桃胶(peach gum)是蔷薇科植物桃或山桃等树干或果实受到多蒂氏球孢杆菌、罗地那芽孢杆菌、钝角芽孢杆菌等微生物侵袭或机械损伤后分泌的胶状物质[1-3]。从文献记载中可以知道,桃树最先是分布在长江中下游地区,后逐步发展至全国[4]。桃树的大量种植,一是为了采摘其果实,二是为了桃胶的采集。桃胶具有很高的食用价值和药用价值[5]。桃胶食用价值不仅体现在其作为食品添加剂用于制作糕点、面包以及巧克力等食品,还能作为抗氧化“美容食品”[6]。桃胶的主要成分是多糖,另外还有酚类和黄酮等,具有调节血糖、降血脂以及抗氧化等药用价值[7-9]。特别是近年来,桃胶作为一种具有滋补养颜及抗皱嫩肤等保健功能的食品受到广大爱美人士的追捧[10-11]。但是在防治桃树的病虫害过程中,杀虫剂和杀菌剂等农药的使用,致使其在桃胶上蓄积残留,进而增加通过食物链进入人体内的风险。虽然一直以来桃胶作为食品和药品在应用[12],但是目前仍未被列入国家普通食品名单以及药品名单中,仅作为一种普通食用农产品在市场上流通,且关于桃胶中农药残留的国家检测标准和限量标准缺失,在GB 2763—2021《食品安全国家标准食品中农药最大残留限量》和《中华人民共和国药典(2020年版)》中也没有涉及桃胶产品的农药残留限量和检测方法的信息。因此,建立一种快捷、污染小以及可靠性强的桃胶中多农药残留的定性和定量检测方法,进而明确桃胶中农药残留情况,对保护生态环境、保障人民健康具有重要意义。
近年来,关于桃胶中农药残留检测方面文献较少发表,如陈妙金等[13]基于气相色谱法,对喷洒农药后桃胶农药残留进行了多时期检测分析,但并未涉及相关分析方法参数。官金艳等[14]基于QuEChERS样品前处理技术,结合气相色谱-火焰光度法(gas chromatography-flame photometric detector,GC-FPD),建立了一种桃胶中16种有机磷类农药残留的分析方法,该方法分析时间长且易出现假阳性。周建峰等[15]基于凝胶色谱仪辅助分散型固相萃取前处理技术,结合高效液相色谱-串联质谱法(liquid chromatography-triple quadrupole tandem mass spectrometry,LC-MS/MS),建立了一种桃胶中10种杀虫剂和杀菌剂的检测方法,该方法所需溶剂量大且覆盖农药种类较少。田菊等[16]基于LC-MS/MS建立桃胶中44种农药残留的定量分析方法,该方法所包含农药均为中极性或高极性农药,缺少常见的低极性农药且包含农药种类相对较少。
μ-QuEChERS前处理技术是原始QuEChERS[17](Original QuEChERS)方法的小型化,与原始QuEChERS相比,它更快,使用更少的溶剂、样品、提取盐和吸附材料,显著降低了分析成本,提高了检测通量,且对环境更为友好[18-20]。当目标分析物通过气相色谱柱时,非常容易被活性位点吸附。这会导致峰拖尾、保留时间偏移,在极端情况下,峰甚至会完全丢失[21]。相对于普通的气相色谱柱,超高惰性色谱柱在保持高选择性的基础上,具有更高的灵敏度、更低的拖尾因子以及超低的组分流失和降解等特点,且其较小内径(0.18 mm)配置,提高了样品分析速度,可以实现更高样品通量[22]。与典型低压气相色谱柱(low pressure-gas chromatography column,LP-GC)相比,超高惰性色谱柱在实现较快分析的同时,其色谱柱结构更加简单和高效[23-24]。本文以桃胶为研究对象,采用μ-QuEChER前处理技术结合超高惰性气相色谱柱以及气相色谱-串联三重四极杆质谱仪,建立了桃胶中农药残留的快速检测方法,并对市售桃胶中农药残留情况进行定量分析,为桃胶中多农药残留的快速检测和风险评估提供技术支持和数据支撑。
1 材料与方法
1.1 材料与仪器
乙烯菌核利、甲基对硫磷、狄氏剂、二嗪磷、三氯杀螨醇、苯醚甲环唑等106种农药混合标准溶液(100 mg/L),天津阿尔塔公司;乙腈、丙酮、甲醇(色谱纯),美国Merck公司;实验用水(超纯水),美国Milli-Q公司;十八烷基硅烷键合硅胶(octadecylsilane bonded silica gel,C18),苏州纳谱分析科技有限公司;无水硫酸镁,上海凌峰化学试剂有限公司;石墨化炭黑(graphitized carbon black,GCB),天津博纳艾杰尔公司;0.22 μm尼龙66(Nylon66)滤膜,深圳野之马公司;QuEChERS提取套装(4 g MgSO4+1 g NaCl+1 g Na3C6H5O7+0.5 g Na2C6H6O7);乙二胺-N-丙基烷(N-propyl ethylenediamine,PSA),深圳逗点公司;小陶瓷均质子,美国Agilent公司。
Agilent 7890B/7010B气相色谱-串联三重四极杆质谱仪,美国Agilent公司;Auto EVA-60多功能全自动氮吹仪、V20垂直振荡器,厦门RayKol公司;Velocity 14高速离心机,日本HITACHI公司;BCM2500多功能混匀仪,深圳逗点公司;Dispensette S Organic瓶口分液器,德国Brand公司;SOCOREX 825微量移液器(100~1 000 μL)和(20 μL~200 μL),瑞士SOCOREX公司;BCD-440 WDPG冰箱,中国海尔集团有限公司。
1.2 实验方法
1.2.1 样品前处理
1.2.1.1 样品的提取
准确称取制备后的样品(0.500±0.002) g于15 mL 的聚乙烯(polyethylene,PE)离心管中,加入一颗小陶瓷均质子,准确加入2.00 mL乙腈,加入QuEChERS萃取盐(200 mg MgSO4+50 mg NaCl+50 mg Na3C6H5O7+25 mg Na2C6H6O7),在垂直振荡器剧烈振荡5 min,9 500 r/min离心3 min,上层清液待净化。
1.2.1.2 提取液的净化
将无水150 mg MgSO4、50 mg C18加至15 mL的PE离心管中,然后加入1.2.1.1节中上清液,以2 000 r/min涡旋振荡1 min,9 500 r/min离心3 min,准确取出0.500 mL至玻璃氮吹管中,40 ℃氮吹浓缩至近干,用移液枪精密加入0.500 mL含分析物保护剂的丙酮溶液,涡旋30 s,过尼龙66 μm滤膜,待GC-MS/MS检测。
