鼠伤寒沙门氏菌(Salmonella typhimurium, S.typhimurium)是一种革兰氏阴性菌,也是兼性的细胞内病原体,属于非伤寒沙门氏菌的一种,对人畜有严重危害性。鼠伤寒沙门氏菌病主要通过“粪口”途径传播,通常是自我限制的,但也可能导致长期的并发症[1-2]。
鼠伤寒沙门氏菌感染是全球都在关注的重要健康问题。据估计,非伤寒沙门氏菌病在人类中造成的疾病负担每年高达1.295亿例,导致全球每年10万~100万人死亡,这其中就包括由鼠伤寒沙门氏菌引起的感染[3-5]。在撒哈拉以南的非洲地区,鼠伤寒沙门氏菌是引起腹泻病和侵袭性疾病的主要病因,且人类免疫缺陷病毒(human immunodeficiency virus, HIV)感染者、患有疟疾、贫血和营养不良的婴儿和幼儿更容易被感染[6-7]。除此以外,中国地区的很多省份(如浙江省、福建省)鼠伤寒沙门氏菌的感染有所增加,再加上多药耐药菌株的出现,使其治疗受到严峻的挑战[8-9]。本文主要对鼠伤寒沙门氏菌的感染过程以及治疗方法进行综述,为更深入了解鼠伤寒沙门氏菌的感染机制并开发新的治疗方法提供参考。
1 鼠伤寒沙门氏菌的感染
鼠伤寒沙门氏菌是非伤寒沙门氏菌的一种,可引起非伤寒沙门氏菌病,发病率较高,且严重感染导致的死亡主要局限于发展中国家[10]。鼠伤寒沙门氏菌拥有广泛的宿主,包括人类、各种家畜、野生动物和食物基质等,主要通过污染的食物或水源进行传播[10-11]。临床上人类感染鼠伤寒沙门氏菌的症状大部分表现为自限性急性肠胃炎、水样腹泻、恶心、呕吐、腹痛和发烧等,一般在摄入病原体后6~12 h出现症状,持续时间接近10 d。但在症状消失后,患者还会持续携带鼠伤寒沙门氏菌。调查显示成年人在感染后粪便中含有沙门氏菌的持续时间约为1个月,而5岁以下儿童的持续时间约为7周[10]。鼠伤寒沙门氏菌在免疫缺陷性人群中更易引起侵袭性感染,尤其是HIV患者,疟疾患者和营养不良的婴幼儿,临床表现为高热,肝脾肿大,并有呼吸系统并发症,进一步发展为败血症。同时,这类患者通常没有肠道症状,正如对撒哈拉以南的非洲地区的报道[6-7,10]。近年来,鼠伤寒沙门氏菌感染发病率在澳大利亚、印度等地呈上升趋势,而在以色列、马拉维等地呈下降趋势,其发病率存在地域性差异。但随着耐药菌株的出现,鼠伤寒沙门氏菌感染已严重威胁公共卫生安全[12-17]。
鼠伤寒沙门氏菌能够在宿主体内存活、繁殖和扩散,这离不开许多毒力因子的协助,其主要毒力基因存在于沙门氏菌毒力岛(Salmonella pathogenicity islands,SPIs)上。目前,鼠伤寒沙门氏菌已有多个毒力岛被研究报道,其中SPI-1和SPI-2是研究最深入的[2]。鼠伤寒沙门氏菌SPI-1和SPI-2含有2种Ⅲ型分泌系统(type 3 secretion system,T3SS)的基因,分别在其入侵宿主细胞和在宿主细胞内存活复制中起作用。T3SS是革兰氏阴性细菌所特有的,很可能是从鞭毛基体进化而来的。每个T3SS包含一个马达、一个针复合体和一个易位子,分泌的效应蛋白通过易位子被输送到宿主细胞,有助于鼠伤寒沙门氏菌的感染并刺激宿主细胞免疫反应[18]。鼠伤寒沙门氏菌的其他毒力因子如pSLT毒力质粒、粘附素、鞭毛和生物膜等也有助于其侵入宿主并在宿主体内生存和繁殖,这些毒力因子在沙门氏菌的不同感染阶段发挥不同的作用[19]。
鼠伤寒沙门氏菌经口进入宿主体内,需要突破的第一道防线是胃酸环境。到达胃部后,其耐酸反应被激活,维持细胞内pH值高于细胞外pH值,使细菌能够在这种环境中生存[2]。随后,鼠伤寒沙门氏菌进入肠道,依靠几种粘附素和菌毛粘附于肠上皮细胞,并使用几种不同的机制穿过肠道上皮(图1)。其中一条主要的进入途径是通过由SPI-1编码的T3SS-1分泌效应蛋白参与宿主细胞肌动蛋白的重排,来诱导其内化到非吞噬细胞的肠上皮细胞中[20]。鼠伤寒沙门氏菌还可以通过M(micro-fold)细胞存在于滤泡相关上皮(follicle-associated epithelium,FAE)并覆盖派尔斑(Peyer’s patches,PPs),可以将抗原和细菌从肠道内腔输送到固有层[20]。除此之外,有研究报道鼠伤寒沙门氏菌可直接通过表达CD18的巨噬细胞和CXC3R+树突状细胞从胃肠道进入血液[21-22]。透过肠上皮细胞之后,鼠伤寒沙门氏菌能够被巨噬细胞或树突状细胞吞噬。进入细胞后,鼠伤寒沙门氏菌依靠其SPI-2编码的T3SS-2形成含沙门氏菌的液泡(Salmonella containing vacuole,SCV),并在细胞内大量繁殖,从而引发局部炎症[23]。大量的鼠伤寒沙门氏菌被这些免疫细胞运输到肠系膜淋巴结,继而通过Caspase-1/8依赖的细胞凋亡途径;TNF(tumor necrosis factor)受体、Ⅰ型干扰素受体以及TLR(toll-like receptor)信号通路介导的RIPI-RIP3依赖的细胞程序性坏死途径刺激巨噬细胞裂解,导致大量病原菌进入血液循环和淋巴循环,在肝脏脾脏中定植,引发全身系统性感染[24]。

