;GM-CSF(granulocyte-macrophage colony stimulating factor),粒细胞-巨噬细胞集落刺激因子;Th17(T helper cell 17),辅助性T细胞17;Treg cell(Regulatory cells),调节性T细胞;5-HT(5-hydroxytryptamine),5-羟色胺;Pancreatic b cell,胰腺b细胞;Mø,巨噬细胞;plasma cell,浆细胞;M1,M1型巨噬细胞;M2,M2型巨噬细胞;CD8+T,细胞毒性T淋巴细胞。
3.2 IBD
IBD为累及回肠、直肠、结肠的一种特发性肠道炎症性疾病,常见为非特异性溃疡性结肠炎与克罗恩病。老年人肠道菌群中有益菌群的减少而潜在病原体的增加改变了促炎细胞和免疫调节细胞之间的平衡,特别是Th17和Treg细胞,从而引发IBD[44]。目前专门针对老年人IBD的研究很少,多是覆盖各年龄段的研究,这些研究已经充分证明益生菌可以通过调节肠道微生物群来减轻IBD[45],还可通过调节宿主的免疫反应来缓解IBD[46];SHARMA等[47]发现在小鼠模型中长双歧杆菌Bif10和短双歧杆菌Bif11可显著降低TNF-α和脂质运载蛋白水平;增加抗炎标志物(IL-10和IL-22),并改善盲肠含量中的SCFAs;AGRAIB等[48]同样发现溃疡性结肠炎患者补充含副干酪乳杆菌A234、加氏乳杆菌A237、鼠李糖乳杆菌A119等的益生菌胶囊会增加抗炎因子IL-10的水平,降低C反应蛋白、免疫球蛋白G(immunoglobulin G,IgG)和免疫球蛋白M(immunoglobulin M,IgM)的水平,并对血细胞计数有所改善;值得注意的是,XIA等[49]评估了8种不同益生菌株缓解小鼠结肠炎的能力,发现植物乳杆菌AR113和干酪乳杆菌AR342在减轻上皮损伤指标、改善结肠长度(+10%~30%)和保持上皮屏障完整性方面比其他菌株有效2~3倍,同时还下调了促炎细胞因子(TNF-α、IL-1β、IL-6)的表达以及上调了结肠抗炎细胞因子(IL-10)的表达。总体来说,使用益生菌作为膳食补充剂可诱导抗炎反应,显著降低IL-1、TNF-α和IL-8水平,恢复肠道稳态,缓解IBD症状[50],但缓解效果具有菌株特异性。
益生菌缓解IBD的主要机制可能有:调控炎症因子;增强肠道屏障;增强SCFAs的产生;诱导AMP的表达;嗜中粒细胞募集(图2-b)。SCFAs诱导的B细胞内和T细胞内微生物传感诱导的分泌型免疫球蛋白A(secretory immunoglobulin A, sIgA)反应促进肠道稳态,分泌的SIgA通过与肠道微生物结合和防止病菌侵入而促进屏障功能;此外,SCFAs作用于固有层的树突状细胞(dendritic cell, DC)上,以刺激包括IL-10和TGF-β在内的细胞因子的分泌,并诱导native T细胞分化为Th17细胞和Treg细胞,Th17细胞分泌的IL-17可刺激嗜中粒细胞消除根尖上皮表面附近的炎症组织[51],Treg细胞分泌的IL-10可以抑制炎症;益生菌复合物联合药物可以通过激活巨噬细胞诱导ILC3分泌IL-22和GM-CSF,同时IL-22诱导了AMP的表达和嗜中粒细胞募集从而阻止了病菌和炎症的全身性传播,GM-CSF增强了IL-10的DC表达[52],从而促进Treg细胞分化和在结肠中扩散。
3.3 癌症
