过去几十年,不可再生化石燃料的过度消费造成了环境污染、全球变暖和气候变化。因此,目前需要通过生物途径获取可再生替代能源(生物燃料),以降低化石燃料的消耗[1]。而微藻具有光合效率高、生长速度快、容易实现大规模培养、生长不受季节和气候的限制等优点,同时微藻资源丰富,油脂含量高,以微藻作为生物柴油生产的潜在新原料,具有很好的应用前景。此外,微藻还能产生蛋白质、活性多糖、氨基酸、色素、维生素和矿物质等高价值生物活性化合物,它们在许多工业,如食品、保健品、畜牧业、水产饲料和化妆品等领域都有广泛应用。根据最新分析,预计2022年,全球微藻市场将达到33.18亿美元,主要来源于制药和营养品行业需求的驱动[2]。
微藻种类繁多,其细胞组成和生长速度各异,而且微藻的生长速度与其代谢产物的积累却是不同步的。因此,为了从工程策略上促进各种高价值代谢产物的积累,就需要引入适当的环境压力,其中温度、pH、光照、各种营养物浓度以及盐度等环境因素[3]均被认为是影响微藻代谢产物积累的主要因素。光照会影响微藻的生长发育、光合作用、细胞形态和代谢产物的合成等,它主要从光质、光强度和光周期等几个方面影响藻类的生长。因为光强度和光周期更容易进行调节,光照对微藻生理代谢影响的研究主要集中在光强和光周期,而光质对微藻生长及代谢产物积累影响的研究较少。
尽管太阳光是微藻生产最具成本效益的能源,但当生物质被用于生产高价值产品时,如食品、添加剂或营养药品等(如类胡萝卜素和多不饱和脂肪酸),人工光源在经济上仍然是可行的。人工光还可以更好地调节微藻生产中的光子通量密度、光周期和光谱,这可以提高其生物量的产率和质量[4]。微藻研究和生产中的人工光源通常采用荧光灯,其具有较宽的发射光谱[4];也可以使用发光二极管(light emitting diode,LED)。LED是长时间(±50 000 h)、无汞和快速响应(纳秒级)的人工光源,通过固态电子技术在各种波长下发射单色光[5]。与其他人工光源相比, LED具有较长的使用寿命周期、较低的发热量和较高的转换效率。由于其窄光谱发射,将微藻暴露于LED可导致最大的光吸收和较高的生物质转换效率,以及微藻生物质组成的控制。许多研究发现,对于不同的微藻物种,存在支持高生产率和有利成分的最佳波长[6-7]。本文对现有文献进行整理,重点综述了LED不同光质对微藻生长及代谢产物积累的影响及LED光质影响微藻代谢产物积累的分子机制,并对LED光质在微藻生产中的发展趋势进行了展望,以期为微藻大规模生产高值化代谢产物提供理论依据。
1 光质对微藻生物量的影响
微藻的生物量是培养优化时优先考虑的重要因素,这是因为任何微藻产品,如脂质、碳水化合物和蛋白质等,都由生物量决定。微藻可吸收利用的主要是可见光谱,根据波长分为紫光(380~450 nm)、蓝光(450~485 nm)、青光(485~500 nm)、绿光(500~565 nm)、黄光(565~590 nm)、橙光(590~625 nm)和红光(625~740 nm)[8]。表1列出了LED不同光质下各种微藻的生物量。
据报道,LED红光可以加速细胞生长[9],这是因为红光可以提供叶绿素a或叶绿素b所需的能量,以促进电子通量并进一步刺激光收集天线复合体[10];此外,红光可以增强微藻光系统Ⅱ,而蓝光可以改善微藻光系统I [11]。根据DE DOOIJ等[12]的报道,蓝光在光合生物的发育中起着关键作用,它可以激活对大规模培养稳定性至关重要的代谢调节机制;用黄光和蓝光培养的莱茵衣藻显示出更好的生产力和光系统Ⅱ功能[12];在强光下生长时,蓝光也可以激活三角褐指藻的光保护[13]。在一些研究中,发现LED红蓝混合光(红光和蓝光按比例同时照射,以下用红蓝光表示,其他混合光类似)的性能优于单色光[14]。具体见表1。
微藻能利用叶绿素及藻胆色素、类胡萝卜素等其他光合辅助色素进行光能的吸收及捕获,不同的微藻有各自的光合色素及补光色素复合物,其吸收光谱存在较大差异,所以不同微藻可吸收利用的光谱范围是不相同的;而所有微藻都含有叶绿素a,红光和蓝光是有效支持光合作用的主要波长。
2 光质对微藻代谢产物积累的影响
2.1 脂质
脂质是微藻中除蛋白质和碳水化合物外的主要成分。然而,脂质的增加往往与蛋白质、碳水化合物或两者的减少有关。一些微藻会产生更多的脂质来应对环境压力,这是因为脂质有助于微藻细胞的结构维持和恢复。光照、温度、二氧化碳、营养饥饿、盐胁迫和金属胁迫等策略都可以用来提高微藻的脂质生产力。在众多因素中,波长可能是最重要的参数,因为光谱可以影响微藻的细胞组成。许多研究者使用LED照明作为微藻生产脂质的关键工具,这是因为LED的波长具有特定的光谱,可以刺激微藻细胞分子组成的改变,从而有选择地增强脂质生产所需的生化成分[53]。
表1 LED不同光质对微藻生物量的影响
Table 1 Effects of LED different light quality on microalgae biomass

