1 杂环胺概述
杂环芳香胺(heterocyclic aromatic amines,HAAs)起初是从热加工高蛋白质食物中分离出来的一类致癌、致突变性的有害物质[1-2]。后来,它们从各种环境来源中分离出来,如河水、汽车尾气颗粒、香烟烟雾、人类排泄物和雨水等。HAAs是由2~5个含氮杂环和环外氨基组成的低分子有机胺类化合物,以游离态和结合态2种不同的形式存在[3]。目前在热加工食品中已经鉴定出了30多种HAA,主要分为两大类(表1):一类是氨基咪唑氮杂芳烃类杂环胺(amino-imidazo-azaarenes,AIAs),是在加热温度为100~250 ℃,由碳水化合物、氨基酸和肌酐生成的,主要包括喹啉类(IQ)、喹喔啉类(IQx)、吡啶类(PhIP)、吡啶呋喃类(IFP)等,另一类是氨基咔类杂环胺(amino-carbolines,ACs),是在加热温度高于250 ℃,通过谷氨酸或色氨酸直接热裂解形成的,主要包括α-咔啉类、β-咔啉类、γ-咔啉类、δ-咔啉类等[4-6],常见HAA结构如图1所示。HAAs由于其分子中含有苯环或其他芳香基团,因此具有芳香性[7]。另外,由于其分子中含有多种官能团,同时具有较高的反应活性[8]。
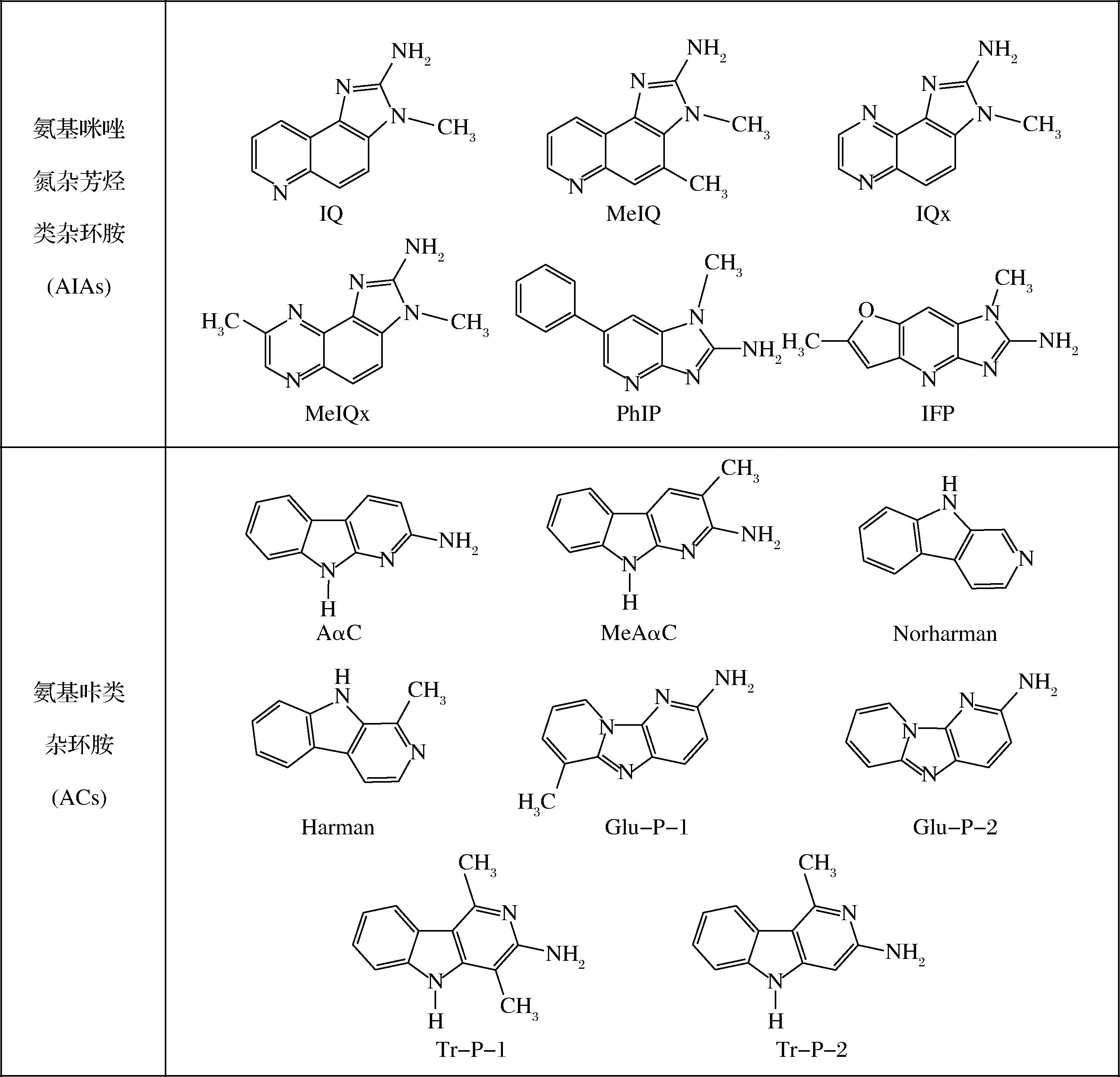
图1 常见HAAs的化学结构式
Fig.1 Chemical formula of common HAAs
表1 HAAs的分类
Table 1 Classification of HAAs
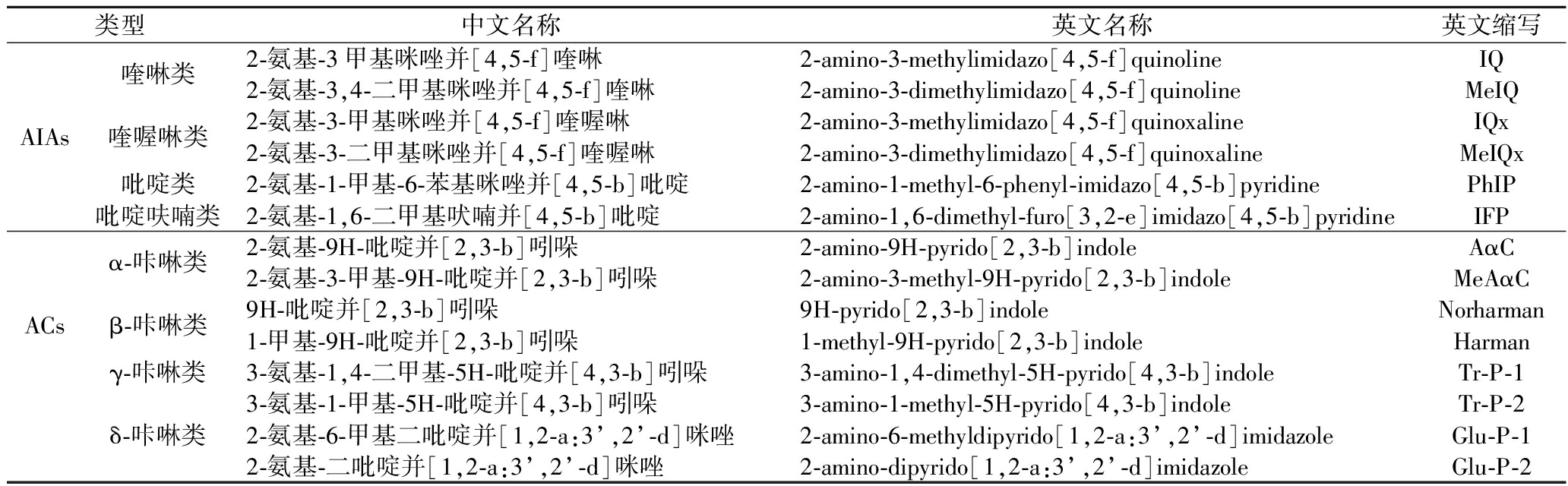
类型中文名称英文名称英文缩写AIAs喹啉类喹喔啉类吡啶类吡啶呋喃类2-氨基-3甲基咪唑并[4,5-f]喹啉2-amino-3-methylimidazo[4,5-f]quinolineIQ2-氨基-3,4-二甲基咪唑并[4,5-f]喹啉2-amino-3-dimethylimidazo[4,5-f]quinolineMeIQ2-氨基-3-甲基咪唑并[4,5-f]喹喔啉2-amino-3-methylimidazo[4,5-f]quinoxalineIQx2-氨基-3-二甲基咪唑并[4,5-f]喹喔啉2-amino-3-dimethylimidazo[4,5-f]quinoxalineMeIQx2-氨基-1-甲基-6-苯基咪唑并[4,5-b]吡啶2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridinePhIP2-氨基-1,6-二甲基吠喃并[4,5-b]吡啶2-amino-1,6-dimethyl-furo[3,2-e]imidazo[4,5-b]pyridineIFPACsα-咔啉类β-咔啉类γ-咔啉类2-氨基-9H-吡啶并[2,3-b]吲哚2-amino-9H-pyrido[2,3-b]indoleAαC2-氨基-3-甲基-9H-吡啶并[2,3-b]吲哚2-amino-3-methyl-9H-pyrido[2,3-b]indoleMeAαC9H-吡啶并[2,3-b]吲哚9H-pyrido[2,3-b]indoleNorharman1-甲基-9H-吡啶并[2,3-b]吲哚1-methyl-9H-pyrido[2,3-b]indoleHarman3-氨基-1,4-二甲基-5H-吡啶并[4,3-b]吲哚3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indoleTr-P-13-氨基-1-甲基-5H-吡啶并[4,3-b]吲哚3-amino-1-methyl-5H-pyrido[4,3-b]indoleTr-P-2δ-咔啉类2-氨基-6-甲基二吡啶并[1,2-a:3’,2’-d]咪唑2-amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazoleGlu-P-12-氨基-二吡啶并[1,2-a:3’,2’-d]咪唑2-amino-dipyrido[1,2-a:3’,2’-d]imidazoleGlu-P-2
2 杂环胺在食品中的含量及检测方法
目前检测HAA的方法主要有高效液相色谱法、高效液相色谱-质谱联用法、气相色谱-质谱联用法、荧光免疫检测、酶联免疫吸附测定法等。由于食品中HAA含量较低,较难检出,并存在耗时、消耗大量有机试剂的问题。因此研究快速、简便、安全的检测方法至关重要。不同食品中HAA的含量及检测方法如表2所示。
表2 不同食品中HAA的含量及检测方法
Table 2 HAA content and detection methodsin different foods
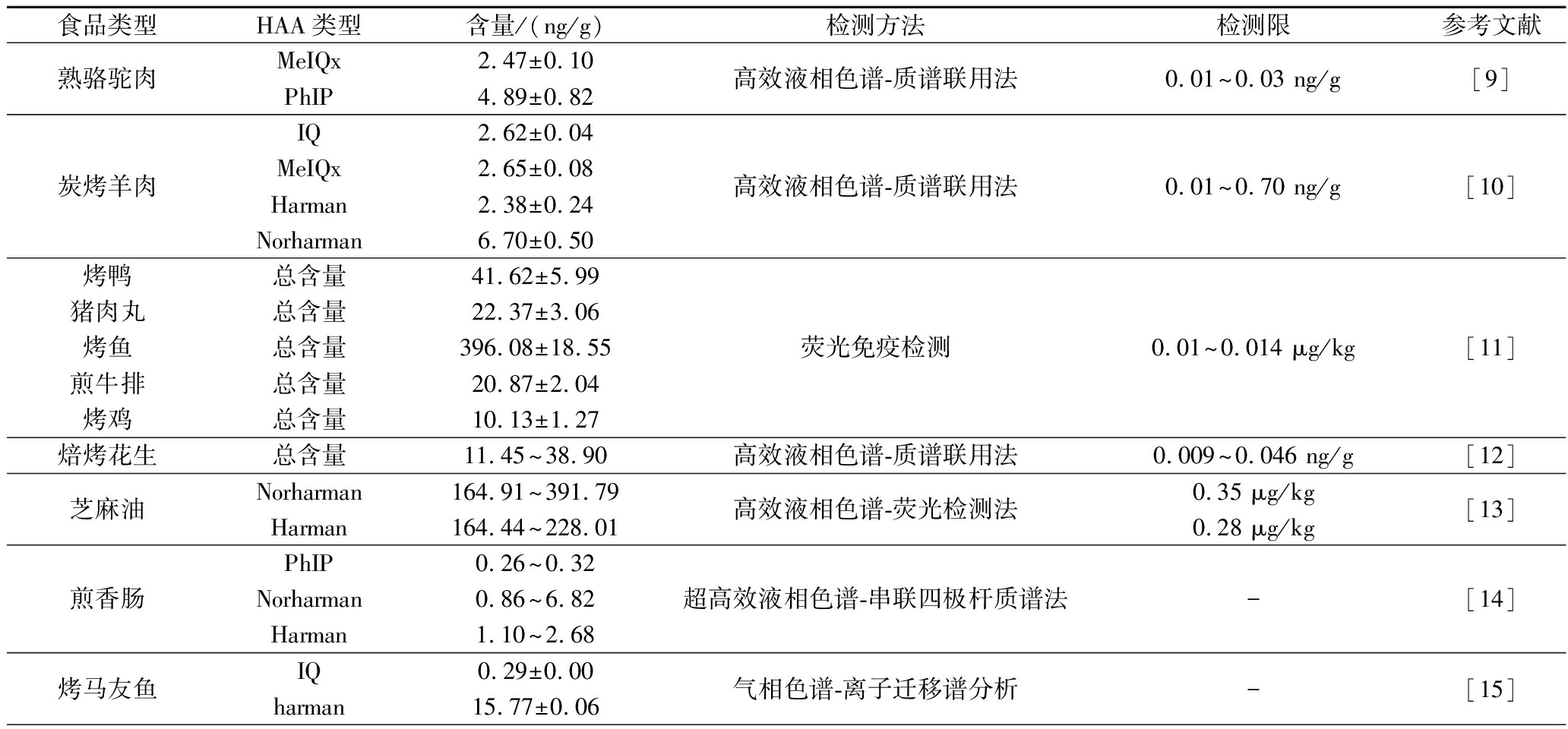
