β-胡萝卜素属于类胡萝卜素家族,存在于植物、水果和微生物中[1],它可以作为维生素原、功能性化妆品、食品着色剂和饲料补充剂,因此被广泛应用于制药、保健品、化妆品和食品工业等领域,是一类具有重要生物功能和商业价值的色素[2-3]。另外,β-胡萝卜素还是一种最常见的维生素A补充剂,维生素A对于人体视觉发育至关重要,如果身体缺少维生素A,视力就会出现问题,甚至会导致夜盲症[4]。尽管90%的商业化β-胡萝卜素是通过化学合成方法得到的[5],但考虑到化学品的安全合成问题,以及消费者对天然添加剂、微生物的偏好,利用微生物异源表达合成β-胡萝卜素受到越来越多的关注[6]。
由于遗传背景清楚、基因操作工具丰富、营养需求简单、对高糖低pH等耐受性强、安全性高等特点,酿酒酵母不仅是生物化学和分子生物学等研究的首选模式真核微生物,也是传统发酵食品生产和现代生物技术领域的重要工业微生物[7-9]。通过对酿酒酵母的系统改造,已经实现多种以乙酰辅酶A作为主要前体高效合成重要化合物,如中长链脂肪酸、长链醇、萜类、黄酮等[10-11]。在酿酒酵母中构建的β-胡萝卜素的合成途径可以分为两部分,上游甲羟戊酸途径合成前体香叶基香叶基焦磷酸,以及下游β-胡萝卜素的合成。类胡萝卜素合成途径非常复杂需要多种酶的协同作用,包括合成酶、脱氢酶、环化酶、羟化酶和酮化酶等。此外,大多数类胡萝卜素在胞内大量积累后,因其疏水性会损伤细胞膜结构并危害细胞的正常生理功能[12]。而脂质体的积累可以缓解其产物毒性,但由于微生物底盘细胞中有限的膜结构在一定程度上限制了类胡萝卜素产量提升空间。
本研究室前期研究发现,以酿酒酵母YPH499为底盘细胞,过量表达关键基因dga1、pah1以及与脂滴形成关联紧密的ldp1基因,得到一株脂质体含量提高的菌株LFD18,番茄红素单位产量为109.3 mg/g CDW。本研究以LFD18为出发菌株,在实验中偶然得到一株β-胡萝卜素产量提高的突变株,通过转录组分析发现hxk1、dpp1、lpp1、fdh1、hmg2和gre2等基因转录水平提高。进一步研究改造后得到工程菌ZS20,其β-胡萝卜素产量提高到194.3 mg/L。
1 材料与方法
1.1 质粒和菌株和所用引物
研究过程中所用的菌株及质粒如表1所示,所用引物如表2所示,实验所用引物均由苏州金唯智生物科技有限公司合成。
表1 本实验使用的菌株和质粒
Table 1 Strains and plasmids used in this study

菌株和质粒相关特征来源JM109E.coli, used for plasmids construction本实验室YPH499MATa, his3-Δ200, leu2-Δ1, trp1-Δ63, ura3-52, lys2-801, ade2-101本实验室YthmgⅠas YPH499;Δgal80::loxp;GAL1pr-thmg1-CYC1ter::adh5本实验室LFD18as YthmgⅠ;Δgal10::ldp1;Δexg1::GAL1,10pr-pah1,dga1-ADH1ter;Δypl062w::GAL10pr-acs1L641P-ADH1ter;Δgal7::pap1本研究ZS19As LFD18;Δyjl064w::CYC1ter-CarRA-TEFprGPDpr-CarB-ADH1ter,Δrox1::CYC1ter-CarRA-TEFprGPD-pr-CrtE-ADH1ter本研究ZS19-HXK1As ZS19;Δyer187w::HXK1pr-hxk1-ADH1ter本研究ZS19-HMG2As ZS19;Δyer187w::HMG2pr-hmg2-ADH1ter本研究ZS19-FDH1As ZS19;Δyer187w::FDH1pr-fdh1-ADH1ter本研究ZS19-DPP1As