光质藻种生物量文献B, R, G, WArthrospira maxima, Chlorella minutissima, Rhodomonas salina, Nannochloropsis oceanicaR>G>W>B[5]R, RBScenedesmus obliquusR>RB[6]W, R, B, RB, BWChlorella sp.W>B>BW>RB>R[7]W, R, B, G, YArthrospira platensisR>W>Y>G>B[15]W, R, B, G, YChlorella vulgarisW>R>B>Y>G[16]W, R, B, G, YDiacronema lutheriB>R>G>Y[16]W, R, B, G, YPorphyridium purpureumG>B>Y>R [16]W, R, BAuxenochlorella pyrenoidosa,Scenedesmus quadricaud,Tetradesmus obliquusB>R>W[17]W, R, YPhaeodactylum tricornutumR>W>Y[18]W, R, B, GArthrospira platensisR>W>G>B[19]R, G, BAcutodesmus obliquusR>G>B[20]B, R, W, RB, BWChlorella sp.B>BW>RB>R>W[21]W, R, B, GPorphyridium cruentum不显著[22]W, R, B, GRhodomonas sp.G>W>B>R[23]W, R, Y, OAcutodesmus obliquusW>Y>O>R[24]W, R, BDunaliella salinaR>B>W[25]W, R, B, RBDunaliella salinaRB>W>B>R[26]W, R, B, G, YTetradesmus sp.B>W>R>G>Y[27]R, RBHaematococcus pluvialisRB>R[28]G, R, YSynechococcus nidulansY>R>G[29]B, R, G, WGracilaria lemaneiformisG>W>R>B[30]B, R, G, WUlva lactucaB>W>G>R[30]R, RBDunaliella salinaRB>R[31]B, R, G, WArthrospira platensisR>W>Y>B[32]W, R, GGloeothece membranaceaW>G>R[33]B, R, W, RBTetraselmis sp.B>RB>W>R[34]B, R, W, RBNannochloropsis oceanicaB>RB>R>W[34]B, Y, WIsochrysis galbanaW>Y>B[35]B, R, WOdontella auritaR>B>W[36]B, R, WEustigmatos cf.polyphemR>W>B[37]B, WChlorella vulgarisB>W[38]B, R, W, GScenedesmus sp.W>R>B>G[39]G, R, YAcutodesmus obliquusR>G>Y[40]B, R, WDunaliella salinaR>W>B[41]B, RHaematococcus pluvialisR>B[42]B, R, W, GChlorella sorokinianaB>R>W>G[43]B, R, WSynechococcus sp.B>W>R[44]B, R, GNannoclzloropsis oceanicaR>B>G[45]B, R, W, GScenedesmus obliquusB>R>W>G[46]B, R, WChlorella sp.W>R>B[47]R, WNeochloris oleoabundansR>W[48]W, R, B, G, YChlorella vulgarisB>W>Y>G>R[49]B, R, W, RBChlorella pyrenoidsaB>W>RB>R[50]R, RBSpirulina platensisRB>R[51]B, R, W, RBChlamydomonas sp.B>RB>W>R[52]
注:W:白光;R:红光;B:蓝光;G:绿光;Y:黄光;O:橙光;RB:红蓝光;BW:蓝白光;RO:红橙光;WY:白黄光;下同。
LED蓝光常用来提高微藻中的脂质产量,这是因为LED蓝光影响了微藻的酶(二磷酸核酮糖羧化酶/加氧酶和碳酸酐酶)的活性,引发了甘油三酯的更高积累[38]。有报道称蓝光可以促进氮代谢,激活脂质积累酶。乙酰辅酶a和NADPH是脂质生物合成过程中必不可少的2个成分,已知是由ATP柠檬酸裂解酶(ATP citrate lyase,ACL)、苹果酸酶(malic enzyme,ME)和脂肪酸合成酶(fatty acid synthetase,FAS)协同作用产生的[54]。这3种物质(ACL、ME、FAS)在蓝光下比较活跃。然而,对于一些微藻物种来说,将整个培养过程暴露于单色蓝光下往往会导致较低的生物量,尽管干重中脂质含量较高。具体见表2。
表2 LED不同光质对微藻脂质积累的影响
Table 2 Effects of LED different light quality on lipid accumulation in microalgae

光质藻种脂质文献R, RBScenedesmus obliquusR>RB[6]W, R, B, RB, BWChlorella sp.R>RB>W>B>BW[7]W, R, B, G, YArthrospira platensisB>G>W>R>Y[15]W, R, YPhaeodactylum tricornutumR>W[18]R, G, BAcutodesmus obliquusG>B>R [20]B, R, W, RB, BWChlorella sp.W>B>RB>R>BW[21]W, R, B, GPorphyridium cruentumB>R>G>W[22]W, R, Y, OAcutodesmus obliquusW>Y>O>R[24]W, R, BDunaliella salinaR>W>B[25]W, R, B, RBDunaliella salinaB>RB>W>R[26]G, R, YSynechococcus nidulansG>R>Y[29]B, R, W, RBTetraselmis sp.B>W>R>RB[34]B, R, W, RBNannochloropsis