氨基糖苷类药物(aminoglycosides,AGs)是一类广谱抗生素,常见的AGs包括链霉素(streptomycin,STR)、新霉素(neomycin,NEO)、卡那霉素(kanamycin,KAN)、妥布霉素(tobramycin,TOB)、庆大霉素(gentamicin,GEN)和阿米卡星(amikacin,AMK)等。其化学结构由一个氨基环醇和一个或多个氨基糖分子通过糖苷键连接而成,具有高极性和亲水性,极易溶于水,微溶于甲醇,不溶于非极性有机溶剂。AGs可以通过抑制70S起始复合物的形成,选择性地与30S亚基上的目标蛋白结合并诱导密码子错配,抑制细菌的蛋白质合成而起到抗菌作用[1-2]。AGs的广谱活性、快速杀菌作用以及良好的理化稳定性和药代动力学特性使它们成为临床上广泛使用的一类药物,可用于防治多种感染类型,如肠杆菌、克雷伯菌、变形杆菌、铜绿假单胞菌和葡萄球菌等引起的各种中重度呼吸道感染、泌尿道感染、肠道感染和皮肤软组织感染[3]。
AGs价格低廉,抗菌作用良好,在临床医学和畜牧养殖中作为抗感染和促生长剂被广泛使用[4],AGs在动物体内难以代谢降解,会以原形经肾排泄,大量AGs释放到环境中会导致某些菌株的耐药性增加[5],许多国家和地区都对动物产品中的AGs残留设定了最大残留限量(maximum residue limits,MRLs),具体见表1。残留的AGs还会在动物组织中积累,影响动物源性食品安全,严重危害人体健康。AGs容易被耳蜗细胞和肾近端小管细胞摄取,通过诱导线粒体基因突变、破坏传入神经元之间的突触以及诱导细胞内溶酶体病变造成耳蜗功能障碍、肾小球滤过率下降,严重时甚至导致听力丧失和肾衰竭[6-8]。因此,迫切需要研究简便快捷、灵敏度高、准确性好的AGs检测方法。
表1 不同动物产品中AGs的最大残留限量
Table 1 AGs MRLs in different animal products

AGs种类动物样品种类最大残留限量(mg/kg)美国欧盟中国日本JECFA新霉素猪牛/羊鸡肌肉1200500500500500脂肪-500500500500肝脏3600500500500500肾7200500090001000010000肌肉1200500500500500脂肪7200500500500500肝脏36005005500500500肾7200500090001000010000牛奶1501500150020001500肌肉/脂肪/肝脏/鸡蛋-500500500500肝脏-5005500500500肾-500090001000010000卡那霉素猪牛羊鸡肌肉/脂肪-10010040-肝脏-600600900-肾-250025004000-肌肉/脂肪-10010040-肝脏-6006001000-肾-2500250013000-牛奶-150150700-肌肉/脂肪-100100100-肝脏-600600600-肾-250025003000-肌肉-100100200-脂肪-100100300-肝脏-60060013000-肾-2500250025000-鸡蛋--10200-庆大霉素猪牛鸡肌肉10050100100100脂肪40050100100100肝脏300200200020002000肾400750500050005000肌肉/脂肪-50100100100肝脏-200200020002000肾-750500050005000牛奶-100200200200肌肉/脂肪/肝脏/肾100-100--
由于AGs极性很强,大多数不含有发色团或荧光团,除非进行柱前或柱后衍生,否则难以用紫外或荧光检测器检测,因此AGs的检测一直面临许多困难。常用检测方法包括微生物法、免疫分析法以及仪器分析法,其中微生物法相对简单且成本较低,但测定时间长,结果误差较大,抗菌谱相似的药物会干扰结果甚至出现假阳性,难以达到定量检测的需求[9]。以酶联免疫吸附测定为代表的免疫分析方法背景信号高、检测时间长且人工抗原、抗体的制备过程复杂冗长,稳定性差[10-11],容易出现假阳性或假阴性。高效液相色谱、高效液相色谱串联质谱、毛细管电泳和表面等离子体共振等仪器分析方法也是AGs检测的常用方法[12-21],虽然这些方法具有极高的准确性与灵敏度,但所需仪器昂贵,操作耗时,需要熟练的操作人员。与上述方法相比,光学和电化学传感器灵敏度高、检测限低、操作简单、便于携带且成本低廉[22],在AGs残留检测方面具有广阔应用前景。本文简要介绍了AGs光学和电化学传感器的构建原理与分类,总结了荧光法、化学发光法、比色法、表面增强拉曼光谱(surface-enhanced Raman spectroscopy,SERS)、电致发光以及电化学传感器的构建与应用,最后对AGs光电化学传感器的应用范围与缺陷进行了总结与讨论。
1 光电化学传感器的构建原理与组成
光电化学传感器通常由识别元件和信号转换元件构成,当目标分子存在时,识别元件对其进行特异性识别,并由信号转换元件将识别元件与目标分子之间发生的理化变化转换为可识别的光信号或电信号,从而实现目标分子的特征识别。