癌是指起源于上皮组织的恶性肿瘤,是恶性肿瘤中最常见的一类。随着人们年龄增长,先天免疫反应中的关键免疫细胞变得反应迟钝或无法发挥应有的作用,因此老年人的患癌率越来越高[53]。研究表明,乳酸杆菌属及其复合益生菌属可在治疗癌症期间作为辅助手段,帮助恢复肠道功能并减少感染性并发症。ZAHARUDDIN等[26]发现在结直肠癌患者手术后食用复合益生菌(嗜酸乳杆菌BCMC12310、乳酸乳杆菌BCMC12451、干酪乳杆菌亚种BCMC12313、长双歧杆菌BCMC02120等)有助于改变肠道微环境并且促炎细胞因子(TNF-α、IL-6、IL-10、IL-12、IL-17A、IL-17C和IL-22)水平显著降低;LIU等[54]同样发现在胃癌患者手术前1周至手术后7天或出院日服用含长双歧杆菌、嗜酸乳杆菌和粪肠球菌的益生菌胶囊同样可以降低术后感染,改善短期临床结果,并降低常见炎症指标的水平。动物实验发现植物乳杆菌YYC-3可以阻止结肠癌小鼠结肠肿瘤和黏膜损伤的发生,并下调促炎细胞因子IL-6、IL-17F和IL-22的表达[55]。在胰腺癌小鼠模型中,PANEBIANCO等[56]发现丁酸盐通过增加SCFAs的产量显著减少癌症相关的基质(细胞外基质、纤维化、血管和巨噬细胞标志物水平)生成,同时,减少促炎微生物的生成,保持肠黏膜完整性。综上,在动物实验中探究发现益生菌通过调控炎症因子和刺激SCFAs来预防和改善癌症,而在临床试验中则充分验证了服用益生菌可改善肠道微环境,降低炎症,使患者能够耐受各种化疗和随后的高侵入性手术,并在术后安全有效地辅助患者恢复健康。
益生菌辅助干预癌症治疗的可能机制如图2-c所示。在抗癌症疗法的主作用下,益生菌通过刺激肠道菌群产生SCFAs作用于固有层的DC,刺激包括IL-6和IL-12在内的细胞因子的分泌,同时促进native T细胞分化为Th1细胞和Th17细胞,Th1细胞分泌的IFN-γ和DC分泌的IL-12诱导了B淋巴细胞的增殖和分化[57],分化的浆细胞所分泌的IgA、IgG通过与肠道微生物结合和防止病菌侵入而促进屏障功能;乳杆菌静脉注射可直接刺激细胞毒性T淋巴细胞分泌能识别恶性肿瘤细胞的抗体,与其抗原特异性结合进而控制和根除恶性肿瘤生长[58];益生菌混合物还可诱导M2巨噬细胞极化为M1巨噬细胞吞噬引发癌症的病原体,并激活淋巴球和其他免疫细胞,使其对癌症的病原体做出反应,进而达到预防癌症的作用[59]。
3.4 神经退行性疾病
神经退行性疾病是大脑和脊髓的细胞神经元丧失的疾病状态。肠道微生物群在“肠-微生物-脑”(gut-microbiota-brain,GMB)轴中与中枢神经系统相互作用,衰老会诱导肠神经元的丧失和中枢神经系统老化,导致防御功能下降,因此老年人中神经退行性疾病患病率高,同时肠神经元的丧失会改变肠道动力学和黏膜屏障,引起肠道菌群失衡,从而导致肠道炎症反应,通过GMB轴加剧老年人患神经退行性疾病的风险[60]。在老年人中常见的神经退行性疾病是AD和PD。
AD与大脑神经元退化有关,以进行性认知障碍和行为损害为特征,而越来越多的研究证明补充益生菌可以改善认知缺陷,增强肌肉力量和功能,减轻外周组织的氧化应激和炎症,并改善肠道屏障功能[31, 61]。XIAO等[62]发现每天服用短双歧杆菌A1的认知障碍老年患者即时记忆、视觉空间/结构和延迟记忆的领域评分均有显著改善。但更多研究证明多菌株益生菌联合使用对于改善AD患者症状更有效,在动物模型中,BONFILI等[63]发现给予小鼠SLAB51混合物(包括嗜热链球菌、双歧杆菌和乳酸杆菌等)可有效抑制小鼠皮质萎缩,脑损伤和β淀粉样蛋白聚集体积累减少;同样地,TAMTAJI等[30]发现嗜酸乳杆菌、两双歧杆菌、长双歧杆菌与硒共同补充不仅可改善AD患者的认知缺陷,还显著降低TNF-α的基因表达,同时增加了过氧化物酶体增殖体激活受体γ和低密度脂蛋白受体(low density lipoprotein receptor,LDL-R)的基因表达。LIU等[64]发现聚甘露糖醛酸和鼠李糖乳杆菌GG联合使用比单独的聚甘露糖醛酸或鼠李糖乳杆菌GG具有更好的神经保护作用,且通过SCFAs介导的抗炎和抗细胞凋亡机制提供神经保护。