oceanicaB>RB>R>W[34]B, Y, WIsochrysis galbanaY>B>W[35]B, R, WEustigmatos cf.polyphemR>W>B[37]B, WChlorella vulgarisB>W[38]G, R, YAcutodesmus obliquusR>G>Y[40]B, R, W, GChlorella sorokinianaR>B>G>W[43]B, R, W, GScenedesmus obliquusB>R>W>G[46]B, R, WChlorella sp.W>R>B[47]R, WNeochloris oleoabundansR>W[48]W, R, B, G, YChlorella vulgarisB>W>G>Y>R[49]B, R, W, RBChlorella pyrenoidsaRB>W>R>B[50]B, R, W, RBChlamydomonas sp.B>RB>W>R[52]B, R, RBScenedesmus obliquusB>R>RB[55]R, B, RGBPhaeodactylum tricornutumB>RGB>R[56]
注:RGB:红绿蓝光。
2.2 蛋白质
微藻蛋白质含量也与光照质量有关。据报道,蓝光对大豆等陆生植物的蛋白质合成有促进作用。对于藻类,也有报道LED蓝光能促进Dunaliella salina[25-26,57]、Arthrospira platensis[19,32]、Chlamydomonas reinhardtii[58]]等蛋白质含量的增加。在某些情况下,LED红光和其他光也有利于蛋白质合成,如Arthrospira platensis[15]、Porphyridium cruentum[22]、Ulva lactuca[30]。也有研究表明,混合光比单色光更有利于Chlorella sp.蛋白质的积累,特别是红蓝光[7]。尽管最佳光照质量会因微藻品种和光照强度而异,但在大多数情况下蓝光被证明是最佳的,如表3所示。处于指数阶段且生长速度快的藻类通常具有高蛋白质含量和低碳水化合物含量的特点。据报道,蛋白质含量在前5个细胞周期内逐渐增加[59];在后期稳定阶段,营养缺乏会抑制蛋白质合成[60],它可能导致蛋白质合成转向碳水化合物或脂质合成以积累能量。
表3 LED不同光质对微藻蛋白质积累的影响
Table 3 Effects of LED different light quality on protein accumulation in microalgae
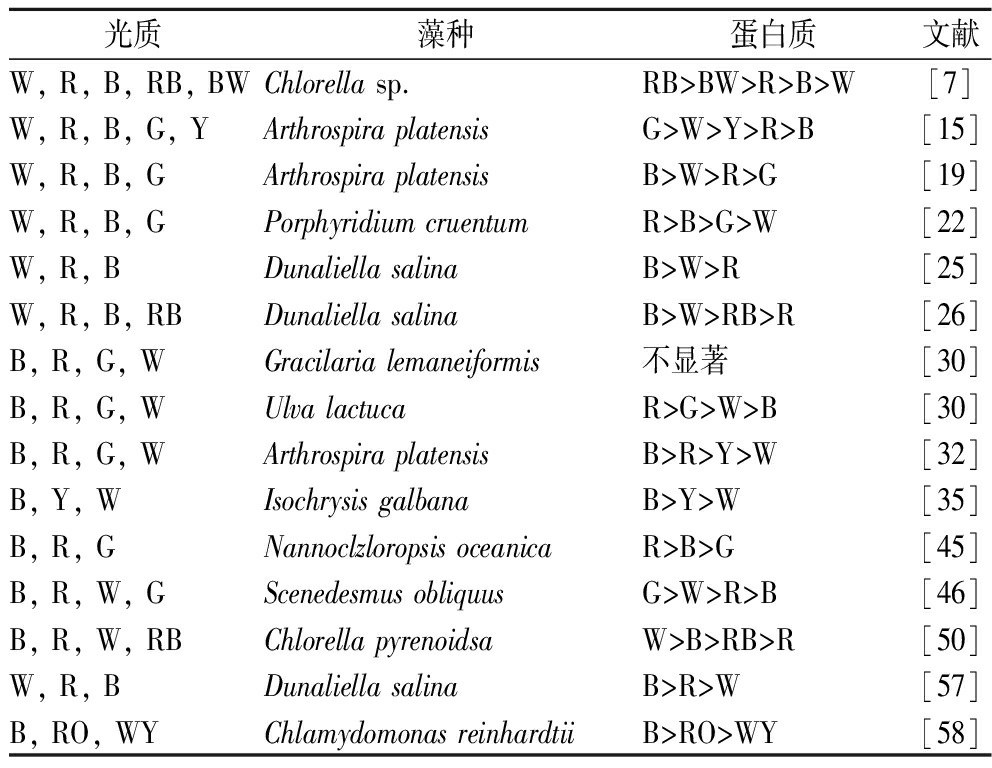
光质藻种蛋白质文献W, R, B, RB, BWChlorella sp.RB>BW>R>B>W[7]W, R, B, G, YArthrospira platensisG>W>Y>R>B[15]W, R, B, GArthrospira platensisB>W>R>G[19]W, R, B, GPorphyridium cruentumR>B>G>W[22]W, R, BDunaliella salinaB>W>R[25]W, R, B, RBDunaliella salinaB>W>RB>R[26]B, R, G, WGracilaria lemaneiformis不显著[30]B, R, G, WUlva lactucaR>G>W>B[30]B, R, G, WArthrospira platensisB>R>Y>W[32]B, Y, WIsochrysis galbanaB>Y>W[35]B, R, GNannoclzloropsis oceanicaR>B>G[45]B, R, W, GScenedesmus obliquusG>W>R>B[46]B, R, W, RBChlorella pyrenoidsaW>B>RB>R[50]W, R, BDunaliella salinaB>R>W[57]B, RO, WYChlamydomonas reinhardtiiB>RO>WY[58]
2.3 碳水化合物
众所周知,红光作为光合作用的反馈调节,可以刺激碳水化合物的合成和积累,但这种影响也取决于物种。据报道,LED红光能促进很多微藻碳水化合物的合成和积累,如Chlorella sp.[7,47]、Chlorella sorokiniana[43]、Chlamydomonas sp.[52];但在某些情况下,LED蓝光和其他光也有利于碳水化合物的合成,如Arthrospira platensis[15]、Rhodomonas sp.[23]、Dunaliella salina[25]。也有研究表明,混合光比单色光更有利于微藻碳水化合物的积累,如红蓝光、红橙光[7,58]。大多数情况下,白光不利于微藻积累大分子有机物,合成有机物与分解有机物的生化过程均比较活跃。这可能是因为白色LED光源中包含了红蓝绿光,白光表现出红蓝绿光质的混合效应。尽管不同微藻的最佳光照质量有差异,但大多数情况下红光最有利于碳水化合物的合成和积累,如表4所示。
表4 LED不同光质对微藻碳水化合物积累的影响
Table 4 Effects of LED different light quality on carbohydrate accumulation in microalgae
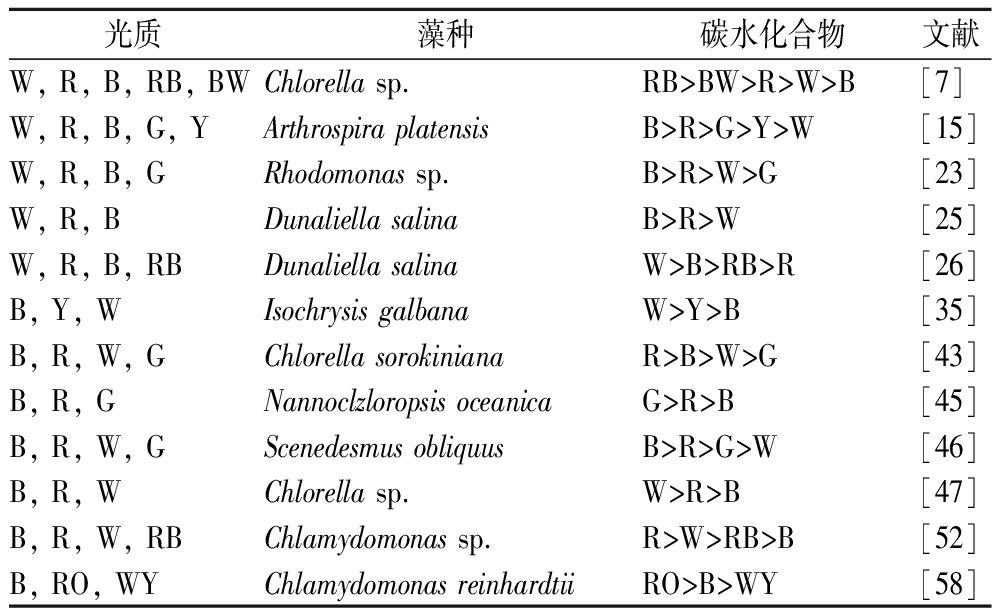