图1 沙门氏菌入侵肠道上皮示意图[20]
Fig.1 Schematic diagram of Salmonella invading intestinal epithelium[20]
在鼠伤寒沙门氏菌感染过程中会产生许多炎症反应代谢产物,其可以适应并利用这种炎症环境,为自己的生长获得优势,这也是其感染性强的原因之一。硝酸盐是鼠伤寒沙门氏菌肠道感染过程中产生的最丰富的代谢物之一。LI等[25]发现,鼠伤寒沙门氏菌在低氧条件下表现出利用硝酸盐的生长优势,全球调节因子FNR和硝酸盐传感双组分系统NarX-NarL都激活了该优势,促进鼠伤寒沙门氏菌的毒力,有助于细菌的细胞内复制和全身感染。鼠伤寒沙门氏菌也可以使用PhoP/PhoQ双组分系统促进细菌包膜的重塑,从而增加对抗菌肽的抵抗力。BADER等[26]的研究表明,亚致死浓度的阳离子抗菌肽能够激活PhoP/PhoQ双组分调节系统和RpoS毒力调节因子,增强对不同抗菌肽的耐药性,以利于其在巨噬细胞中存活。此外,在鼠伤寒沙门氏菌诱导的炎症过程中,硫代硫酸盐被活性氧(reactive oxygen species,ROS)氧化成四硫酸盐,随后,鼠伤寒沙门氏菌利用四硫酸盐作为厌氧呼吸的电子受体,使其能够利用从宿主组织释放的乙醇胺,进而在发炎的肠道中与微生物群竞争[27]。
2 鼠伤寒沙门氏菌的治疗
鼠伤寒沙门氏菌的感染性和危害性使其治疗措施显得尤为重要。传统治疗鼠伤寒沙门氏菌的方法是抗生素疗法,但由于抗生素的滥用,耐多药沙门氏菌对抗生素如环丙沙星、头孢曲松等已经表现出广泛的耐药性,因此开发新的治疗手段迫在眉睫[28]。近年来,小分子疗法、噬菌体疗法、减毒疫苗疗法等研究渐渐兴起,有望成为治疗鼠伤寒沙门氏菌感染的有效方法(图2)。
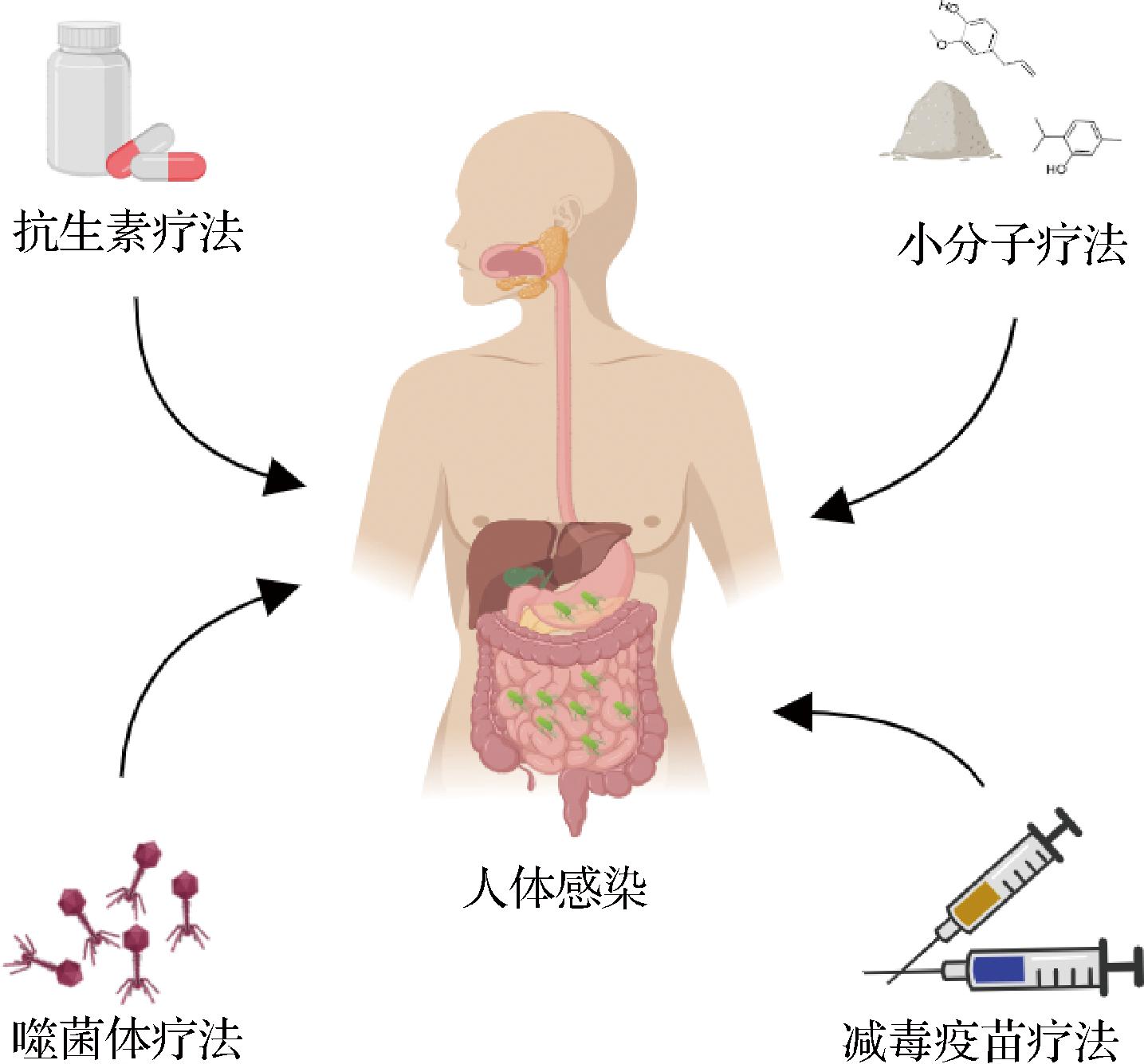
图2 鼠伤寒沙门氏菌治疗方法示意图
Fig.2 Schematic diagram of treatment methods for Salmonella typhimurium
2.1 抗生素疗法
抗生素是临床上用于治疗肠道感染的首选药物。1947年,氯霉素被发现并用于治疗沙门氏菌的感染[28]。随后,喹诺酮类药物环丙沙星、氧氟沙星和培氟沙星等也渐渐被投入使用。这些抗生素主要通过抑制DNA旋转酶,从而抑制细菌DNA的复制、螺旋和超螺旋,达到杀死细菌的目的。第三代头孢菌素如头孢哌酮、头孢噻肟和头孢曲松等也是鼠伤寒沙门氏菌的有效治疗选择[28]。除此之外,大环内酯类药物克拉霉素能够抑制鼠伤寒沙门氏菌生物膜的形成。克拉霉素治疗显著降低了鼠伤寒沙门氏菌生物膜激活剂CsgD的转录和表达,同时csgB和adrA等生物膜基因下调,这为临床上使用克拉霉素治疗鼠伤寒沙门氏菌感染提供了参考[29]。
抗生素具有杀菌活性强、毒性低、适应症广、治疗效果好的优点,但其最大的缺陷是过度使用可以导致病原菌耐药。ZHANG等[30]研究发现,随着环丙沙星浓度的提高,鼠伤寒沙门氏菌GyrA突变(G81D)对环丙沙星的敏感性降低,且另一种突变(ParC,G78D)导致高水平的环丙沙星耐药性。除此之外,鼠伤寒沙门氏菌对氨苄西林、氟苯尼考、头孢曲松等抗生素也产生不同程度的耐药性[31-33]。不仅耐药性,抗生素的持久性也是一个关键性问题。它可以使敏感的细菌在抗生素致死浓度下存活,这就延长了抗生素的治疗时间,能够促进抗生素耐药病原菌的进化。一但停止使用抗生素,残留在组织中的病原菌就会大量繁殖,导致病情复发[34-35]。
为了应对单一抗生素治疗带来的耐药性,抗生素联合疗法应运而生。DAWAN等[36]的研究结果表明,卡那霉素与青霉素或红霉素联用可以表现出对鼠伤寒沙门氏菌的协同治疗活性,减缓鼠伤寒沙门氏菌的耐药突变。相比于使用单一抗生素,抗生素联用杀菌作用更强,抗菌谱更广。此外,头孢噻吩和托普霉素的联合治疗也被证明能够增加对鼠伤寒沙门氏菌的杀菌活性,并且不会导致细胞损伤[37]。但是抗生素的联合治疗仍存在争议,还需进一步研究以使其能够在临床上应用。
2.2 小分子疗法
鉴于抗生素疗法存在一些弊端,人们将目光转移到了小分子药物上,开始研究能够替代抗生素的新型抗菌剂。目前,对于治疗鼠伤寒沙门氏菌感染的新药研究,主要有靶向T3SS的小分子天然产物及化合物;靶向细胞自噬清除病原菌的小分子药物;靶向生物膜形成或破坏革兰氏阴性菌细胞膜的小分子药物等。
2.2.1 靶向T3SS的小分子药物