识别元件的性能直接决定了传感器特异性识别能力,是传感检测的核心,常见的构建材料包括适配体(aptamer,Apt)、分子印迹聚合物(molecularly imprinted polymers,MIPs)以及各类纳米材料。核酸适配体是由大约10~100个碱基组成的单链脱氧核糖核酸(single-stranded DNA,ssDNA)或RNA序列,它们折叠成三维结构可识别几乎所有类型的靶标,包括金属离子、药物小分子、蛋白质、病毒和动物细胞[23]。与天然抗体相比,适配体具有体外合成简单、无需实验动物、易于修饰、热稳定性好、温度和pH范围广以及无毒性等特点[24]。适配体还可以与纳米材料和荧光探针等信号放大标记物相结合,极大提高检测灵敏度,缩短检测时间,拓宽其应用范围[25]。MIPs是一种通过分子印迹技术制备的高选择性聚合物,在表面具有多个结合位点,可以选择性地识别目标分子[26]。与生物分子相比,MIPs具有特异性强、制备简单、稳定性好和可重复使用等特点,广泛用于痕量物质的分析检测[27]。
在识别元件的构建过程中,增敏材料修饰是信号放大的重要途径,碳纳米管、石墨烯、金属纳米粒子和量子点等纳米材料具有高比表面积、高导电性、良好的生物相容性和独特的物理化学性质,常作为增敏材料修饰于各类传感器,以减少非特异性信号的干扰,提高传感检测灵敏度[28]。
2 光学传感器
2.1 荧光传感器
荧光传感器识别元件通常由3部分组成,包括识别基团、荧光探针和偶联结构。识别基团可以特异性地识别底物,与被检测物质发生反应,它决定了荧光传感器的选择性;荧光团可以发生相应的光物理性质变化,产生感应信号,如荧光发射波长、荧光强度和荧光寿命等[29]。根据反应前后的荧光变化,荧光传感器主要分为荧光淬灭型(“关闭”效应)、荧光增强型(“开启”效应)和比率型荧光传感器。
2.1.1 荧光淬灭型传感器
荧光淬灭型传感器可以发出强荧光,当检测到待测物质后,荧光发射大大减弱甚至淬灭。GENG等[30]将KAN适配体组装在金纳米簇(gold nanoclusters,AuNCs)修饰的牛血清白蛋白(bovine serum albumin,BSA)表面,此时荧光发生淬灭。检测到KAN后,特异性适配体从BSA-AuNCs表面分离,形成适配体-KAN复合物,红色荧光重新恢复,该传感器检测限可达0.032 nmol/L。但由于其高背景信号,当待测物的浓度接近检测限时,很难观察到荧光的信号变化。
通过筛选并制备多种适配体荧光探针可有效降低背景信号的干扰。TANG等[31]设计了一种荧光淬灭型消逝波适配体传感器用于AGs检测,原理如图1所示。靶标不存在时,荧光标记的适配体(Cy3-Apt)形成分子间多聚体;当检测到KAN时,多聚体结构被破坏形成具有荧光淬灭作用的单链发夹结构(KAN-Apt)。游离单链对氧化石墨烯(graphene oxide,GO)的结合能力高于KAN-Apt和多聚体结构,GO会优先与荧光探针和靶标结合,以淬灭游离单链的荧光,降低传感器的背景信号,最后GO将KAN-Apt与多聚体结构分离,并进一步淬灭KAN-Apt的荧光,其检测限约为26 nmol/L。这种基于消逝波的荧光传感器不易受外界电磁场干扰,稳定性强,可在线连续检测目标,为自动化和小型化AGs传感器的构建提供了可能性。
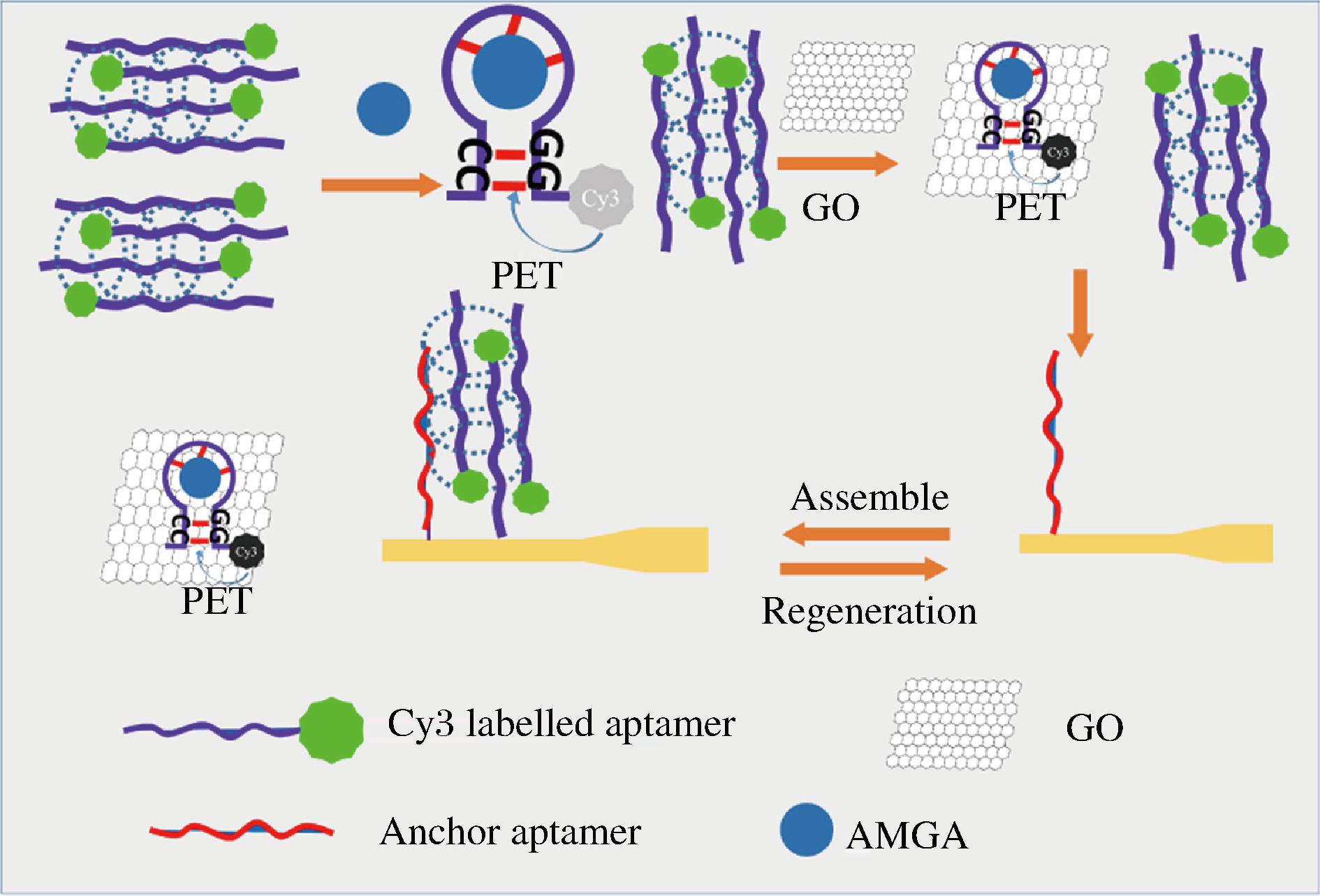
图1 基于荧光淬灭的消逝波适配体传感器原理图[31]
Fig.1 Scheme of the fluorescence quenching-based evanescent wave aptasensor[31]
2.1.2 荧光增强型传感器
荧光增强型传感器本身没有荧光或荧光较弱,当识别到底物之后,荧光会明显增强。与荧光淬灭型相比,该类传感器不仅可以放大目标信号,还能降低背景信号,从而提高信噪比,降低检测限,提高传感器的灵敏度和分辨率[32]。SOFIA等[33]将叶绿素作为疏水近红外荧光团,开发了用于检测NEO的荧光增强型传感器。叶绿素、十二烷基硫酸钠(阴离子)和AGs(阳离子)在溶剂中自发聚集形成三元结构,利用正负离子间的非共价键对靶标进行特异性识别。这种基于正负离子间非共价键的自组装纳米结构探针,无需复杂的合成流程,拓展了高性能荧光探针的制备方式。
量子点作为新兴的纳米材料,具有传统有机染料所不具备的一些独特光学性能,包括荧光激发光谱宽、斯托克斯位移大、荧光寿命长和光稳定性良好等,广泛用于食品中农兽药残留物检测[34-36]。GENG等[37]将MIPs与适配体相结合,以CdSe量子点为载体,巯基修饰适配体和甲基丙烯酸为双功能单体,构建了新型分子印迹荧光传感器,检测限为0.013 mg/mL。与表面印迹法相比,GENG等[37]使用硫醇-烯点击反应构建了双识别荧光传感系统,无需用复杂的硅烷化试剂对量子点进行功能化,极大优化了传感器构建流程。基于Cd的量子点尽管具有优良的光学性能,但这种量子点毒性较强、表面修饰方法复杂且成本高昂,因此研究开发毒性低、性能良好的新型金属量子点可拓展其应用范围。
2.1.3 比率型荧光传感器
比率型荧光传感器通常会发出双荧光,当识别待测物质之后,其中一种荧光发射的强度逐渐降低,而另一种荧光发射的强度逐渐增强,并且比率荧光探针可以同时分析2到多个不同荧光发射峰强度的变化,以不同荧光发射峰强度的比率作为输出信号,提高了探针的特异性和可靠性[38]。PINTO等[36]从一系列金属离子中筛选出对NEO具有强亲和力的Fe3+,用谷胱甘肽(glutathione,GSH)对石墨烯量子点(graphene quantum dots,GQDs)进行氨基化,构建了GQDs-GSH-Fe3+探针,Fe3+存在时,GQDs-GSH发生荧光淬灭,检测到NEO后,即恢复原始信号,其检测限可达7.4×10-8 mol/L[36]。比率型荧光传感器不易受生物自发荧光、溶液的浓度和极性、激发光强度以及光漂白等外部因素干扰,在定量检测方面具有明显优势。
虽然与其他光学传感技术相比,荧光传感灵敏度高、特异性强且准确性良好在实际检测中应用广泛。但是大多数传统的荧光传感仪器需要复杂的光学元件和系统(尤其是光谱仪),难以满足现场检测的需求,进一步开发便携式荧光传感器是解决上述问题的有效途径[39]。