1.2.2 仪器条件
1.2.2.1 气相色谱条件
气相色谱条件色谱柱:Agilent Technologies HP-5MS UI(20 m×0.18 mm,0.18 μm);进样口温度:280 ℃;柱流量:1 mL/min;程序升温:T始=60 ℃,0~0.5 min,保持60 ℃;0.5~1.875 min,以80 ℃/min速率,从60~170 ℃;1.875~8.875 min,170~310 ℃;8.875~10.0 min,保持310 ℃,总用时10.0 min;进样方式:分流进样;分流比:3∶1;进样体积:0.5 μL;载气:高纯氦气,纯度≥99.999%;碰撞气:高纯氮气,纯度≥99.999%。
1.2.2.2 质谱条件
质谱条件质谱电离方式:电子轰击电离源;接口温度:280 ℃;离子源温度:280 ℃;电离能量:70 eV;数据采集:多反应监测模式;定量方式:外标法;106种农药的质谱参数见表1。
表1 各农药保留时间以及质谱参数
Table 1 The retention time and MRM parameters of pesticides

序号名称离子对(m/z)保留时间/min序号名称离子对(m/z)保留时间/min1甲胺磷141.0>95.0∗,141.0>80.02.26954杀扑磷144.9>85.0∗,144.9>58.15.1872速灭磷127.0>94.9∗,127.0>109.02.789552,4′-滴滴伊246.0>176.2∗,248.0>176.25.2153乙酰甲胺磷136.0>42.0∗,136.0>94.02.80756多效唑236.0>125.1∗,236.0>167.15.2314异丙威121.0>77.1∗,136.0>121.13.10557丁草胺188.1>160.2∗,236.9>160.25.2905氧乐果156.0>110.0∗,110.0>79.03.29658α-硫丹194.9>159.0∗,194.9>125.05.2946残杀威110.0>63.0∗,110.0>64.03.32559己唑醇231.0>175.0∗,256.0>82.15.3917灭线磷157.9>97.0∗,157.9>114.03.40160稻瘟灵162.1>85.0∗,162.1>134.05.4228脱乙基莠去津145.0>97.0∗,145.0>67.93.48961抑霉唑214.9>173.0∗,216.8>175.05.4429百治磷127.0>109.0∗,127.0>95.03.52162丙溴磷338.8>268.7∗,207.9>63.05.44410治螟磷201.8>145.9∗,237.8>145.93.56263咯菌腈248.0>154.1∗,248.0>182.15.46711久效磷127.1>109.0∗,127.1>95.03.572644,4′-滴滴伊246.1>176.2∗,315.8>246.05.47512甲拌磷230.9>128.9∗,260.0>75.03.60765狄氏剂262.9>191.0∗,277.0>241.05.51313α-六六六216.9>181.0∗,218.9>183.03.65866腈菌唑179.0>125.1∗,179.0>90.05.53914六氯苯283.8>213.9∗,283.8>248.83.713672,4′-滴滴滴235.0>165.2∗,237.0>165.25.55215乐果87.0>46.0∗,142.9>111.03.73568醚菌酯116.0>89.0∗,116.0>63.05.58416莠去津214.9>58.1∗,214.9>200.23.78769吡氟禾草灵281.9>91.0∗,281.9>238.05.66417γ-六六六216.9>181.0∗,181.0>145.03.83670异狄氏剂262.8>193.0∗,244.8>173.05.69518β-六六六216.9>181.1∗,181.0>145.03.87771β-硫丹206.9>172.0∗,194.9>124.95.77319特丁硫磷230.9>129.0∗,230.9>175.03.89572倍硫磷亚砜278.0>109.0∗,278.0>169.05.79720地虫硫膦109.0>80.9∗,246.1>137.03.92873烯唑醇267.9>232.1∗,269.9>232.05.80521二嗪磷137.1>84.0∗,137.1>54.03.962742,4′-滴滴涕235.0>165.2∗,237.0>165.25.82922δ-六六六217.0>181.1∗,181.1>145.14.04975倍硫磷砜309.9>105.0∗,135.9>92.05.84023氯唑磷161.0>119.1∗,161.0>146.04.062764,4′-滴滴滴234.9>165.1∗,236.9>165.25.85924野麦畏268.0>184.1∗,142.9>83.04.07277乙硫磷230.9>129.0∗,230.9>175.05.86625百菌清265.9>133.0∗,265.9>168.04.08178三唑磷161.2>134.2∗,161.2>106.15.97726抗蚜威238.0>166.2∗,166.0>55.14.15579肟菌酯116.0>89.0∗,131.0>89.06.13927乙草胺222.9>132.2∗,222.9>147.24.310804,4′-滴滴涕235.0>165.2∗,237.0>165.26.14528乙烯菌核利187.0>124.0∗,197.9>145.04.32081丙环唑172.9>145.0∗,172.9>74.06.14929甲基毒死蜱285.9>93.0∗,287.9>92.94.33082戊唑醇250.0>125.0∗,250.0>153.06.27030甲基对硫磷262.9>109.0∗,232.9>109.04