丁香酚是一种苯丙烷类的酚类化合物,具有很强的抗炎、镇痛、抗氧化作用,亦具有抗感染的作用[38]。ZHAO等[39-40]研究发现,丁香酚可以显著下调鼠伤寒沙门氏菌T3SS相关效应蛋白的mRNA表达水平,包括sipA、sipC、spiC、misL、hilA、hilD。其中,HilA转录激活因子诱导许多侵袭基因的表达,且其Ⅰ型菌毛相关粘附因子(fimA、fimH、fimD、fimY、fimZ和stm0551)的mRNA表达水平也受到了显著下调。不仅如此,丁香酚还破坏了鼠伤寒沙门氏菌细胞膜的完整性,导致细菌裂解死亡。更重要的是,丁香酚可以影响NF-κB(nuclear factor kappa-B)信号通路并稳定肠道屏障完整性,减轻肠道炎症和损伤。这些研究使得丁香酚在治疗鼠伤寒沙门氏菌感染方面具有广阔的前景。
百里香酚是从唇形科植物中分离出的主要单萜苯酚,具有抗氧化、抗炎、局部麻醉等活性,特别是其抗菌和抗真菌的特性已经被研究报道[41]。根据ZHANG等[42-43]的研究结果显示,百里香酚能够显著抑制鼠伤寒沙门氏菌T3SS-1介导的效应蛋白SipA进入HeLa细胞。百里香酚可以与SipA的至少2个区域(分别参与伴侣结合和肌动蛋白成核)相互作用,且这种相互作用仅在蛋白质翻译和折叠时发生,导致SipA的构象改变,继而这种蛋白被递送到Lon蛋白酶处进行降解,达到抑制鼠伤寒沙门氏菌入侵的目的。GIOVAGNONI等[44]也证明了百里香酚作用能够下调鼠伤寒沙门氏菌毒力基因的表达,亚致死浓度的百里香酚和香芹酚可以抵消鼠伤寒沙门氏菌对Caco-2细胞的攻击。
除了丁香酚和百里香酚外,能够靶向T3SS的天然来源小分子化合物还有很多,如肉桂醛、丹皮酚、杨梅素、骆驼蓬碱等(表1)。
表1 治疗鼠伤寒沙门氏菌感染的小分子天然化合物
Table 1 Small molecule natural compounds for the treatment of Salmonella typhimurium infection

名称功能文献来源丁香酚下调T3SS相关效应蛋白的mRNA表达水平[39-40]百里香酚抑制T3SS-1介导的效应蛋白SipA进入Hela细胞[42-43]肉桂醛抑制SPI-1相关基因(sipA, sipB等)的转录[45]丹皮酚通过hilA调节途径抑制SPI-1相关基因的表达[46]丁香醛抑制SPI-1相关基因的转录[47]杨梅素通过hilD-hilC-rstA-hilA调控途径抑制SPI-1效应蛋白表达[48]甘草黄酮醇抑制相关效应蛋白分泌,调控SicA/InvF基因的转录和效应蛋白SipC的转运[49]骆驼蓬碱通过hilA调节途径抑制SPI-1相关基因的表达[50]柚皮素以pstS/hilD依赖性方式抑制SPI-1[51]槲皮苷阻断SipA易位,抑制SPI-1基因(hilA和sopA)和效应子(SipA和SipC)的表达[52]
除此之外,一些非天然来源的小分子化合物对T3SS也有抑制作用。例如,HEGAZY等[53]研究报道了特拉唑嗪可以显著下调SPI-2相关基因的表达,包括ssrB、ssaE、sseF、ssaJ、sseI、sseF、sseJ和sscA,从而降低了鼠伤寒沙门氏菌在巨噬细胞中的复制。一些真菌来源的小分子化合物如环孢菌素B、镰刀菌酸等,也可以靶向T3SS,达到治疗鼠伤寒沙门氏菌的目的[54]。
2.2.2 靶向细胞自噬清除病原菌的小分子药物
自噬是一个自我降解的过程,靶向诱导细胞自噬以清除病原菌是潜在的抗沙门氏菌感染药物开发的机制。CHIU等[55]研究发现塞来昔布衍生物AR-12在早期阶段可以通过诱导自噬调节蛋白的表达来达到杀菌目的,而在后期阶段则通过抑制Akt来抑制鼠伤寒沙门氏菌在巨噬细胞中的存活。AMMANATHAN等[56]证明了阿卡西汀可以诱导TFEB(转录因子EB,被认为是自噬和溶酶体基因的主要调节因子)的去磷酸化,去磷酸化的TFEB易位到细胞核,激活溶酶体和自噬基因,从而限制细胞内沙门氏菌的复制。除此之外,黄苓苷和白藜芦醇也被证明能够促进宿主细胞内鼠伤寒沙门氏菌的自噬依赖性清除,但两者的调节通路不尽相同[57-58]。在细胞中,调节自噬的信号通路有很多,这些通路为研究靶向细胞自噬的小分子药物提供了多种选择。
2.2.3 靶向生物膜形成或破坏病原菌细胞膜的小分子药物