2.2 化学发光传感器
化学发光是物质在进行化学反应过程中伴随的一种光辐射现象[40-41],化学发光法通过检测发光强度间接测定目标物含量,该方法无需激发光源、背景干扰小、灵敏度高、检出限低、线性范围广且重现性良好[42]。ZENG等[43]通过将抗原固定在硝酸纤维素膜上,将辣根过氧化物酶(horseradish peroxidase,HRP)标记的山羊抗小鼠抗体与单壁碳纳米管结合来制备多酶纳米颗粒,建立了增强型化学发光微阵列免疫测定法。STR、KAN、GEN和NEO的检测限分别为1.25、0.64、0.38和0.39 ng/mL,与传统化学发光方法相比发光强度分别提高了3倍、7倍、11倍和7倍。
鲁米诺及其衍生物是当前应用最广泛的化学发光试剂,其反应速率慢,发光强度低,严重影响检测灵敏度。吖啶酯类在强碱性条件下发光稳定且强度高,以该体系构建的传感器,检测限可达0.116 pg/mL[44-45]。NAKAZONO等[46]在吖啶酯类化合物中引入吸电子基团,使其在中性条件下同样具有良好的发光强度;过氧草酸酯类在非水性介质中发光时间长且效率高,发射量子产率高达100%[47]。目前这些体系尚未应用于AGs传感器的构建,探索并优化这些发光强度高且持续时间长的化学发光体系,可以极大拓展AGs化学发光传感器的效能。
2.3 比色传感器
比色法是一种经典化学分析方法,通过比较和测定待测溶液的特征峰或颜色变化以达到定性和定量检测的目的[48]。基于比色法构建的传感器通常分为两类,第一类可以不依赖于任何仪器实现裸眼检测,凭借裸眼观察显色底物的深浅变化,此类比色传感器简单便捷,但是准确度低且易受环境影响;第二类需要借助紫外可见分光光度计和光电比色器等设备,此类传感器的构建通常与纳米粒子材料相结合,具有检测范围宽、快速和稳定等优势[49-50]。
金纳米粒子(gold nanoparticles,AuNPs)具有独特的光学特性和较高的摩尔消光系数,易与生物材料偶联,是构建探针的良好材料[51]。YAN等[52]开发了一种无标记ssDNA-AuNPs偶联探针的比色传感器阵列,用以同时检测多种AGs。在高盐浓度溶液中ssDNA可以保护AuNPs不聚集,且不同AGs触发AuNPs聚集的方式不同,溶液会呈现出不同的颜色。可在120~280 nmol/L范围内,对TOB、STR、GEN和KAN进行区分和定量。ALHARBI等[53]和SARATALE等[54]合成了一种基于AgNPs-金霉素涂层的比色探针。由于相邻纳米粒子之间的表面等离子共振作用,AGs可以迅速诱导AgNPs聚集,同时颜色从黄色变为橙红色。在该方法中,带负电的AgNPs和带正电的AGs相互作用,导致AgNPs探针在水溶液中因表面等离子共振而产生聚集,产生从黄色到红色的显著颜色变化,STR和KAN的检测限分别为2 nmol/L和120 pmol/L。
但比色传感器在实际样品检测时易受蛋白和油脂等样品基质的干扰,在前处理过程中,减少基质干扰,开发适用于比色法的样品前处理技术是十分必要的。其次,为了避免由其他因素引起的聚集,用于修饰的纳米粒子应只与目标分析物产生特异性结合,例如在修饰时可以考虑与适配体或MIPs相结合。
2.4 表面增强拉曼光谱传感器
SERS是利用贵金属纳米粒子局域表面等离子体共振来放大分子的拉曼散射信号,与传统的分析方法相比,SERS具有出色的灵敏度和目标识别能力[55]。在SERS测量期间,分析物的信号不会被水分子干扰,在检测水中靶标时具有独特优势,并且SERS可以深入复杂食品基质内部[56],因此基于SERS的传感器在AGs残留检测中具有独特优势。SHI等[57]以侧向流动免疫分析(lateral flow immunoassay,LFI)试纸条为基础,开发了一种由NEO-单克隆抗体和拉曼信号分子4-氨基苯硫酚双标记的免疫探针,其原理如图2所示。首先将AuNPs与NEO-单克隆抗体、4-氨基苯硫酚和BSA偶联合成免疫探针,并将免疫探针固定在硝化纤维素膜上,封闭干燥后制得结合垫,最后将其与样品垫和吸收垫进行组装得到SERS-LFI试纸条。游离NEO和包被抗原NEO-卵白蛋白与免疫探针相互竞争,NEO存在时,免疫探针上的结合位点被其占据,并且免疫探针不会被包被抗原捕获,T线没有变红;当溶液中没有NEO时,免疫探针被包被抗原捕获后T线显色,该方法的半抑制浓度和检出限分别为0.04 ng/mL和0.216 pg/mL。
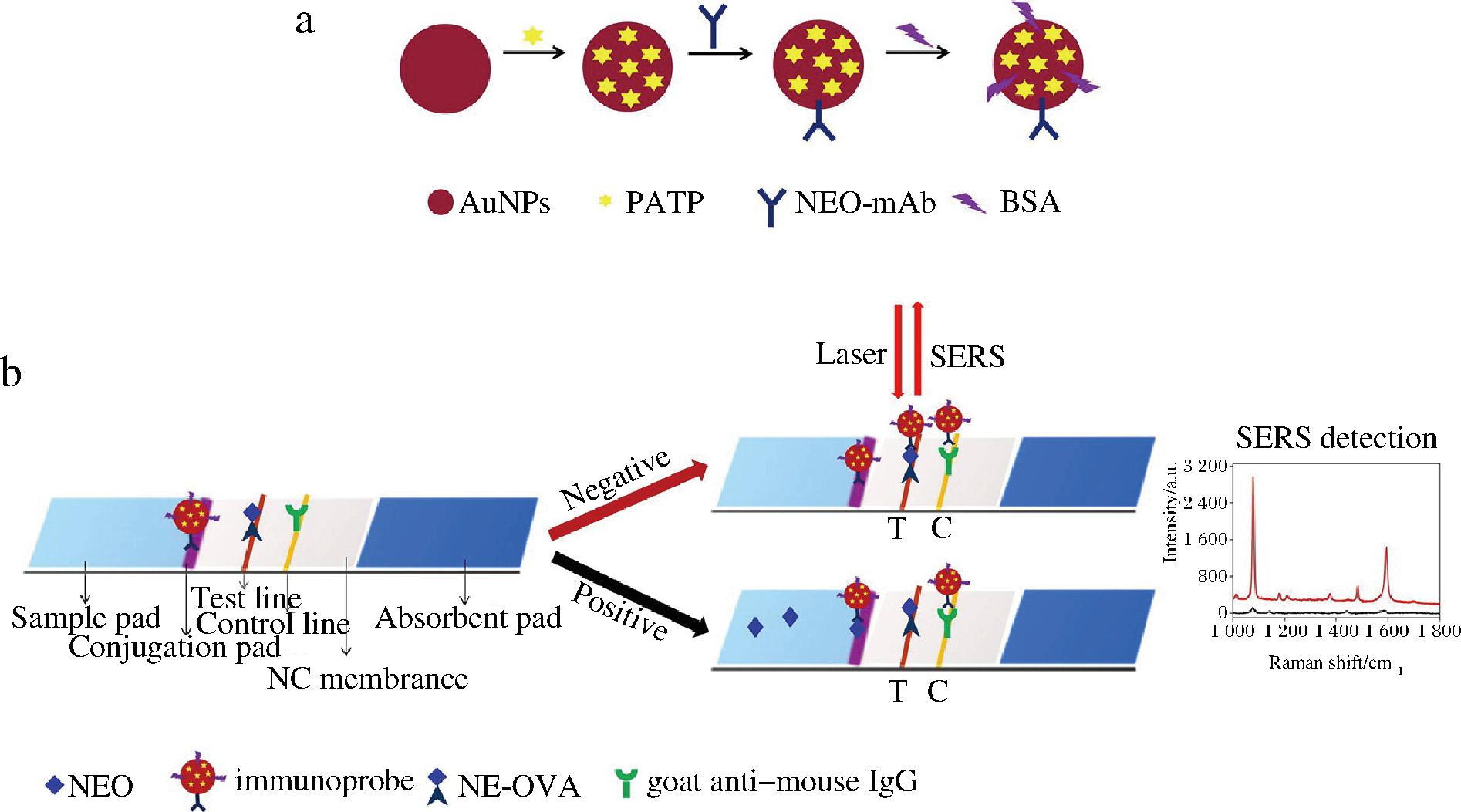
a-NEO免疫探针制备过程;b-LFI试纸条的组装及SERS-LFI检测
图2 SERS-LFI试纸条制备示意图[57]
Fig.2 Scheme for the fabrication of the SERS-LFI strip[57]
碳量子点修饰的SERS传感器不仅可以增强信号,还能对荧光背景进行淬灭[58]。WANG等[59]将掺杂Zn的碳量子与适配体结合,构建了用于检测KAN的双增强信号SERS传感器,检测限可达0.303 pg/mL[59]。此外,云母和二氧化硅等材料也具有类似作用,若将这些材料用于修饰AGs传感器,可以提高其检测性能,拓展其应用范围[60-61]。
优良的SERS基底是获得稳定信号的基础,理想的基底应具备良好的SERS活性、稳定性和重复性,在检测复杂样品基质时,如何合成形态均匀和物理性能稳定的SERS基底是限制其应用的主要障碍。通过对SERS基底进行官能化[62],制备自组装膜[63],将半导体与纳米材料结合使用有望改善此问题[64]。