食品类型HAA类型含量/(ng/g)检测方法检测限参考文献熟骆驼肉MeIQx2.47±0.10PhIP4.89±0.82高效液相色谱-质谱联用法0.01~0.03 ng/g[9]炭烤羊肉IQ2.62±0.04MeIQx2.65±0.08Harman2.38±0.24Norharman6.70±0.50高效液相色谱-质谱联用法0.01~0.70 ng/g[10]烤鸭总含量41.62±5.99猪肉丸总含量22.37±3.06烤鱼总含量396.08±18.55煎牛排总含量20.87±2.04烤鸡总含量10.13±1.27荧光免疫检测0.01~0.014 μg/kg[11]焙烤花生总含量11.45~38.90高效液相色谱-质谱联用法0.009~0.046 ng/g[12]芝麻油Norharman164.91~391.79Harman164.44~228.01高效液相色谱-荧光检测法0.35 μg/kg0.28 μg/kg[13]煎香肠PhIP0.26~0.32Norharman0.86~6.82Harman1.10~2.68超高效液相色谱-串联四极杆质谱法-[14]烤马友鱼IQ0.29±0.00harman15.77±0.06气相色谱-离子迁移谱分析-[15]
续表2
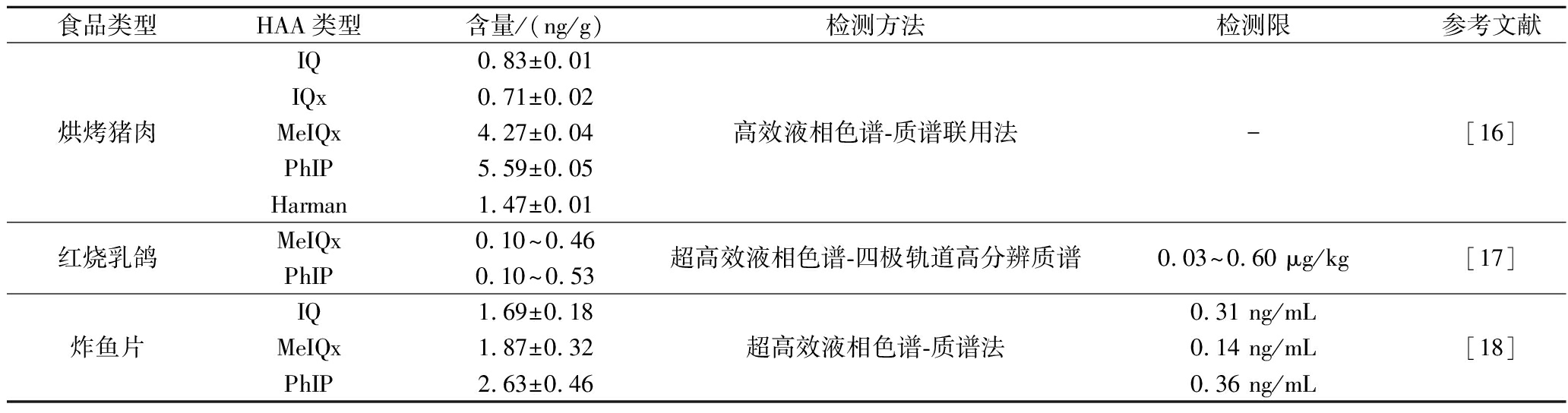
食品类型HAA类型含量/(ng/g)检测方法检测限参考文献烘烤猪肉IQ0.83±0.01IQx0.71±0.02MeIQx4.27±0.04PhIP5.59±0.05Harman1.47±0.01高效液相色谱-质谱联用法-[16]红烧乳鸽MeIQx0.10~0.46PhIP0.10~0.53超高效液相色谱-四极轨道高分辨质谱0.03~0.60 μg/kg[17]炸鱼片IQ1.69±0.18MeIQx1.87±0.32PhIP2.63±0.46超高效液相色谱-质谱法0.31 ng/mL0.14 ng/mL0.36 ng/mL[18]
3 杂环胺在机体中的代谢机制
通常食品中HAA的含量以ng/g为单位进行计量,但长期摄入会对人体造成极大威胁,因此研究HAA在机体中的代谢对其毒性控制具有重要意义。根据已有研究报道,膳食摄入的不同类型HAA在机体中通过多种酶代谢,包括细胞色素 P450酶(cytochrome P450s,CYPs)、过氧化物酶(peroxidase,POD)、N-乙酰转移酶(N-acetyltransferases,NATs)、磺基转移酶(sulfotransferases,SULTs),尿甘二磷酸葡醛酸转移酶(uridine diphosphate-glucuronosyltransferases,UGTs)以及谷胱甘肽S转移酶(glutathione S-transferases,GSTs),它们的主要代谢物会进一步与DNA、蛋白质、脂肪结合,从而产生毒性作用,或通过尿液和粪便的形式排出体外[18]。以MeIQx为例,其代谢途径如图2所示。
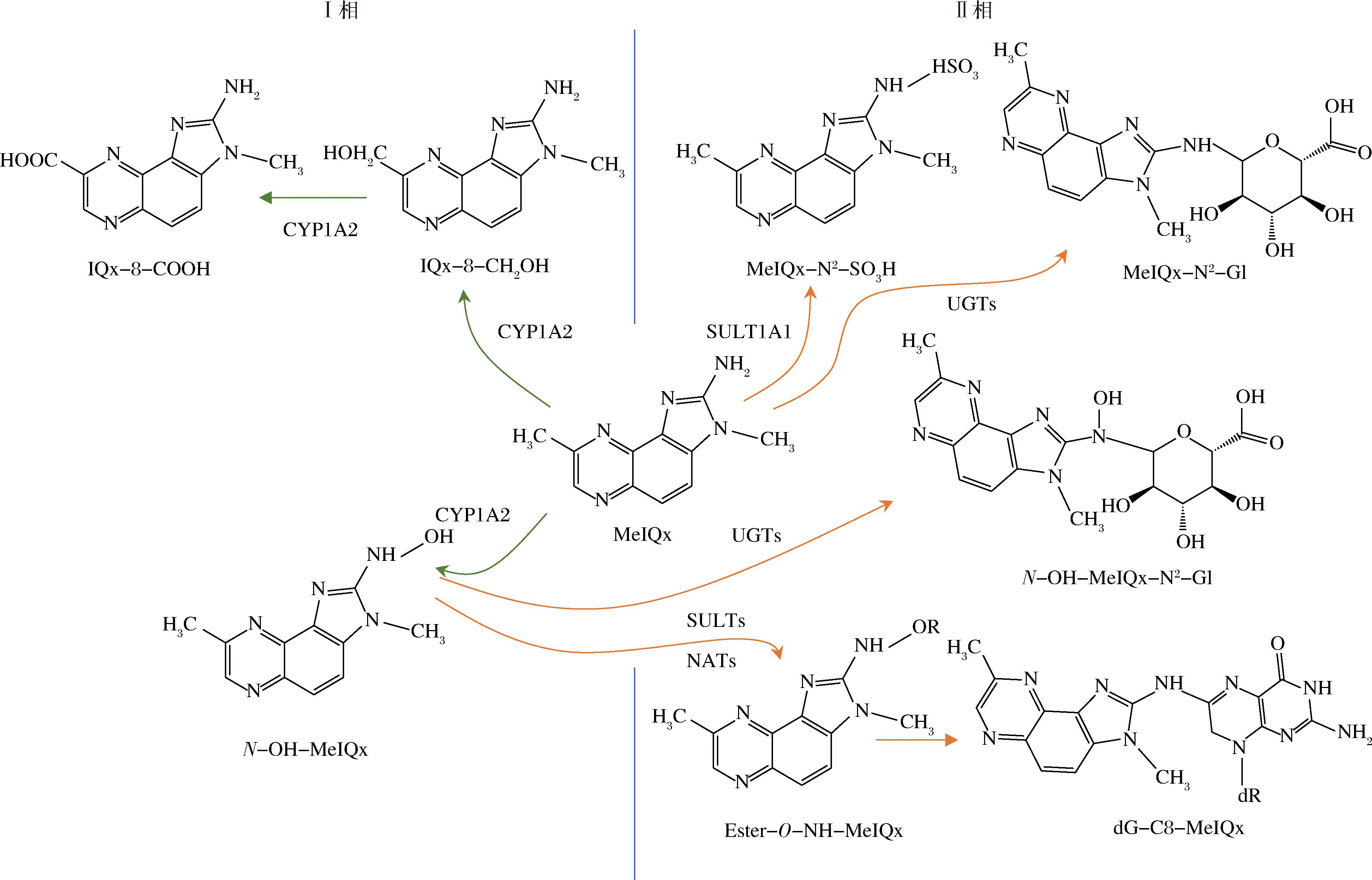
图2 MeIQx的代谢途径
Fig.2 Metabolic pathway of MeIQx
HAAs的代谢主要包括解毒和活化[19]。首先是Ⅰ相酶的代谢,HAAs在CYPs催化作用下环外氨基的N发生氧化反应形成氮-羟基-杂环胺(N-OH-HAAs)衍生物[20]。N-OH-HAAs是一种具有遗传毒性的代谢物,它与DNA共价结合形成易于突变的DNA加合物[21]。肝脏中CYP1A2、CYP1A1和CYP1B1是催化人类HAA 环外氨基N氧化的主要CYP[22]。一项药物动力学研究,CYP1A2在人体中参与了91%的MeIQx代谢和70%的PhIP代谢[23]。PhIP被CYP1A2代谢的主要途径是通过N氧化形成具有遗传毒性的N-OH-PhIP,而MeIQx被CYP1A2代谢的主要途径是通过8-甲基氧化形成解毒产物IQx-8-COOH[24]。在啮齿类动物、非人灵长类动物和人类中,CYP对HAAs的代谢存在种间差异,这主要归因于CYP表达水平的不同、催化活性的差异以及CYP对HAA的区位选择性[25-26]。
II期酶在HAAs代谢中具有双重作用,有助于解毒和生物活化[9]。II期酶(包括NATs、SULTs和UGTs)会与一些HAA环外氨基直接结合进行解毒[27]。NAT1和NAT2是两种不同的N-乙酰转移酶同工酶。NAT2主要在肝脏中表达,而NAT1主要在肝外组织中表达。许多具有致癌芳香胺结构的物质会经过NATs的代谢,例如Glu-P-1和AαC会经历NATs的N-乙酰化,而AIAs则不会被NATs代谢[28]。人体中SULTs属于一个基因超家族,分为两个亚家族:苯酚磺基转移酶(SULT1)和羟基类固醇磺基转移酶(SULT2)[29]。IQ和MeIQx在大鼠体内由SULT1A1催化形成氨基磺酸衍生物,是主要的解毒代谢物[30]。在人尿中也检测到MeIQx-N2-SO3H,也证实了SULTs的解毒作用[31]。而其他HAAs在啮齿类动物或人类中则不发生磺化作用。UGTs在机体中会参与环境中的化学物质、致癌物等葡萄糖醛酸结合反应,从而清除或灭活外源和内源性化合物。有研究报道,UGTs会通过催化IQ、PhIP、AαC等环外氨基葡萄糖醛酸化发挥解毒作用[32]。另外,II期酶也会发挥生物活化作用。在啮齿动物和非人灵长类动物体内N-OH-HAAs会被NATs或SULTs催化酯化形成活化产物,进一步异裂生成易与大分子物质(如蛋白质、DNA等)形成加合物的芳基氮离子中间物,最终导致癌症的发生[21]。大多数N-OH-HAAs可被NAT2生物激活,例如HONH-MeIQx、HONH-IQ、HONH-AαC等, SULT1A1、SULT1A2可以催化IQ、PhIP、AαC 等N-羟基代谢产物结合DNA形成加成物[33]。
4 杂环胺的毒性作用
4.1 致突变作用