光质藻种碳水化合物文献W, R, B, RB, BWChlorella sp.RB>BW>R>W>B[7]W, R, B, G, YArthrospira platensisB>R>G>Y>W[15]W, R, B, GRhodomonas sp.B>R>W>G[23]W, R, BDunaliella salinaB>R>W[25]W, R, B, RBDunaliella salinaW>B>RB>R[26]B, Y, WIsochrysis galbanaW>Y>B[35]B, R, W, GChlorella sorokinianaR>B>W>G[43]B, R, GNannoclzloropsis oceanicaG>R>B[45]B, R, W, GScenedesmus obliquusB>R>G>W[46]B, R, WChlorella sp.W>R>B[47]B, R, W, RBChlamydomonas sp.R>W>RB>B[52]B, RO, WYChlamydomonas reinhardtiiRO>B>WY[58]
2.4 类胡萝卜素
微藻在光胁迫下会生产过量的类胡萝卜素,有时与光质有关,例如LED红光[31]。β-胡萝卜素和叶黄素等类胡萝卜素在光系统Ⅱ中起着核心作用,它们能收集蓝光并将能量传递给光系统反应中心,同时保护光合系统免受活性氧引起的光氧化损伤[31]。很多研究表明,微藻类胡萝卜素的产生强烈地受到光源的影响,特别是在指数早期和稳定期。
如表5所示,在所有单色光中,LED红光似乎在微藻类胡萝卜素的生产中起着主要作用,如Phaeodactylum tricornutum[56]、Tetradesmus sp.[27]、Ulva lactuca[30]、Dunaliella salina[41]等,这主要是由于诱导类胡萝卜素的产生与光敏色素的激活和反应有关。也有报道称,LED蓝光能促进Gracilaria lemaneiformis[30]、Eustigmatos cf.polyphem[37]、Haematococcus pluvialis[42]等微藻类胡萝卜素的积累。也有研究表明,混合光特别是红蓝光下,Scenedesmus obliquus[6]、Chlorella sp.[7]、Chlorella pyrenoidsa[50] 等微藻类胡萝卜素的产量更高,这可能与β-胡萝卜素在PS Ⅱ中的作用、蓝光的捕获以及光合系统的保护有关。
一些研究表明,蓝光特别能诱导Haematococcus pluvialis积累虾青素[61];而另一些研究表明,红光更有利于微藻叶黄素的生成。然而,针对Dunaliella salina的研究表明,红蓝光下β-胡萝卜素和叶黄素的积累增加[62]。这种现象的可能解释是,微藻与植物的相似,它们具有不同的感光体/结构域,一些受蓝光调节,另一些受红光调节,而蓝光信号传导可能与红光信号传导不同。
表5 LED不同光质对微藻类胡萝卜素积累的影响
Table 5 Effects of LED different light quality on carotenoid accumulation in microalgae

光质藻种类胡萝卜素文献B, R, G, WArthrospira maxima, Chlorellaminutissima, Rhodomonas salina, Nannochloropsis oceanicaR>G>W>B[5]R, RBScenedesmus obliquusRB>R[6]W, R, B, RB, BWChlorella sp.RB>BW>B>R>W[7]W, R, B, G, YArthrospira platensisR>W>Y>G>B[15]W, R, BDunaliella salinaB>R>W[25]W, R, B, RBDunaliella salinaW>B>RB>R[26]W, R, B, G, YTetradesmus sp.R>W>B>G>Y[27]R, RBHaematococcus pluvialisRB>R[28]G, R, YSynechococcus nidulansG>R>Y[29]B, R, G, WGracilaria lemaneiformisB>W>G>R[30]B, R, G, WUlva lactucaR>W>B>G[30]R, RBDunaliella salinaRB>R[31]B, R, G, WArthrospira platensis不显著[32]B, R, WOdontella auritaR>B>W[36]B, R, WEustigmatos cf.polyphemB>W>R[37]B, R, WDunaliella salinaR>B>W[41]B, RHaematococcus pluvialisB>R[42]B, R, W, GChlorella sorokinianaB>R>W>G[43]B, R, WSynechococcus sp.B>W>R[44]B, R, GNannoclzloropsis oceanicaG>B>R[45]B, R, WChlorella sp.B>R>W[47]B, R, W, RBChlorella pyrenoidsaRB>W>R>B[50]R, RBSpirulina platensisRB>R[51]B, R, W, RBChlamydomonas sp.B>RB>W>R[52]B, R, RBScenedesmus obliquusRB>B>R[55]R, B, RGBPhaeodactylum tricornutumR>B>RGB [56]W, R, BDunaliella salina不显著[57]R, BHaematococcus pluvialisB>R[61]B, R, W, RBDunaliella salinaRB>R>W>B[62]
3 光质影响微藻代谢产物积累的分子机制