PD是老年人中最常见的中枢神经系统变性疾病,由于脑中多巴胺神经元的丧失而导致运动缺陷,并伴有肠道炎症的迹象。大量的研究证明服用益生菌可以显著缓解PD小鼠模型中的运动缺陷,长期服用更是对多巴胺神经元具有神经保护作用[65]。LIAO等[66]发现植物乳杆菌PS128可以改善PD小鼠的运动缺陷,减少多巴胺神经元死亡,并减少神经炎症和氧化应激;此外CASTELLI等[67]研究发现SLAB51制剂(包括嗜热链球菌、双歧杆菌和乳酸杆菌等)在PD小鼠中能够保护多巴胺能神经元并改善行为障碍,还能够抵消神经炎症和氧化应激;同样的ALIPOUR NOSRANI等[68]发现嗜酸乳杆菌、双歧杆菌、罗伊氏乳杆菌和发酵乳杆菌的益生菌混合物可以改善6-羟基多巴胺诱导的PD小鼠的旋转行为,认知功能,脂质过氧化和神经元损伤;而MEHRABANI等[34]在临床上证明了补充嗜酸乳杆菌LAA-5、鼠李糖乳杆菌LAR-7、植物乳杆菌LAP-10等益生菌混合物,可以显著提高PD患者的总抗氧化能力,降低丙二醛的浓度和减少PD患者的氧化应激标志物,并且显著改善DQ-39幸福感和认知障碍领域。综上这些研究表明补充益生菌可以显著改善神经退行性疾病的认知障碍、神经炎症和氧化应激,且多菌株益生菌联合治疗效果更显著。
益生菌及其代谢产物可通过巨噬细胞极化、促进神经信号物质产生、调节内分泌细胞激素等作用缓解神经退行性疾病(图2-d)。益生菌LPS作用于固有层的DC上诱发native T细胞分化为Th2和Treg细胞,进而分泌的IL-4、IL-10和TGF-β随外周血液循环系统迁移,通过血脑屏障进入大脑,发挥抗炎功能;益生菌能够促进巨噬细胞分化为促炎性M1型巨噬细胞和抗炎型M2型巨噬细胞并分泌相应的促炎细胞因子;此外,巨噬细胞可以迁移到大脑并成为小胶质细胞[60],最终发挥抗炎和促炎功能;益生菌及其代谢产物还可刺激肠道内分泌细胞释放多巴胺、γ-氨基丁酸、乙酰胆碱作为神经递质,通过肠神经系统与迷走神经连接,影响中枢神经系统中的大脑皮层[69];同时,SCFAs刺激EEC和ECC分泌神经肽YY、GLP-1和5-HT,进一步调节下丘脑-垂体-肾上腺轴从而影响脑功能。
4 结论与展望
随着全球老龄化的加剧,老年人健康问题成为重要的公共卫生问题。随着年龄增长,肠道微生物菌群组成改变导致的肠道生理变化、炎症反应和免疫衰老可能增加了老年人罹患IBD、代谢综合征、癌症、神经退行性疾病等疾病的风险。针对此类疾病,传统的药物治疗效果有诸多弊端,新兴的FMT技术还有很多问题有待解决,益生菌作为新兴辅助治疗手段,已有许多临床试验证明其在老年人疾病治疗中的效果显著,但目前数据较少且益生菌的效果具有高度的菌株特异性。关于益生菌改善老年人肠道菌群失衡相关疾病的机制,绝大多数集中于体外实验和动物模型中。益生菌可通过干预调节脂联素、GIP等内分泌细胞激素,抑制病原菌侵入增强肠道屏障,诱导AMP和嗜中粒细胞表达以及调控炎症因子,增强巨噬细胞免疫调节作用,减少炎症,促进多巴胺、乙酰胆碱等信号物质产生等方式有效缓解老年人代谢综合征、IBD、癌症和神经退行性疾病。因此,在未来的研究中,益生菌疗法应从益生菌菌株水平的功效出发,向着根据老年人的病症来确定饮食和特定益生菌补充剂的摄入量方向研究,同时,需要更多的大样本、多中心、随机及双盲对照的老年人临床试验去验证益生菌的疗效和安全性,为老年人提供健康指导。
[1] 潘锋. 促进老年健康从疾病预防开始[J].中国医药导报, 2022, 19(24):1-4.PAN F.Promoting the health of the elderly begins with disease prevention[J].China Medical Herald, 2022, 19(24):1-4.