细菌感染之所以难以治疗,有一部分原因是其配备了许多防御系统。外膜屏障的存在阻止了许多抗生素进入细胞,且细菌生活在生物膜群落内,分泌的胞外多糖、核酸和蛋白质会形成保护性细胞外基质,使它们能够强烈地粘附在生物膜内表面和其他细胞上,保护包裹的细菌免受免疫和抗生素的干扰[59-62]。为了克服鼠伤寒沙门氏菌的这种保护屏障,人们对抗生物膜抑制剂和能够破坏菌膜的小分子药物进行了研究。MOSHIRI等[63]鉴定了一种被命名为T315的小分子化合物,T315以WrbA为靶标,推测是通过抑制WrbA的表达从而抑制生物膜的形成。DOMBACH等[64]发现了一种芳香族小分子JD1,通过增加膜流动性、破坏屏障功能并导致膜变形的形成来破坏细菌细胞质膜,且JD1不会破坏哺乳动物的细胞膜,没有明显的细胞毒性。近期,VILLANUEVA等[65]报道了2种能够破坏鼠伤寒沙门氏菌细胞内膜的小分子化合物JAV1和JVA2,JVA2透化细菌内膜并迅速杀菌,JAV1增加了膜流动性,改变了还原电位。但这些破坏细胞膜的小分子并不会透化细胞外膜,需要以宿主可溶性先天免疫因子透化细菌外膜为前提,再利用外膜损伤进入细菌细胞发挥作用。除此之外,白术氯仿提取物[主要成分为苍术酮、(R)-紫檀素B、6-羟基-2-冰片酮葡糖苷、邻氨基苯甲酸芳樟酯和一些单体]也能抑制鼠伤寒沙门氏菌生物膜的形成,并且显著抑制鞭毛(filA,filC)、菌毛(fimA,fimH)和生物膜(csgA,csgB)等多个粘附相关基因的表达,从而抑制其在细胞表面的定植能力[66]。
2.3 噬菌体疗法
噬菌体作为一种可能的抗生素替代品受到了广泛关注,因为它具有特异性和自我复制特性,对有益微生物群落和人类细胞没有影响[67-68]。早在1926年,噬菌体对肠道沙门氏菌的裂解活性在体外就得到了证实[69]。随着研究的不断深入,其在体内杀灭鼠伤寒沙门氏菌的活性也得到了验证。JUNG等[70]评价了几种裂解性噬菌体的形态学特性、热稳定性、pH稳定性、最佳感染多重性和对鼠伤寒沙门氏菌的裂解活性,发现噬菌体P22能够有效灭活鼠伤寒沙门氏菌ATCC 19585,噬菌体PBST10、PBST13、PBST32和PBST35也都显示出对临床分离的抗生素耐药性鼠伤寒沙门氏菌的溶解活性。
此外,多种噬菌体混合物在治疗鼠伤寒沙门氏菌方面的效果也得到了证实。![]() 等[71]研究发现,噬菌体vB_Sen-TO17和vB_SenM-2可以有效抑制培养基中细菌的生长并减少活细胞的数量,且2种噬菌体混合物的治疗效果也在蜡螟动物感染模型中得到了良好的体现。除此之外,LIANG等[72]也研究了噬菌体混合物对鼠伤寒沙门氏菌的裂解活性,用噬菌体F118P13和裂解性噬菌体PLL1的噬菌体组合,在腹腔内注射,可以完全保护小鼠免受沙门氏菌诱导而死亡,并抑制细菌在各个器官中的快速增殖。噬菌体可以作为抗生素药物的替代品,也可以联合抗生素治疗鼠伤寒沙门氏菌的感染。有研究报道,噬菌体与盐酸左氧氟沙星联用有利于鼠伤寒沙门氏菌生物膜的根除,同样与盐酸左氧氟沙星或环丙沙星联用也对浮游状态的鼠伤寒沙门氏菌有治疗作用,其给药顺序会影响治疗效果[73]。
等[71]研究发现,噬菌体vB_Sen-TO17和vB_SenM-2可以有效抑制培养基中细菌的生长并减少活细胞的数量,且2种噬菌体混合物的治疗效果也在蜡螟动物感染模型中得到了良好的体现。除此之外,LIANG等[72]也研究了噬菌体混合物对鼠伤寒沙门氏菌的裂解活性,用噬菌体F118P13和裂解性噬菌体PLL1的噬菌体组合,在腹腔内注射,可以完全保护小鼠免受沙门氏菌诱导而死亡,并抑制细菌在各个器官中的快速增殖。噬菌体可以作为抗生素药物的替代品,也可以联合抗生素治疗鼠伤寒沙门氏菌的感染。有研究报道,噬菌体与盐酸左氧氟沙星联用有利于鼠伤寒沙门氏菌生物膜的根除,同样与盐酸左氧氟沙星或环丙沙星联用也对浮游状态的鼠伤寒沙门氏菌有治疗作用,其给药顺序会影响治疗效果[73]。
噬菌体疗法具有特异性强,安全性好等优点,对抗生素过敏患者更友好,但噬菌体是蛋白质实体,会触发宿主免疫系统导致治疗效果降低。其次,噬菌体的鉴定和分离,确定给药途径与最佳剂量也需要花费大量时间,并且细菌也会不断发展出对噬菌体产生抗性的生物机制[28,74]。在噬菌体治疗鼠伤寒沙门氏菌感染方面,还需进一步研究,使噬菌体疗法更好地应用于临床。
2.4 减毒疫苗疗法
疫苗接种是人获得病原菌免疫力的有效工具,目前有3种许可的沙门氏菌疫苗:减毒活疫苗Ty21a、非偶联Vi多糖疫苗、与破伤风类毒素结合的Vi多糖[75]。随着沙门氏菌病的发展,人们愈发意识到开发多价针对沙门氏菌血清型疫苗的重要性,比如鼠伤寒沙门氏菌、肠炎沙门氏菌、副伤寒沙门氏菌等[75]。
在马里兰大学医学院疫苗开发中心,研究人员开发评估了各种沙门氏菌减毒活疫苗,其中包括针对鼠伤寒沙门氏菌的疫苗:CVD1921和CVD1931,且CVD1921已被证明在免疫功能低下的非人灵长类动物中安全且耐受性良好[75-77]。除此之外,还有疫苗LH1160、WT05,分别通过删除phoP/phoQ基因,aroC和ssaV基因使毒力减弱。2种疫苗都具有高度免疫原性,志愿者在接种后均未观察到菌血症[78-81]。最近,膜抗原广义模块(generalized modules for membrane antigens,GMMA)技术被开发成为非伤寒沙门氏菌疫苗递送新策略,该技术以天然构象呈现表面多糖和外膜蛋白[82]。