在人体中,HAA可激活CYP1A1和1A2的氧化作用,形成N-羟基衍生物,而这一类物质可与DNA形成加合物,从而导致 DNA受损,如DNA中的链式氢键断裂、位点突变以及DNA中的缺失和插入等,对人体的肝脏、淋巴组织等器官产生致突变性[34]。与食品中的其他诱变剂相比,HAAs具有较强的致突变性,其致突变性比黄曲霉毒素 B1高100倍,比苯并芘高2 000倍[35]。不同类型HAA的致突变能力不同,据TOTSUKA等[36]报道,AIA是高温加工肉食中存在的主要诱变剂,并证明了AIA 是沙门氏菌中迄今为止最有效的诱变剂。王一然等[37]通过Ames 氏预培养法对采用不同烹调方法的高蛋白食物进行致突变检测,发现采用不同烹调方法的食物致突变性不同,烹调温度、加热时间与致突变性成正比。LYNCH等[38]通过对Muta小鼠持续4天经口灌胃0.2、2 和 20 mg/kg剂量的PhIP,来研究PhIP在体内的致突变性,发现PhIP仅在20 mg/kg剂量时对小鼠大肠和肝脏具有致突变性。GI等[39]持续4周给雄性 F344 gpt delta 转基因大鼠分别灌胃0、0.1、1、10 或 100 mg/kg剂量的IQ,结果发现在10 和 100 mg/kg 组中,肝脏中gpt转基因突变的频率显着增加。此外,突变频率在 1、10 和 100 mg/kg组中呈剂量依赖性。
4.2 致癌作用
流行病学研究调查表明,膳食摄入HAAs与结肠癌、食道癌、胃癌、胰腺癌、前列腺癌、乳腺癌和其他癌症的风险增加相关[40]。结直肠癌的发病率取决于生活方式、环境因素、遗传和饮食[41]。接触食品源性 HAA被认为是结直肠癌发生的一个重要因素。研究发现,MeIQx与结直肠癌风险之间存在统计学上存在显著的正相关关系[42]。食道癌被认为是全世界最常见的癌症类型之一[43]。在 CROSS 等[44]的研究中,食用肉类中两种高浓度的 HAA(MeIQx 和 PhIP)与食道癌风险增加相关。另外,越来越多的证据表明饮食习惯也会影响前列腺癌的发病率[9]。在动物模型中,杂环胺与前列腺癌的风险呈正相关[45]。然而,与流行病学研究的结果并不一致,在SANDER等[45]研究发现,HAA摄入量或大量食用熟肉与前列腺癌之间不存在相关性。BOGEN等[46]研究中,在接受根治性前列腺切除术的男性中,前列腺肿瘤细胞中有较高水平的PhIP-DNA加合物,并且PhIP 增加前列腺特异性抗原水平。过度煮熟的食物产生的杂环胺在许多体外和体内测试系统中具有极强的致突变性,其中一种诱变剂PhIP可诱发大鼠乳腺肿瘤,饮食流行病学研究表明它也会增加人类患乳腺癌的风险[47-48]。
4.3 神经毒性
目前大多数神经退行性疾病没有有效的治疗方法,识别和预防导致病因的可改变危险因素是减少神经退行性疾病的关键方法[49]。流行病学研究发现HAA与神经退行性疾病风险之间的存在关联性[50-51],因此研究HAA神经毒性机制具有重要意义。相关研究发现HAA主要会通过造成特定神经元群的功能障碍、活性氧的形成、氧化应激、线粒体生物能受损、线粒体功能障碍和DNA损伤等增加神经退行性疾病的患病风险[52-55]。HAA神经毒性的研究主要集中在β-咔啉类(Harman及其衍生物)。据报道,在震颤和帕金森病患者中,血液中Harman及其衍生物的浓度显着升高[56-57]。Harman会在特发性震颤患者的大脑中积聚,表明它可以穿过血脑屏障进入大脑,从而造成神经退行性疾病[58]。另外,有研究表明AIA类中 PhIP及其代谢物和其他 HAA 也具有神经毒性[59-60]。SYEDA等[49]研究团队发现HAA的暴露在细胞和动物模型中会产生与帕金森病和阿尔茨海默病相关的神经毒性。
4.4 心肌毒性
HAA除了具有致突变、致癌、神经毒性作用外,它还会造成心肌毒性,但相关研究较少[61]。DAVIS等[62]通过10 d给大鼠灌胃剂量为100 mg/kg IQ或PhIP,发现与对照组大鼠对比,IQ或PhIP处理组大鼠的心脏异常,伴有慢性炎症病灶、肌细胞坏死、肌原纤维溶解和紊乱等症状。另外,他们研究团队还发现将大鼠心肌细胞暴露N-OH-IQ或N-OH-PhIP后,心肌细胞显示异常,如肌丝损失、肌浆网肿胀和线粒体异常等[63]。这些结果表明,HAA可能在心脏退化中发挥作用。HAA毒性作用具体见图3。
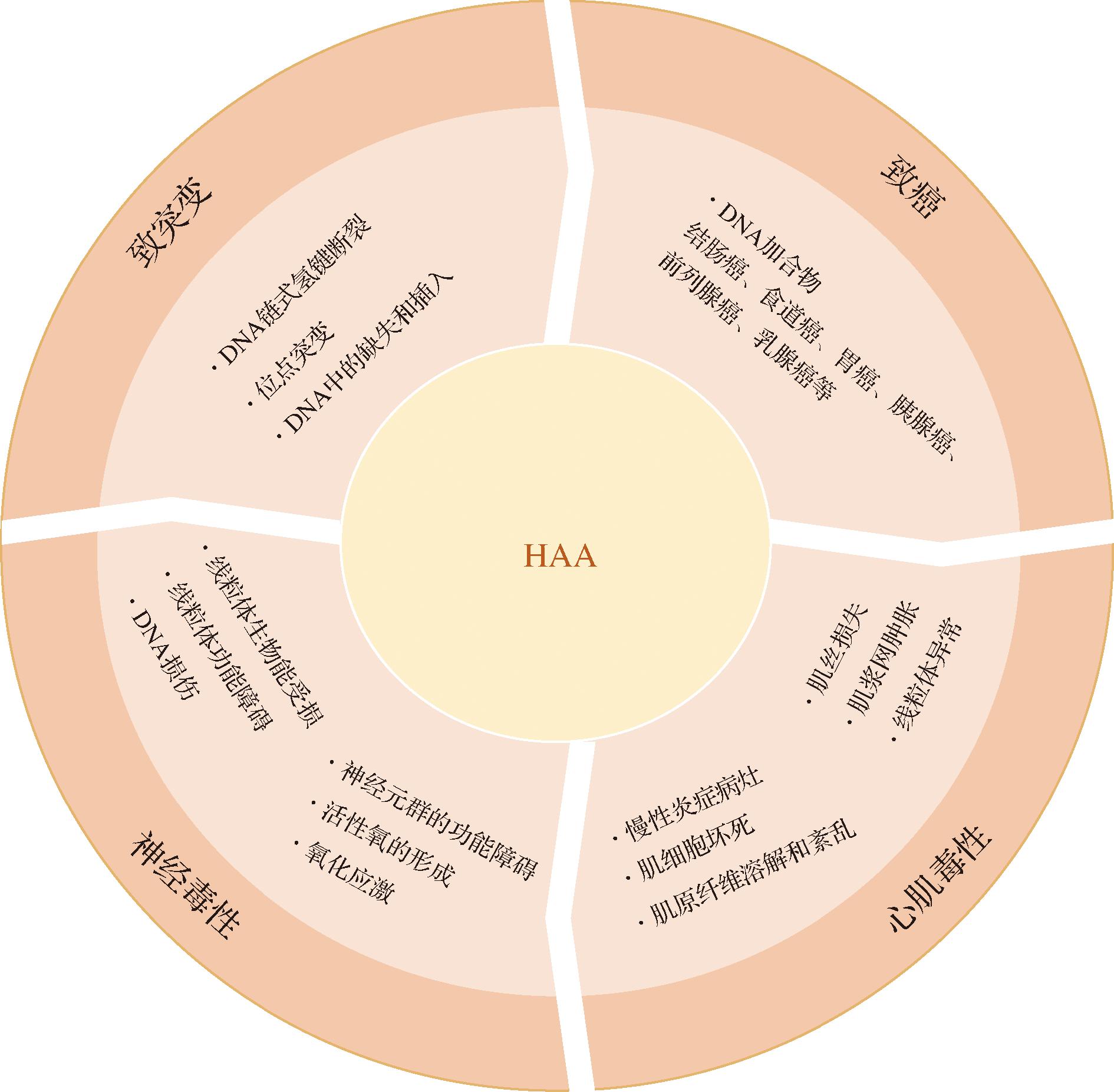
图3 HAA的毒性作用
Fig.3 Toxic effects of HAA
5 杂环胺的减控措施
目前,已有研究发现加工温度、加工时间以及加工方式会在不同程度上影响食物中HAA的形成。另外,通过添加天然或合成的外源物,也会在一定程度上减少食物中HAA的含量[64]。根据相关报道,多种癌症与HAAs有关,因此应在食品加工过程中有效减少HAAs的形成,并从膳食摄入中加以控制,以促进人体健康[40]。
5.1 改善加工条件