3 电化学传感器
电化学传感是一种将化学信号转化为电信号进行传感检测的技术,它以电极作为转换元件或固定载体,通过将电极界面的化学变化转化为电位、电导率和电流等电信号参数,实现对目标物的定性或定量分析。在电极表面修饰适配体和MIPs等材料是提高电化学传感器灵敏度和选择性的有效途径之一,表2中列出了一些用于检测AGs的电化学传感器实例。
表2 用于检测AGs的电化学传感器
Table 2 Electrochemical sensors for AGs detection
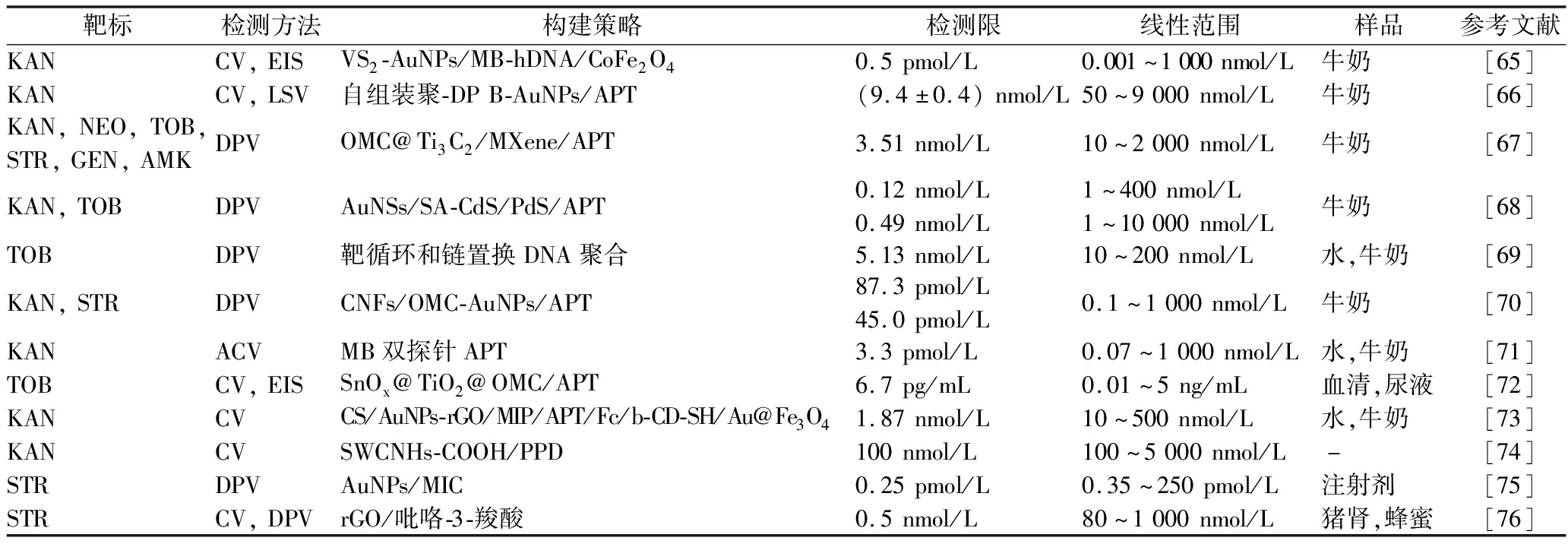
靶标检测方法构建策略检测限线性范围样品参考文献KANCV,EISVS2-AuNPs/MB-hDNA/CoFe2O40.5pmol/L0.001~1000nmol/L牛奶[65]KANCV,LSV自组装聚-DPB-AuNPs/APT(9.4±0.4)nmol/L50~9000nmol/L牛奶[66]KAN,NEO,TOB,STR,GEN,AMKDPVOMC@Ti3C2/MXene/APT3.51nmol/L10~2000nmol/L牛奶[67]KAN,TOBDPVAuNSs/SA-CdS/PdS/APT0.12nmol/L0.49nmol/L1~400nmol/L1~10000nmol/L牛奶[68]TOBDPV靶循环和链置换DNA聚合5.13nmol/L10~200nmol/L水,牛奶[69]KAN,STRDPVCNFs/OMC-AuNPs/APT87.3pmol/L45.0pmol/L0.1~1000nmol/L牛奶[70]KANACVMB双探针APT3.3pmol/L0.07~1000nmol/L水,牛奶[71]TOBCV,EISSnOx@TiO2@OMC/APT6.7pg/mL0.01~5ng/mL血清,尿液[72]KANCVCS/AuNPs-rGO/MIP/APT/Fc/b-CD-SH/Au@Fe3O41.87nmol/L10~500nmol/L水,牛奶[73]KANCVSWCNHs-COOH/PPD100nmol/L100~5000nmol/L-[74]STRDPVAuNPs/MIC0.25pmol/L0.35~250pmol/L注射剂[75]STRCV,DPVrGO/吡咯-3-羧酸0.5nmol/L80~1000nmol/L猪肾,蜂蜜[76]
注:CV:循环伏安法(cyclic voltammetry),EIS:交流阻抗法(electrochemical impedance spectroscopy),DPV:差分伏安脉冲法(differential pulse voltammetry),LSV:线性扫描伏安法(linear sweep voltammetry),ACV:交流伏安法(alternate current voltammetry),MB:亚甲基蓝(methylene blue),DPB:邻苯二甲酸二丁酯(dibutyl phthalate),MIC:分子印迹复合物(molecularly imprinted composite),AuNSs:金纳米壳(gold nanoshells),SA:链霉亲和素(streptavidin),CNFs:碳纤维(carbonfibers),-:未注明。
3.1 电化学发光传感器
电化学发光传感器结合了电化学和光学的特点,具有灵敏度高、背景信号低和操作简单等优势,广泛应用于食品检测、临床诊断和环境监测等领域[77]。HUANG等[78]合成了一种还原型AuNPs,通过Au价态的改变淬灭荧光,实现了对KAN的精准定量,检测限为1.5 pmol/L。
黑磷量子点(black phosphorus quantum dots,BPQDs)由于其独特的量子尺寸效应和边缘效应,具有良好的生物相容性、低毒性和丰富的活性位点等优势,可以替代有毒的重金属量子点,由BPQDs修饰的复合荧光探针具有高迁移率和适宜的能带水平,是构建光学传感器的理想材料[79]。WEN等[80]将银纳米粒子(silver nanoparticles,AgNPs)修饰的高发光聚多巴胺纳米微球(high-luminescence polydopamine nanospheres,HLPNs)与BPQDs相结合作为发光材料修饰电极检测KAN,原理如图3所示。AgNPs可以有效地增加电子传输速率并且与BPQDs结合产生协同增强效应,该传感器表现出优异的电化学发光性能,线性范围为10-12~10-7 mol/L,检测限为0.17 pmol/L。目前大部分电化学发光体系的发光信号及效率普遍较低,还需进一步研究电化学发光机理,提高电化学发光信号,开发新型高效的发光体系,从而提升电化学发光传感器的检测能力。
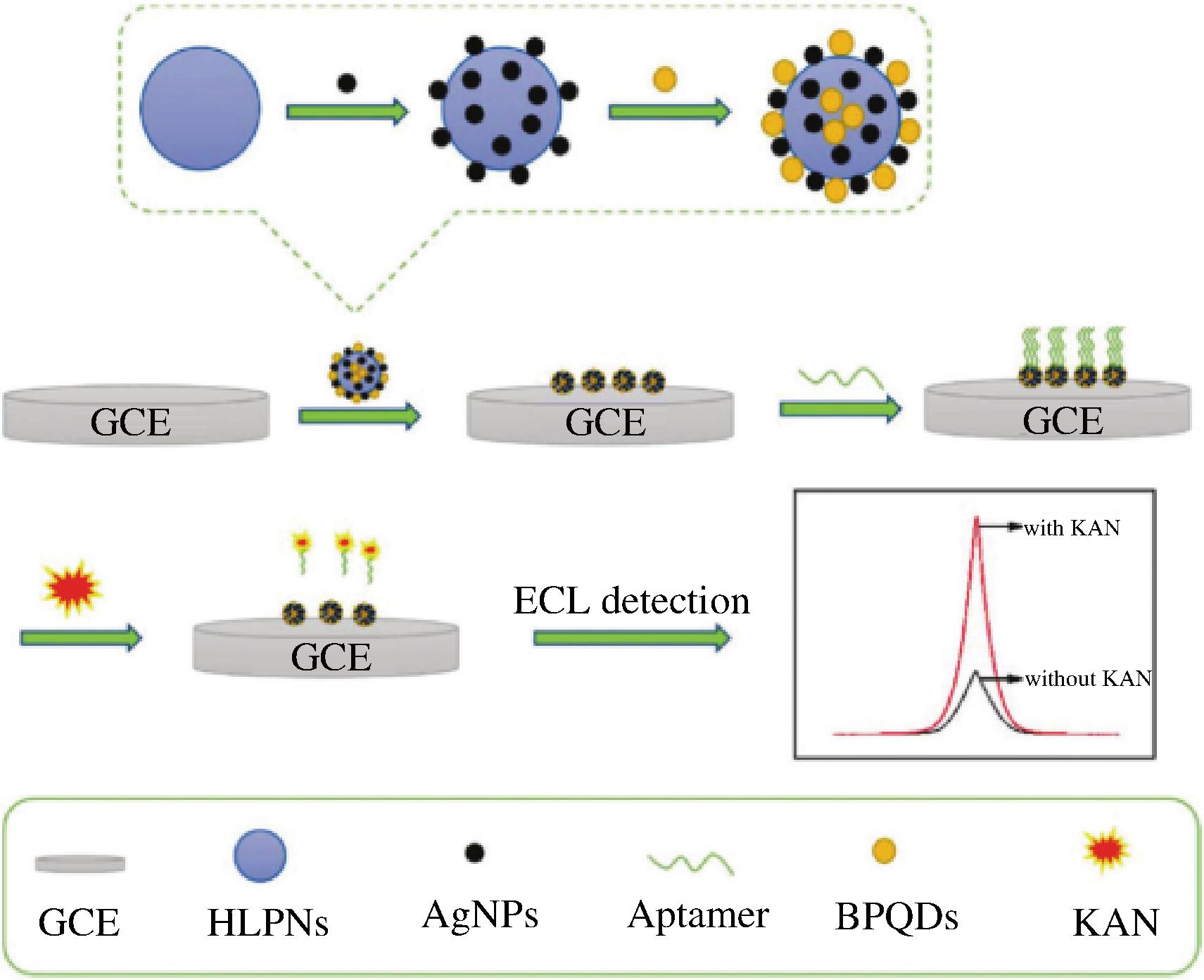
图3 HLPNs@Ag/BPQDs传感器构建示意图[80]
Fig.3 Scheme for the fabrication of HLPNs@Ag/BPQDs sensor[80]
3.2 适配体电化学传感器
适配体电化学传感器与其他传感器相比,具有操作简单、灵敏度高、成本低、便携等优点,是目前最常用的传感器之一[81-82]。YUE等[67]使用有序介孔碳(ordered mesoporous carbon,OMC)、Ti3C2和MXene复合材料修饰电极,以广谱适配体为识别元件构建了可同时测定多种AGs的适配体电化学传感器,构建方式如图4所示。OMC的加入有效提高了其稳定性,还避免了Ti3C2在电极表面堆积,提高了传感性能。合成的OMC@Ti3C2 MXene不仅可以促进电极表面的电子转移从而放大信号,同时还能作为纳米载体容纳大量的适配体来识别和捕获目标。该传感器的线性范围为10~2 000 nmol/L,检测限可达3.51 nmol/L。