.33183禾草灵253.0>162.1∗,339.9>252.96.27931莠灭净227.0>58.1∗,227.0>170.14.38584增效醚176.1>103.1∗,176.1>131.16.33232甲草胺188.1>160.1,160.1>132.14.38685氟环唑192.0>138.1∗,192.0>111.06.42033甲霜灵220.0>192.1∗,234.0>174.14.42586异菌脲313.8>244.9∗,313.8>55.96.49934杀螟硫磷277.0>260.1∗,277.0>109.04.53687亚胺硫磷160.0>77.1∗,160.0>133.16.57435甲基嘧啶磷290.0>125.0∗,232.9>151.04.53988联苯菊酯181.2>166.2∗,181.2>165.26.59936马拉硫磷172.9>99.0∗,126.9>99.04.60989甲氧滴滴涕227.0>141.1∗,227.0>169.16.64137甲拌磷亚砜96.9>64.9∗,199.0>142.94.61490甲氰菊酯207.9>181.0∗,264.9>210.06.65838甲拌磷砜153.0>97.0∗,124.9>96.94.66691乙螨唑141.0>113.0∗,141.0>63.16.67739艾氏剂254.9>220.0∗,262.9>192.94.67792伏杀硫磷182.0>111.0∗,182.0>102.16.90540倍硫磷278.0>109.0∗,278.0>169.04.68593吡丙醚136.1>96.0∗,136.1>78.16.93441毒死蜱198.9>171.0∗,196.9>169.04.70494高效氯氟氰菊酯197.0>141.1∗,197.0>161.17.04042对硫磷139.0>109.0∗,291.0>109.04.71095氯菊酯183.1>168.1∗,183.1>153.07.45443三唑酮208.0>181.1∗,208.0>111.04.73096哒螨灵147.2>117.1∗,147.2>132.27.48744三氯杀螨醇139.0>111.0∗,139.0>75.04.73297蝇毒磷210.0>182.0∗,361.9>109.07.53545水胺硫磷135.9>108.0∗,135.9>69.04.76298腈苯唑128.9>102.1∗,197.9>129.07.71346噻唑膦195.0>103.0∗,195.0>60.04.85099氟氯氰菊酯226.0>206.0∗,198.9>170.17.77147甲基异柳磷199.0>121.0∗,241.1>199.14.914100啶酰菌胺140.0>112.0∗,140.0>76.07.88948二甲戊灵251.8>162.2∗,251.8>161.14.962101氯氰菊酯163.0>127.0∗,163.0>91.07.91149戊菌唑248.0>192.1∗,248.0>157.14.981102氟氰戊菊酯156.9>107.1∗,198.9>157.08.03050特丁硫磷砜153.0>97.0∗,199.0>97.04.982103氰戊菊酯167.0>125.1∗,224.9>119.08.36551氟虫腈366.8>212.8∗,368.8>214.85.040104氟胺氰菊酯250.0>55.0∗,250.0>200.08.42052三唑醇168.0>70.0∗,128.0>65.05.067105苯醚甲环唑322.8>264.8∗,264.9>202.08.54853腐霉利282.8>96.0∗,282.8>68.05.115106溴氰菊酯252.9>93.0∗,250.7>172.08.616
注:*表示定量离子。
1.2.3 标准溶液及其他相关溶液的配制
1.2.3.1 混合标准中间液及基质标准曲线的配制
准确移取一定量农药混合标准溶液(100 mg/L),用丙酮定容至刻度,配制成1.00 mg/L混合标准中间液1。基质标准曲线配制:分别准确吸取适量的混合标准中间液1,用桃胶基质空白溶液逐级稀释,配制得到质量浓度分别为:0.002 00、0.005 00、0.020 0、0.050 0、0.100、0.200、0.300、0.400 mg/L的基质标准曲线。
1.2.3.2 其他相关溶液的配制
含分析物保护剂的丙酮溶液的配制:a)L-古洛糖酸-γ-内酯储备液:称取5.00 g L-古洛糖酸-γ-内酯于100 mL容量瓶中,40 mL水超声溶解并用丙酮定容至刻度。b)D-山梨醇储备液:称取2.50 g D-山梨醇于50 mL容量瓶中,25 mL水超声溶解并用丙酮定容至刻度。c)分析物保护剂(AP)溶液:将100 mL 的L-古洛糖酸内酯储备液和50 mL的D-山梨醇储备液加入250 mL容量瓶中,并用丙酮定容至刻度。d)含分析物保护剂的丙酮溶液:将50 mL的分析物保护剂溶液加入至1 L的容量瓶中,并用丙酮定容至刻度。
1.3 数据处理
本研究采用美国Agilent公司的Masshunter软件建立采集方法,并进行数据采集及定量处理,导出原始数据后使用Microsoft Excel进行计算、整理及分析,用Origin进行表格和图的制作。前处理和分析条件优化中的回收率的计算均采用6个平行样品取平均值计算,精密度由低、中、高3个浓度且每个浓度梯度取6个平行样品的浓度数据之间的RSD值来表示。
2 结果与分析
2.1 样品前处理优化
2.1.1 提取溶剂的优化
为了研究样品的最佳提取溶剂,以桃胶基质空白样品加标回收率作为评价标准(加标质量浓度为0.200 mg/kg,n=6),比较了正己烷、丙酮、乙酸乙酯以及乙腈4种提取溶剂对桃胶中106种农药残留的提取效果。如图1所示:采用正己烷作为提取溶剂时,各种农药的平均回收率为71%,这可能是因为正己烷极性较弱,难以溶解中等极性及较强极性的农药;采用乙酸乙酯提取时,106种农药的平均回收率为74%,一些脂溶性农药回收较好,但是其他干扰成分也容易提取出来,造成干扰。而采用乙腈和丙酮作为提取溶剂时,目标组分有较好的溶解性,106种农药的平均回收率分别达了90%和83%,且采用乙腈作为提取溶剂时,106种农药的平均回收率最高,误差线也较小。因此本实验选择乙腈作为桃胶中农药多残留的提取溶剂。