ZS19;Δyer187w::DPP1pr-dpp1-ADH1ter本研究ZS19-LPP1As ZS19;Δyer187w::LPP1pr-lpp1-ADH1ter本研究ZS19-GRE2As ZS19;Δyer187w::GRE2pr-gre2-ADH1ter本研究ZS20As ZS19 HXK1;Δydr476cR500P::DPP1pr-dpp1-ADH1ter-LPP1pr-lpp1-ADH1ter-FDH1pr-fdh1-ADH1ter本研究pY26Shuttle plasmid with TEF and GPD promoters, Ampr, Δura3本研究pHCas9Expressing Cas9 protein, Noursr本研究Ts-BY(GAL1prGAL10pr)CYC1ter-CarRA-GAL1, 10pr-CarB-ADH1ter,possessing yjl064w homologous arm本实验室Ts-BR(GAL1prGAL10pr)CYC1ter-CarRA-GAL1,10pr-CrtE-ADH1ter, possessing rox1 homologous arm本实验室yjl064w-rox1(gRNA)Double knockout plasmid carrying the PAM sequence of the gene yjl064w and rox1本实验室yer187w(gRNA)Single knockout plasmid carrying the PAM sequence of the gene yer187w本研究yhr180w(gRNA)Single knockout plasmid carrying the PAM sequence of the gene yhr180w本研究ydr476c(gRNA)Single knockout plasmid carrying the PAM sequence of the gene ydr476c本研究Ts-HXK1HXK1pr-hxk1-ADH1ter,possessing yer187w homologous arm本研究Ts-HMG2HMG2pr-hmg2-ADH1ter,possessing yer187w homologous arm本研究Ts-FDH1FDH1pr-fdh1-ADH1ter,possessing yer187w homologous arm本研究Ts-DPP1DPP1pr-dpp1-ADH1ter,possessing yer187w homologous arm本研究Ts-LPP1LPP1pr-lpp1-ADH1ter,possessing yer187w homologous arm本研究Ts-GRE2GRE2pr-gre2-ADH1ter,possessingyer187w homologous arm本研究Ts-3DPP1pr-dpp1-ADH1ter-LPP1pr-lpp1-ADH1ter-FDH1pr-fdh1-ADH1ter,possessing ydr476c homologous arm本研究Vector 19T(simple)Ampicillin Sulfate本实验室BTSAmpicillin Sulfate本实验室302Ampicillin Sulfate本实验室149Ampicillin Sulfate本实验室
表2 本实验使用的引物
Table 2 Primers used in this study