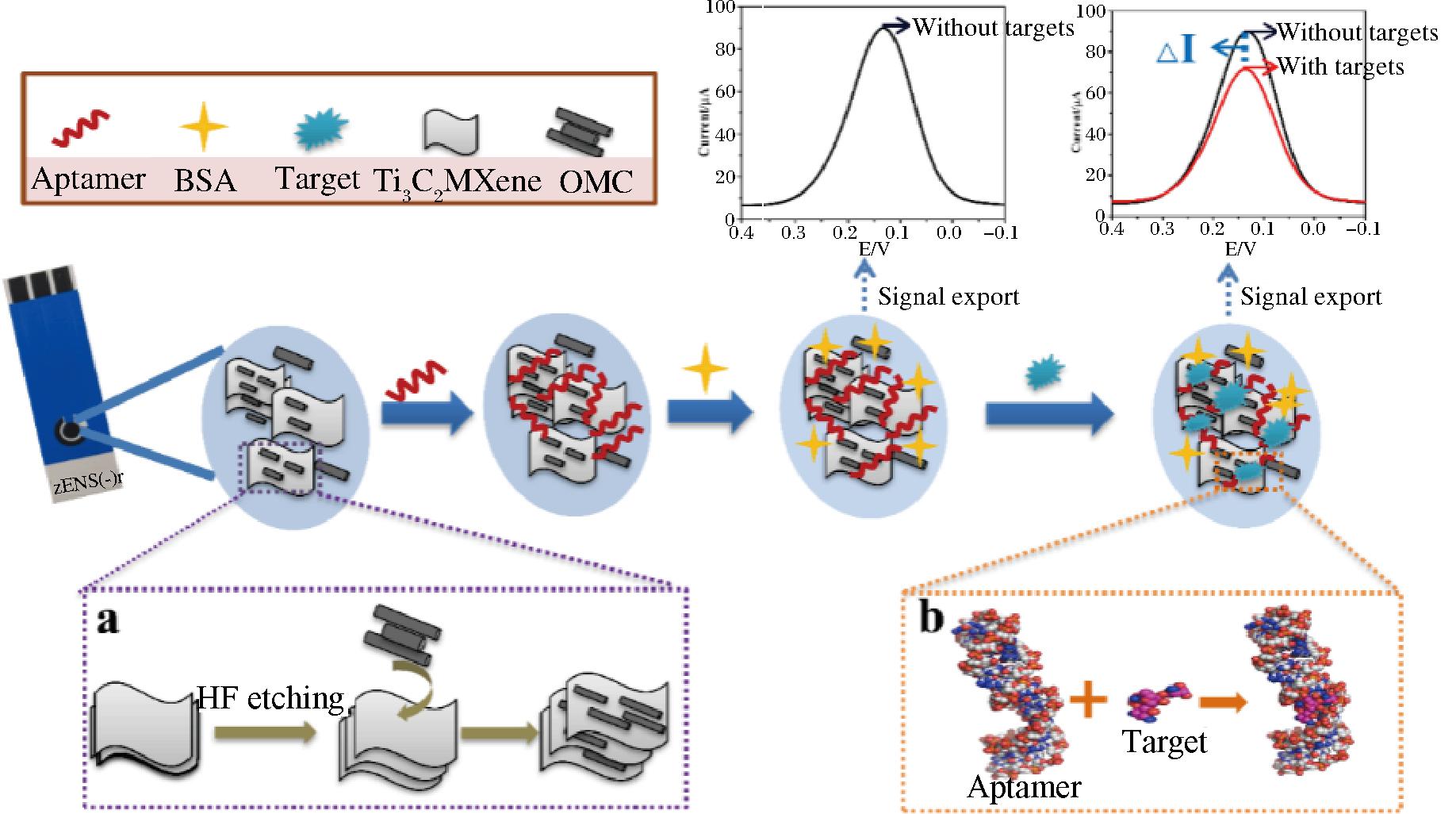
a-OMC@Ti3C2 MXene制备过程;b-适配体与靶标结合模式
图4 适配体电化学传感器构建示意图[67]
Fig.4 Scheme for the fabrication of the aptamer electrochemical sensor[67]
平衡解离常数(equilibrium dissociation constant,KD)常用来表征适配体与靶标的结合能力,KD值越低,表明适配体与靶标的结合越牢固,即适配体的亲和力越高,反之亦然。在已开发的适配体电化学传感器中,大多数都选择使用DNA适配体,只有少数使用RNA适配体。相比于DNA适配体,RNA适配体具有更低的KD值,其对靶标的亲和力更高,可以降低环境和食品样品中复杂基质对其的影响,对构建特异性更强的适配体电化学传感器具有重要意义。此外,用于同时检测多种AGs的适配体传感器通常需要Cd、Pb以及其他金属纳米粒子标记,筛选能够同时识别多种氨基酸的广谱适配体,有利于简化传感器的制备工艺,实现多AGs靶标的同时检测。
3.3 仿生印迹电化学传感器
仿生印迹电化学传感器以MIPs为识别元件,当目标分析物与固定在电极表面的受体相互作用时,即会产生化学或物理信号以供分析检测。纳米材料在仿生印迹电化学传感器的构建中应用极为广泛,其不仅可以通过放大电极表面、增加电子转移、催化电化学反应来提高传感器灵敏度,还可以间接引入官能团修饰载体表面,从而加强载体与纳米颗粒之间的相互作用,增强印迹界面的特异性,提高传感器的稳定性[83-84]。BI等使用壳聚糖将AuNPs-rGO固定在玻碳电极上,并在其表面合成MIPs。之后通过将适配体与二茂铁(Ferrocene,Fc)、巯基-β-环糊精(β-cyclodextrin-sulfhydryl,β-CD-SH)和铁、金纳米粒子等材料相结合,合成了APT/Fc/β-CD-SH/Au@Fe3O4杂化纳米材料来放大电信号,制备流程如图5所示。KAN浓度与电化学信号强度在10~500 nmol/L内呈良好的线性关系,其检测限为1.87 nmol/L[73]。ZHANG等[85]利用AGs与Cu2+之间的配位作用制备了磁性纳米复合物电化学传感器,采用差分伏安脉冲法记录Cu2+溶出峰电流信号,间接测定AGs浓度,KAN、TOB和GEN的检测限分别为4.88、1.28和1.07 nmol/L。基于纳米材料构建的仿生印迹电化学传感器检测限低、稳定性高并且可以重复使用,为AGs现场检测提供了简便、高效的途径。
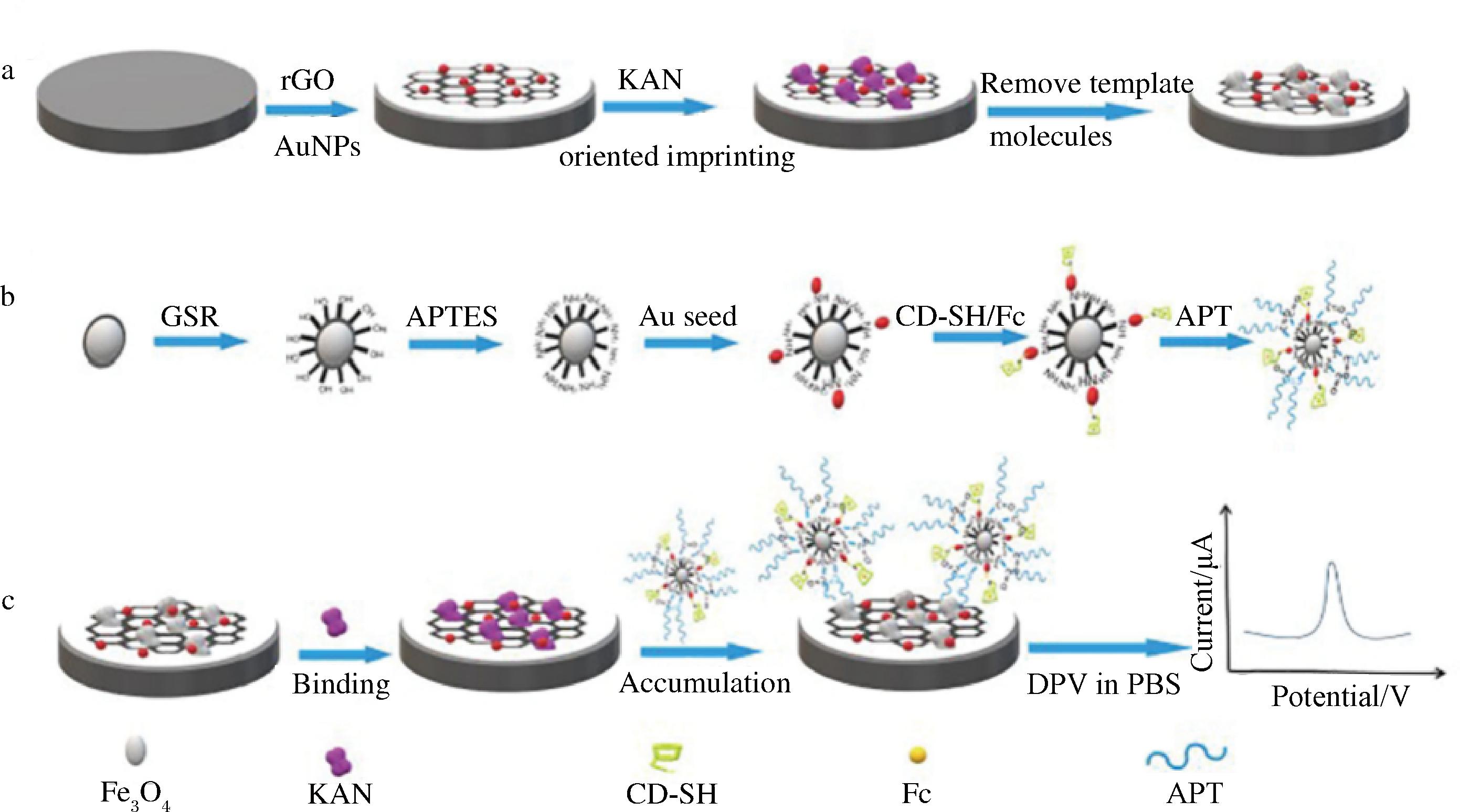
a-KAN分子印迹电化学传感器的研制;b-APT/Fc/b-CD-SH/Au@Fe3O4杂化纳米材料制备过程;c-KAN电化学方法传感检测
图5 双功能适配体生物传感器构建示意图[73]
Fig.5 Scheme for the fabrication of the dual functional aptamer biosensor[73]
虽然纳米材料的修饰可以极大提高仿生印迹电化学传感器的灵敏度,但选择性差、结合力弱的问题仍限制了其应用,采用先进的MIPs制备技术有望改善这些缺陷。SHAO等[86]将MIPs与AuNPs和MXene结合,通过对Fe3O4纳米颗粒进行多次修饰,最后使用可逆加成-断裂链转移聚合制备了高选择性的磁性MIPs,该传感器检测限可达0.014 4 nmol/L。此外,在制备过程中使用多功能单体可以提高传感器的印迹能力,LI等[87]以苯胺和3-氨基苯硼酸为功能单体,在壳聚糖与GO修饰的电极表面制备了仿生MIPs,该传感器检测限可达0.254 pmol/L;XIA等[88]以苯乙烯和4-乙烯基苯甲酸为功能单体,采用半共价印迹进行偶联合成了双功能单体的MIPs,该方法同时增强了印迹界面的选择性与结合力,拓展了仿生印迹传感器的构建思路。
4 总结与讨论
本文主要介绍了近年来各种光学和电化学传感器在AGs检测方面的研究进展及在实际样品检测中的应用。对于定性和半定量检测,比色传感器由于其操作简单和快速响应的特点成为适合的方法;荧光、化学发光和电化学传感器因具有优秀特异性和极低的检测限在定量分析方面是更优的选择;而SERS传感器的应用则更加灵活,可根据检测需求制备侧重不同的增强基底,在进行定量检测中,应充分考虑基底的均匀性与重现性,在痕量检测中,应尽可能提高基底的增强因子,以增强其灵敏度。
尽管这些光电化学传感器相对于传统的检测方法具有一定的优势,但在实际样品的检测中仍存在一些挑战。首先,传感器的特异性与灵敏度取决于识别元件的性能,深入研究传感机理并开发新型的修饰材料是构建高性能传感器的迫切需要。其次,AGs通常是在水相中进行检测,各类干扰组分(脂质、蛋白质、维生素和碳水化合物)、离子强度、pH和黏度都会影响传感器的灵敏度和选择性,如何制备出在水相中性能稳定的传感器是亟待解决的问题。最后,样品检测前的处理过程大多复杂冗长,如何开发出样品损失低且微型化的样品前处理技术还需要进一步研究。相信随着对传感机理与构建方法的深入研究,光电化学传感器在AGs检测领域的应用将进一步拓展。