目前,有关光质影响微藻代谢产物积累的分子机制方面的研究较少,其分子机制尚不清晰。有研究发现,红光下Chlorella sorokiniana固定CO2的碳酸酐酶的基因表达量升高,合成脂肪酸的乙酰辅酶A羧化酶和脂肪酸合成酶的基因表达量也升高;同时,参与TCA循环的丙酮酸羧化酶和磷酸戊糖途径的葡萄糖-6-磷酸脱氢酶的基因表达量降低,即红光下C.sorokiniana合成碳水化合物和脂质等能量物质要更活跃且C.分解碳水化合物的过程变缓[43],从而导致碳水化合物和脂质的积累。对于Isochrysis galbana,蓝光会抑制脂肪酸的积累,转录组分析发现脂肪酸去饱和酶可能是影响蓝光下I.galbana合成脂肪酸的关键酶[63]。也有研究表明,蓝光会影响微藻中二磷酸核酮糖羧化酶/加氧酶和碳酸酐酶的活性,促进甘油三酯的积累[48]。综上,不同光质下微藻积累碳水化合物、脂质的响应机制是不同的,而且不同的藻种其响应机制也有差别,可能主要是通过调控碳水化合物、脂质代谢通路中关键酶的表达量来调节其积累。
李元翔[64]研究发现,红光和蓝光条件下Dunaliella salina类胡萝卜素的含量都有所升高,是因为编码光感受器隐花色素基因和类胡萝卜素生物合成相关基因PSY、LCY在红光和蓝光条件下都表达上调;而且蓝光下活性氧清除酶相关基因表达也上调,能保护藻细胞免受氧化胁迫损伤。蔡学花[65]研究发现,蓝光可以促进Dunaliella salina β-胡萝卜素的积累,蓝光使psbS及外周天线蛋白Lhca3、Lhcb1、Lhcb2等表达下调,这种影响可能是通过提高光能捕获效率或者调控不同的光合放氧相关蛋白的表达变化,从而影响杜氏盐藻的光合作用(图1)。对于Isochrysis galbana,蓝光下类胡萝卜素代谢途径中的PDS、Z-ISO、ZDS、LCYB、ZEP等基因表达下调,抑制岩藻黄素的积累[63]。综上,不同藻种及相同藻种不同光质下对类胡萝卜素积累的响应机制是不同,可能主要是通过调控类胡萝卜素生物合成途径中关键酶及光合放氧相关蛋白的表达量来调控其积累(图1)。
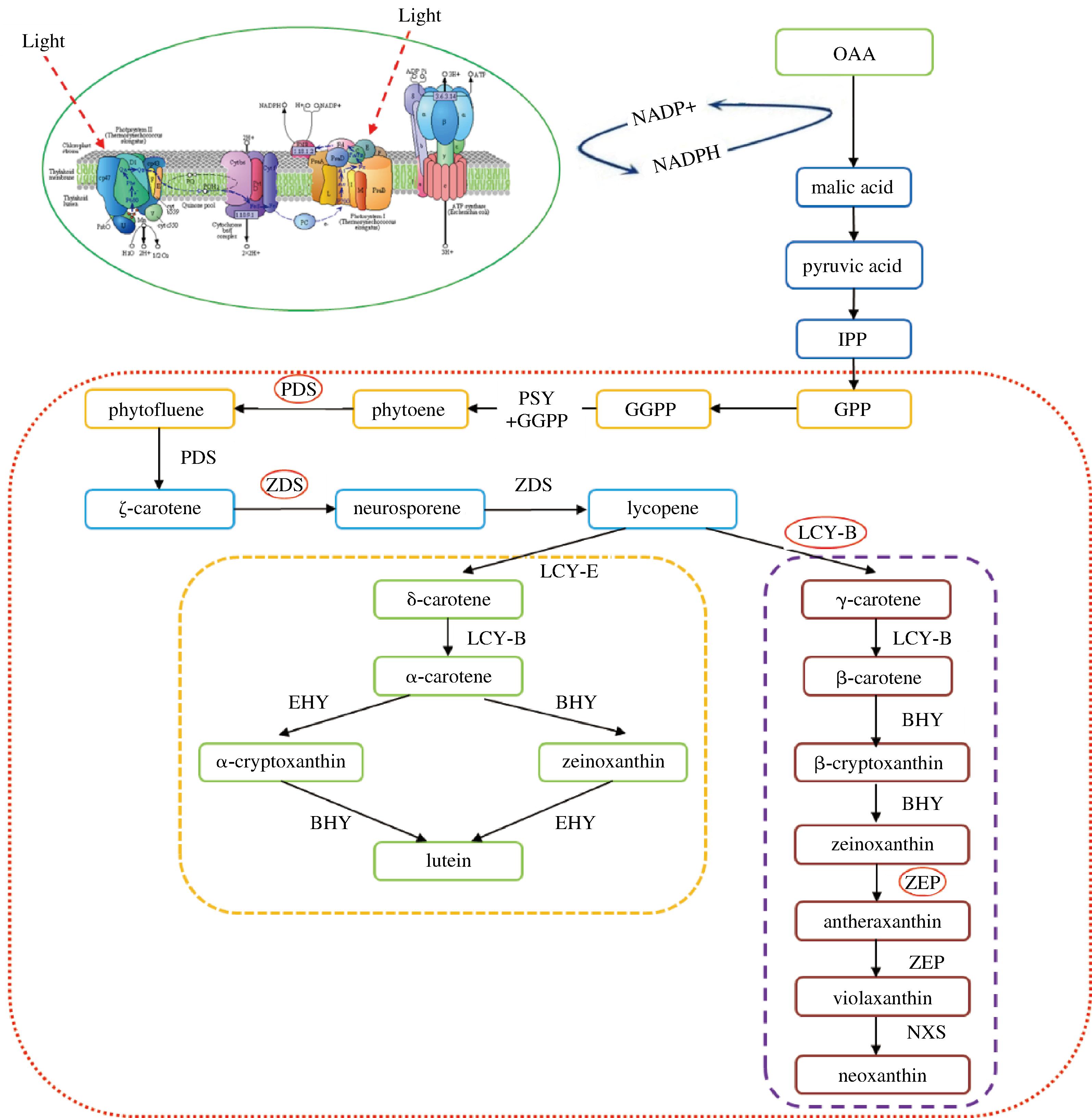
图1 微藻中类胡萝卜素的生物合成途径
Fig.1 Biosynthetic pathways of carotenoids in microalgae
注:OAA:草酰乙酸;malic acid:苹果酸;pyruvic acid:丙酮酸;IPP:异戊二烯焦磷酸;GPP:牻牛儿基二磷酸;GGPP:牻牛基牻牛基焦磷酸;PSY:八氢 番茄红素合酶;phytoene:八氢番茄红素;PDS:八氢番茄红素脱氢酶;phytofluene:六氢番茄红素;ζ-carotene:ζ-胡萝卜素;ZDS:ζ-胡萝卜素脱氢酶; neurosporene:链孢红素;lycopene:番茄红素;LCY-B:番茄红素β-环化酶;LCY-E:番茄红素化酶;γ-carotene:γ-胡萝卜素;β-carotene:β-胡萝卜素; BHY:胡萝卜素β-环羟化酶;β-cryptoxanthin:β-玉米黄质;zeinoxanthin:玉米黄素;ZEP:玉米黄质环化酶;antheraxanthin:环氧玉米黄质; violaxanthin:紫黄质;neoxanthin:新黄质;NXS:新黄质合成酶;δ-carotene:δ-胡萝卜素;α-carotene:α-胡萝卜素;EHY:胡萝卜素ε-环羟化酶; α-cryptoxanthin:α-玉米黄质;lutein:叶黄素。
4 结论与展望
光是微藻自养生长的先决条件,而光质可以作为调节微藻生长、新陈代谢变化以及高价值代谢产物积累的有效工具。微藻研究和生产中使用的人工光源已经越来越多地采用具有很多优点的LED,LED可以使用特殊的光谱成分改变微藻细胞的代谢过程以及细胞代谢产物的组成。目前,已经有很多采用LED光质调控微藻生长及代谢产物积累的研究,使用的单色光有LED红光、蓝光、绿光、黄光和橙光,其中以红光和蓝光对不同类群微藻生物量和代谢产物积累的提升最为显著;也有研究LED混合光对微藻生长及代谢产物积累的影响,其中红蓝光往往比单色光更能增加不同类群微藻生物量和代谢产物的积累。
目前,已经有越来越多的研究者和企业关注LED光质调控在微藻生产中的优势。而且,采用LED光质调控Haematococcus pluvialis高效生产虾青素和Odontella aurita高效生产岩藻黄素已经分别实现中试生产[36]。尽管LED光质在微藻培养中具有广阔的应用前景,但在实际生产中,这种培养技术仍然面临着生产成本较高的挑战。此外,微藻的光合作用,生长,脂质、类胡萝卜素、蛋白质、碳水化合物等代谢产物积累的最佳光质在不同类群和同一物种的不同藻株中均有所不同。
考虑到上述情况及该领域的现有发展,为了更加高效地利用光质调控技术促进微藻高价值代谢产物的大规模生产,结合现有文献报道,笔者认为今后LED光质在微藻生产中的应用应该在以下4个方向重点发展:a)采用转录组学、代谢组学等多组学技术解析不同光质影响微藻代谢产物积累的分子机制,为开发生产高价值代谢产物的菌株提供理论依据;b)采用新型光电材料开发新型高效LED光源,以降低热损耗,提高电能向光能、光能向生物质转化的效率,实现微藻的经济、高效生产;c)根据微藻在不同生长阶段及代谢产物积累对光质的不同需求,开发基于LED光质调节的高效光生物反应器,以促进微藻的生长及代谢产物积累;d)利用LED光质调控技术,实现微藻可持续的大规模培养,以提高微藻生物量和高价值代谢产物的产量。
[1] HASSAN M H, KALAM M A. An overview of biofuel as a renewable energy source: Development and challenges[J]. Procedia Engineering, 2013, 56:39-53.