在食品加工过程中,加工温度和时间是两个关键因素,直接影响HAAs的产生和加工食品的感官属性[64]。已有研究表明,加工温度和时间与HAAs的形成呈线性正相关,通过控制加工温度和加工时间是降低食品中HAAs含量的有效途径[65]。早期毒理学研究表明,控制预先设计的加工温度和时间,可以降低从培根样品中提取的HAAs的致突变活性[66]。此外,通过热加工前微波预处理也可以降低HAAs含量。早期的研究报道,微波预处理降低了炸牛肉饼中HAA前体(包括脂肪、肌酸、肌酐、各种氨基酸和葡萄糖)的浓度,使其致突变性远低于未接受预处理的牛肉饼[67]。最近一项研究发现,在烧烤前使用微波处理相比未经微波处理,显著降低了HAA浓度,其原因可能是微波处理过程中造成水分损失,使小分子前体无法到达肉表面,无法作为HAA形成的反应物质[68]。另外,杨钊宇等[69]研究了不同种类植物油(大豆油、葵花籽油、高油酸葵花籽油、花生油、菜籽油、棕榈油)对油炸鸡肉中HAA的含量的影响,发现在相同加工温度及时间条件下,大豆油对HAA形成的促进作用大于其他5种植物油。
5.2 添加外源物
5.2.1 益生菌
在食品中添加益生菌可以起到发酵作用,提高食品品质,以及利用各种机制,如活的菌株产生特定酶、菌株细胞壁的吸附特性等去除食品中产生的有毒污染物,从而降低有毒污染物对人类身体健康的风险[70]。乳酸菌菌株作为众所周知的益生菌,可以作为抗致癌化合物的良好食品添加剂。有研究通过胃肠道模型、动物试验和临床研究发现,一些乳酸菌菌株细胞壁的肽聚糖可以通过直接物理结合,从介质环境中去除致癌化合物,并避免致癌化合物降解产生有毒次级代谢产物的再度污染。王惠汀等[71]研究结果表明,乳酸菌主要通过细胞壁上各种官能团物理吸附PhIP,并探究了乳酸菌吸附效果最佳条件为菌株浓度1010CFU/mL、pH 6、温度37 ℃、吸附时间1.5 h。
5.2.2 调味料
食品调味料经常在食品加工过程中使用,调味料的使用不仅可以提高感官属性和消费者接受度,而且有助于减少加工过程中HAAs。一些常用调味料,如黑胡椒粉、洋葱、大蒜、生姜、辣椒粉、八角和迷迭香等,已被证明可以减轻HAAs的形成。JINAP等[72]已证明当生姜添加量为4% (4 g/100 g牛肉)时可以有效地抑制烤牛肉中MeIQx和IQ的水平。王可心等[15]也研究了生姜对HAA形成的影响,发现生姜添加量为1.5%对烘烤猪肉中HAA形成抑制率最大。张漫漫[73]研究发现当八角、良姜和黑胡椒的添加量为1.5%时,对Norharman的抑制率分别为 66%、66%和 63%,可有效减少山黑猪叉烧肉中HAAs含量。华玲等[74]研究,发现添加黑胡椒可显著降低烤草鱼中HAA的生成量,但黑胡椒的添加量与其对HAA的抑制效果不存在相关性。另外,李星雨等[75]探究了5种香辛料(丁香、桂皮、花椒、草果、黑胡椒)及其主效成分对煎制猪肉中杂环胺生成的影响,发现丁香水提物及其主效成分的抑制效果最为显著,且发现香辛料对HAA的抑制作用与与其主效成分有关,但其影响程度各不相同,可能是与其他成分共同作用,抑制HAA的形成。
5.2.3 天然活性物质
多酚广泛存在于植物性食物中,具有良好的抗氧化及抗菌等生物活性,具有极高的潜在应用价值[76]。DING等[77]通过研究绿原酸、表儿茶素、芦丁、槲皮素和奎宁酸对炭烤羊肉中 HAA形成的抑制作用,发现绿原酸和表儿茶素通过与HAA前体物(葡萄糖、核糖、果糖、异亮氨酸、缬氨酸和赖氨酸)发生竞争性化学反应,极大地抑制了IQ、MeIQx、Norharman、Harman和PhIP的形成,且表儿茶素的抑制效果优于绿原酸。绿茶中富含酚类化合物,可以起到抗衰老和抗癌等作用。杨爍等[78]通过研究发现0.5%绿茶提取物对炸羊肉丸中PhIP、IQ、MeIQx、4,8-DiMeIQx有显著抑制作用,但对Harman、Norharman表现出促进作用,另外,随着绿茶提取物添加量的增加,HAA前体物的含量呈下降趋势,表明绿茶提取物可前体物的转化,抑制HAA的形成。光皮木瓜中也富含多酚,其中原花青素所占比例最高,具有强抗氧化活性。孔婉青[79]探究了光皮木瓜原花青素对煎炸鸡肉和豆腐中HAA的抑制作用,结果表明原花青素可以通过减缓食物脂质氧化,降低葡萄糖、肌酸以及氨基酸等前体物质的消耗对杂环胺的抑制呈剂量依赖性。周娜等[80]研究发现矢车菊-3-O-葡萄糖苷及其代谢物原儿茶酸可以通过减少DNA损伤和细胞凋亡修复IQ和PhIP诱导的细胞损伤,细胞损伤特别严重时则可促进细胞凋亡,减少癌变,抑制HAA毒性作用的发挥。基于桑叶中富含多种多酚化合物,XU等[81]研究了桑叶提取物对红烧番鸭中HAA形成的影响,发现酚类化合物显著抑制HAA的形成。其中,四羟基异黄酮对游离HAA具有显著抑制作用,抑制率为64.43%。另外,有研究发现山药提取物的添加也可以阻碍前体物向HAA的转化,降低烤马友鱼过程中脂肪氧化及蛋白氧化[14]。具体见表3。
表3 添加外源物对HAA毒性作用的影响
Table 3 Effect of adding foreign substances on toxicity of HAA
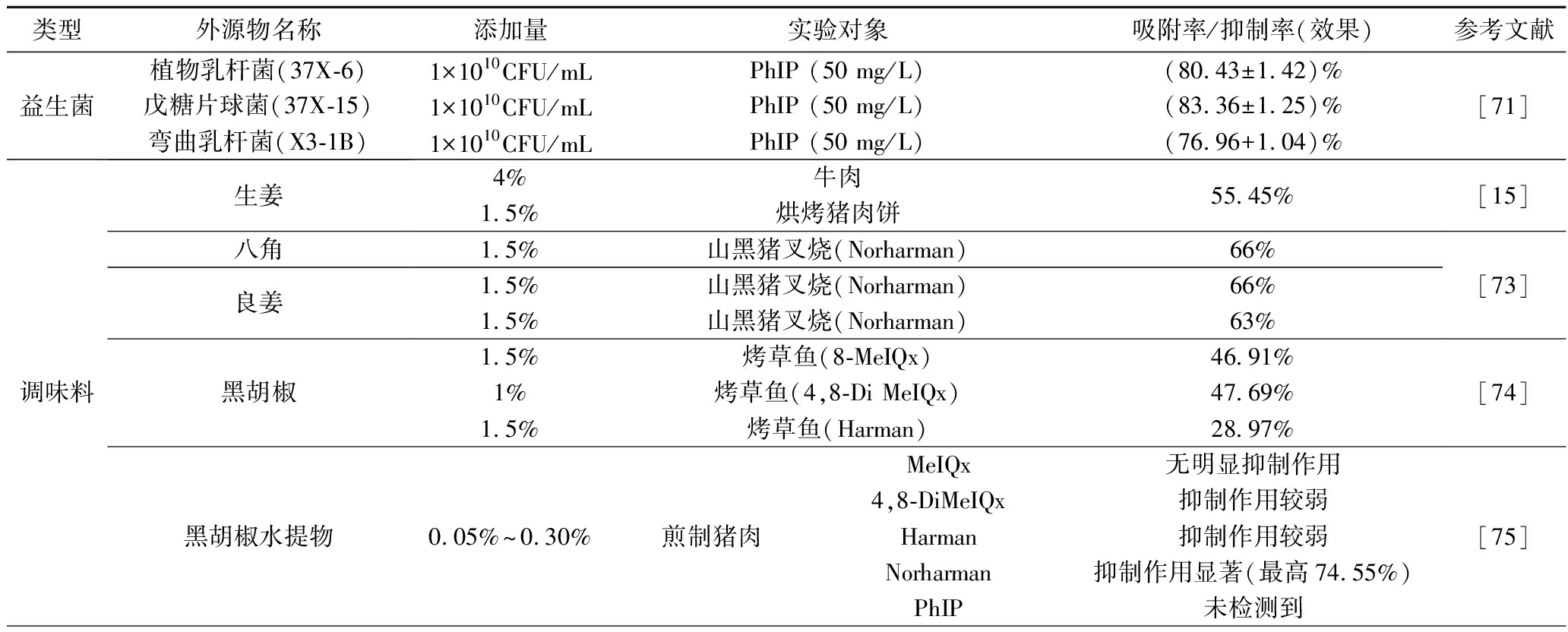
类型外源物名称添加量实验对象吸附率/抑制率(效果)参考文献益生菌植物乳杆菌(37X-6)1×1010CFU/mLPhIP (50 mg/L)(80.43±1.42)%戊糖片球菌(37X-15)1×1010CFU/mLPhIP (50 mg/L)(83.36±1.25)%弯曲乳杆菌(X3-1B)1×1010CFU/mLPhIP (50 mg/L)(76.96+1.04)%[71]调味料生姜八角良姜黑胡椒黑胡椒水提物4%牛肉1.5%烘烤猪肉饼55.45%1.5%山黑猪叉烧(Norharman)66%1.5%山黑猪叉烧(Norharman)66%1.5%山黑猪叉烧(Norharman)63%1.5%烤草鱼(8-MeIQx)46.91%1%烤草鱼(4,8-Di MeIQx)47.69%1.5%烤草鱼(Harman)28.97%0.05%~0.30%煎制猪肉MeIQx无明显抑制作用4,8-DiMeIQx抑制作用较弱Harman抑制作用较弱Norharman抑制作用显著(最高74.55%)PhIP未检测到[15][73][74][75]