[2] BARONE M, D’AMICO F, RAMPELLI S, et al.Age-related diseases, therapies and gut microbiome:A new frontier for healthy aging[J].Mechanisms of Ageing and Development, 2022, 206:111711.
[3] TARANTINI S, SUBRAMANIAN M, BUTCHER J T, et al.Revisiting adipose thermogenesis for delaying aging and age-related diseases:Opportunities and challenges[J].Ageing Research Reviews, 2023, 87:101912.
[4] ANWAR H, IFTIKHAR A, MUZAFFAR H, et al.Biodiversity of gut microbiota:Impact of various host and environmental factors[J].BioMed Research International, 2021, 2021:5575245.
[5] LI N, YAN F F, WANG N N, et al.Distinct gut microbiota and metabolite profiles induced by different feeding methods in healthy Chinese infants[J].Frontiers in Microbiology, 2020, 11:714.
[6] STOJANOV S, BERLEC A,  TRUKELJ B.The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease[J].Microorganisms, 2020, 8(11):1715.
TRUKELJ B.The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease[J].Microorganisms, 2020, 8(11):1715.
[7] MILLION M, RAOULT D.The role of the manipulation of the gut microbiota in obesity[J].Current Infectious Disease Reports, 2013, 15(1):25-30.
[8] HARAN J P, MCCORMICK B A.Aging, frailty, and the microbiome—How dysbiosis influences human aging and disease[J].Gastroenterology, 2021, 160(2):507-523.
[9] YOSHIMOTO S, MITSUYAMA E, YOSHIDA K, et al.Enriched metabolites that potentially promote age-associated diseases in subjects with an elderly-type gut microbiota[J].Gut Microbes, 2021, 13(1):1-11.[10] HARAN J P, BUCCI V, DUTTA P, et al.The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location[J].Journal of Medical Microbiology, 2018, 67(1):40-51.
[11] KONTUREK P C, HAZIRI D, BRZOZOWSKI T, et al.Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases[J].Journal of Physiology and Pharmacology:an Official Journal of the Polish Physiological Society, 2015, 66(4):483-491.
[12] CONWAY J, DUGGAL N A.Ageing of the gut microbiome:Potential influences on immune senescence and inflammageing[J].Ageing Research Reviews, 2021, 68:101323.
[13] JACKSON M A, JEFFERY I B, BEAUMONT M, et al.Signatures of early frailty in the gut microbiota[J].Genome Medicine, 2016, 8(1):8.
[14] VOGT N M, KERBY R L, DILL-MCFARLAND K A, et al.Gut microbiome alterations in Alzheimer’s disease[J].Scientific Reports, 2017, 7(1):13537.
[15] ROMANO S, SAVVA G M, BEDARF J R, et al.Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation[J].NPJ Parkinson’s Disease, 2021, 7(1):27.
[16] CARDOSO M H, MENEGUETTI B T, OLIVEIRA-J NIOR N G, et al.Antimicrobial peptide production in response to gut microbiota imbalance[J].Peptides, 2022, 157:170865.
NIOR N G, et al.Antimicrobial peptide production in response to gut microbiota imbalance[J].Peptides, 2022, 157:170865.