[1] FOSSO M Y,LI Y J,GARNEAU-TSODIKOVA S.New trends in the use of aminoglycosides[J].MedChemComm,2014,5(8):1075-1091.
[2] HUTCHINGS M I,TRUMAN A W,WILKINSON B.Antibiotics:Past,present and future[J].Current Opinion in Microbiology,2019,51:72-80.
[3] KRAUSE K M,SERIO A W,KANE T R,et al.Aminoglycosides:an overview[J].Cold Spring Harbor perspectives in medicine,2016,6(6):a027029.
[4] GLINKA M,WOJNOWSKI W,WASIK A.Determination of aminoglycoside antibiotics:Current status and future trends[J].TrAC Trends in Analytical Chemistry,2020,131:116034.
[5] ESVAC.Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2021[M].The European Medicines Agency CEE:Antibiotic Use in Meat 2021.
[6] JIANG M Y,KARASAWA T,STEYGER P S.Aminoglycoside-induced cochleotoxicity:A review[J].Frontiers in Cellular Neuroscience,2017,11:308.
[7] OLIVEIRA J F P,CIPULLO J P,BURDMANN E A.Aminoglycoside nephrotoxicity[J].Brazilian Journal of Cardiovascular Surgery,2006,21:444-452.
[8] FU X L,WAN P F,LI P P,et al.Mechanism and prevention of ototoxicity induced by aminoglycosides[J].Frontiers in Cellular Neuroscience,2021,15:692762.
[9] ENDIMIANI A,RAMETTE A,RHOADS D D,et al.The evolving role of the clinical microbiology laboratory in identifying resistance in gram-negative bacteria:An update[J].Infectious Disease Clinics of North America,2020,34(4):659-676.
[10] TIAN Y F,CHEN G H,GUO L H,et al.Methodology studies on detection of aminoglycoside residues[J].Food Analytical Methods,2015,8(7):1842-1857.
[11] CONZUELO F,RUIZ-VALDEPE AS MONTIEL V,CAMPUZANO S,et al.Rapid screening of multiple antibiotic residues in milk using disposable amperometric magnetosensors[J].Analytica Chimica Acta,2014,820:32-38.
AS MONTIEL V,CAMPUZANO S,et al.Rapid screening of multiple antibiotic residues in milk using disposable amperometric magnetosensors[J].Analytica Chimica Acta,2014,820:32-38.
[12] MOROVJ N G,CSOK
N G,CSOK N P P,NÉMETH-KONDA L.HPLC determination of colistin and aminoglycoside antibiotics in feeds by post-column derivatization and fluorescence detection[J].Chromatographia,1998,48(1):32-36.
N P P,NÉMETH-KONDA L.HPLC determination of colistin and aminoglycoside antibiotics in feeds by post-column derivatization and fluorescence detection[J].Chromatographia,1998,48(1):32-36.
[13] STEAD D A.Current methodologies for the analysis of aminoglycosides[J].Journal of Chromatography B:Biomedical Sciences and Applications,2000,747(1-2):69-93.