续表3
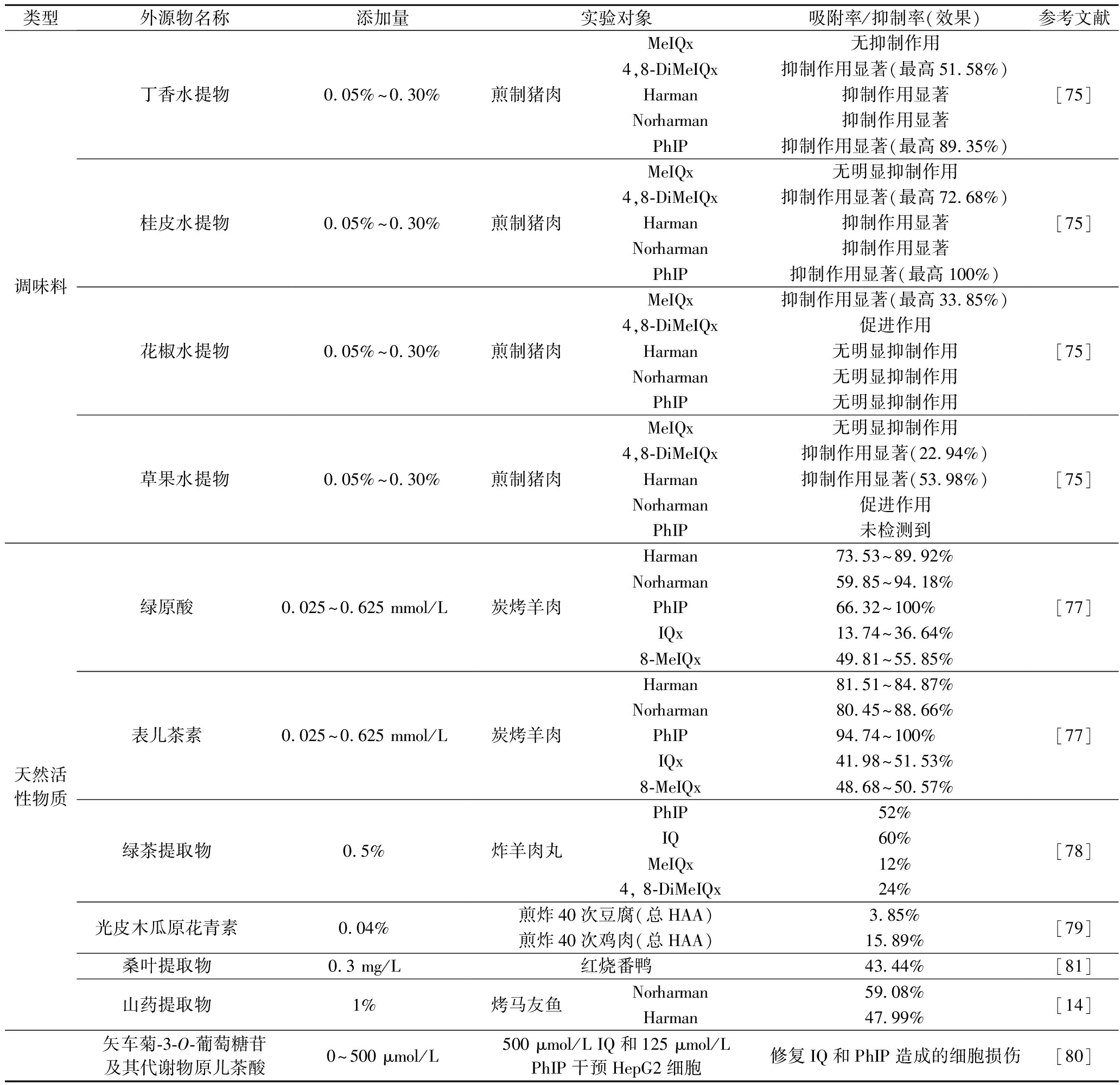
类型外源物名称添加量实验对象吸附率/抑制率(效果)参考文献调味料丁香水提物桂皮水提物花椒水提物草果水提物0.05%~0.30%煎制猪肉MeIQx无抑制作用4,8-DiMeIQx抑制作用显著(最高51.58%)Harman抑制作用显著Norharman抑制作用显著PhIP抑制作用显著(最高89.35%)0.05%~0.30%煎制猪肉MeIQx无明显抑制作用4,8-DiMeIQx抑制作用显著(最高72.68%)Harman抑制作用显著Norharman抑制作用显著PhIP抑制作用显著(最高100%)0.05%~0.30%煎制猪肉MeIQx抑制作用显著(最高33.85%)4,8-DiMeIQx促进作用Harman无明显抑制作用Norharman无明显抑制作用PhIP无明显抑制作用0.05%~0.30%煎制猪肉MeIQx无明显抑制作用4,8-DiMeIQx抑制作用显著(22.94%)Harman抑制作用显著(53.98%)Norharman促进作用PhIP未检测到[75][75][75][75]天然活性物质绿原酸0.025~0.625 mmol/L表儿茶素0.025~0.625 mmol/L绿茶提取物0.5%光皮木瓜原花青素0.04%桑叶提取物0.3 mg/L山药提取物1%炭烤羊肉Harman 73.53~89.92%Norharman59.85~94.18%PhIP66.32~100% IQx13.74~36.64%8-MeIQx49.81~55.85%炭烤羊肉Harman 81.51~84.87%Norharman80.45~88.66%PhIP94.74~100% IQx41.98~51.53%8-MeIQx48.68~50.57%炸羊肉丸PhIP52%IQ60%MeIQx12%4, 8-DiMeIQx24%煎炸40次豆腐(总HAA)3.85%煎炸40次鸡肉(总HAA)15.89%红烧番鸭43.44%烤马友鱼Norharman59.08%Harman47.99%[77][77][78][79][81][14]矢车菊-3-O-葡萄糖苷及其代谢物原儿茶酸0~500 μmol/L500 μmol/L IQ和125 μmol/L PhIP干预HepG2细胞修复IQ和PhIP造成的细胞损伤[80]
6 总结与展望
本文详细综述了杂环胺的分类、在不同食品中的含量及检测方法、代谢机制、毒性作用及其减控措施,为未来杂环胺相关研究提供参考。由于食品中HAA的含量微少较难测得,相关检测技术仍需进一步改进,以提高灵敏度和分析结果准确度,为其相关研究奠定基础。通过改善加工条件和添加外源抑制物都可有效抑制或减少有害物生成量,未来可以继续挖掘新型抑制剂,并探究其对HAAs的形成和代谢的影响,深入研究相关机理,为开发新型减控措施提供参考。在日常饮食中,我们除了要合理膳食、均衡营养外,还需根据食物的特性选取合适的加工方式,以减少有害物的形成,避免有害物对人体产生危害。
[1] 陈敬敬, 韩金花, 张永胜, 等.肉制品中杂环胺的研究进展[J].中国食品卫生杂志, 2022, 34(1):168-174.CHEN J J, HAN J H, ZHANG Y S, et al.Research progress of heterocyclic amines in meat products[J].Chinese Journal of Food Hygiene, 2022, 34(1):168-174.
[2] SHEN X, CHEN Y, OJOBI OMEDI J, et al.Effects of volatile organic compounds of smoke from different woods on the heterocyclic amine formation and quality changes in pork patty[J].Food Research International, 2023, 173(Pt 1):113262.
[3] 王惠汀, 孙学颖, 王丹, 等.肉制品中杂环胺类化合物形成及控制措施的研究进展[J].食品研究与开发, 2022, 43(5):195-203.WANG H T, SUN X Y, WANG D, et al.Research progress of formation and control measures of heterocyclic aromatic amines in meat products[J].Food Research and Development, 2022, 43(5):195-203.