[2] BALASUBRAMANIAM V, GUNASEGAVAN R D N, MUSTAR S, et al. Isolation of industrial important bioactive compounds from microalgae[J]. Molecules, 2021, 26(4):943.
[3] 黄箫喃, 葛志强. 微藻生产生物燃料的工程策略研究进展[J]. 现代化工, 2015, 35(9):21-25.
HUANG X N, GE Z Q. Research progress of engineering strategies for biofuels from microalgae[J]. Modern Chemical Industry, 2015, 35(9):21-25.
[4] SCHULZE P S C, GUERRA R, PEREIRA H, et al. Flashing LEDs for microalgal production[J]. Trends in Biotechnology, 2017, 35(11):1088-1101.
[5] LJUBIC A, HOLDT S L, JAKOBSEN J, et al. Fatty acids, carotenoids, and tocopherols from microalgae: Targeting the accumulation by manipulating the light during growth[J]. Journal of Applied Phycology, 2021, 33(5):2783-2793.
[6] PAGELS F, AMARO H M, TAVARES T G, et al. Effects of irradiance of red and blue: Red LEDs on Scenedesmus obliquus M2-1 optimization of biomass and high added-value compounds[J]. Journal of Applied Phycology, 2021, 33(3):1379-1388.
[7] LIU X Y, HONG Y, GU W P. Influence of light quality on Chlorella growth, photosynthetic pigments and high-valued products accumulation in coastal saline-alkali leachate[J]. Journal of Water Reuse and Desalination, 2021, 11(2):301-311.
[8] 张虎, 朱瑞鸿, 谭英南, 等. LED光质调控微藻生长及目标产物积累的研究进展[J]. 生物加工过程, 2022, 20(2):125-136. ZHANG H, ZHU R H, TAN Y N, et al. Research progress on LED quality regulating cell growth and target chemicals accumulation in microalgae[J]. Chinese Journal of Bioprocess Engineering, 2022, 20(2):125-136.
[9] PANG N, FU X, FERNANDEZ J S M, et al. Multilevel heuristic LED regime for stimulating lipid and bioproducts biosynthesis in Haematococcus pluvialis under mixotrophic conditions[J]. Bioresource Technology, 2019, 288:121525.
[10] GLEMSER M, HEINING M, SCHMIDT J, et al. Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: Current state and perspectives[J]. Applied Microbiology and Biotechnology, 2016, 100(3):1077-1088.
[11] YOU T, BARNETT S M. Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum[J]. Biochemical Engineering Journal, 2004, 19(3):251-258.
[12] DE MOOIJ T, DE VRIES G, LATSOS C, et al. Impact of light color on photobioreactor productivity[J]. Algal Research, 2016, 15:32-42.
[13] SCHELLENBERGER COSTA B, JUNGANDREAS A, JAKOB T, et al. Blue light is essential for high light acclimation and photoprotection in the diatom Phaeodactylum tricornutum[J]. Journal of Experimental Botany, 2013, 64(2):483-493.
[14] Pattanaik A, Sukla L B, Pradhan D. Effect of LED lights on the growth of microalgae[J]. Inglomayor, 2018, 14: 14-24.
[15] MARKOU G. Effect of various colors of light-emitting diodes (LEDs) on the biomass composition of Arthrospira platensis cultivated in semi-continuous mode[J]. Applied Biochemistry and Biotechnology, 2014, 172(5):2758-2768.
[16] KIM S H, SUNWOO I Y, HONG H J, et al. Lipid and unsaturated fatty acid productions from three microalgae using nitrate and light-emitting diodes with complementary LED wavelength in a two-phase culture system[J]. Bioprocess and Biosystems Engineering, 2019, 42(9):1517-1526.
[17] ZHONG Y, JIN P, CHENG J J. A comprehensive comparable study of the physiological properties of four microalgal species under different light wavelength conditions[J]. Planta, 2018, 248(2):489-498.
[18] SHARMA N, FLEURENT G, AWWAD F, et al. Red light variation an effective alternative to regulate biomass and lipid profiles in Phaeodactylum tricornutum[J]. Applied Sciences, 2020, 10(7):2531.
[19] JUNG C H G, WALDECK P, SYKORA S, et al. Influence of different light-emitting diode colors on growth and phycobiliprotein generation of Arthrospira platensis[J]. Life, 2022, 12(6):895.
[20] HELAMIEH M, GEBHARDT A, REICH M, et al. Growth and fatty acid composition of Acutodesmus obliquus under different light spectra and temperatures[J]. Lipids, 2021, 56(5):485-498.
[21] LIU X Y, HONG Y, LIU Y. Cultivation of Chlorella sp. HQ in inland saline-alkaline water under different light qualities[J]. Frontiers of Environmental Science &Engineering, 2021, 16(4):1-10.