引物名称引物序列(5′-3′)CarB-FAGATCTTTAAATTCTAATATCATTAGAATTTTGAACTTG(Bgl Ⅱ)CarB-RTTCAATATAAGCGGCCGCACarRA-FGGATCCATGTCTATTTTGACTTATTTGGCarRA-RGAATTCTTAATCAACAAAAAAAGATTTAATTTTTCTAGC(EcoR Ⅰ)GAL1,10-F1TGCGGCCGCTTATATTGAATTTTCAAAAATTCTTACTTTTTTTTTGGATGGACGAL1,10-R1GTCAAAATAGACATGGATCCTATAGTTTTTTCTCCTTGACGTTAAAGADH1-F1TTTTTGTTGATTAAGAATTCAATCTTCGCCAGAGGTTTGADH-R1GCACTCTTCTGACGGCGCTATTACGCCAGCTGAATTGCYC1-F1CATCGCCTTCATAAAAGCCCAAAGCCTTCGAGCGTCCCYC1-R1ATATTAGAATTTAAAGATCTCATGTAATTAGTTATGTCACGCTTACATTCYJL064 W-up-arm-FCCAAGCTTGGCCATGGCAAGTGCACTATC(Hind Ⅲ)YJL064 W-up-arm-RGACGCTCGAAGGCTTTGGGCTTTTATGAAGGCGATG
续表2

引物名称引物序列(5′-3′)YJL064 W-down-arm-FTTCAGCTGGCGTAATAGCGCCGTCAGAAGAGTGCACAYJL064 W-down-arm-RCCCCCGGGGGAATGATCCAGAATATATGGACACTTCG(Sma Ⅰ)CrtE-FTTAGTTTTGCCTGAAAGCGATGCrtE-RATGGCTTATACCGCAATGGGAL1,10-F2GCGGTATAAGCCATTTATATTGAATTTTCAAAAATTCTTACTTTTTTTTTGGATGGGAL1,10-R2CAAAATAGACATGGATCCTATAGTTTTTTCTCCTTGACGTTAAAGTATAGADH1-R2AAGAAATGGAAAAAAAAAAGCTATTACGCCAGCTGAATTGCYC1-F2TCAACAAAAGCCTTCGAGCGTCCYC1-R2TCAGGCAAAACTAACATGTAATTAGTTATGTCACGCTTACROX1-up-arm-FCCCCCGGGGG ACTCTTGCATTTTCCTTTTCTGC(Sma Ⅰ)ROX1-up-arm-RGACGCTCGAAGGCTTTTGTTGATTGTCTAACTGCGTTCROX1-down-arm-FAATTCAGCTGGCGTAATAGCTTTTTTTTTTCCATTTCTTCTTTCCGROX1-down-arm-RCCAAGCTTGGATTTGTGAGGTGAATATATACGTG(Hind Ⅲ)471-FAAGGAGGTATTCTGGGCCTCCATGTC471-RTCTGCAGAATTCGTCGACGAGCTCGGTAC699-FATGTACGGGCGACAGTCAC699-RAGTGAGCTGATACCGCTCGLPP1-FAATAAAATTTTTTAAAGATTGTCCATATTCCTCGATCCACACLPP1-RGTGACATAACTAATTACATGCTAAACACTAACCGGTGAAGGAADPP1-FGATGTCCCAGACTTGCAGTTCTCTTCAAGGDPP1-RGACAATCTTTAAAAAATTTTATTTATACATAGTATGTGTTAAGGGGAACGFDH1-FTTGGGACGCTCGAAGGCTTTGTCACCACTCGAGGATAGGFDH1-RTTCCTCTTTTTTTGAAGTTACTTGCTATTACGCCAGCTGAATTGHXK1-FACCACATACGCGCTGATTGGGTGGGGTGATTATCTAGACCATGGGHXK1-RTTAAGCGCCAATGATACCAAGAGACHMG2-FCGATGCATATATTGGCTGAGAGTACTHMG2-RTTGAGGCAGGTTGAAGGTTCCTCGRE2-FCAACGCATTTCTTGGCTAACCATCGRE2-RAGTTGCTGCTCCGGTCATTG