[4] XUE T, JIANG Q Q, XIANG L W, et al.Effect of chemical modification of κ-carrageenan on its inhibitory effect against heterocyclic amine (HAs) formation in roasted tilapia fish patties[J].International Journal of Biological Macromolecules, 2023, 253(Pt 1):126586.
[5] 郝麒麟, 黄先智, 丁晓雯.食品中杂环胺的危害与控制措施研究进展[J].食品与发酵工业, 2019, 45(13):275-280.HAO Q L, HUANG X Z, DING X W.Threat of heterocyclic amines in foods and their controlling measures[J].Food and Fermentation Industries, 2019, 45(13):275-280.
[6] 洪燕婷, 王盼, 朱雨辰, 等.肉制品中杂环胺形成与控制的研究进展[J].中国食品学报, 2014, 14(11):149-156.HONG Y T, WANG P, ZHU Y C, et al.Development in controlling and formation of heterocyclic amines in meat[J].Journal of Chinese Institute of Food Science and Technology, 2014, 14(11):149-156.
[7] 赵东霞, 李丹慧, 隋明航, 等.用分子形貌理论探讨杂环化合物的键长、极化率和芳香性[J].中国科学:化学, 2016, 46(1):101-113; 1-6.ZHAO D X, LI D H, SUI M H, et al.Molecular face theory being applied to investigate the bond length, polarizability and aromaticity in the heterocyclic compounds[J].Scientia Sinica Chimica, 2016, 46(1):101-113; 1-6.
[8] BELLAMRI M, WALMSLEY S J, TURESKY R J.Metabolism and biomarkers of heterocyclic aromatic amines in humans[J].Genes and Environment, 2021, 43(1):29.
[9] RIZWAN KHAN M, NAUSHAD M, ABDULLAH ALOTHMAN Z.Presence of heterocyclic amine carcinogens in home-cooked and fast-food camel meat burgers commonly consumed in Saudi Arabia[J].Scientific Reports, 2017, 7(1):1707.
[10] 丁晓倩.多酚对羊肉炭烤过程杂环胺形成的抑制作用[D].北京:中国农业科学院,2021. DING X Q. Inhibitory effect of polyphenols on heterocyclic amines formation during mutton char roasting[D]. Beijing:Chinese Academy of Agricultural Sciences,2021.
[11] 张彪. 食品中生物胺与杂环胺高通量荧光免疫检测方法研究[D].天津:天津科技大学,2023. ZHANG B. Study on high-throughput fluorescence immunoassay for biogenic amines and heterocyclic amines in food[D]. Tianjin: Tianjin University of Science and Technology,2023.
[12] 于小慧. 焙烤花生中糖基化终产物和杂环胺的形成与控制[D].郑州:河南工业大学,2022. YU X H. Formation and control of glycosylation end products and heterocyclic amines in roasted peanuts[D]. Zhengzhou: Henan University of Technology,2022.
[13] 张晨霞, 盛冰莹, 张咪, 等.不同产地芝麻所制芝麻油中杂环胺的分析测定[J].中国油脂, 2023, 48(4):87-93.ZHANG C X, SHENG B Y, ZHANG M, et al.Determination and analysis of heterocyclic aromatic amines in sesame oil extracted from sesame of different producing areas[J].China Oils and Fats, 2023, 48(4):87-93.