[17] MARSOOL M D M, VORA N, MARSOOL A D M, et al.Ulcerative colitis:Addressing the manifestations, the role of fecal microbiota transplantation as a novel treatment option and other therapeutic updates[J].Disease-a-Month, 2023, 69(11):101606.
[18] DAILEY F E, TURSE E P, DAGLILAR E, et al.The dirty aspects of fecal microbiota transplantation:A review of its adverse effects and complications[J].Current Opinion in Pharmacology, 2019, 49:29-33.
[19] MISHRA J, STUBBS M, KUANG L X, et al.Inflammatory bowel disease therapeutics:A focus on probiotic engineering[J].Mediators of Inflammation, 2022, 2022:9621668.
[20] TOEJING P, KHAMPITHUM N, SIRILUN S, et al.Influence of Lactobacillus paracasei HII01 supplementation on glycemia and inflammatory biomarkers in type 2 diabetes:A randomized clinical trial[J].Foods, 2021, 10(7):1455.
[21] MOBINI R, TREMAROLI V, STÅHLMAN M, et al.Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes:A randomized controlled trial[J].Diabetes, Obesity &Metabolism, 2017, 19(4):579-589.
[22] CANCELLO R, TURRONI S, RAMPELLI S, et al.Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women[J].Nutrients, 2019, 11(12):3011.
[23] IVEY K L, HODGSON J M, KERR D A, et al.The effect of yoghurt and its probiotics on blood pressure and serum lipid profile;a randomised controlled trial[J].Nutrition, Metabolism and Cardiovascular Diseases, 2015, 25(1):46-51.
[24] CICERO A F G, FOGACCI F, BOVE M, et al.Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients:A randomized placebo-controlled clinical trial[J].European Journal of Nutrition, 2021, 60(2):655-663.
[25] BELLIKCI-KOYU E, SARER-YUREKLI B P, KARAGOZLU C, et al.Probiotic kefir consumption improves serum apolipoprotein A1 levels in metabolic syndrome patients:A randomized controlled clinical trial[J].Nutrition Research, 2022, 102:59-70.
[26] ZAHARUDDIN L, MOKHTAR N M, NAWAWI K N M, et al.A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer[J].BMC Gastroenterology, 2019, 19(1):131.
[27] HUANG F, LI S J, CHEN W J, et al.Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients[J].Nutrients, 2023, 15(2):356.
[28] FRANKO J, RAMAN S, PATEL S, et al.Survival and cancer recurrence after short-course perioperative probiotics in a randomized trial[J].Clinical Nutrition ESPEN, 2024, 60:59-64.
[29] PARK I J, LEE J H, KYE B H, et al.Effects of PrObiotics on the symptoms and surgical ouTComes after anterior REsection of colon cancer (POSTCARE):A randomized, double-blind, placebo-controlled trial[J].Journal of Clinical Medicine, 2020, 9(7):2181.
[30] TAMTAJI O R, HEIDARI-SOURESHJANI R, MIRHOSSEINI N, et al.Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease:A randomized, double-blind, controlled trial[J].Clinical Nutrition, 2019, 38(6):2569-2575.
[31] TON A M M, CAMPAGNARO B P, ALVES G A, et al.Oxidative stress and dementia in Alzheimer’s patients:Effects of synbiotic supplementation[J].Oxidative Medicine and Cellular Longevity, 2020, 2020:2638703.
[32] TAN A H, LIM S Y, CHONG K K, et al.Probiotics for constipation in parkinson disease:A randomized placebo-controlled study[J].Neurology, 2021, 96(5):e772-e782.
[33] IBRAHIM A, ALI R A R, MANAF M R A, et al.Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease:A randomised controlled trial[J].PLoS One, 2020, 15(12):e0244680.
[34] MEHRABANI S, KHORVASH F, HEIDARI Z, et al.The effects of synbiotic supplementation on oxidative stress markers, mental status, and quality of life in patients with Parkinson’s disease:A double-blind, placebo-controlled, randomized controlled trial[J].Journal of Functional Foods, 2023, 100:105397.
[35] HANDAJANI Y S, TURANA Y, YOGIARA Y, et al.Effects of tempeh probiotics on elderly with cognitive impairment[J].Frontiers in Aging Neuroscience, 2022, 14:891773.