注:限制性酶切位点标在表格右边。
1.2 培养基
大肠杆菌培养基LB (g/L):蛋白胨10、酵母粉5、NaCl 10、固体培养基添加琼脂粉18,121 ℃、20 min灭菌。酵母培养基YPD (g/L):蛋白胨20、酵母粉10、葡萄糖20、若需固体培养基添加琼脂粉18,115 ℃、20 min灭菌。筛选培养基(g/L):蛋白胨20、酵母粉10、葡萄糖20、5-FOA 1,115 ℃、20 min灭菌。氨基酸补足液(μg/mL):亮氨酸100、赖氨酸100、色氨酸80、尿嘧啶30、组氨酸30,超净台过滤除菌。
1.3 实验材料
Phanta® Max Super-Fidelity DNA polymerase、HiScript®Ⅲ All-in-one RT SuperMix Perfect for qPCR、质粒提取试剂盒、DNA纯化试剂盒、DNA胶回收试剂盒,南京诺唯赞生物科技有限公司;Hieff® qPCR SYBR Green Master Mix(No Rox),羿圣生物科技上海有限公司;Fast Digested TM快速限制性内切酶,美国Thermo公司;T4 DNA连接酶、pY26质粒,大连TaKaRa公司;氨苄青霉素、诺尔斯菌素、潮霉素, Sigma-Aldrich公司;Simply P 总RNA提取试剂盒,杭州博日科技股份有限公司;蛋白胨、酵母粉,索莱宝科技有限公司。
1.4 重组菌发酵实验
将平板活化得到的单克隆工程菌株挑取单菌落接种于装有50 mL YPD液体培养基的250 mL锥形瓶中,30 ℃、200 r/min振荡培养至对数生长中期(30 h左右)得到种子液,然后以体积分数3%转接于含50 mL YPD液体培养基的250 mL锥形瓶中,30 ℃、200 r/min培养,每隔12 h取样测定。
1.5 β-胡萝卜素的提取和产量测定
对发酵过程中提取的发酵液样品进行快速提取,具体方法如下所述:先配制β-胡萝卜素标样梯度制作标曲,之后分别各取两等份1 mL发酵液,离心收集菌体,无菌水清洗后,其中一份菌体80 ℃烘干至恒重,称重计算菌体干重;另一份用于产物提取。向待提取β-胡萝卜素的离心管中加入等体积的玻璃珠(0.5 mm)和1 mL氯仿,振荡破碎5 min,冰浴1 min,10 000 r/min离心1 min,取上清液直到样品无色,合并上清液;用N2吹干至色素析出,1 mL丙酮复溶后用酶标仪测定,计算产物浓度。
1.6 RT-qPCR测定目的基因相对表达量
首先,采用Simply P总RNA提取试剂盒提取总RNA;然后,将提取好的RNA反转录成cDNA,经微量紫外分光光度计定量,使浓度约在200 ng/μL左右,以cDNA为模板,以act1为内参基因。之后采用SYBR Green Master Mix在荧光定量PCR仪检测系统CFX96上进行qPCR。其中退火温度为60 ℃,条件如下:循环1次(95 ℃,5 min),循环40次(95 ℃,10 s;60 ℃,30 s),结束时采集数据。采用2-ΔΔCq法计算目的基因的相对表达量[13]。
1.7 β-胡萝卜素定性检测
将待测菌株平板划线活化平后板单菌落接种到装有100 mL YPD液体培养基的500 mL锥形瓶中,30 ℃、200 r/min 摇瓶培养,培养4 d后收集菌体,用无菌水洗涤两次收集菌体后以三氯甲烷为溶剂提取色素,N2吹干为粉末状,送往无锡瑞峰检测技术有限公司检测。