GMMA是纳米级颗粒,显示高密度抗原并含有细菌病原体相关分子模式(pathogen-associated molecular patterns,PAMPs),有可能引发强烈的免疫反应[83]。DE等[84]研究了GMMA作为鼠伤寒沙门氏菌和肠炎沙门氏菌O抗原递送系统的用途。革兰氏阴性菌在自然脱落外膜的过程中形成囊泡,删除tolR基因会使脱落水平大大提高,再通过对脂多糖的脂质A部分进行解毒。之后,将进一步的基因修饰引入产生GMMA的菌株中,以降低反应原性,这些基因修饰会导致鼠伤寒沙门氏菌表面O抗原结构的变化。当在小鼠中进行测试时,GMMA诱导了高水平的抗O抗原特异性IgG功能抗体。这些免疫原性数据表明,GMMA有望成为鼠伤寒沙门氏菌疫苗开发的有效策略。
3 展望
鼠伤寒沙门氏菌作为一种食源性致病菌,严重威胁人类的生命健康。而多药耐药菌株的出现,为治疗鼠伤寒沙门氏菌感染带来了巨大的挑战。随着抗生素的滥用,传统抗生素治疗已不能满足需求,新兴的小分子疗法、噬菌体疗法、减毒疫苗疗法等有望成为治疗鼠伤寒沙门氏菌感染的有效方法。此外,微生物群和益生菌在改善健康和防止感染方面发挥着重要作用。据报道,大部分益生菌可缓解肠道微生物生态失调,维持代谢和免疫稳态。这表明益生菌或可作为鼠伤寒沙门氏菌感染的干预剂和治疗剂,有望成为鼠伤寒沙门氏菌治疗方法的新方向[85-86]。开发鼠伤寒沙门氏菌感染的治疗方法还面临重重困难,包括菌株耐药性、药物靶向特异性、对人类的毒副作用等等。因此,了解鼠伤寒沙门氏菌的感染机制及治疗措施十分重要,相信在未来会有更多的疗法出现,更好地应用于临床造福人类。
[1] HAVELAAR A H, KIRK M D, TORGERSON P R, et al.World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010[J].PLoS Medicine, 2015, 12(12):e1001923.
[2] DOS SANTOS A M P, FERRARI R G, CONTE-JUNIOR C A.Virulence factors in Salmonella typhimurium:The sagacity of a bacterium[J].Current Microbiology, 2019, 76(6):762-773.
[3] MAJOWICZ S E, MUSTO J, SCALLAN E, et al. The global burden of nontyphoidal Salmonella gastroenteritis[J]. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 2010, 50(6):882-889.
[4] KIRK M D, PIRES S M, BLACK R E, et al.World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010:A data synthesis[J].PLoS Medicine, 2015, 12(12):e1001921.
[5] BRANCHU P, BAWN M, KINGSLEY R A.Genome variation and molecular epidemiology of Salmonella enterica serovar Typhimurium pathovariants[J].Infection and Immunity, 2018, 86(8):e00079-e00018.
[6] GILCHRIST J J, MACLENNAN C A.Invasive nontyphoidal Salmonella disease in Africa[J].EcoSal Plus, 2019, 8(2):ecosalplus.ESP-0007-2018.
[7] UCHE I V, MACLENNAN C A, SAUL A.A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014)[J].PLoS Neglected Tropical Diseases, 2017, 11(1):e0005118.
[8] HE Y, WANG J K, ZHANG R H, et al.Epidemiology of foodborne diseases caused by Salmonella in Zhejiang Province, China, between 2010 and 2021[J].Frontiers in Public Health, 2023, 11:1127925.