[22] CASTRO-VARELA P A, CELIS-PL P S M, ABDALA-D
P S M, ABDALA-D AZ R, et al. Photobiological effects on biochemical composition in Porphyridium cruentum (rhodophyta) with a biotechnological application[J]. Photochemistry and Photobiology, 2021, 97(5):1032-1042.
AZ R, et al. Photobiological effects on biochemical composition in Porphyridium cruentum (rhodophyta) with a biotechnological application[J]. Photochemistry and Photobiology, 2021, 97(5):1032-1042.
[23] LATSOS C, HOUCKE J, BLOMMAERT L, et al. Effect of light quality and quantity on productivity and phycoerythrin concentration in the cryptophyte Rhodomonas sp[J]. Journal of Applied Phycology, 2021, 33(2):729-741.
[24] HELAMIEH M, REICH M, BORY S, et al. Blue-green light is required for a maximized fatty acid unsaturation and pigment concentration in the microalga Acutodesmus obliquus[J]. Lipids, 2022, 57(4-5):221-232.[PubMed]
[25] NWOBA E G, ROHANI T, RAEISOSSADATI M, et al. Monochromatic light filters to enhance biomass and carotenoid productivities of Dunaliella salina in raceway ponds[J]. Bioresource Technology, 2021, 340:125689.
[26] JIN C L, YU B Q, QIAN S Y, et al. Impact of combined monochromatic light on the biocomponent productivity of Dunaliella salina[J]. Journal of Renewable and Sustainable Energy, 2021, 13(2): e023101.
[27] GONÇALVES V D, FAGUNDES-KLEN M R, TRIGUEROS D E G, et al. Combination of light emitting diodes (LEDs) for photostimulation of carotenoids and chlorophylls synthesis in Tetradesmus sp[J]. Algal Research, 2019, 43:101649.
[28] LI K, YE Q, LI Q Y, et al. Effects of the spatial and spectral distribution of red and blue light on Haematococcus pluvialis growth[J]. Algal Research, 2020, 51:102045.
[29] DUARTE J H, DE SOUZA C O, DRUZIAN J I, et al. Light emitting diodes applied in Synechococcus nidulans cultures: Effect on growth, pigments production and lipid profiles[J]. Bioresource Technology, 2019, 280:511-514.
[30] GONG J Y, LIU Z W, ZOU D H. Growth and photosynthetic characteristics of Gracilaria lemaneiformis (Rhodophyta) and Ulva lactuca (Chlorophyta) cultured under fluorescent light and different LED light[J]. Journal of Applied Phycology, 2020, 32(5):3265-3272.
[31] FU W Q, ![]()
 , PAGLIA G, et al. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution[J]. Applied Microbiology and Biotechnology, 2013, 97(6):2395-2403.
, PAGLIA G, et al. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution[J]. Applied Microbiology and Biotechnology, 2013, 97(6):2395-2403.
[32] TAYEBATI H, PAJOUM SHARIATI F, SOLTANI N, et al. Effect of various light spectra on amino acids and pigment production of Arthrospira platensis using flat-plate photobioreactor[J]. Preparative Biochemistry &Biotechnology, 2021:1-12.
[33] MOHSENPOUR S F, RICHARDS B, WILLOUGHBY N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production[J]. Bioresource Technology, 2012, 125:75-81.
[34] TEO C L, ATTA M, BUKHARI A, et al. Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths[J]. Bioresource Technology, 2014, 162:38-44.
[35] SUHAIMI N B, KWAN P P, BANERJEE S, et al. Influence of blue and yellow light-emitting diodes on the lipid and carbohydrate composition of Isochrysis galbana[J]. Aquaculture Research, 2021, 52(7):3226-3232.
[36] ZHANG H, GONG P Y, CAI Q H, et al. Maximizing fucoxanthin production in Odontella aurita by optimizing the ratio of red and blue light-emitting diodes in an auto-controlled internally illuminated photobioreactor[J]. Bioresource Technology, 2022, 344:126260.
[37] ZHANG H, CHEN A L, HUANG L D, et al. Transcriptomic analysis unravels the modulating mechanisms of the biomass and value-added bioproducts accumulation by light spectrum in Eustigmatos Cf. Polyphem (Eustigmatophyceae)[J]. Bioresource Technology, 2021, 338:125523.
[38] ATTA M, IDRIS A, BUKHARI A, et al. Intensity of blue LED light: A potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris[J]. Bioresource Technology, 2013, 148:373-378.
[39] KIM T H, LEE Y, HAN S H, et al. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment[J]. Bioresource Technology, 2013, 130:75-80.
[40] HURTADO D X, GARZ N-CASTRO C, CORTÉS-ROMERO J, et al. Using different wavelengths and irradiance on the microalgae\n Acutodesmus obliquus\n batch culture [J]. J Chem Technol Biotechnol, 2019, 94(7) : 2141-2147.
N-CASTRO C, CORTÉS-ROMERO J, et al. Using different wavelengths and irradiance on the microalgae\n Acutodesmus obliquus\n batch culture [J]. J Chem Technol Biotechnol, 2019, 94(7) : 2141-2147.
[41] LI Y X, CAI X H, GU W H, et al. Transcriptome analysis of carotenoid biosynthesis in Dunaliella salina under red and blue light[J]. Journal of Oceanology and Limnology, 2020, 38(1):177-185.
[42] PEREIRA S, OTERO A. Haematococcus pluvialis bioprocess optimization: Effect of light quality, temperature and irradiance on growth, pigment content and photosynthetic response[J]. Algal Research, 2020, 51:102027.
[43] 甘钰华, 魏群, 马湘蒙, 等. 光质对小球藻(Chlorella sorokiniana)生长和代谢机制的调控[J]. 中国环境科学, 2022, 42(9):4296-4303.
GAN Y H, WEI Q, MA X M, et al. Regulation of light quality on Chlorella sorokiniana growth and metabolism[J]. China Environmental Science, 2022, 42(9):4296-4303.
[44] 李凯, 宫一玮, 李兴康, 等. 不同光质对嗜热蓝细菌Synechococcus sp. PCC6715生长的影响[J]. 北京大学学报(自然科学版), 2020, 56(4):739-744.
LI K, GONG Y W, LI X K, et al. Effects of different light qualities on the growth of thermophilic cyanobacteria Synechococcus sp. PCC6715[J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2020, 56(4):739-744.