1.8 发酵罐发酵
工程菌在YPD板上划线活化获得单个菌落,将该单菌落置于含有100 mL YPD的500 mL摇瓶中,220 r/min,30 ℃,培养16~18 h。将2.5 L的YPD培养基置于5 L发酵罐中,0.5 mol/L硫酸和5 mol/L氨水的自动加入使pH值恒定在6.5,气流为2 vvm,发酵温度为30 ℃,转速500 r/min。
2 结果与分析
2.1 β-胡萝卜素生产菌株的构建
在酿酒酵母中利用诱导型GAL启动子表达外源类胡萝卜素合成酶效果更好[14]。本文利用含有GAL启动子的质粒Ts-BY(GAL1prGAL10pr)和Ts-BR(GAL1prGAL10pr)通过Sma Ⅰ和Hind Ⅲ 酶切线性化,如图1-a所示,醋酸锂转化法转入实验室已有的酿酒酵母LFD18得到工程菌ZS19,涂布YPD平板发现平板上出现两种颜色菌落,一种颜色偏红命名为ZS19 reddish,一种橘红色较浅命名为ZS19,如图1-b所示。经核磁共振定性检测两株菌落产物都是β-胡萝卜素。将ZS19 reddish和ZS19分别挑取到装有50 mL YPD培养基的250 mL锥形瓶中发酵96 h,结果偏红菌株ZS19 reddish在84 h产量更高为160.4 mg/L,如图1-c所示。
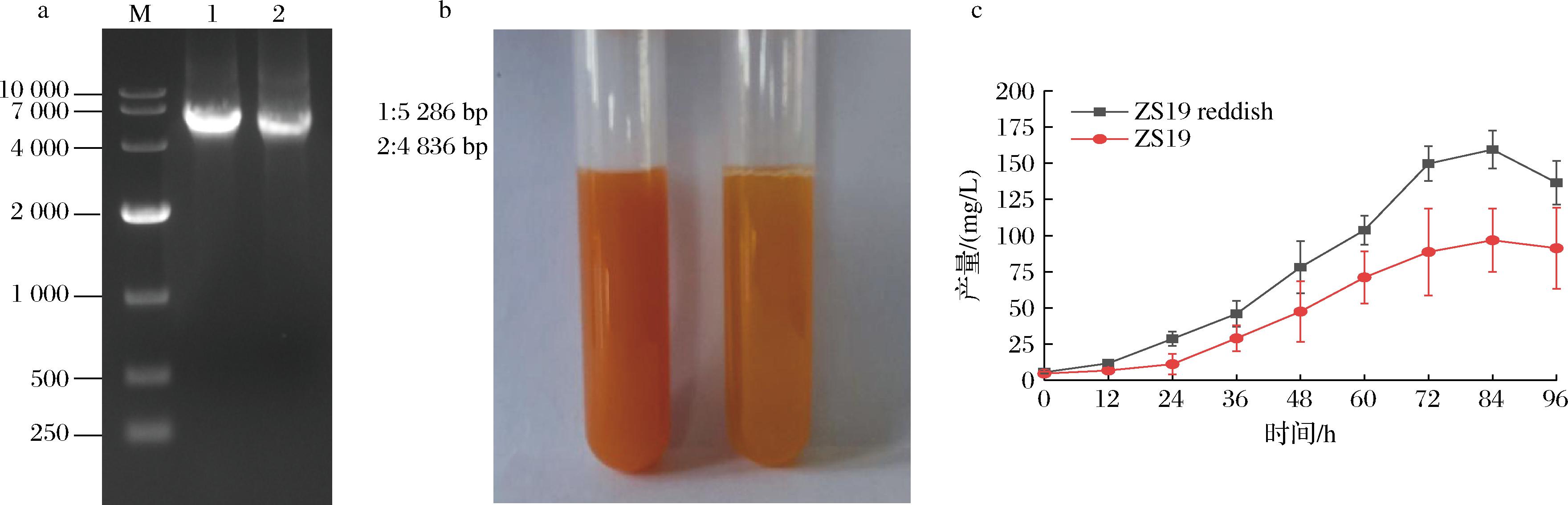
a-质粒TS-BY(GAL1prGAL10pr)和TS-BR(GAL1prGAL10pr)通过Sma Ⅰ和Hind Ⅲ酶切电泳图; b-ZS19 reddish和ZS19发酵84 h对比图;c-ZS19 reddish和ZS19发酵产物产量
图1 构建转化片段的验证,ZS19 reddish和ZS19发酵84 h对比及发酵产量对比图
Fig.1 Build validation of the transformation fragment, Comparison of ZS19 reddish and ZS19 fermentation at 84 h and production concentration
2.2 转录组结果分析
为了进一步研究菌株ZS19 reddish和ZS19表现型差异的原因,分别挑取单菌落到装有50 mL YPD培养基的250 mL锥形瓶中,摇瓶培养72 h后收集5 mL菌体送样进行转录组测定。结果表明,ZS19 reddish对比ZS19共有84个基因发生下调,12个基因上调,如图2-a所示。对上述差异基因进行GO功能富集分析,结果如图3所示。转录水平变化的基因主要与各种生物过程有关,如:生物调控(biological process)、细胞过程(cellular process)、代谢过程(metabolic process)等。此外,与大分子复合体(macromolecular complex)、膜组分(membrane part)、催化活性(catalytic activity)等也密切相关。
根据上述结果,选取与葡萄糖代谢、能量代谢和脂质代谢有关的hxk1、dpp1、lpp1、fdh1、hmg2和gre2等6个基因,进行定量PCR验证。结果表明,上述基因均发生显著上调,特别是hxk1上调125.2倍,hmg2上调31.3倍,结果如表3所示。
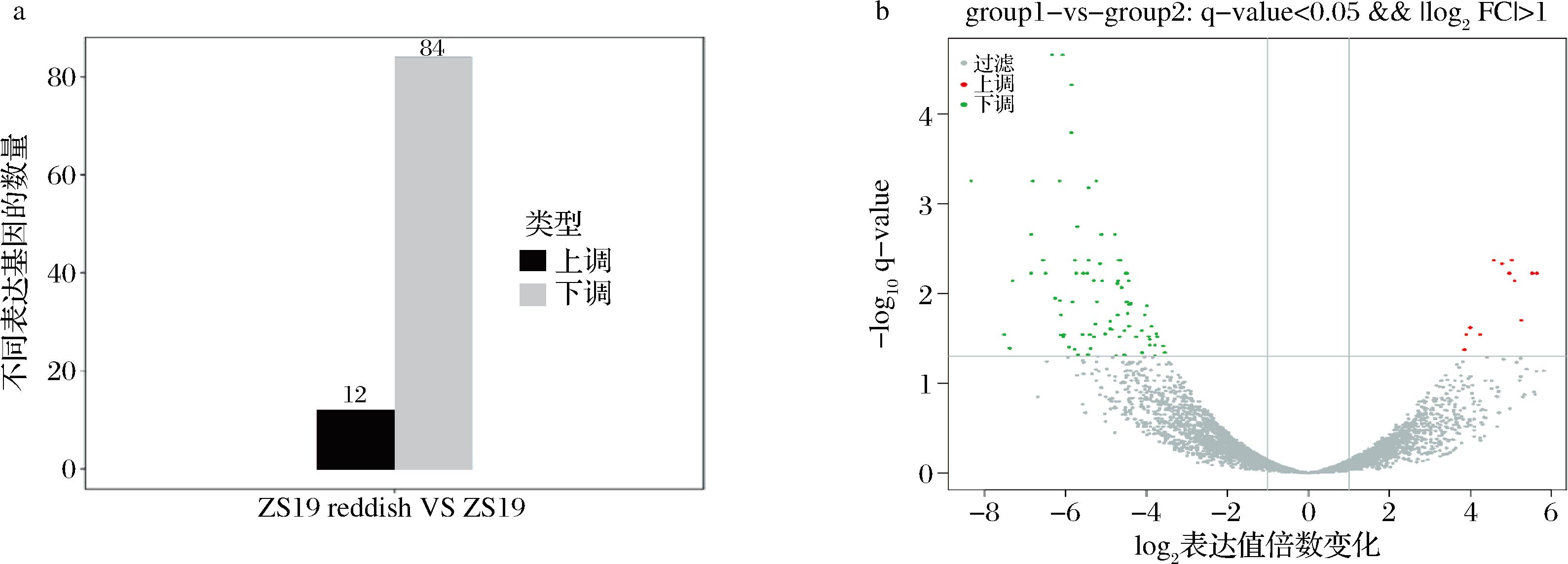
a-ZS19 reddish和ZS19显著性差异比较图;b-ZS19 reddish和ZS19差异情况火山图
图2 ZS19 reddish和ZS19显著性差异比较图和差异情况火山图
Fig.2 Comparison of ZS19 reddish and ZS19 significant differences and volcanic map