[9] CHEN H Y, QIU H H, ZHONG H, et al.Non-typhoidal Salmonella infections among children in Fuzhou, Fujian, China:A 10-year retrospective review from 2012 to 2021[J].Infection and Drug Resistance, 2023, Volume 16:2737-2749.
[10] GAL-MOR O, BOYLE E C, GRASSL G A.Same species, different diseases:How and why typhoidal and non-typhoidal Salmonella enterica serovars differ[J].Frontiers in Microbiology, 2014, 5:391.
[11] FERRARI R G, ROSARIO D K A, CUNHA-NETO A, et al. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis[J]. Applied and Environmental Microbiology, 2019, 85(14): e00591-e00519.
[12] PARISI A, CRUMP J A, STAFFORD R, et al.Increasing incidence of invasive nontyphoidal Salmonella infections in Queensland, Australia, 2007-2016[J].PLoS Neglected Tropical Diseases, 2019, 13(3):e0007187.
[13] FORD L, GLASS K, VEITCH M, et al.Increasing incidence of Salmonella in Australia, 2000-2013[J].PLoS One, 2016, 11(10):e0163989.
[14] JACOB J J, SOLAIMALAI D, RACHEL T, et al.A secular trend in invasive non-typhoidal Salmonella in South India, 2000-2020:Identification challenges and antibiogram[J].Indian Journal of Medical Microbiology, 2022, 40(4):536-540.
[15] BASSAL R, DAVIDOVICH-COHEN M, YAKUNIN E, et al.Trends in the epidemiology of non-typhoidal Salmonellosis in Israel between 2010 and 2021[J].International Journal of Environmental Research and Public Health, 2023, 20(9):5626.
[16] WILSON C N, CHUNGA A, MASESA C, et al.Incidence of invasive non-typhoidal Salmonella in Blantyre, Malawi between January 2011-December 2019[J].Wellcome Open Research, 2022, 7:143.
[17] CRUMP J A, SJÖLUND-KARLSSON M, GORDON M A, et al.Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections[J].Clinical Microbiology Reviews, 2015, 28(4):901-937.
[18] SRIKANTH C V, MERCADO-LUBO R, HALLSTROM K, et al.Salmonella effector proteins and host-cell responses[J].Cellular and Molecular Life Sciences: CMLS, 2011, 68(22):3687-3697.
[19] 赵泽慧, 李强, 何小丽, 等.鼠伤寒沙门氏菌致病机理的研究进展[J].黑龙江畜牧兽医, 2017(5):71-75.ZHAO Z H, LI Q, HE X L, et al.Research progress on pathogenic mechanism of Salmonella typhimurium[J].Heilongjiang Animal Science and Veterinary Medicine, 2017(5):71-75.
[20] BROZ P, OHLSON M B, MONACK D M.Innate immune response to Salmonella typhimurium, a model enteric pathogen[J].Gut Microbes, 2012, 3(2):62-70.
[21] VAZQUEZ-TORRES A, JONES-CARSON J, B UMLER A J, et al.Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes[J].Nature, 1999, 401(6755):804-808.
UMLER A J, et al.Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes[J].Nature, 1999, 401(6755):804-808.
[22] NIESS J H, BRAND S, GU X B, et al.CX3 CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance[J].Science, 2005, 307(5707):254-258.
[23] AZIMI T, ZAMIRNASTA M, SANI M A, et al.Molecular mechanisms of Salmonella effector proteins:A comprehensive review[J].Infection and Drug Resistance, 2020,13:11-26.
[24] 战仁慧, 张建.Toll样受体在伤寒沙门氏菌感染过程中的作用机制[J].中国免疫学杂志, 2013, 29(10):1098-1102.ZHAN R H, ZHANG J.Mechanism of toll-like receptor in Salmonella typhoid infection[J].Chinese Journal of Immunology, 2013, 29(10):1098-1102.
[25] LI W W, LI L X, YAN X L, et al.Nitrate utilization promotes systemic infection of Salmonella typhimurium in mice[J].International Journal of Molecular Sciences, 2022, 23(13):7220.
[26] BADER M W, NAVARRE W W, SHIAU W, et al.Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides[J].Molecular Microbiology, 2003, 50(1):219-230.
[27] TAYLOR S J, WINTER S E.Salmonella finds a way:Metabolic versatility of Salmonella enterica serovar Typhimurium in diverse host environments[J].PLoS Pathogens, 2020, 16(6):e1008540.
[28] GANGATHRAPRABHU B, KANNAN S, SANTHANAM G, et al.A review on the origin of multidrug-resistant Salmonella and perspective of tailored phoP gene towards avirulence[J].Microbial Pathogenesis, 2020, 147:104352.
[29] ZAFAR M, JAHAN H, SHAFEEQ S, et al.Clarithromycin exerts an antibiofilm effect against Salmonella typhimurium rdar biofilm formation and transforms the physiology towards an apparent oxygen-depleted energy and carbon metabolism[J].Infection and Immunity, 2020, 88(11):e00510-e00520.
[30] ZHANG C Z, REN S Q, CHANG M X, et al.Resistance mechanisms and fitness of Salmonella typhimurium and Salmonella Enteritidis mutants evolved under selection with ciprofloxacin in vitro[J].Scientific Reports, 2017, 7(1):9113.
[31] BAUCHERON S, TYLER S, BOYD D, et al.AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar typhimurium DT104[J].Antimicrobial Agents and Chemotherapy, 2004, 48(10):3729-3735.
[32] WEI Z, XU X B, YAN M Y, et al.Salmonella typhimurium and Salmonella enteritidis infections in sporadic diarrhea in children:Source tracing and resistance to third-generation cephalosporins and ciprofloxacin[J].Foodborne Pathogens and Disease, 2019, 16(4):244-255.
[33] TACK B, PHOBA M F, BARBÉ B, et al.Non-typhoidal Salmonella bloodstream infections in Kisantu, DR Congo:Emergence of O5-negative Salmonella typhimurium and extensive drug resistance[J].PLoS Neglected Tropical Diseases, 2020, 14(4):e0008121.
[34] DIARD M, SELLIN M E, DOLOWSCHIAK T, et al.Antibiotic treatment selects for cooperative virulence of Salmonella typhimurium[J].Current Biology: CB, 2014, 24(17):2000-2005.