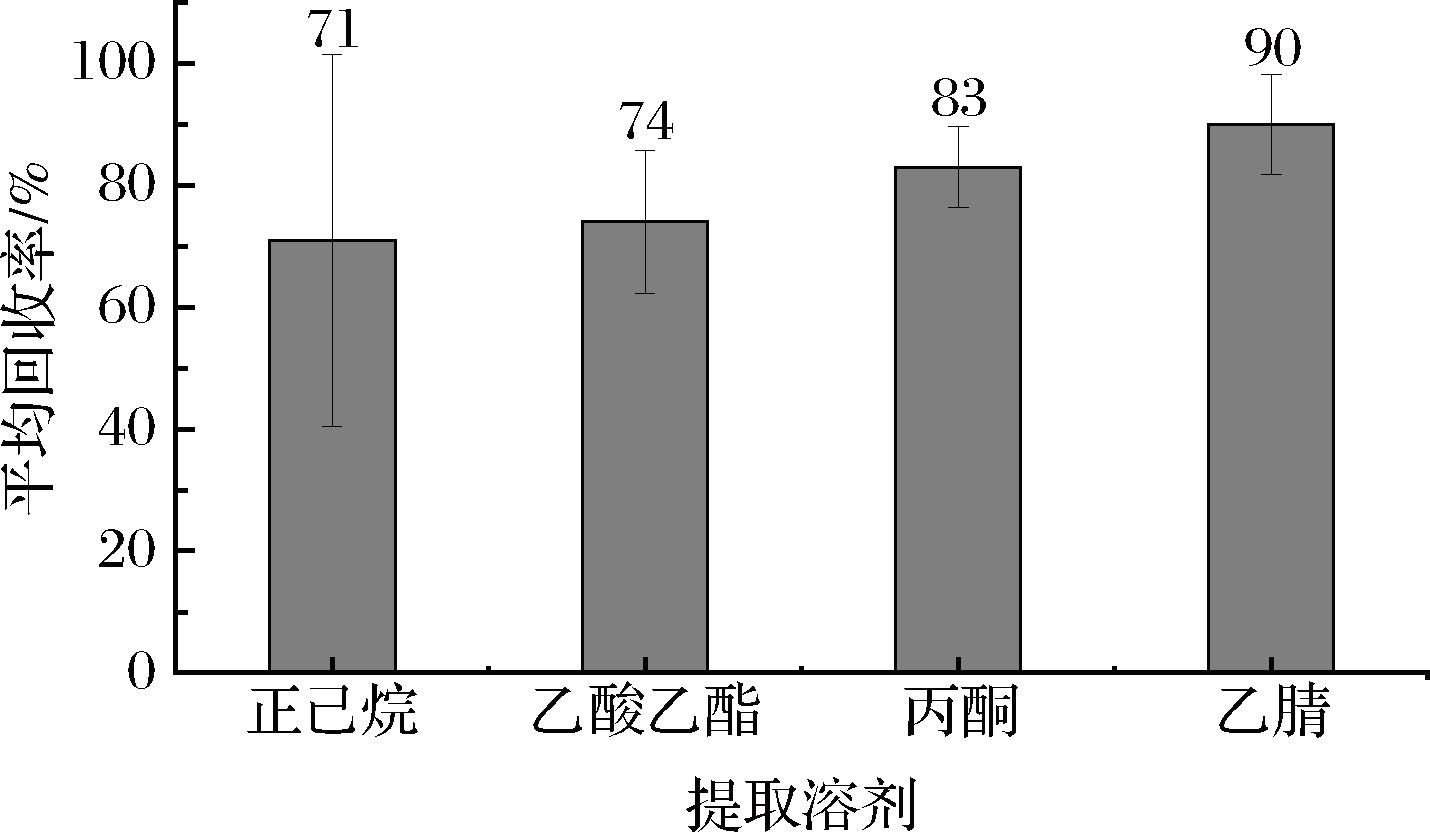
图1 不同提取溶剂样品的平均回收率
Fig.1 Average recovery of samples with different extraction solvents
2.1.2 提取体积的优化
提取溶剂体积的大小对实验效果有着重要影响。本实验从桃胶基质空白加标回收率的角度,考察了不同提取体积(1、2、3、4、5 mL)对各种农药回收率的影响(加标质量浓度为 0.200 mg/kg,n=6)。如图2所示:当提取体积为2 mL时,桃胶基质空白加标样品的平均回收率最高(为94%),且误差线最小(即标准偏差最小)。为了达到最优提取效果以及降低试验成本和避免有机试剂的浪费,因此选择乙腈的提取体积为2 mL。
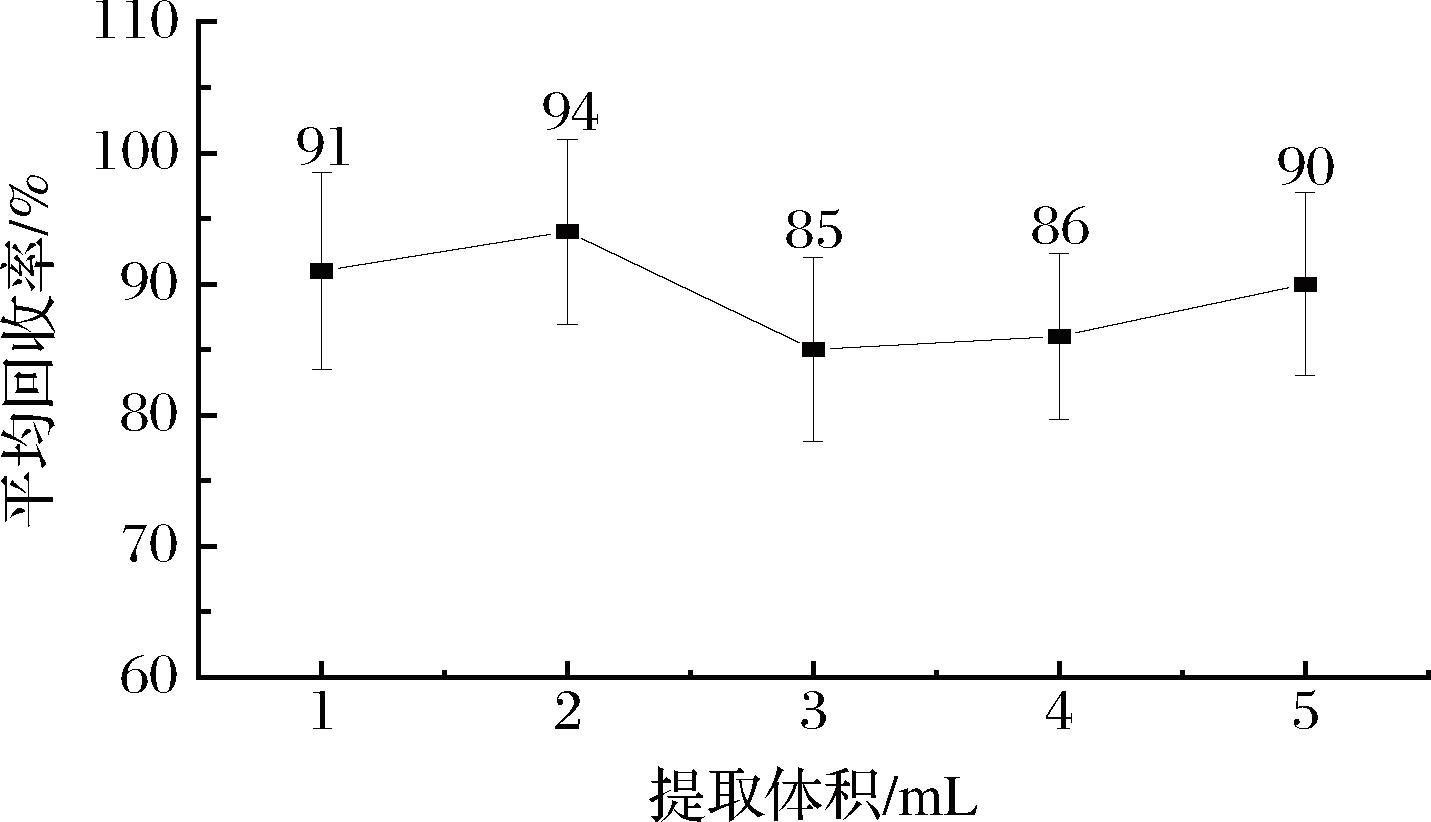
图2 不同提取体积样品的平均回收率
Fig.2 Average recovery of samples with different extraction volumes
2.1.3 净化方法的优化
参考Original QuEChERS前处理方法中净化材料配比,本实验以1 mL提取液为净化目标,比较了5种不同的分散固相萃取净化方案,方案1:150 mg无水MgSO4;方案2:150 mg无水MgSO4、50 mg C18;方案3:150 mg无水MgSO4、50 mg PSA;方案4:150 mg无水MgSO4、50 mg C18、50 mg PSA;方案5:150 mg无水MgSO4、50 mg C18、50 mg PSA、25 mg GCB。如图3所示:方案1、方案2、方案3、方案4及方案5的空白基质样品加标(加标质量浓度为0.200 mg/kg,n=6)平均回收率分别为:92%、99%、95%、98%和77%。方案2中乙酰甲胺磷、百菌清、二甲戊灵、亚胺硫磷和蝇毒磷的回收率均优于其他4种方案(图3)。由实验结果可知:方案2优于其他方案,所以最终采用方案2 作为最终净化方案。
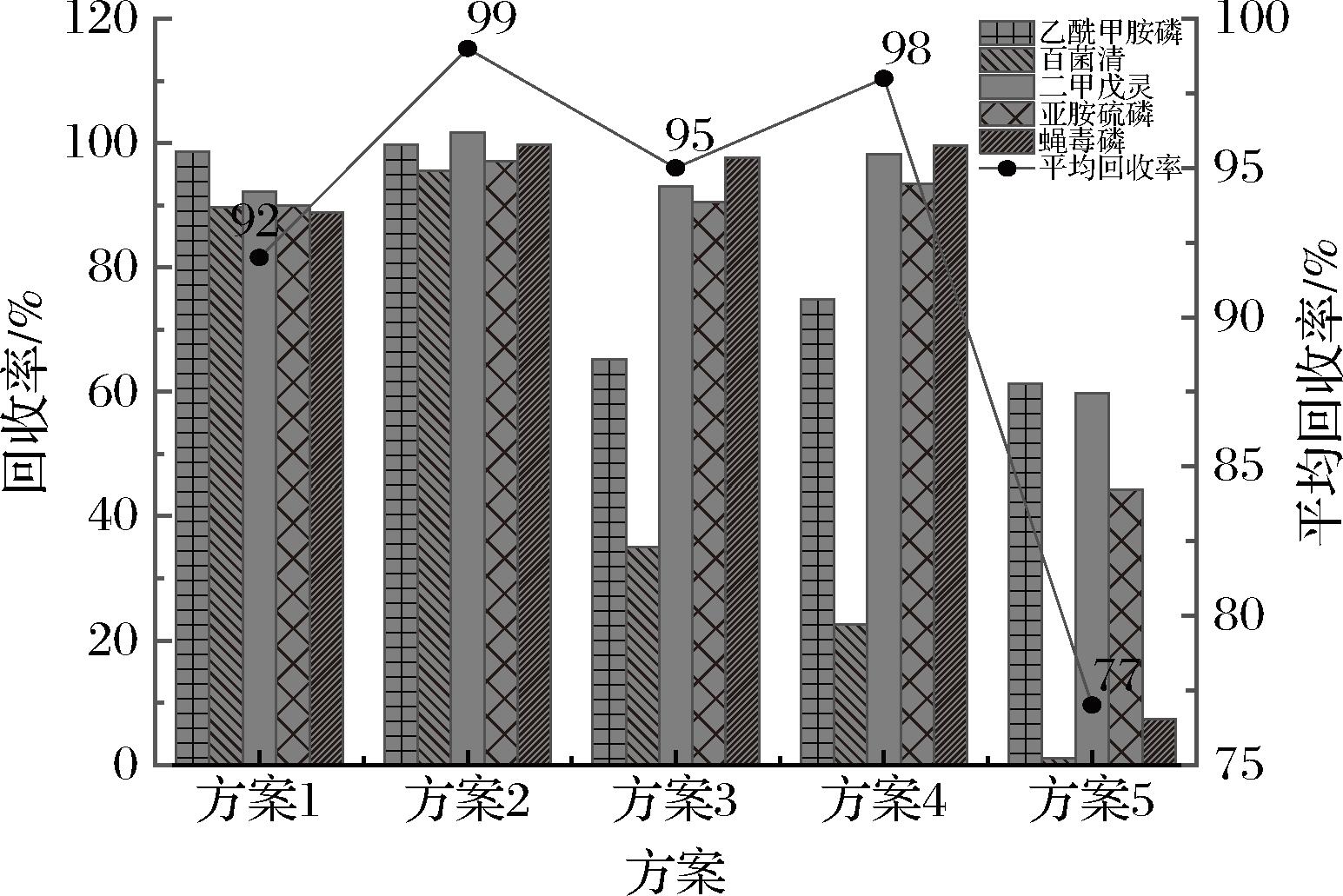
图3 不同净化方案对目标农药回收率的影响
Fig.3 Effects of different purification schemes on the recovery rate of target pesticidess
2.2 仪器条件优化
2.2.1 气相色谱条件优化
本实验参考以往研究,以超高惰性色谱柱:Agilent Technologies HP-5MS UI(20 m×0.18 mm,0.18 μm)作为色谱柱分离结合梯度升温程序,不仅使得目标化合物获得了较好的预分离,而且极大地提高了分析的速度和效率。
2.2.2 质谱条件优化
在EI电离模式下,结合谱库进行全扫描,确定各目标化合的保留时间及母离子;对母离子进行子离子扫描,选择响应最好的2个离子分别作为定量离子和定性离子,并分别与母离子组成对应的定量离子对和定性离子对;优化定量离子对和定性离子对碰撞电压等参数;按照保留时间对目标化合物进行分段。106种目标化合物的离子对信息等质谱参数见表1。
2.3 方法的线性范围、检出限及定量限
在0.002 00、0.005 00、0.020 0、0.050 0、0.100、0.200、0.300、0.400 mg/L系列基质标准曲线质量浓度范围内,各种农药的线性相关系数均大于0.995。根据空白基质加标样品的3倍信噪比(S/N=3)确定方法的检出限,10倍信噪比(S/N=10)确定方法的定量限。各农药线性相关系数、检出限和定量限和基质效应见表2。
表2 各农药线性参数、检出限、定量限以及基质效应
Table 2 The linear parameter,limits of detection,and limits of quantificationand matrix effect of pesticides
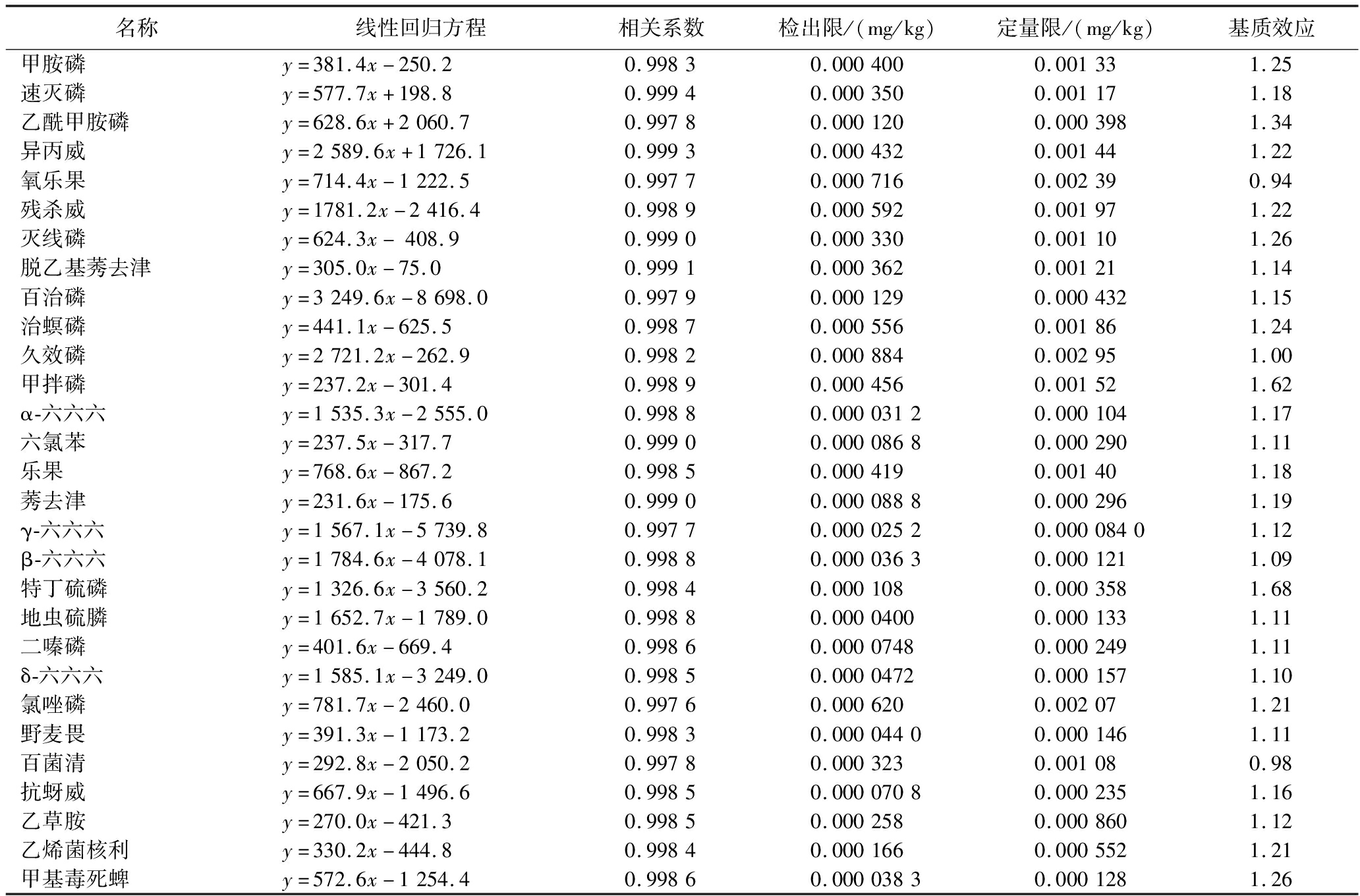
名称线性回归方程相关系数检出限/(mg/kg)定量限/(mg/kg)基质效应甲胺磷y=381.4x-250.20.99830.0004000.001331.25速灭磷y=577.7x+198.80.99940.0003500.001171.18乙酰甲胺磷y=628.6x+2060.70.99780.0001200.0003981.34异丙威y=2589.6x+1726.10.99930.0004320.001441.22氧乐果y=714.4x-1222.50.99770.0007160.002390.94残杀威y=1781.2x-2416.40.99890.0005920.001971.22灭线磷y=624.3x-408.90.99900.0003300.001101.26脱乙基莠去津y=305.0x-75.00.99910.0003620.001211.14百治磷y=3249.6x-8698.00.99790.0001290.0004321.15治螟磷y=441.1x-625.50.99870.0005560.001861.24久效磷y=2721.2x-262.90.99820.0008840.002951.00甲拌磷y=237.2x-301.40.99890.0004560.001521.62α-六六六y=1535.3x-2555.00.99880.00003120.0001041.17六氯苯y=237.5x-317.70.99900.00008680.0002901.11乐果y=768.6x-867.20.99850.0004190.001401.18莠去津y=231.6x-175.60.99900.00008880.0002961.19γ-六六六y=1567.1x-5739.80.99770.00002520.00008401.12β-六六六y=1784.6x-4078.10.99880.00