[45] 张元博, 田娇娇, 叶凌志, 等. 几种环境因子对微拟球藻营养物质积累的影响[J]. 核农学报, 2022, 36(6):1273-1283.
ZHANG Y B, TIAN J J, YE L Z, et al. Effects of several environmental factors on nutrient accumulations of Nannochloropsis oceanica[J]. Journal of Nuclear Agricultural Sciences, 2022, 36(6):1273-1283.
[46] ABOMOHRA A E F, SHANG H, EL-SHEEKH M, et al. Night illumination using monochromatic light-emitting diodes for enhanced microalgal growth and biodiesel production[J]. Bioresource Technology, 2019, 288:121514.
[47] LI D J, YUAN Y Z, CHENG D J, et al. Effect of light quality on growth rate, carbohydrate accumulation, fatty acid profile and lutein biosynthesis of Chlorella sp. AE10[J]. Bioresource Technology, 2019, 291:121783.
[48] HWANG J H, MAIER N. Effects of LED-controlled spatially-averaged light intensity and wavelength on Neochloris oleoabundans growth and lipid composition[J]. Algal Research, 2019, 41:101573.[知网学术搜索]
[49] 尹继龙, 唐小红, 郑洪立, 等. 不同光质对小球藻光自养培养积累油脂的影响[J]. 生物加工过程, 2014, 12(5):62-68.
YIN J L, TANG X H, ZHENG H L, et al. Effect of light wavelengths on lipid accumulation of Chlorella vulgaris in photoautotrophic culture[J]. Chinese Journal of Bioprocess Engineering, 2014, 12(5):62-68.
[50] 唐青青, 方治国, 嵇雯雯, 等. 光质对蛋白核小球藻(Chlorella pyrenoidosa)生长特征及生化组成的影响研究[J]. 环境科学, 2014, 35(11):4212-4217.
TANG Q Q, FANG Z G, JI W W, et al. Effects of light quality on the growth characteristics and biochemical component of Chlorella pyrenoidosa[J]. Environmental Science, 2014, 35(11):4212-4217.
[51] 毛瑞鑫, 郭双生. LED红蓝光对钝顶螺旋藻生长及光合放氧的影响[J]. 航天医学与医学工程, 2017, 30(3):225-229.
MAO R X, GUO S S. Effects of red and blue LED light on growth and oxygen-releasing capacity of Spirulina platensis[J]. Space Medicine &Medical Engineering, 2017, 30(3):225-229.
[52] 孙建瑞, 赵君峰, 符丹丹, 等. 不同光质对衣藻(Chlamydomonas sp. 212)生长及油脂积累的影响[J]. 应用与环境生物学报, 2020, 26(4):1016-1022.
SUN J R, ZHAO J F, FU D D, et al. Effects of different lights on the growth and lipid accumulation of Chlamydomonas sp. 212[J]. Chinese Journal of Applied and Environmental Biology, 2020, 26(4):1016-1022.
[53] KUMAR M S, HWANG J H, ABOU-SHANAB R A I, et al. Influence of CO2 and light spectra on the enhancement of microalgal growth and lipid content[J]. Journal of Renewable and Sustainable Energy, 2014, 6(6): e63107.
[54] ZHU X G, LONG S P, ORT D R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass?[J]. Current Opinion in Biotechnology, 2008, 19(2):153-159.
[55] AMARO H M, PAGELS F, AZEVEDO I C, et al. Light-emitting diodes—a plus on microalgae biomass and high-value metabolite production[J]. Journal of Applied Phycology, 2020, 32(6):3605-3618.
[56] DUARTE B, FEIJ O E, GOESSLING J W, et al. Pigment and fatty acid production under different light qualities in the diatom Phaeodactylum tricornutum[J]. Applied Sciences, 2021, 11(6):2550.
O E, GOESSLING J W, et al. Pigment and fatty acid production under different light qualities in the diatom Phaeodactylum tricornutum[J]. Applied Sciences, 2021, 11(6):2550.
[57] SUI Y X, MAZZUCCHI L, ACHARYA P, et al. A comparison of β-carotene, phytoene and amino acids production in Dunaliella salina DF 15 (CCAP 19/41) and Dunaliella salina CCAP 19/30 using different light wavelengths[J]. Foods, 2021, 10(11):2824.
[58] LI X P, MANUEL J, SLAVENS S, et al. Interactive effects of light quality and culturing temperature on algal cell size, biomass doubling time, protein content, and carbohydrate content[J]. Applied Microbiology and Biotechnology, 2021, 105(2):587-597.
[59] COOKSON N A, COOKSON S W, TSIMRING L S, et al. Cell cycle-dependent variations in protein concentration[J]. Nucleic Acids Research, 2010, 38(8):2676-2681.
[60] GAMEIRO P A, STRUHL K. Nutrient deprivation elicits a transcriptional and translational inflammatory response coupled to decreased protein synthesis[J]. Cell Reports, 2018, 24(6):1415-1424.
[61] KATSUDA T, LABABPOUR A, SHIMAHARA K, et al. Astaxanthin production by Haematococcus pluvialis under illumination with LEDs[J]. Enzyme and Microbial Technology, 2004, 35(1):81-86.
[62] LI Y F, LI L, LIU J G, et al. Light absorption and growth response of Dunaliella under different light qualities[J]. Journal of Applied Phycology, 2020, 32(2):1041-1052.
[63] 陈璐瑶. 蓝光诱导球等鞭金藻岩藻黄素及脂肪酸合成转录组研究[D]. 福州: 福建师范大学, 2021.
CHEN L Y. Transcriptome analysis of fucoxanthin and fatty acid synthesis induced by blue light in Isochrysis globosa[D]. Fuzhou: Fujian Normal University, 2021.
[64] 李元翔. 杜氏盐藻类胡萝卜素代谢对光强和光质变化的响应机制[D]. 青岛: 中国科学院大学(中国科学院海洋研究所), 2019.
LI Y X. The response mechanism of carotenoid biosynthesis pathway under different intensities and wavelengths of light in Dunaliella salina[D]. Qingdao: Institute of Oceanology, Chinese Academy of Sciences, 2019.
[65] 蔡学花. 杜氏盐藻Dunaliella salina对不同浓度CO2与光质的代谢响应[D]. 青岛: 中国科学院大学(中国科学院海洋研究所), 2018.
CAI X H. The metabolic response in Dunaliella salina to different concentration of CO2 and light quality[D]. Qingdao: Institute of Oceanology, Chinese Academy of Sciences, 2018.