[36] ALJUMAAH M R, BHATIA U, ROACH J, et al.The gut microbiome, mild cognitive impairment, and probiotics:A randomized clinical trial in middle-aged and older adults[J].Clinical Nutrition, 2022, 41(11):2565-2576.
[37] FEI Y Z, WANG R R, LU J C, et al.Probiotic intervention benefits multiple neural behaviors in older adults with mild cognitive impairment[J].Geriatric Nursing, 2023, 51:167-175.
[38] OLIVEIRA L V A, DOS SANTOS B N S, MACHADO  E, et al.Prevalence of the metabolic syndrome and its components in the Brazilian adult population[J].Ciencia &Saude Coletiva, 2020, 25(11):4269-4280.
E, et al.Prevalence of the metabolic syndrome and its components in the Brazilian adult population[J].Ciencia &Saude Coletiva, 2020, 25(11):4269-4280.
[39] SALARI A, GHODRAT S, GHEFLATI A, et al.Effect of kefir beverage consumption on glycemic control:A systematic review and meta-analysis of randomized controlled clinical trials[J].Complementary Therapies in Clinical Practice, 2021, 44:101443.
[40] FORD A L, NAGULESAPILLAI V, PIANO A, et al.Microbiota stability and gastrointestinal tolerance in response to a high-protein diet with and without a prebiotic, probiotic, and synbiotic:A randomized, double-blind, placebo-controlled trial in older women[J].Journal of the Academy of Nutrition and Dietetics, 2020, 120(4):500-516.e10.
[41] BELLIKCI-KOYU E, SARER-YUREKLI B P, AKYON Y, et al.Effects of regular kefir consumption on gut microbiota in patients with metabolic syndrome:A parallel-group, randomized, controlled study[J].Nutrients, 2019, 11(9):2089.
[42] 朱建丰, 徐英.益生菌对老年2型糖尿病患者的影响[J].中国微生态学杂志, 2021, 33(11):1299-1303.ZHU J F, XU Y.Effects of probiotics on elderly patients with type 2 diabetes[J].Chinese Journal of Microecology, 2021, 33(11):1299-1303.
[43] 王丹萍, 徐珒昭, 张晓航, 等.糖尿病与宿主肠道菌群的关系及饮食介导的菌群调控作用研究进展[J].食品科学, 2023, 44(15):379-389.WANG D P, XU J Z, ZHANG X H, et al.Progress in understanding the relationship between diabetes and host intestinal microbiota and diet-mediated microbiota regulation[J].Food Science, 2023, 44(15):379-389.
[44] BRITTON G J, CONTIJOCH E J, MOGNO I, et al.Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice[J].Immunity, 2019, 50(1):212-224.e4.
[45] XU H Y, MA C, ZHAO F Y, et al.Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome[J].European Journal of Nutrition, 2021, 60(5):2553-2565.
[46] FENG C J, ZHANG W Q, ZHANG T, et al.Oral administration of pasteurized probiotic fermented milk alleviates dextran sulfate sodium-induced inflammatory bowel disease in rats[J].Journal of Functional Foods, 2022, 94:105140.
[47] SHARMA S, BHATIA R, DEVI K, et al.A synbiotic combination of Bifidobacterium longum Bif10 and Bifidobacterium breve Bif11, isomaltooligosaccharides and finger millet Arabinoxylan prevents dextran sodium sulphate induced ulcerative colitis in mice[J].International Journal of Biological Macromolecules, 2023, 231:123326.
[48] AGRAIB L M, YAMANI M I, TAYYEM R, et al.Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients:A pilot, randomized, double-blind, placebo-controlled study[J].Clinical Nutrition ESPEN, 2022, 51:83-91.
[49] XIA Y J, CHEN Y, WANG G Q, et al.Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition[J].Journal of Functional Foods, 2020, 67:103854.
[50] EMAMIE A D, RAJABPOUR M, GHANAVATI R, et al.The effects of probiotics, prebiotics and synbiotics on the reduction of IBD complications, a periodic review during 2009-2020[J].Journal of Applied Microbiology, 2021, 130(6):1823-1838.