[14] 邓鹏,胡璐曼,凌菁,等.煎香肠中杂环胺的生成规律研究[J].食品安全质量检测学报, 2022, 13 (11): 3423-3430.DENG P, HU L M, LING J, et al. Study on the formation of heterocyclic amines in fried sausage[J]. Journal of Food Safety and Quality Inspection,2022,13(11):3423-3430.
[15] 李梦, 林松毅, 王睿纯, 等.山药提取物对烤马友鱼中杂环胺的抑制作用[J].中国食品学报, 2023, 23(1):194-203.LI M, LIN S Y, WANG R C, et al.Inhibitory effect of yam extract on heterocyclic amines in roasted Eleutheronema tetradactylum[J].Journal of Chinese Institute of Food Science and Technology, 2023, 23(1):194-203.
[16] 王可心, 王华丽, 单艳琴, 等.生姜对烘烤猪肉饼品质及杂环胺形成的影响[J].食品研究与开发, 2023, 44(5):9-14.WANG K X, WANG H L, SHAN Y Q, et al.Effects of ginger on the quality of roasted pork patties and the formation of heterocyclic aromatic amines[J].Food Research and Development, 2023, 44(5):9-14.
[17] 叶惠萍, 曾晓房, 白卫东, 等.红烧乳鸽中11种极性杂环胺的UHPLC-Q-Orbitrap HRMS检测[J].轻工学报, 2023, 38(2):56-62.YE H P, ZENG X F, BAI W D, et al.Determination of 11 polar heterocyclic aromatic amines in braised squab By UHPLC-Q-Orbitrap HRMS[J].Journal of Light Industry, 2023, 38(2):56-62.
[18] ALI KHAN I, SHI B P, SHI H B, et al.Attenuation of heterocyclic amine formation and lipid and protein oxidation in air-fried fish fillets by marination with selected legume seed extracts[J].Food Chemistry, 2024, 435:137592.
[19] 薛桂中, 黄现青, 宋莲军, 等.高温肉制品杂环胺防控及体内代谢调控研究进展[J].食品科学, 2022, 43(13):256-266.XUE G Z, HUANG X Q, SONG L J, et al.Progress in the prevention of the formation of heterocyclic amines in high-temperature meat products and the regulation of their metabolism in the human body[J].Food Science, 2022, 43(13):256-266.
[20] SCHUT H A, SNYDERWINE E G.DNA adducts of heterocyclic amine food mutagens:Implications for mutagenesis and carcinogenesis[J].Carcinogenesis, 1999, 20(3):353-368.
[21] TURESKY R J, LE MARCHAND L.Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies:Lessons learned from aromatic amines[J].Chemical Research in Toxicology, 2011, 24(8):1169-1214.
[22] KODA M, IWASAKI M, YAMANO Y, et al.Association between NAT2, CYP1A1, and CYP1A2 genotypes, heterocyclic aromatic amines, and prostate cancer risk:A case control study in Japan[J].Environmental Health and Preventive Medicine, 2017, 22(1):72.
[23] BOOBIS A R, LYNCH A M, MURRAY S, et al.CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans[J].Cancer Research, 1994, 54(1):89-94.
[24] LANGOUЁT S, WELTI D H, KERRIGUY N, et al.Metabolism of 2-amino-3, 8-dimethylimidazo[4, 5-f]quinoxaline in human hepatocytes:2-amino-3-methylimidazo[4, 5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2[J].Chemical Research in Toxicology, 2001, 14(2):211-221.
[25] OLALEKAN ADEYEYE S A, ASHAOLU T J.Heterocyclic amine formation and mitigation in processed meat and meat products:A mini-review[J].Journal of Food Protection, 2021, 84(11):1868-1877.
[26] TURESKY R J, CONSTABLE A, RICHOZ J, et al.Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2[J].Chemical Research in Toxicology, 1998, 11(8):925-936.
[27] AIROLDI L, MAGAGNOTTI C, PASTORELLI R, et al.Enzyme polymorphisms influencing the metabolism of heterocyclic aromatic amines[J].Journal of Chromatography.B, Analytical Technologies in the Biomedical and Life Sciences, 2004, 802(1):175-181.
[28] HEIN D W.Molecular genetics and function of NAT1 and NAT2:Role in aromatic amine metabolism and carcinogenesis[J].Mutation Research, 2002, 506-507:65-77.
[29] YUAN Z X, JHA G, MCGREGOR M A, et al.Metabolites of the carcinogen 2-amino-alpha-carboline formed in male Sprague-Dawley rats in vivo and in rat hepatocyte and human HepG2 cell incubates[J].Chemical Research in Toxicology, 2007, 20(3):497-503.
[30] BELLAMRI M, LE HEGARAT L, TURESKY R J, et al.Metabolism of the tobacco carcinogen 2-amino-9H-pyrido[2, 3-b]indole (AαC) in primary human hepatocytes[J].Chemical Research in Toxicology, 2017, 30(2):657-668.
[31] TURESKY R J, GARNER R C, WELTI D H, et al.Metabolism of the food-borne mutagen 2-amino-3, 8-dimethylimidazo[4, 5-f]quinoxaline in humans[J].Chemical Research in Toxicology, 1998, 11(3):217-225.
[32] TANG Y J, LEMASTER D M, NAUWELAЁRS G, et al.UDP-glucuronosyltransferase-mediated metabolic activation of the tobacco carcinogen 2-amino-9H-pyrido[2, 3-b]indole[J].The Journal of Biological Chemistry, 2012, 287(18):14960-14972.
[33] 付瑜锋, 胡少东, 段鹍, 等.主要杂环胺类化合物研究进展[J].轻工学报, 2018, 33(1):13-25.FU Y F, HU S D, DUAN K, et al.Research progress of the major HAAs[J].Journal of Light Industry, 2018, 33(1):13-25.
[34] 王守涛. 亚临界乙醇/水提取木瓜原花青素及其对油炸鸡肉中杂环胺的抑制作用[D].郑州:河南工业大学,2022.WANG S T.Extraction of papaya proanthocyanidins by subcritical ethanol/water and its inhibitory effect on heterocyclic amines in fried chicken[D].Zhengzhou:Henan University of Technology,2022.
[35] NADEEM H R, AKHTAR S, ISMAIL T, et al.Heterocyclic aromatic amines in meat:Formation, isolation, risk assessment, and inhibitory effect of plant extracts[J].Foods, 2021, 10(7):1466.
[36] TOTSUKA Y, WAKABAYASHI K.Biological significance of aminophenyl-β-carboline derivatives formed from co-mutagenic action of β-carbolines and aniline and o-toluidine and its effect on tumorigenesis in humans:A review[J].Mutation Research.Genetic Toxicology and Environmental Mutagenesis, 2020, 850-851:503148.
[37] 王一然, 刘磊.某些富含蛋白质烹调食品中致突变物形成的研究[J].哈尔滨医科大学学报, 2011, 45(5):427-430.WANG Y R, LIU L.Study on the forming of mutagen in some protein-rich cooking food[J].Journal of Harbin Medical University, 2011, 45(5):427-430.
[38] LYNCH A M, GOODERHAM N J, BOOBIS A R.Organ distinctive mutagenicity in MutaMouse after short-term exposure to PhIP[J].Mutagenesis, 1996, 11(5):505-509.
[39] GI M, FUJIOKA M, TOTSUKA Y, et al.Quantitative analysis of mutagenicity and carcinogenicity of 2-amino-3-methylimidazo[4, 5-f]quinoline in F344 gpt delta transgenic rats[J].Mutagenesis, 2019, 34(3):279-287.
[40] BULANDA S, JANOSZKA B.Consumption of thermally processed meat containing carcinogenic compounds (polycyclic aromatic hydrocarbons and heterocyclic aromatic amines) versus a risk of some cancers in humans and the possibility of reducing their formation by natural food additives: A literature review[J].International Journal of Environmental Research and Public Health, 2022, 19(8):4781.
[41] 郑蒙, 林雪, 谢华兵, 等.杂环胺摄入与结直肠癌关系的剂量-反应meta分析[J].胃肠病学, 2020, 25(6):348-357.ZHENG M, LIN X, XIE H B, et al.Association between heterocyclic amines intake and colorectal cancer:A dose-response meta-analysis[J].Chinese Journal of Gastroenterology, 2020, 25(6):348-357.
[42] MART NEZ G
NEZ G NGORA V, MATTHES K L, CASTA
NGORA V, MATTHES K L, CASTA O P R, et al.Dietary heterocyclic amine intake and colorectal adenoma risk:A systematic review and meta-analysis[J].Cancer Epidemiology, Biomarkers &Prevention:a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 2019, 28(1):99-109.
O P R, et al.Dietary heterocyclic amine intake and colorectal adenoma risk:A systematic review and meta-analysis[J].Cancer Epidemiology, Biomarkers &Prevention:a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 2019, 28(1):99-109.
[43] TORRE L A, BRAY F, SIEGEL R L, et al.Global cancer statistics, 2012[J].CA:A Cancer Journal for Clinicians, 2015, 65(2):87-108.
[44] CROSS A J, FREEDMAN N D, REN J S, et al.Meat consumption and risk of esophageal and gastric cancer in a large prospective study[J].The American Journal of Gastroenterology, 2011, 106(3):432-442.
[45] SANDER A, LINSEISEN J, ROHRMANN S.Intake of heterocyclic aromatic amines and the risk of prostate cancer in the EPIC-Heidelberg cohort[J].Cancer Causes &Control:CCC, 2011, 22(1):109-114.