[35] NEWSON J P, GAISSMAIER M S, MCHUGH S C, et al.Studying antibiotic persistence in vivo using the model organism Salmonella typhimurium[J].Current Opinion in Microbiology, 2022, 70:102224.
[36] DAWAN J, UDDIN M J, AHN J.Development of de novo resistance in Salmonella typhimurium treated with antibiotic combinations[J].FEMS Microbiology Letters, 2019, 366(10):fnz127.
[37] DAWAN J, AHN J.Effectiveness of antibiotic combination treatments to control heteroresistant Salmonella typhimurium[J].Microbial Drug Resistance, 2021, 27(4):441-449.
[38] ULANOWSKA M, OLAS B.Biological properties and prospects for the application of Eugenol-a review[J].International Journal of Molecular Sciences, 2021, 22(7):3671.
[39] ZHAO X, WEI S M, TIAN Q M, et al.Eugenol exposure in vitro inhibits the expressions of T3SS and TIF virulence genes in Salmonella typhimurium and reduces its pathogenicity to chickens[J].Microbial Pathogenesis, 2022, 162:105314.
[40] ZHAO X, ZHENG S M, WEI S M, et al.The protective effect and potential mechanisms of eugenol against Salmonella in vivo and in vitro[J].Poultry Science, 2022, 101(5):101801.
[41] MARCHESE A, ORHAN I E, DAGLIA M, et al.Antibacterial and antifungal activities of thymol:A brief review of the literature[J].Food Chemistry, 2016, 210:402-414.
[42] ZHANG Y, LIU Y, QIU J Z, et al.The herbal compound thymol protects mice from lethal infection by Salmonella typhimurium[J].Frontiers in Microbiology, 2018, 9:1022.
[43] ZHANG Y, LIU Y, LUO J J, et al.The herbal compound thymol targets multiple Salmonella typhimurium virulence factors for lon protease degradation[J].Frontiers in Pharmacology, 2021, 12:674955.
[44] GIOVAGNONI G, ROSSI B, TUGNOLI B, et al.Thymol and carvacrol downregulate the expression of Salmonella typhimurium virulence genes during an in vitro infection on Caco-2 cells[J].Microorganisms, 2020, 8(6):862.
[45] LIU Y, ZHANG Y, ZHOU Y L, et al.Cinnamaldehyde inhibits type three secretion system in Salmonella enterica serovar Typhimurium by affecting the expression of key effector proteins[J].Veterinary Microbiology, 2019, 239:108463.
[46] LV Q H, LI S F, WEI H L, et al.Identification of the natural product paeonol derived from peony bark as an inhibitor of the Salmonella enterica serovar Typhimurium type Ⅲ secretion system[J].Applied Microbiology and Biotechnology, 2020, 104(4):1673-1682.
[47] LV Q H, CHU X, YAO X Y, et al.Inhibition of the type Ⅲ secretion system by syringaldehyde protects mice from Salmonella enterica serovar Typhimurium[J].Journal of Cellular and Molecular Medicine, 2019, 23(7):4679-4688.
[48] LYU Q H, LV Y Z, DOU X Y, et al.Myricetin inhibits the type Ⅲ secretion system of Salmonella enterica serovar typhimurium by downregulating the Salmonella pathogenic island Ⅰ gene regulatory pathway[J].Microbial Pathogenesis, 2021, 150:104695.
[49] GUO Z X, LI X L, LI J F, et al.Licoflavonol is an inhibitor of the type three secretion system of Salmonella enterica serovar Typhimurium[J].Biochemical and Biophysical Research Communications, 2016, 477(4):998-1004.
[50] SHI Y J, CHEN X D, SHU J Y, et al.Harmine, an inhibitor of the type Ⅲ secretion system of Salmonella enterica serovar Typhimurium[J].Frontiers in Cellular and Infection Microbiology, 2022, 12:967149.
[51] VIKRAM A, JESUDHASAN P R, JAYAPRAKASHA G K, et al.Citrus flavonoid represses Salmonella pathogenicity island 1 and motility in S.typhimurium LT2[J].International Journal of Food Microbiology, 2011, 145(1):28-36.
[52] LI Q J, WANG L P, XU J W, et al.Quercitrin is a novel inhibitor of Salmonella enterica serovar typhimurium type Ⅲ secretion system[J].Molecules, 2023, 28(14):5455.
[53] HEGAZY W A H, SALEM I M, ALOTAIBI H F, et al.Terazosin interferes with quorum sensing and type three secretion system and diminishes the bacterial espionage to mitigate the Salmonella typhimurium pathogenesis[J].Antibiotics, 2022, 11(4):465.
[54] HUSSAIN S, OUYANG P, ZHU Y K, et al.Type 3 secretion system 1 of Salmonella typhimurium and its inhibitors:A novel strategy to combat salmonellosis[J].Environmental Science and Pollution Research International, 2021, 28(26):34154-34166.
[55] CHIU H C, KULP S K, SONI S, et al.Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent[J].Antimicrobial Agents and Chemotherapy, 2009, 53(12):5236-5244.
[56] AMMANATHAN V, MISHRA P, CHAVALMANE A K, et al.Restriction of intracellular Salmonella replication by restoring TFEB-mediated xenophagy[J].Autophagy, 2020, 16(9):1584-1597.
[57] ZHANG L, SUN Y, XU W, et al.Baicalin inhibits Salmonella typhimurium-induced inflammation and mediates-autophagy through TLR4/MAPK/NF-κB signalling pathway[J].Basic &Clinical Pharmacology &Toxicology, 2021, 128(2):241-255.
[58] AL AZZAZ J, RIEU A, AIRES V, et al.Resveratrol-induced xenophagy promotes intracellular bacteria clearance in intestinal epithelial cells and macrophages[J].Frontiers in Immunology, 2019, 9:3149.
[59] ZGURSKAYA H I, RYBENKOV V V.Permeability barriers of Gram-negative pathogens[J].Annals of the New York Academy of Sciences, 2020, 1459(1):5-18.