[51] IYER N, CORR S C.Gut microbial metabolite-mediated regulation of the intestinal barrier in the pathogenesis of inflammatory bowel disease[J].Nutrients, 2021, 13(12):4259.
[52] SUGIHARA K, KAMADA N.Diet-microbiota interactions in inflammatory bowel disease[J].Nutrients, 2021, 13(5):1533.
[53] VIDIT K, PATEL A S.Geriatric assessments:Tools for every oncologist to stage the aging when caring for older adults with cancer[J].Advances in Oncology, 2022, 2(1):81-97.
[54] LIU G, CAO S G, LIU X D, et al.Effect of perioperative probiotic supplements on postoperative short-term outcomes in gastric cancer patients receiving neoadjuvant chemotherapy:A double-blind, randomized controlled trial[J].Nutrition, 2022, 96:111574.
[55] YUE Y C, YE K, LU J, et al.Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment[J].Biomedicine &Pharmacotherapy, 2020, 127:110159.
[56] PANEBIANCO C, VILLANI A, PISATI F, et al.Butyrate, a postbiotic of intestinal bacteria, affects pancreatic cancer and gemcitabine response in in vitro and in vivo models[J].Biomedicine &Pharmacotherapy, 2022, 151:113163.
[57] GOCHER A M, WORKMAN C J, VIGNALI D A A.Interferon-γ:Teammate or opponent in the tumour microenvironment?[J].Nature Reviews.Immunology, 2022, 22(3):158-172.
[58] ABDOLALIPOUR E, MAHOOTI M, SALEHZADEH A, et al.Evaluation of the antitumor immune responses of probiotic Bifidobacterium bifidum in human papillomavirus-induced tumor model[J].Microbial Pathogenesis, 2020, 145:104207.
[59] GUHA D, BANERJEE A, MUKHERJEE R, et al.A probiotic formulation containing Lactobacillus bulgaricus DWT1 inhibits tumor growth by activating pro-inflammatory responses in macrophages[J].Journal of Functional Foods, 2019, 56:232-245.
[60] CHENG Y F, CHEN C, ZHANG F.Immunity orchestrates a bridge in gut-brain axis of neurodegenerative diseases[J].Ageing Research Reviews, 2023, 85:101857.
[61] NI Y H, YANG X, ZHENG L J, et al.Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota[J].Molecular Nutrition &Food Research, 2019, 63(22):e1900603.
[62] XIAO J Z, KATSUMATA N, BERNIER F, et al.Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment:A randomized, double-blind, placebo-controlled trial[J].Journal of Alzheimer’s Disease:JAD, 2020, 77(1):139-147.
[63] BONFILI L, CECARINI V, BERARDI S, et al.Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels[J].Scientific Reports, 2017, 7(1):2426.
[64] LIU X, DU Z R, WANG X, et al.Polymannuronic acid prebiotic plus Lacticaseibacillus rhamnosus GG probiotic as a novel synbiotic promoted their separate neuroprotection against Parkinson’s disease[J].Food Research International, 2022, 155:111067.
[65] HSIEH T H, KUO C W, HSIEH K H, et al.Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson’s disease[J].Brain Sciences, 2020, 10(4):206.
[66] LIAO J F, CHENG Y F, YOU S T, et al.Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse models of Parkinson’s disease[J].Brain, Behavior, and Immunity, 2020, 90:26-46.
[67] CASTELLI V, D’ANGELO M, LOMBARDI F, et al.Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models[J].Aging, 2020, 12(5):4641-4659.
[68] ALIPOUR NOSRANI E, TAMTAJI O R, ALIBOLANDI Z, et al.Neuroprotective effects of probiotics bacteria on animal model of Parkinson’s disease induced by 6-hydroxydopamine:A behavioral, biochemical, and histological study[J].Journal of Immunoassay &Immunochemistry, 2021, 42(2):106-120.
[69] YADAV H, JALDHI, BHARDWAJ R, et al.Unveiling the role of gut-brain axis in regulating neurodegenerative diseases:A comprehensive review[J].Life Sciences, 2023, 330:122022.