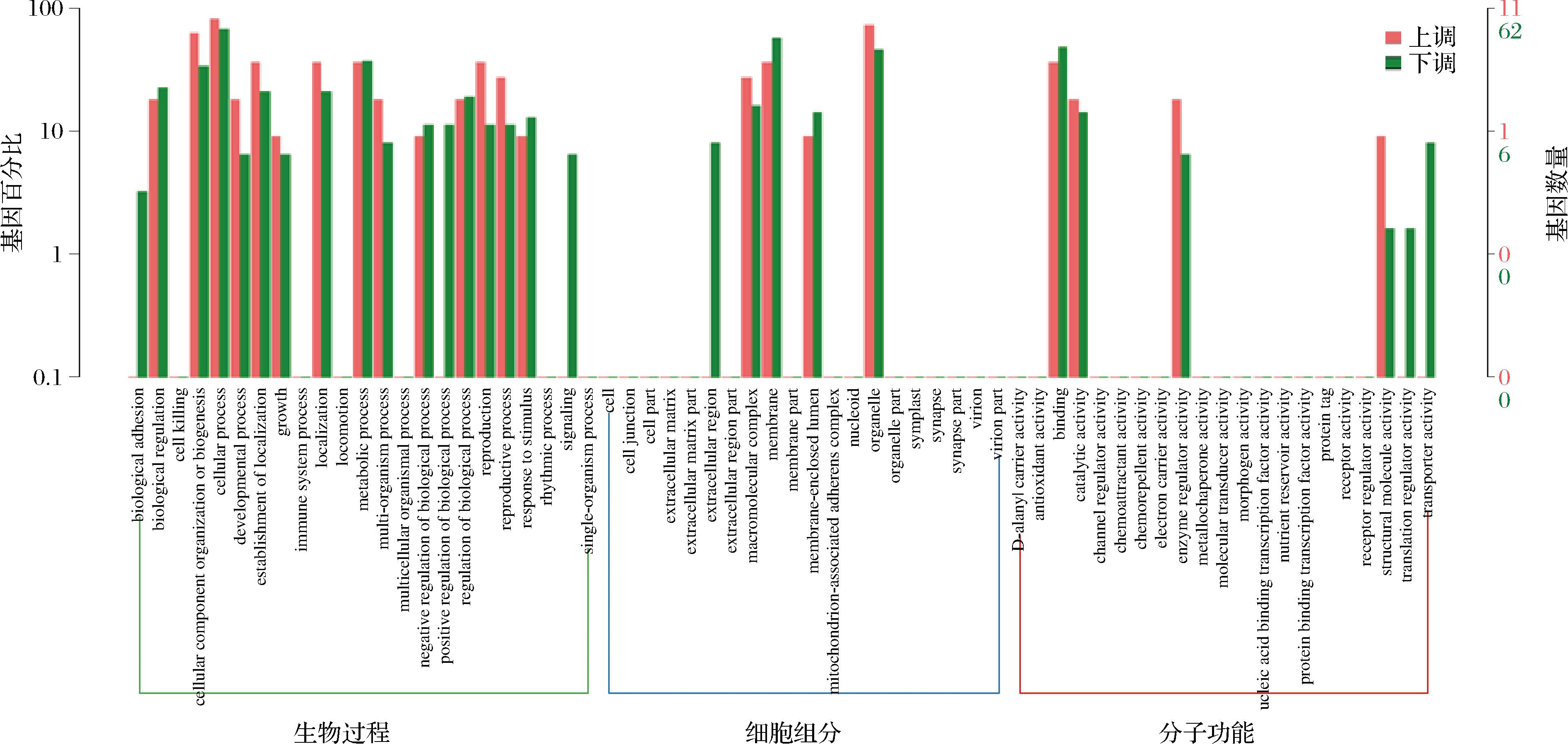
图3 ZS19 reddish和ZS19比较基因分类图
Fig.3 Comparison of ZS19 reddish and ZS19 gene ontology classification
表3 qPCR验证转录结果
Table 3 qPCR verify transcription results
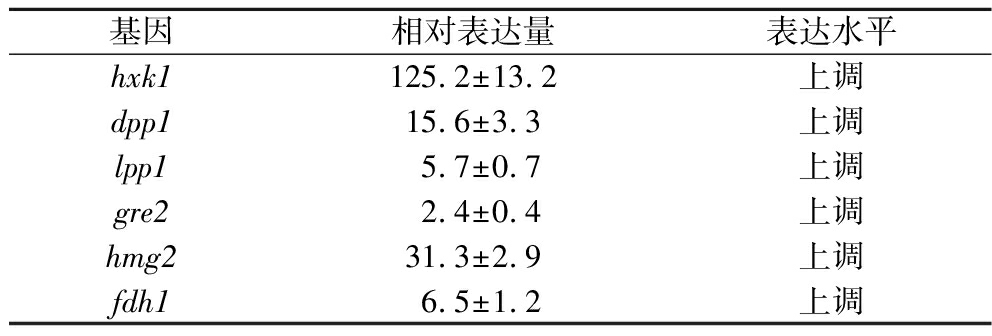
基因相对表达量表达水平hxk1125.2±13.2上调dpp115.6±3.3上调lpp15.7±0.7上调gre22.4±0.4上调hmg231.3±2.9上调fdh16.5±1.2上调
2.3 β-胡萝卜素生产菌的改造
将hxk1、dpp1、lpp1、fdh1、hmg2和gre2基因分别过量表达,结果表明相比突变株ZS19 reddish β-胡萝卜素产量有所下降,但菌株ZS19-DPP1、ZS19-LPP1、ZS19-HXK1、ZS19-FDH1细胞干重有明显提高。并且上述4株菌生产性状相比ZS19 reddish更为稳定,平板传代5次后,菌落仍然全部一致呈现橙红色,而ZS19 reddish传代5次后出现大量白色菌落。根据图4的结果,hxk1、dpp1、lpp1和fdh1基因的发酵产量相对较高,而且细胞干重也有所提高,因此将这4个基因进行联合过表达得到的新菌株命名为ZS20。摇瓶发酵结果表明β-胡萝卜素的产量在84 h达到194.3 mg/L,此时ZS19 reddish产量为154.7 mg/L,提高约26%;相比出发菌株ZS19提高约110%。后续可以尝试将hxk1、dpp1、lpp1、fdh1、hmg2和gre2基因进行联合过表达得到新的改造菌株,或者可以进一步提高重组菌β-胡萝卜素的产量。
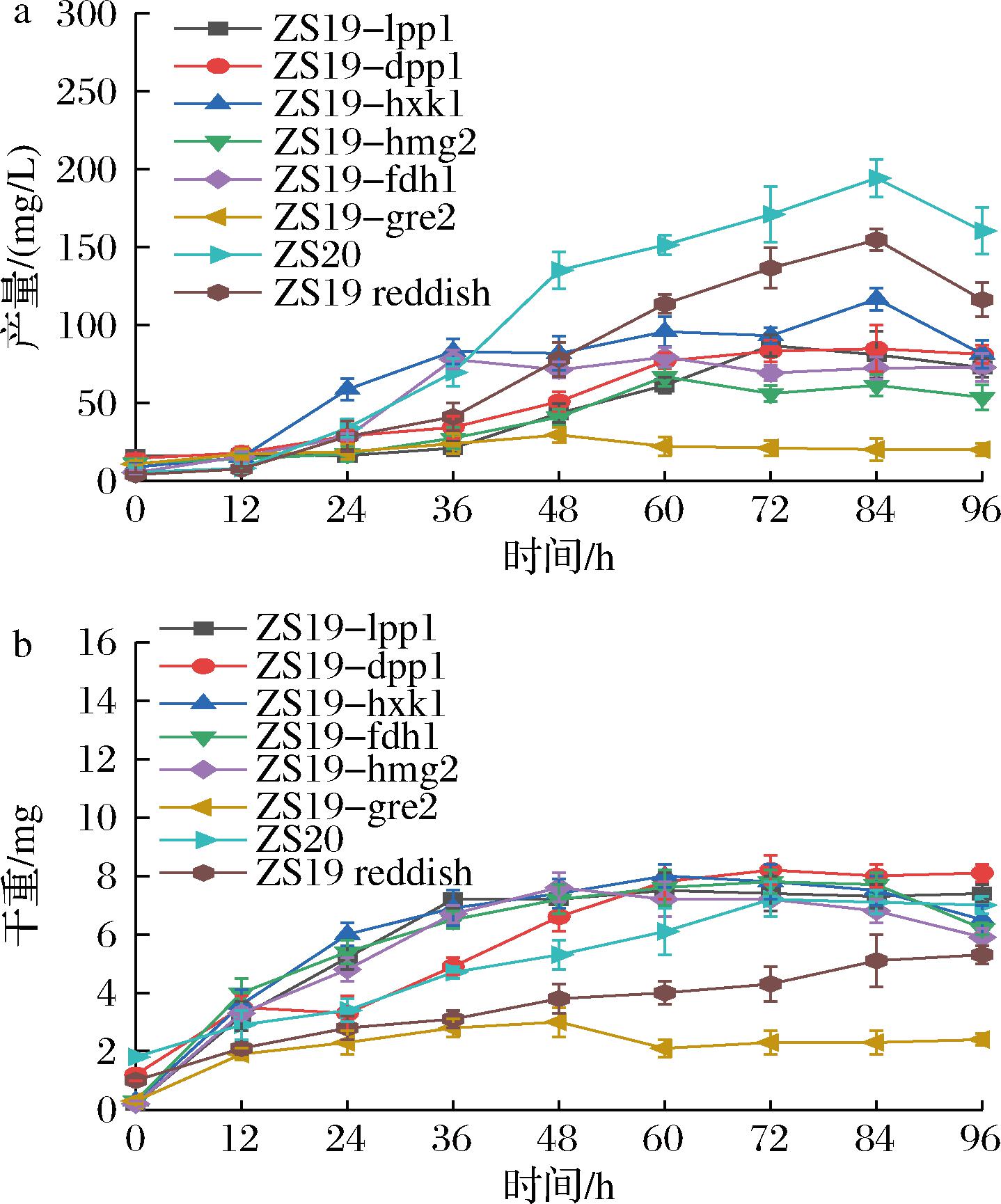
a-各过表达菌株发酵产量;b-各过表达菌株发酵细胞干重
图4 过表达基因转化菌株摇瓶发酵产量及干重
Fig.4 Strain fermentation concentration and dry cell weight
2.4 发酵罐发酵培养
使用上海迪必尔公司的5 L发酵罐,对重组菌ZS20进行发酵。发酵条件为:温度30 ℃,转速500 r/min,pH 6.5。2.5 L培养基高压灭菌后降温至30 ℃加入种子液,发酵总时长为96 h,发酵28 h时溶氧降至最低,此时开始以F1(0)为0.006进行指数流加500 g/L葡萄糖至48 h(发酵罐体积因素),β-胡萝卜素在84 h达到最高为259.8 mg/L,细胞干重在84 h为7.9 mg,单位产量为32.9 mg/g CDW。
对ZS20菌株进行补加氨基酸发酵优化,本研究以酿酒酵母YthmgⅠ菌株作为出发菌株构建代谢改造重组菌,Ythmg1菌株中组氨酸、赖氨酸、亮氨酸、色氨酸和尿嘧啶被改造破坏,因此对ZS20菌株补加氨基酸培养。2.5 L培养基高压灭菌后降温至30 ℃,加入种子液并添加氨基酸补足液,发酵24.5 h时溶氧降至最低,此时以F1(0)为0.006进行指数流加500 g/L葡萄糖到48 h,继续发酵培养至96 h,发酵图片如图5-a所示;每12 h取样后处理样品提取β-胡萝卜素后测产量在84 h达到最高为314.3 mg/L,相比优化之前产量提高约21%,单位产量为38.8 mg/g CDW,结果如图5-b所示;对胞外代谢产物进行液相测定,葡萄糖和乙醇、乙酸、甘油等发酵副产物含量如图5-c所示。

a-ZS20菌株发酵96 h发酵图;b-ZS20菌株发酵产量及干重;c-ZS20菌株发酵液中葡萄糖、乙醇、乙酸和甘油质量浓度