003630.0001211.09特丁硫磷y=1326.6x-3560.20.99840.0001080.0003581.68地虫硫膦y=1652.7x-1789.00.99880.00004000.0001331.11二嗪磷y=401.6x-669.40.99860.00007480.0002491.11δ-六六六y=1585.1x-3249.00.99850.00004720.0001571.10氯唑磷y=781.7x-2460.00.99760.0006200.002071.21野麦畏y=391.3x-1173.20.99830.00004400.0001461.11百菌清y=292.8x-2050.20.99780.0003230.001080.98抗蚜威y=667.9x-1496.60.99850.00007080.0002351.16乙草胺y=270.0x-421.30.99850.0002580.0008601.12乙烯菌核利y=330.2x-444.80.99840.0001660.0005521.21甲基毒死蜱y=572.6x-1254.40.99860.00003830.0001281.26
续表2

名称线性回归方程相关系数检出限/(mg/kg)定量限/(mg/kg)基质效应甲基对硫磷y=717.6x-2195.20.99810.00003880.0001291.22莠灭净y=312.2x-466.40.99840.0001800.0006001.17甲草胺y=730.1x-565.30.99840.0006080.002021.20甲霜灵y=232.4x+1632.80.99850.0003190.001061.25杀螟硫磷y=396.2x-1786.70.99740.00009840.0003281.07甲基嘧啶磷y=287.0x-1055.50.99780.0001010.0003361.09马拉硫磷y=818.8x-3366.80.99750.0004200.001401.11甲拌磷亚砜y=423.3x-1556.50.99750.001160.003861.10甲拌磷砜y=1782.9x-3259.40.99800.0003810.001271.07艾氏剂y=143.2x-505.00.99830.002810.009361.09倍硫磷y=483.8x-1586.60.99760.00004080.0001361.04毒死蜱y=612.0x+1355.80.99810.0008560.002851.14对硫磷y=446.1x-1567.40.99790.0004000.001341.09三唑酮y=415.3x-544.60.99810.0006160.002041.08三氯杀螨醇y=996.4x-5796.50.99770.0003250.001082.10水胺硫磷y=1378.7x-3691.50.99800.0009040.003021.21噻唑膦y=510.3x-2007.30.99720.0001990.0006641.00甲基异柳磷y=1851.8x-5024.80.99790.00006280.0002101.14二甲戊灵y=272.3x-1124.00.99820.0001000.0003341.07戊菌唑y=574.8x-257.70.99760.0002280.0007561.12特丁硫磷砜y=2893.5x+2214.70.99740.0002830.0009441.13氟虫腈y=205.6x+426.50.99590.0001610.0005361.15三唑醇y=484.0x+2455.20.99890.0001720.0005721.18腐霉利y=439.1x+1673.80.99960.00009040.0003011.07杀扑磷y=1834.6x+9959.30.99760.0001320.0004401.452,4′-滴滴伊y=1149.3x+36.10.99800.00004880.0001621.17多效唑y=638.4x+44732.90.99720.00002760.00009201.31丁草胺y=308.4x-705.70.99750.0001890.0006281.13α-硫丹y=47.7x-70.90.99760.0009400.003141.19己唑醇y=122.4x-399.10.99730.0008680.002891.10稻瘟灵y=601.4x-1868.90.99880.0001000.0003331.11抑霉唑y=431.2x-874.90.99760.0003030.001011.16丙溴磷y=156.4x-350.60.99790.0001340.0004480.99咯菌腈y=515.0x-161.70.99950.0001380.0004600.854,4′-滴滴伊y=1993.1x-5309.10.99820.00003300.0001101.03狄氏剂y=152.0x-892.60.99560.0007200.002390.88腈菌唑y=772.7x-1708.00.99820.0001220.0004080.842,4′-滴滴滴y=1584.1x-3889.40.99790.0001370.0004561.05醚菌酯y=1700.4x-6182.70.99720.0006360.002120.86吡氟禾草灵y=446.4x-1901.00.99690.00008040.0002681.00异狄氏剂y=235.4x-877.40.99760.002000.006641.08β-硫丹y=81.5x-232.80.99780.0007000.002331.01倍硫磷亚砜y=381.4x-250.20.99830.0002370.0007880.82烯唑醇y=816.0x+1010.30.99950.0001720.0005720.992,4′-滴滴涕y=577.7x+198.80.99940.00002740.00009121.30倍硫磷砜y=628.6x+2060.70.99780.0006160.002050.964,4′-滴滴滴y=2589.6x+1726.10