[14] LIU Q Y,LI J F,SONG X Q,et al.Simultaneous determination of aminoglycoside antibiotics in feeds using high performance liquid chromatography with evaporative light scattering detection[J].RSC Advances,2017,7(3):1251-1259.
[15] BLANCHAERT B,HUANG S Y,WACH K,et al.Assay development for aminoglycosides by HPLC with direct UV detection[J].Journal of Chromatographic Science,2017,55(3):197-204.
[16] CURIEL H,VANDERAERDEN W,VELEZ H,et al.Analysis of underivatized gentamicin by capillary electrophoresis with UV detection[J].Journal of Pharmaceutical and Biomedical Analysis,2007,44(1):49-56.
[17] LIN Y F,WANG Y C,CHANG S Y.Capillary electrophoresis of aminoglycosides with Argon-ion laser-induced fluorescence detection[J].Journal of Chromatography A,2008,1188(2):331-333.
[18] MORENO-GONZ LEZ D,LARA F J,JURGOVSK
LEZ D,LARA F J,JURGOVSK N,et al.Determination of aminoglycosides in honey by capillary electrophoresis tandem mass spectrometry and extraction with molecularly imprinted polymers[J].Analytica Chimica Acta,2015,891:321-328.
N,et al.Determination of aminoglycosides in honey by capillary electrophoresis tandem mass spectrometry and extraction with molecularly imprinted polymers[J].Analytica Chimica Acta,2015,891:321-328.
[19] HENDRIX M,PRIESTLEY E S,JOYCE G F,et al.Direct observation of aminoglycoside-RNA interactions by surface plasmon resonance[J].Journal of the American Chemical Society,1997,119(16):3641-3648.
[20] SANTOS H S,DE FRANÇA G M,ROMANI E C,et al.Selective determination of tobramycin in the presence of streptomycin through the visible light effect on surface plasmon resonance of gold nanoparticles[J].Microchemical Journal,2014,116:206-215.
[21] MCKEATING K S,COUTURE M,DINEL M P,et al.High throughput LSPR and SERS analysis of aminoglycoside antibiotics[J].The Analyst,2016,141(17):5120-5126.
[22] CAPIT N-VALLVEY L F,PALMA A J.Recent developments in handheld and portable optosensing—A review[J].Analytica Chimica Acta,2011,696(1-2):27-46.
N-VALLVEY L F,PALMA A J.Recent developments in handheld and portable optosensing—A review[J].Analytica Chimica Acta,2011,696(1-2):27-46.
[23] TOMBELLI S,MINUNNI M,MASCINI M.Aptamers-based assays for diagnostics,environmental and food analysis[J].Biomolecular Engineering,2007,24(2):191-200.
[24] YUE F L,LI F L,KONG Q Q,et al.Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications[J].Science of the Total Environment,2021,762:143129.
[25] LUAN Y X,WANG N,LI C,et al.Advances in the application of aptamer biosensors to the detection of aminoglycoside antibiotics[J].Antibiotics,2020,9(11):787.
[26] TARANNUM N,HENDRICKSON O D,KHATOON S,et al.Molecularly imprinted polymers as receptors for assays of antibiotics[J].Critical Reviews in Analytical Chemistry,2020,50(4):291-310.
[27] CHEN C C,LUO J X,LI C L,et al.Molecularly imprinted polymer as an antibody substitution in pseudo-immunoassays for chemical contaminants in food and environmental samples[J].Journal of Agricultural and Food Chemistry,2018,66(11):2561-2571.
[28] LI F Q,YU Z G,HAN X D,et al.Electrochemical aptamer-based sensors for food and water analysis:A review[J].Analytica Chimica Acta,2019,1051:1-23.
[29] MART NEZ-M
NEZ-M
 EZ R,SANCEN
EZ R,SANCEN N F.Fluorogenic and chromogenic chemosensors and reagents for anions[J].Chemical Reviews,2003,103(11):4419-4476.
N F.Fluorogenic and chromogenic chemosensors and reagents for anions[J].Chemical Reviews,2003,103(11):4419-4476.
[30] GENG Y M,ZHANG S Z,WANG Y X,et al.Aptamer act as fluorescence switching of bovine serum albumin stabilized gold nanoclusters for ultrasensitive detection of kanamycin in milk[J].Microchemical Journal,2021,165:106145.
[31] TANG Y F,GU C M,WANG C,et al.Evanescent wave aptasensor for continuous and online aminoglycoside antibiotics detection based on target binding facilitated fluorescence quenching[J].Biosensors and Bioelectronics,2018,102:646-651.
[32] CHEN C,TIAN R,ZENG Y,et al.Activatable fluorescence probes for “turn-on” and ratiometric biosensing and bioimaging:From NIR-Ⅰ to NIR-Ⅱ[J].Bioconjugate Chemistry,2020,31(2):276-292.
[33] ZAKHARENKOVA S A,DOBROVOLSKII A A,GARSHEV A V,et al.Chlorophyll-based self-assembled nanostructures for fluorescent sensing of aminoglycoside antibiotics[J].ACS Sustainable Chemistry &Engineering,2021,9(9):3408-3415.
[34] BUKOWSKI T J,SIMMONS J H.Quantum dot research:Current state and future prospects[J].Critical Reviews in Solid State and Materials Sciences,2002,27(3-4):119-142.
[35] GARC A DE ARQUER F P,TALAPIN D V,KLIMOV V I,et al.Semiconductor quantum dots:Technological progress and future challenges[J].Science,2021,373(6555):eaaz8541.
A DE ARQUER F P,TALAPIN D V,KLIMOV V I,et al.Semiconductor quantum dots:Technological progress and future challenges[J].Science,2021,373(6555):eaaz8541.
[36] PINTO I A,TOLOZA C A T,ALMEIDA J M S,et al.Quantification of neomycin in Rubella vaccine by off/on metal ion mediated photoluminescence from functionalized graphene quantum dots[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2019,220:117139.
[37] GENG Y Y,GUO M L,TAN J A,et al.A fluorescent molecularly imprinted polymer using aptamer as a functional monomer for sensing of kanamycin[J].Sensors and Actuators B:Chemical,2018,268:47-54.
[38] WANG Q,LIAO M,LIN Q M,et al.A review on fluorescence intensity ratio thermometer based on rare-earth and transition metal ions doped inorganic luminescent materials[J].Journal of Alloys and Compounds,2021,850:156744.
[39] HO S Y,TERESA GUTIERREZ WING M,WOO C J.Review—Recent progress in portable fluorescence sensors[J].Journal of the Electrochemical Society,2021,168(1):17502.
[40] LIU M L,LIN Z,LIN J M.A review on applications of chemiluminescence detection in food analysis[J].Analytica Chimica Acta,2010,670(1-2):1-10.
[41] DODEIGNE C,THUNUS L,LEJEUNE R.Chemiluminescence as diagnostic tool.A review[J].Talanta,2000,51(3):415-439.
[42] ZHANG Z Y,ZHANG S C,ZHANG X R.Recent developments and applications of chemiluminescence sensors[J].Analytica Chimica Acta,2005,541(1-2):37-46.
[43] ZENG K,ZHANG Y Y,MENG H,et al.Chemiluminescence microarray immunoassay for multiple aminoglycoside antibiotics based on carbon nanotube-assisted signal amplification[J].Analytical and Bioanalytical Chemistry,2022,414(5):1819-1828.
[44] ZHAO H,LIN Q F,HUANG L,et al.Ultrasensitive chemiluminescence immunoassay with enhanced precision for the detection of cTnI amplified by acridinium ester-loaded microspheres and internally calibrated by magnetic fluorescent nanoparticles[J].Nanoscale,2021,13(5):3275-3284.
[45] ZHAN Z X,DAI Y C,LI Q Y,et al.Small molecule-based bioluminescence and chemiluminescence probes for sensing and imaging of reactive species[J].TrAC Trends in Analytical Chemistry,2021,134:116129.
[46] NAKAZONO M,OSHIKAWA Y,NAKAMURA M,et al.Strongly chemiluminescent acridinium esters under neutral conditions:Synthesis,properties,determination,and theoretical study[J].Journal of Organic Chemistry,2017,82(5):2450-2461.
[47] CABELLO M C,BARTOLONI F H,BAADER W J.An update on general chemiexcitation mechanisms in cyclic organic peroxide decomposition and the chemiluminescent peroxyoxalate reaction in aqueous media[J].Photochemistry and Photobiology,2023,99(2):235-250.
[48] YOO S M,LEE S Y.Optical biosensors for the detection of pathogenic microorganisms[J].Trends in Biotechnology,2016,34(1):7-25.
[49] PIRIYA V S A,JOSEPH P,DANIEL S C G K,et al.Colorimetric sensors for rapid detection of various analytes[J].Materials Science and Engineering:C,2017,78:1231-1245.
[50] LIU B,ZHUANG J Y,WEI G.Recent advances in the design of colorimetric sensors for environmental monitoring[J].Environmental Science:Nano,2020,7(8):2195-2213.