图5 葡萄糖指数流加发酵ZS20菌株的β-胡萝卜素产物、干重及副产物测定
Fig.5 Determination of dry weight and byproducts of β-carotene in ZS20 strain fermentation by glucose exponential flow
3 结论与讨论
酿酒酵母是生产类胡萝卜素的常用底盘微生物之一,研究重点主要集中在代谢中心节点乙酰CoA的合成、前体甲羟戊酸(mevalonate,MVA)途径的优化以及下游异源途径的优化等,但类胡萝卜素作为一种外源产物其代谢调控方式仍有待进一步研究。本研究通过同时过量表达hxk1、dpp1、lpp1和fdh1使β-胡萝卜素产量提高26%。本论文过量表达的己糖激酶1(hxk1),可以磷酸化葡萄糖、果糖和甘露糖,在微生物利用葡萄糖生长方面是不可缺少的,调控糖代谢在细胞生长过程中起着重要作用[15]。甲基乙二醛还原酶由gre2(yol151w)编码[16]。hmg2编码酵母中羟甲基戊二酰辅酶A(HMG-CoA)还原酶的两个同工酶之一,HMG-CoA还原酶催化HMG-CoA转化为甲戊酸[17],与MVA途径紧密相关,这种酶的抑制会导致甲羟戊酸下游所有产物的合成减少,包括胆固醇[18]。dpp1(二酰基甘油焦磷酸磷酸酶)基因编码DGPP磷酸酶,在酿酒酵母中转化含有dpp1基因的多拷贝质粒使DGPP磷酸酶活性提高10倍,DGPP磷酸酶是酿酒酵母中的一种膜相关的34-kDa酶,它催化DGPP去磷酸化生成磷酸酯(phosphate ester,PA),再催化PA去磷酸化生成二酰基甘油[19]。lpp1编码的脂质磷酸酶产物是一种膜相关酶,可催化Mg2+ PA、二酰基甘油焦磷酸(diazanium,[[(2R)-2,3-di(octanoyloxy)propoxy]-oxidophosphoryl] hydrogen phosphate,DGPP)和溶血磷酸酯(lysophosphatidic acids,LPA)的独立去磷酸化[12],与脂质载体的合成有关。以上基因或与底物代谢有关,或与MVA途径和脂质载体的合成紧密相关,因此对β-胡萝卜素合成具有重要影响。
值得一提的是虽然分别过量表达hxk1、dpp1、lpp1、fdh1、hmg2和gre2后β-胡萝卜素产量反而下降,但其菌体量相比ZS19有显著提高。对hxk1、dpp1、lpp1和fdh1进行同时过量表达β-胡萝卜素产量显著提高,有可能与dpp1、lpp1的过量表达增加了细胞脂质体含量,可以降低产物毒性,提高了细胞干重有关。酵母代谢调控系统庞大复杂,长期以来大量的代谢途径调控依赖过量表达和基因敲除,难以实现代谢网络的精准调控[20],后续可以利用启动子调控、反义RNA调控和基于CRISPR-Cas9的代谢途径精准调控等策略,精准调控hxk1、dpp1、lpp1、fdh1、hmg2和gre2等基因的表达,并进一步优化培养基和培养方法等使β-胡萝卜素产量继续提高。接下来可以把hxk1、dpp1、lpp1、fdh1、hmg2和gre2同时过表达,或许都可以使β-胡萝卜素产量继续提高,之后进行发酵培养基优化,分批补料优化等策略;同时可以通过进一步PATHWAY分析,研究与产物合成相关的代谢途径及其调控,进一步提高重组菌β-胡萝卜素的产量。
[1] VERWAAL R, WANG J, MEIJNEN J P, et al.High-level production of β-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous[J].Applied and Environmental Microbiology, 2007, 73(13):4342-4350.