[60] JOLIVET-GOUGEON A, BONNAURE-MALLET M.Biofilms as a mechanism of bacterial resistance[J].Drug Discovery Today:Technologies, 2014, 11:49-56.
[61] VAN ACKER H, VAN DIJCK P, COENYE T.Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms[J].Trends in Microbiology, 2014, 22(6):326-333.
[62] HARRELL J E, HAHN M M, D’SOUZA S J, et al.Salmonella biofilm formation, chronic infection, and immunity within the intestine and hepatobiliary tract[J].Frontiers in Cellular and Infection Microbiology, 2021, 10:624622.
[63] MOSHIRI J, KAUR D, HAMBIRA C M, et al.Identification of a small molecule anti-biofilm agent against Salmonella enterica[J].Frontiers in Microbiology, 2018, 9:2804.
[64] DOMBACH J L, QUINTANA J L J, NAGY T A, et al.A small molecule that mitigates bacterial infection disrupts Gram-negative cell membranes and is inhibited by cholesterol and neutral lipids[J].PLoS Pathogens, 2020, 16(12):e1009119.
[65] VILLANUEVA J A, CROOKS A L, NAGY T A, et al.Salmonella enterica infections are disrupted by two small molecules that accumulate within phagosomes and differentially damage bacterial inner membranes[J].mBio, 2022, 13(5):e0179022.
[66] GAO Y Z, CHEN H L, LI W, et al.Chloroform extracts of Atractylodes chinensis inhibit the adhesion and invasion of Salmonella typhimurium[J].Biomedicine &Pharmacotherapy, 2022, 154:113633.
[67] HUANG C X, SHI J C, MA W J, et al.Isolation, characterization, and application of a novel specific Salmonella bacteriophage in different food matrices[J].Food Research International, 2018, 111:631-641.
[68] LAURE N N, AHN J.Phage resistance-mediated trade-offs with antibiotic resistance in Salmonella typhimurium[J].Microbial Pathogenesis, 2022, 171:105732.
[69] STRATHDEE S A, HATFULL G F, MUTALIK V K, et al.Phage therapy:From biological mechanisms to future directions[J].Cell, 2023, 186(1):17-31.
[70] JUNG L S, DING T, AHN J.Evaluation of lytic bacteriophages for control of multidrug-resistant Salmonella typhimurium[J].Annals of Clinical Microbiology and Antimicrobials, 2017, 16(1):66.
[71] ![]() M, GRABOWSKI
M, GRABOWSKI  , et al.Efficacy and safety of phage therapy against Salmonella enterica serovars Typhimurium and Enteritidis estimated by using a battery of in vitro tests and the Galleria mellonella animal model[J].Microbiological Research, 2022, 261:127052.
, et al.Efficacy and safety of phage therapy against Salmonella enterica serovars Typhimurium and Enteritidis estimated by using a battery of in vitro tests and the Galleria mellonella animal model[J].Microbiological Research, 2022, 261:127052.
[72] LIANG L, HUANG J Q, CUI K J, et al.A combination of virulent and non-productive phages synergizes the immune system against Salmonella typhimurium systemic infection[J].International Journal of Molecular Sciences, 2022, 23(21):12830.
[73] LU M, LIU B X, XIONG W B, et al.The combination of Salmonella phage ST-3 and antibiotics to prevent Salmonella typhimurium in vitro[J].Current Microbiology, 2022, 79(12):371.
[74] LOC-CARRILLO C, ABEDON S T.Pros and cons of phage therapy[J].Bacteriophage, 2011, 1(2):111-114.
[75] TENNANT S M, LEVINE M M.Live attenuated vaccines for invasive Salmonella infections[J].Vaccine, 2015, 33:C36-C41.
[76] TENNANT S M, WANG J Y, GALEN J E, et al.Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains[J].Infection and Immunity, 2011, 79(10):4175-4185.
[77] AULT A, TENNANT S M, GORRES J P, et al.Safety and tolerability of a live oral Salmonella typhimurium vaccine candidate in SIV-infected nonhuman primates[J].Vaccine, 2013, 31(49):5879-5888.
[78] DIPETRILLO M D, TIBBETTS T, KLEANTHOUS H, et al. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers[J]. Vaccine, 1999, 18(5-6):449-459.
[79] ANGELAKOPOULOS H, HOHMANN E L.Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers[J].Infection and Immunity, 2000, 68(4):2135-2141.
[80] HINDLE Z, CHATFIELD S N, PHILLIMORE J, et al.Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type Ⅲ secretion system (ssaV) mutations by immunization of healthy volunteers[J].Infection and Immunity, 2002, 70(7):3457-3467.
[81] GALEN J E, BUSKIRK A D, TENNANT S M, et al. Live attenuated human Salmonella vaccine candidates: Tracking the pathogen in natural infection and stimulation of host immunity[J]. EcoSal Plus, 2016, 7(1):10.1128/ecosalplus.ESP-10.1128/ecosalplus0010-2016.
[82] TENNANT S M, MACLENNAN C A, SIMON R, et al.Nontyphoidal Salmonella disease:Current status of vaccine research and development[J].Vaccine, 2016, 34(26):2907-2910.
[83] ELLIS T N, KUEHN M J.Virulence and immunomodulatory roles of bacterial outer membrane vesicles[J].Microbiology and Molecular Biology Reviews: MMBR, 2010, 74(1):81-94.
[84] DE BENEDETTO G, ALFINI R, CESCUTTI P, et al.Characterization of O-antigen delivered by generalized modules for membrane antigens (GMMA) vaccine candidates against nontyphoidal Salmonella[J].Vaccine, 2017, 35(3):419-426.
[85] EL-SHARKAWY H, TAHOUN A, RIZK A M, et al.Evaluation of bifidobacteria and Lactobacillus probiotics as alternative therapy for Salmonella typhimurium infection in broiler chickens[J].Animals: an Open Access Journal from MDPI, 2020, 10(6):1023.
[86] PRADHAN B, GUHA D, NAIK A K, et al.Probiotics L.acidophilus and B.clausii modulate gut microbiota in Th1- and Th2-biased mice to ameliorate Salmonella typhimurium-induced diarrhea[J].Probiotics and Antimicrobial Proteins, 2019, 11(3):887-904.