.99930.0001180.0003940.60乙硫磷y=1179.8x+14081.90.99960.0001570.0005241.14三唑磷y=714.4x-1222.50.99770.001030.003421.39肟菌酯y=1781.2x-2416.40.99890.0002020.0006721.094,4′-滴滴涕y=624.3x-408.90.99900.00006200.0002070.49丙环唑y=305.0x-75.00.99910.0002250.0007481.11戊唑醇y=3249.6x-8698.00.99790.00005560.0001851.10禾草灵y=441.1x-625.50.99870.0001510.0005041.06增效醚y=2721.2x-262.90.99820.0005560.001851.09氟环唑y=237.2x-301.40.99890.00009880.0003290.98异菌脲y=1535.3x-2555.00.99880.00006640.0002221.03亚胺硫磷y=237.5x-317.70.99900.0008000.002661.00联苯菊酯y=768.6x-867.20.99850.0001260.0004201.13甲氧滴滴涕y=231.6x-175.60.99900.0003040.001010.50
续表2
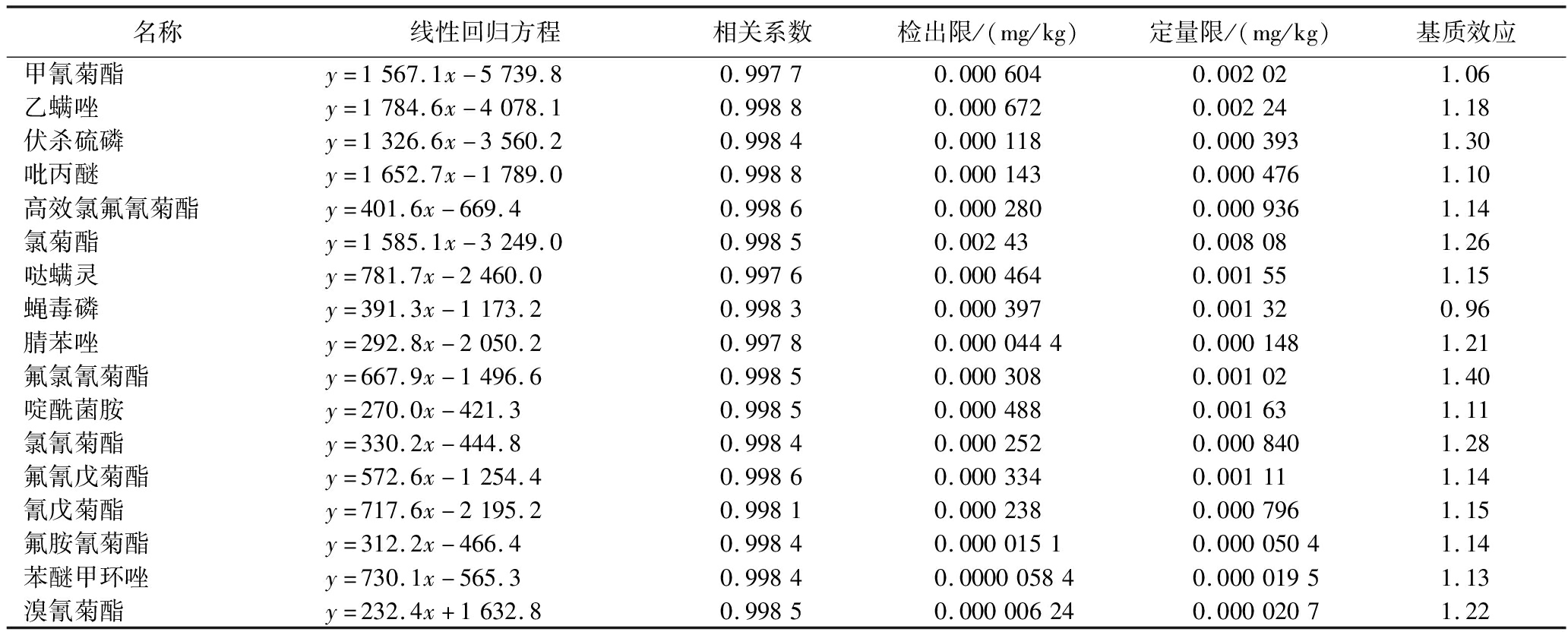
名称线性回归方程相关系数检出限/(mg/kg)定量限/(mg/kg)基质效应甲氰菊酯y=1567.1x-5739.80.99770.0006040.002021.06乙螨唑y=1784.6x-4078.10.99880.0006720.002241.18伏杀硫磷y=1326.6x-3560.20.99840.0001180.0003931.30吡丙醚y=1652.7x-1789.00.99880.0001430.0004761.10高效氯氟氰菊酯y=401.6x-669.40.99860.0002800.0009361.14氯菊酯y=1585.1x-3249.00.99850.002430.008081.26哒螨灵y=781.7x-2460.00.99760.0004640.001551.15蝇毒磷y=391.3x-1173.20.99830.0003970.001320.96腈苯唑y=292.8x-2050.20.99780.00004440.0001481.21氟氯氰菊酯y=667.9x-1496.60.99850.0003080.001021.40啶酰菌胺y=270.0x-421.30.99850.0004880.001631.11氯氰菊酯y=330.2x-444.80.99840.0002520.0008401.28氟氰戊菊酯y=572.6x-1254.40.99860.0003340.001111.14氰戊菊酯y=717.6x-2195.20.99810.0002380.0007961.15氟胺氰菊酯y=312.2x-466.40.99840.00001510.00005041.14苯醚甲环唑y=730.1x-565.30.99840.000005840.00001951.13溴氰菊酯y=232.4x+1632.80.99850.000006240.00002071.22
2.4 方法的准确度和精密度
本实验以空白样品加标实验的回收率作为评价标准来考察方法的准确度,而精密度则通过同一加标水平6次回收率的相对标准偏差来获得,添加浓度选择低、中、高(0.040、0.800、1.600 mg/kg)3个加标水平,来进行方法确认。如表3所示,106种农药在桃胶空白基质中回收率分别为72%~109%,精密度范围分别为0.73%~10%。
表3 回收率和精密度实验结果(n=6)
Table 3 Spiked recoveries and relative standard deviations(RSDs) of pesticides
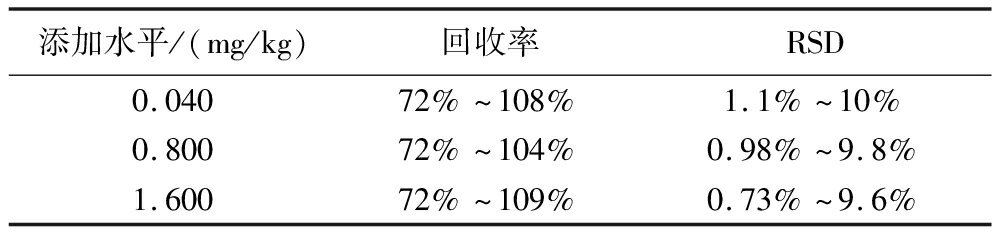
添加水平/(mg/kg)回收率RSD0.04072%~108%1.1%~10%0.80072%~104%0.98%~9.8%1.60072%~109%0.73%~9.6%
2.5 基质效应的研究
基质效应主要指试样中的非待测组分影响目标物浓度或质量测定的准确度,进而引起目标物响应值增加或减少的现象[25-28]。基质效应一般分为基质增强效应和基质抑制效应[29]。桃胶中的化学成分复杂,主要成分是各类多糖,除此之外,还含有蛋白质、多酚类物质、无机元素以及水分等,采用气相色谱-三重四极杆串联质谱仪进行检测分析时容易出现基质效应。本研究采用基质标准曲线斜率与溶剂标准曲线斜率的比值作为评价标准来评价桃胶的基质效应。当基质效应>1.15时,存在基质增强效应;当0.85≤基质效应≤1.15时,基质效应不明显;当基质效应<0.85时,存在基质抑制效应[30]。各种农药在桃胶中基质效应结果如表2所示,结果显示,近35%的农药在桃胶中存在基质增强效应;近60%的农药在桃胶中基质效应不明显;近5%的农药在桃胶中存在基质抑制效应。本实验通过分散固相萃取净化、添加分析物保护剂以及基质标准曲线校正的方式来尽可能消除样品基质效应的干扰。首先,提取液中的糖类、蛋白质、多酚类物质以及水分等通过C18、无水MgSO4分散固相萃取去除。其次,通过添加分析物保护剂的方式来进一步降低样品的基质效应[31]。分析物保护剂工作原理即模仿基质保护作用的单一化合物或简单的混合物。当在纯溶剂标准溶液和样品溶液中加入相同量的保护剂时,它能同等程度地补偿标准溶液和样品溶液的基质效应[32]。最后,通过基质标准曲线校正的方式来消除桃胶样品的基质效应。
2.6 μ-QuEChERS与Original QuEChERS对比研究