[46] BOGEN K T, KEATING G A 2nd, CHAN J M, et al.Highly elevated PSA and dietary PhIP intake in a prospective clinic-based study among African Americans[J].Prostate Cancer and Prostatic Diseases, 2007, 10(3):261-269.
[47] FELTON J S, KNIZE M G, SALMON C P, et al.Human exposure to heterocyclic amine food mutagens/carcinogens:Relevance to breast cancer[J].Environmental and Molecular Mutagenesis, 2002, 39(2-3):112-118.
[48] ZHENG W, LEE S A.Well-done meat intake, heterocyclic amine exposure, and cancer risk[J].Nutrition and Cancer, 2009, 61(4):437-446.
[49] SYEDA T, CANNON J R.Potential role of heterocyclic aromatic amines in neurodegeneration[J].Chemical Research in Toxicology, 2022, 35(1):59-72.
[50] AGIM Z S, CANNON J R.Dietary factors in the etiology of Parkinson’s disease[J].BioMed Research International, 2015, 2015:672838.
[51] PERRONE L, GRANT W B.Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence[J].Journal of Alzheimer’s Disease:JAD, 2015, 45(3):965-979.
[52] LAWANA V, UM S Y, ROCHET J C, et al.Neuromelanin modulates heterocyclic aromatic amine-induced dopaminergic neurotoxicity[J].Toxicological Sciences: An Official Journal of the Society of Toxicology, 2020, 173(1):171-188.
[53] AGIM Z S, CANNON J R.Alterations in the nigrostriatal dopamine system after acute systemic PhIP exposure[J].Toxicology Letters, 2018, 287:31-41.
[54] SYEDA T, FOGUTH R M, LLEWELLYN E, et al.PhIP exposure in rodents produces neuropathology potentially relevant to Alzheimer’s disease[J].Toxicology, 2020, 437:152436.
[55] SAMMI S R, AGIM Z S, CANNON J R.From the cover:Harmane-induced selective dopaminergic neurotoxicity in Caenorhabditis elegans[J].Toxicological Sciences: An Official Journal of the Society of Toxicology, 2018, 161(2):335-348.
[56] LOUIS E D, MICHALEC M, JIANG W, et al.Elevated blood harmane (1-methyl-9H-pyrido[3, 4-b]indole) concentrations in Parkinson’s disease[J].Neurotoxicology, 2014, 40:52-56.
[57] LOUIS E D, ZHENG W, JUREWICZ E C, et al.Elevation of blood beta-carboline alkaloids in essential tremor[J].Neurology, 2002, 59(12):1940-1944.
[58] LOUIS E D, FACTOR-LITVAK P, LIU X H, et al.Elevated brain harmane (1-methyl-9H-pyrido[3, 4-b]indole) in essential tremor cases vs.controls[J].Neurotoxicology, 2013, 38:131-135.
[59] GRIGGS A M, AGIM Z S, MISHRA V R, et al.2-Amino-1-methyl-6-phenylimidazo[4, 5-b]pyridine (PhIP) is selectively toxic to primary dopaminergic neurons in vitro[J].Toxicological Sciences: An Official Journal of the Society of Toxicology, 2014, 140(1):179-189.
[60] CRUZ-HERNANDEZ A, AGIM Z S, MONTENEGRO P C, et al.Selective dopaminergic neurotoxicity of three heterocyclic amine subclasses in primary rat midbrain neurons[J].Neurotoxicology, 2018, 65:68-84.
[61] SUGIMURA T.Food and cancer[J].Toxicology, 2002, 181-182:17-21.
[62] DAVIS C D, FARB A, THORGEIRSSON S S, et al.Cardiotoxicity of heterocyclic amine food mutagens in cultured myocytes and in rats[J].Toxicology and Applied Pharmacology, 1994, 124(2):201-211.
[63] DAVIS C D, SNYDERWINE E G.Protective effect of N-acetylcysteine against heterocyclic amine-induced cardiotoxicity in cultured myocytes and in rats[J].Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 1995, 33(8):641-651.
[64] CHEN X Q, JIA W, ZHU L, et al.Recent advances in heterocyclic aromatic amines:An update on food safety and hazardous control from food processing to dietary intake[J].Comprehensive Reviews in Food Science and Food Safety, 2020, 19(1):124-148.
[65] YANG D D, HE Z Y, GAO D M, et al.Effects of smoking or baking procedures during sausage processing on the formation of heterocyclic amines measured using UPLC-MS/MS[J].Food Chemistry, 2019, 276:195-201.
[66] MILLER A J, BUCHANAN R L.Detection of genotoxicity in fried bacon by the Salmonella/mammalian microsome mutagenicity assay[J].Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 1983, 21(3):319-323.
[67] FELTON J S, FULTZ E, DOLBEARE F A, et al.Effect of microwave pretreatment on heterocyclic aromatic amine mutagens/carcinogens in fried beef patties[J].Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 1994, 32(10):897-903.
[68] BARZEGAR F, KAMANKESH M, MOHAMMADI A.Heterocyclic aromatic amines in cooked food:A review on formation, health risk-toxicology and their analytical techniques[J].Food Chemistry, 2019, 280:240-254.
[69] 杨钊宇,刘伟,李耘.油炸鸡肉中杂环胺的形成及其与色泽的相关性研究[J].河南工业大学学报(自然科学版),2021,42(5):55-63.YANG Z Y, LIU W, LI Y. Study on the formation of heterocyclic amines in fried chicken and their correlation with color[J]. Journal of Henan University of Technology (Natural Science Edition),2021,42(5):55-63.
[70] ZHAO L L, WEI J Y, ZHAO H F, et al.Detoxification of cancerogenic compounds by lactic acid bacteria strains[J].Critical Reviews in Food Science and Nutrition, 2018, 58(16):2727-2742.
[71] 王惠汀, 刘培清, 晓燕, 等.高吸附杂环胺乳酸菌的筛选、吸附特性及机理研究[J].中国食品学报, 2023, 23(6):41-47.WANG H T, LIU P Q, XIAO Y, et al.Screening, adsorption characteristics and mechanism of lactic acid bacteriawith high heterocyclic aromatic amines adsorption capacity[J].Journal of Chinese Institute of Food Science and Technology, 2023, 23(6):41-47.
[72] JINAP S, IQBAL S Z, SELVAM R M P.Effect of selected local spices marinades on the reduction ofheterocyclic amines in grilled beef (satay)[J].LWT - Food Science and Technology, 2015, 63(2):919-926.
[73] 张漫漫. 山黑猪叉烧肉工艺配方优化及产品加工中杂环胺抑制研究[D].长春:吉林大学, 2023.ZHANG M M.Study on optimization of Shanhei pork barbecued meat process formula and inhibition of heterocyclic amines in product processing[D].Changchun:Jilin University, 2023.
[74] 华玲, 王杰.黑胡椒对烤草鱼中杂环胺含量和品质的影响[J].中国调味品, 2023, 48(4):80-84.HUA L, WANG J.Effect of black pepper on heterocyclic aromatic amine content and quality of grilled grass carp[J].China Condiment, 2023, 48(4):80-84.
[75] 李星雨, 徐筱莹, 雷秋琪, 等.香辛料提取物及其主效成分对煎制猪肉中杂环胺生成的影响[J].中国调味品, 2022, 47(3):21-27.LI X Y, XU X Y, LEI Q Q, et al.Effect of spice extract and its main components on the production of heterocyclic amines in fried pork[J].China Condiment, 2022, 47(3):21-27.
[76] 张世钰, 高萌, 王未, 等.植物多酚抑制热加工肉制品中杂环胺形成机制研究进展[J].食品科学, 2023, 44(9):211-220.ZHANG S Y, GAO M, WANG W, et al.Research progress on the inhibitory mechanism of plant polyphenols on the formation of heterocyclic amines in thermally processed meat products[J].Food Science, 2023, 44(9):211-220.
[77] DING X Q, ZHANG D Q, LIU H, et al.Chlorogenic acid and Epicatechin:An efficient inhibitor of heterocyclic amines in charcoal roasted lamb meats[J].Food Chemistry, 2022, 368:130865.
[78] 杨爍, 李佳珊, 尚宏丽.绿茶提取物对炸羊肉丸中杂环胺形成的抑制作用[J].食品工业, 2023, 44(9):123-127.YANG S, LI J S, SHANG H L.Inhibition of heterocyclic amine formation in fried lamb balls in green tea extract[J].The Food Industry, 2023, 44(9):123-127.
[79] 孔婉青. 光皮木瓜原花青素的解聚及其产物对煎炸食物中杂环胺的抑制研究[D].郑州:河南工业大学,2023.KONG W Q.Depolymerization of proanthocyanidins from papaya and their inhibition of heterocyclic amines in fried food[D].Zhengzhou:Henan University of Technology,2023.
[80] 周娜, 张会敏, 潘飞, 等.矢车菊-3-O-葡萄糖苷及其代谢物原儿茶酸对杂环胺诱导细胞损伤的修复机制[J].食品科学, 2022, 43(15):141-149.ZHOU N, ZHANG H M, PAN F, et al.Mechanism by which cyanidin-3-O-glucoside and its metabolite protocatechuic acid repair heterocyclic aromatic amines-induced cell damage[J].Food Science, 2022, 43(15):141-149.
[81] XU Y, HUANG T R, HUANG Y Q, et al.Effect of mulberry leaf (Morus alba L.) extract on the quality and formation of heterocyclic amines in braised Muscovy duck[J].Food Control, 2024, 156:110137.