[51] LIU G Y,LU M,HUANG X D,et al.Application of gold-nanoparticle colorimetric sensing to rapid food safety screening[J].Sensors,2018,18(12):4166.
[52] YAN S,LAI X X,DU G R,et al.Identification of aminoglycoside antibiotics in milk matrix with a colorimetric sensor array and pattern recognition methods[J].Analytica Chimica Acta,2018,1034:153-160.
[53] ALHARBI R,IRANNEJAD M,YAVUZ M.A short review on the role of the metal-graphene hybrid nanostructure in promoting the localized surface plasmon resonance sensor performance[J].Sensors,2019,19(4):862.
[54] SARATALE G D,SARATALE R G,GHODAKE G,et al.Chlortetracycline-functionalized silver nanoparticles as a colorimetric probe for aminoglycosides:Ultrasensitive determination of kanamycin and streptomycin[J].Nanomaterials,2020,10(5):997.
[55] LIANG J F,PENG C,LI P Y,et al.A review of detection of antibiotic residues in food by surface-enhanced Raman spectroscopy[J].Bioinorganic Chemistry and Applications,2021,2021:8180154.
[56] GIRMATSION M,MAHMUD A,ABRAHA B,et al.Rapid detection of antibiotic residues in animal products using surface-enhanced Raman spectroscopy:A review[J].Food Control,2021,126:108019.
[57] SHI Q Q,HUANG J,SUN Y N,et al.Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2018,197:107-113.
[58] YANG D Z,LI H,LI Q L,et al.Highly selective histamine assay via SERS:Based on the signal enhancement of carbon dots and the fluorescence quenching of gold nanoparticles[J].Sensors and Actuators B:Chemical,2022,350:130866.
[59] WANG X M,CHEN C,WATERHOUSE G I N,et al.A novel SERS sensor for the ultrasensitive detection of kanamycin based on a Zn-doped carbon quantum dot catalytic switch controlled by nucleic acid aptamer and size-controlled gold nanorods[J].Food Chemistry,2021,362:130261.
[60] WALTERS C M,PAO C,GAGNON B P,et al.Bright surface-enhanced Raman scattering with fluorescence quenching from silica encapsulated J-aggregate coated gold nanoparticles[J].Advanced Materials,2018,30(5):1705381.
[61] WANG L,YU D Q,HUANG B Q,et al.Large-area ReS2 monolayer films on flexible substrate for SERS based molecular sensing with strong fluorescence quenching[J].Applied Surface Science,2021,542:148757.
[62] LI C C,HUANG Y M,LI X Y,et al.Towards practical and sustainable SERS:A review of recent developments in the construction of multifunctional enhancing substrates[J].Journal of Materials Chemistry C,2021,9(35):11517-11552.
[63] STILES P L,DIERINGER J A,SHAH N C,et al.Surface-enhanced Raman spectroscopy[J].Annual Review of Analytical Chemistry,2008,1:601-626.
[64] AWIAZ G,LIN J,WU A G.Recent advances of Au@Ag core-shell SERS-based biosensors[J].Exploration,2023,3(1):20220072.
[65] TIAN L,ZHANG Y,WANG L B,et al.Ratiometric dual signal-enhancing-based electrochemical biosensor for ultrasensitive kanamycin detection[J].ACS Applied Materials &Interfaces,2020,12(47):52713-52720.
[66] ZHU Y,CHANDRA P,SONG K M,et al.Label-free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite[J].Biosensors and Bioelectronics,2012,36(1):29-34.
[67] YUE F L,LIU M Y,BAI M Y,et al.Novel electrochemical aptasensor based on ordered mesoporous carbon/2D Ti3C2 MXene as nanocarrier for simultaneous detection of aminoglycoside antibiotics in milk[J].Biosensors,2022,12(8):626.
[68] LI F L,WU Y F,CHEN D F,et al.Sensitive dual-labeled electrochemical aptasensor for simultaneous detection of multi-antibiotics in milk[J].International Journal of Hydrogen Energy,2021,46(45):23301-23309.
[69] NIE J J,YUAN L Y,JIN K,et al.Electrochemical detection of tobramycin based on enzymes-assisted dual signal amplification by using a novel truncated aptamer with high affinity[J].Biosensors and Bioelectronics,2018,122:254-262.
[70] LI F L,WANG X Y,SUN X,et al.Multiplex electrochemical aptasensor for detecting multiple antibiotics residues based on carbon fiber and mesoporous carbon-gold nanoparticles[J].Sensors and Actuators B:Chemical,2018,265:217-226.
[71] LI F Q,YU Z G,HAN X D,et al.A signal-on electrochemical aptasensor for highly sensitive and specific detection of kanamycin based on target-induced signaling probe shifting mechanism[J].Sensors and Actuators B:Chemical,2018,273:480-487.
[72] WANG M H,HU B,YANG C,et al.Electrochemical biosensing based on protein-directed carbon nanospheres embedded with SnOx and TiO2 nanocrystals for sensitive detection of tobramycin[J].Biosensors and Bioelectronics,2018,99:176-185.
[73] BI H,WU Y H,WANG Y H,et al.A molecularly imprinted polymer combined with dual functional Au@Fe3O4 nanocomposites for sensitive detection of kanamycin[J].Journal of Electroanalytical Chemistry,2020,870:114216.
[74] HAN S,LI B Q,SONG Z,et al.A kanamycin sensor based on an electrosynthesized molecularly imprinted poly-o-phenylenediamine film on a single-walled carbon nanohorn modified glassy carbon electrode[J].The Analyst,2016,142(1):218-223.
[75] ZAREI K,GHORBANI M.Fabrication of a new ultrasensitive AuNPs-MIC-based sensor for electrochemical determination of streptomycin[J].Electrochimica Acta,2019,299:330-338.
[76] WEN Y P,LIAO X N,DENG C X,et al.Imprinted voltammetric streptomycin sensor based on a glassy carbon electrode modified with electropolymerized poly(pyrrole-3-carboxy acid) and electrochemically reduced graphene oxide[J].Microchimica Acta,2017,184(3):935-941.
[77] LV W X,YE H C,YUAN Z Q,et al.Recent advances in electrochemiluminescence-based simultaneous detection of multiple targets[J].TrAC Trends in Analytical Chemistry,2020,123:115767.
[78] HUANG Z N,LI Z L,CHEN Y,et al.Regulating valence states of gold nanocluster as a new strategy for the ultrasensitive electrochemiluminescence detection of kanamycin[J].Analytical Chemistry,2021,93(10):4635-4640.
[79] GUI R J,JIN H,WANG Z H,et al.Black phosphorus quantum dots:Synthesis,properties,functionalized modification and applications[J].Chemical Society Reviews,2018,47(17):6795-6823.
[80] WEN J,JIANG D,SHAN X L,et al.A novel electrochemiluminescence aptasensor for sensitive detection of kanamycin based on the synergistic enhancement effects between black phosphorus quantum dots and silver-decorated high-luminescence polydopamine nanospheres[J].The Analyst,2021,146(11):3493-3499.
[81] NEGAHDARY M.Electrochemical aptasensors based on the gold nanostructures[J].Talanta,2020,216:120999.
[82] MEHLHORN A,RAHIMI P,JOSEPH Y.Aptamer-based biosensors for antibiotic detection:A review[J].Biosensors,2018,8(2):54.
[83] ANN MARIA C G,VARGHESE A,NIDHIN M.Recent advances in nanomaterials based molecularly imprinted electrochemical sensors[J].Critical Reviews in Analytical Chemistry,2023,53(1):88-97.
[84] ZHONG C J,YANG B,JIANG X X,et al.Current progress of nanomaterials in molecularly imprinted electrochemical sensing[J].Critical Reviews in Analytical Chemistry,2018,48(1):15-32.
[85] ZHANG M S,ZHANG B J,LI T B,et al.Electrochemical detection of aminoglycoside antibiotics residuals in milk based on magnetic molecularly imprinted particles and metal ions[J].Food Chemistry,2022,389:133120.
[86] SHAO Y M,ZHU Y,ZHENG R,et al.Highly sensitive and selective surface molecularly imprinted polymer electrochemical sensor prepared by Au and MXene modified glassy carbon electrode for efficient detection of tetrabromobisphenol A in water[J].Advanced Composites and Hybrid Materials,2022,5(4):3104-3116.
[87] LI Z Z,ZHANG R,NIU H W,et al.Biomimetic imprinted electrochemical sensor for selective detection of streptomycin residue in milk[J].International Journal of Electrochemical Science,2023,18(9):100266.
[88] XIA Y Y,ZHAO F Q,ZENG B Z.A molecularly imprinted copolymer based electrochemical sensor for the highly sensitive detection of L-Tryptophan[J].Talanta,2020,206:120245.