本实验采用的μ-QuEChERS前处理方法是改编自PORTO-FIGUEIRA等[20]采用的方法,是ANASTASSIADES等[17]提出的原始的QuEChERS(即Original QuEChERS)方法的小型化。提取盐按照无水MgSO4、NaCl、柠檬酸钠二水合物以及柠檬酸二钠盐倍半水合物质量比为4∶1∶4∶0.5均匀配制,净化方式采用分散固相萃取并进行优化。为了比较μ-QuEChERS前处理方法与Original QuEChERS前处理方法的差别,本研究以桃胶基质空白样品加标回收率作为评价标准(加标质量浓度为0.020 mg/kg,n=6),比较了2种前处理方法对桃胶中106种农药残留的提取效果。结果表明:采用μ-QuEChERS前处理方法和Original QuEChERS前处理方法的农药平均回收率分别为87%、85%,各种农药的回收率为65%~105%,且无显著性差异。μ-QuEChERS前处理方法相对于Original QuEChERS前处理方法溶剂、耗材消耗更少,对环境更为友好,所以本实验采用μ-QuEChERS前处理方法。
2.6 实际样品的检测
采用优化后的方法对市售的20批次桃胶进行106种农药检测,如表4所示:共有8种农药有检出,检出率为5%~30%,其中苯醚甲环唑和多效唑的检出率相对较高,分别为30%和25%。
表4 桃胶实际样品的检出情况
Table 4 Detection status of peach gum in real samples
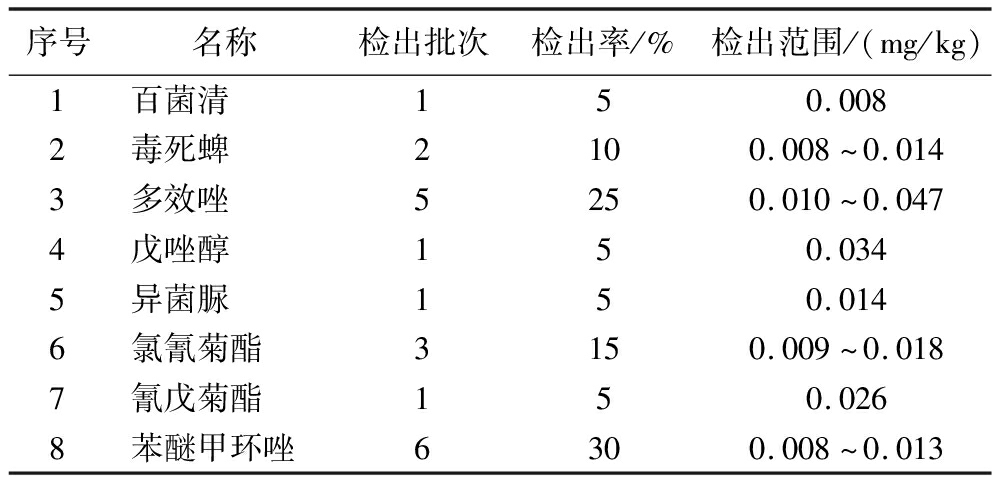
序号名称检出批次检出率/%检出范围/(mg/kg)1百菌清150.0082毒死蜱2100.008~0.0143多效唑5250.010~0.0474戊唑醇150.0345异菌脲150.0146氯氰菊酯3150.009~0.0187氰戊菊酯150.0268苯醚甲环唑6300.008~0.013
3 结论
本实验将μ-QuEChERS前处理方法与超高惰性色谱柱以及气相色谱-三重四极杆串联质谱法相结合,开发了一种适合桃胶中农药多残留的快速检测方法。106种农药在0.002 00~0.400 mg/L内具有良好的线性关系(均大于0.995)且在低、中、高(0.040 0、0.800、1.60 mg/kg)3个加标水平都具有较好的回收率(72%~109%)和良好的精密度(0.73%~10%)。本方法具有分析速度快、灵敏度高和对环境更友好的特点,将该方法应用于市售桃胶的农药多残留检测,达到了良好的检测效果,适用于桃胶中多种农药残留的快速检测。
[1] ZHANG L,KOU X Y,HUANG X,et al.Peach-gum:A promising alternative for retarding the ripening and senescence in postharvest peach fruit[J].Postharvest Biology and Technology,2020,161:111088.
[2] SONG Y H,TAN J S,WANG G,et al.Superior amine-rich gel adsorbent from peach gum polysaccharide for highly efficient removal of anionic dyes[J].Carbohydrate Polymers,2018,199:178-185.
[3] TAN J S,SONG Y H,HUANG X H,et al.Facile functionalization of natural peach gum polysaccharide with multiple amine groups for highly efficient removal of toxic hexavalent chromium (Cr(VI)) ions from water[J].ACS Omega,2018,3(12):17309-17318.
[4] 屈桂波.中国古代桃胶略考[J].科教文汇(中旬刊),2021(5):178-179.QU G B.A brief study on peach gum in ancient China[J].The Science Education Article Collects,2021(5):178-179.
[5] 林慧,代金霞,薛瑾,等.桃胶的理化性质、功效成分及其安全性研究进展[J].食品安全质量检测学报,2023,14(21):219-226.LIN H,DAI J X,XUE J,et al.Research progress on the physical and chemical properties,efficacy composition and safety of peach gum[J].Journal of Food Safety &Quality,2023,14(21):219-226.
[6] YAO X C,CAO Y,WU S J.Antioxidant activity and antibacterial activity of peach gum derived oligosaccharides[J].International Journal of Biological Macromolecules,2013,62:1-3.
[7] 刘启月,李勇,余向阳,等.高效液相色谱-串联质谱法检测原桃胶中左旋肉碱的含量[J].江苏农业科学,2020,48(17):215-218.LIU Q Y,LI Y,YU X Y,et al.Determination of L-carnitine content in raw peach gum by high performance liquid chromatography-tandem mass spectrometry[J].Jiangsu Agricultural Sciences,2020,48(17):215-218.
[8] 徐燕.桃胶的制备、性质及其应用研究[D].无锡:江南大学,2008.XU Y.Study on preparation,properties and application of peach gum[D].Wuxi:Jiangnan University,2008.
[9] QIAN H F,CUI S W,WANG Q,et al.Fractionation and physicochemical characterization of peach gum polysaccharides[J].Food Hydrocolloids,2011,25(5):1285-1290.
[10] 刘启月.桃胶功能成分分析及安全性评价[D].南京:南京财经大学,2021.LIU Q Y.Analysis of functional components and safety evaluation of peach gum[D].Nanjing:Nanjing University of Finance &Economics,2021.
[11] 蔡延渠,董碧莲,陈利秋,等.桃胶多糖体内外抗氧化作用的研究[J].食品工业科技,2020,41(13):53-58.CAI Y Q,DONG B L,CHEN L Q,et al.Antioxidant activity in vivo and in vitro of polysaccharide from peach gum[J].Science and Technology of Food Industry,2020,41(13):53-58.
[12] LIN X B,LAN M Y,XU C,et al.Peach gum polysaccharides promotes epithelial proliferation to attenuate ulcerative colitis by PI3K/AKT pathway[J].Journal of Functional Foods,2023,107:105662.
[13] 陈妙金,孙奇男,谢宝良,等.桃胶多时期采摘的农药残留分析[J].浙江农业科学,2021,62(5):1009-1011; 1015.CHEN M J,SUN Q N,XIE B L,et al.Pesticide residue determination of peach gum in different picking time[J].Journal of Zhejiang Agricultural Sciences,2021,62(5):1009-1011; 1015.
[14] 官金艳,苏小路,胡亦清,等.QuEChERS-气相色谱法快速测定桃胶中16种有机磷农药残留[J].分析科学学报,2022,38(2):260-264.GUAN J Y,SU X L,HU Y Q,et al.Determination of 16 organophosphorus pesticide residues in peach gum by QuEChERS-gas chromatography[J].Journal of Analytical Science,2022,38(2):260-264.
[15] 周建峰,陈鑫兰,颜艳阳,等.GPC/SPE-UPLC-MS/MS凝胶色谱仪辅助分散型固相萃取-快速检测桃胶中10种杀虫剂和杀菌剂农药残留[J].中国测试,2022,48(S2):108-114.ZHOU J F,CHEN X L,YAN Y Y,et al.GPC/SPE-UPLC-MS/MS gel chromatography assisted dispersed solid phase extraction-rapid detection of 10 pesticides and fungicides residues in peach gum[J].China Measurement &Test,2022,48(S2):108-114.
[16] 田菊,李勇,吕春茂,等.桃胶中多农药残留分析及风险评估[J].食品与机械,2023,39(5):55-63; 100.TIAN J,LI Y,LYU C M,et al.Analysis and risk assessment of pesticide residues in peach gum[J].Food &Machinery,2023,39(5):55-63; 100.
[17] ANASTASSIADES M,LEHOTAY S J,STAJNBAHER D,et al.Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce[J].Journal of AOAC International,2003,86(2):412-431.
[18] GARC A-CANSINO L,GARC
A-CANSINO L,GARC A M
A M  ,MARINA M L,et al.Simultaneous microextraction of pesticides from wastewater using optimized μSPEed and μQuEChERS techniques for food contamination analysis[J].Heliyon,2023,9(6):e16742.
,MARINA M L,et al.Simultaneous microextraction of pesticides from wastewater using optimized μSPEed and μQuEChERS techniques for food contamination analysis[J].Heliyon,2023,9(6):e16742.
[19] CASADO N,PERESTRELO R,SILVA C L,et al.An improved and miniaturized analytical strategy based on μ-QuEChERS for isolation of polyphenols.A powerful approach for quality control of baby foods[J].Microchemical Journal,2018,139:110-118.
[20] PORTO-FIGUEIRA P,CAMACHO I,C MARA J S.Exploring the potentialities of an improved ultrasound-assisted quick,easy,cheap,effective,rugged,and safe-based extraction technique combined with ultrahigh pressure liquid chromatography-fluorescence detection for determination of Zearalenone in cereals[J].Journal of Chromatography A,2015,1408:187-196.
MARA J S.Exploring the potentialities of an improved ultrasound-assisted quick,easy,cheap,effective,rugged,and safe-based extraction technique combined with ultrahigh pressure liquid chromatography-fluorescence detection for determination of Zearalenone in cereals[J].Journal of Chromatography A,2015,1408:187-196.
[21] WRIGHT B W,WRIGHT C W.New method for evaluating irreversible adsorption and stationary phase bleed in gas chromatographic capillary columns[J].Journal of Chromatography A,2012,1261:142-150.
[22] CAGLIERO C,BICCHI C,CORDERO C,et al.Analysis of essential oils and fragrances with a new generation of highly inert gas chromatographic columns coated with ionic liquids[J].Journal of Chromatography A,2017,1495:64-75.
[23] RAVINDRA K,DIRTU A C,COVACI A.Low-pressure gas chromatography:Recent trends and developments[J].TrAC Trends in Analytical Chemistry,2008,27(4):291-303.
[24] ARREBOLA F J,MART NEZ VIDAL J L,GONZ
NEZ VIDAL J L,GONZ LEZ-RODR
LEZ-RODR GUEZ M J,et al.Reduction of analysis time in gas chromatography Application of low-pressure gas chromatography-tandem mass spectrometry to the determination of pesticide residues in vegetables[J].Journal of Chromatography A,2003,1005(1-2):131-141.
GUEZ M J,et al.Reduction of analysis time in gas chromatography Application of low-pressure gas chromatography-tandem mass spectrometry to the determination of pesticide residues in vegetables[J].Journal of Chromatography A,2003,1005(1-2):131-141.
[25] 彭汝林,曾婷,朱雨田,等.QuEChERS结合气相色谱-串联三重四极杆质谱快速测定水产品中100种农药残留[J].食品科技,2023,48(8):278-286.PENG R L,ZENG T,ZHU Y T,et al.Rapid determination of multiple pesticide residues in aquatic products by Qu ECh ERS and gas chromatography-tandem mass spectrometry[J].Food Science and Technology,2023,48(8):278-286.
[26] KIM S H,LEE Y H,JEONG M J,et al.LC-MS/MS method minimizing matrix effect for the analysis of bifenthrin and butachlor in Chinese chives and its application for residual study[J].Foods,2023,12(8):1683.
[27] FERRER C,LOZANO A,AGÜERA A,et al.Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables[J].Journal of Chromatography A,2011,1218(42):7634-7639.
[28] LY T K,HO T D,BEHRA P,et al.Determination of 400 pesticide residues in green tea leaves by UPLC-MS/MS and GC-MS/MS combined with QuEChERS extraction and mixed-mode SPE clean-up method[J].Food Chemistry,2020,326:126928.
[29] HUERTAS-PÉREZ J F,BASLÉ Q,DUBOIS M,et al.Multi-residue pesticides determination in complex food matrices by gas chromatography tandem mass spectrometry[J].Food Chemistry,2024,436:137687.
[30] 彭婕,穆迎春,喻亚丽,等.改良QuEChERS技术结合超高效液相色谱-串联质谱法测定水产品中扑草净及其代谢物残留[J].食品科学,2024,45(3):185-192.PENG J,MU Y C,YU Y L,et al.Determination of residues of prometryn and its metabolites in aquatic products by modified QuEChERS method combined with ultra-high performance liquid chromatography-tandem mass spectrometry[J].Food Science,2024,45(3):185-192.
[31] ANASTASSIADES M,MASTOVSK K,LEHOTAY S J.Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides[J].Journal of Chromatography.A,2003,1015(1-2):163-184.
K,LEHOTAY S J.Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides[J].Journal of Chromatography.A,2003,1015(1-2):163-184.
[32] 许秀丽,赵海香,李礼,等.分析保护剂补偿基质效应-气相色谱-质谱法快速测定水果中40种农药残留[J].色谱,2012,30(3):267-272.XU X L,ZHAO H X,LI L,et al.Rapid determination of 40 pesticide residues in fruits using gas chromatography-mass spectrometry coupled with analyte protectants to compensate for matrix effects[J].Chinese Journal of Chromatography,2012,30(3):267-272.