[2] WU T, YE L J, ZHAO D D, et al.Membrane engineering: A novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli[J].Metabolic Engineering, 2017, 43:85-91.
[3] TANG W, WANG Y, ZHANG J, et al.Biosynthetic pathway of carotenoids in Rhodotorula and strategies for enhanced their production[J].Journal of Microbiology and Biotechnology, 2019, 29(4):507-517.
[4] WANG L, LIU Z, JIANG H, et al.Biotechnology advances in β-carotene production by microorganisms[J].Trends in Food Science &Technology, 2021, 111:322-332.
[5] SUN L A, ATKINSON C A, LEE Y G, et al.High-level β-carotene production from xylose by engineered Saccharomyces cerevisiae without overexpression of a truncated HMG1 (tHMG1)[J].Biotechnology and Bioengineering, 2020, 117(11):3522-3532.
[6] ZHUANG Y, YANG G Y, CHEN X H, et al.Biosynthesis of plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme[J].Metabolic Engineering, 2017, 42:25-32.
[7] YU T, ZHOU Y J, WENNING L, et al.Metabolic engineering of Saccharomyces cerevisiae for production of very long chain fatty acid-derived chemicals[J].Nature Communications, 2017, 8:15587.
[8] D’ESPAUX L, GHOSH A, RUNGUPHAN W, et al.Engineering high-level production of fatty alcohols by Saccharomyces cerevisiae from lignocellulosic feedstocks[J].Metabolic Engineering, 2017, 42:115-125.
[9] LU H Z, KERKHOVEN E J, NIELSEN J.Multiscale models quantifying yeast physiology:Towards a whole-cell model[J].Trends in Biotechnology, 2022, 40(3):291-305. [10] SUN L, LIU G Y, LI Y, et al.Metabolic engineering of Saccharomyces cerevisiae for efficient production of endocrocin and emodin[J].Metabolic Engineering, 2019, 54:212-221.
[11] 周琳, 梁轩铭, 赵磊.天然类胡萝卜素的生物合成研究进展[J].生物技术通报, 2022, 38(7):119-127. ZHOU L, LIANG X M, ZHAO L.Biosynthesis of natural carotenoids:Progress and perspective[J].Biotechnology Bulletin, 2022, 38(7):119-127.
[12] FURNEISEN J M, CARMAN G M.Enzymological properties of the LPP1-encoded lipid phosphatase from Saccharomyces cerevisiae[J].Biochimica et Biophysica Acta, 2000, 1484(1):71-82.
[13] 刘翔, 李由然, 张梁, 等.地衣芽孢杆菌中木糖操纵子受葡萄糖胁迫的转录调控特性[J].应用与环境生物学报, 2019, 25(3):695-701. LIU X, LI Y R, ZHANG L, et al.The transcriptional regulation characteristics of xylose-inducible promoter in Bacillus licheniformis[J].Chinese Journal of Applied and Environmental Biology, 2019, 25(3):695-701.
[14] PENG B Y, WOOD R J, NIELSEN L K, et al.An expanded heterologous GAL promoter collection for diauxie-inducible expression in Saccharomyces cerevisiae[J].ACS Synthetic Biology, 2018, 7(2):748-751.
[15] HERRERO P, GAL NDEZ J, RUIZ N, et al.Transcriptional regulation of the Saccharomyces cerevisiae HXK1, HXK2 and GLK1 genes[J].Yeast, 1995, 11(2):137-144.
NDEZ J, RUIZ N, et al.Transcriptional regulation of the Saccharomyces cerevisiae HXK1, HXK2 and GLK1 genes[J].Yeast, 1995, 11(2):137-144.
[16] CHEN C N, PORUBLEVA L, SHEARER G, et al.Associating protein activities with their genes:Rapid identification of a gene encoding a methylglyoxal reductase in the yeast Saccharomyces cerevisiae[J].Yeast, 2003, 20(6):545-554.
[17] GARDNER R, CRONIN S, LEADER B, et al.Sequence determinants for regulated degradation of yeast 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein[J].Molecular Biology of the Cell, 1998, 9(9):2611-2626.
[18] WYSOCKA-KAPCINSKA M, LUTYK-NADOLSKA J, KILISZEK M, et al.Functional expression of human HMG-CoA reductase inSaccharomyces cerevisiae:A system to analyse normal and mutated versions of the enzyme in the context of statin treatment[J].Journal of Applied Microbiology, 2009, 106(3):895-902.
[19] TOKE D A, BENNETT W L, DILLON D A, et al.Isolation and characterization of the Saccharomyces cerevisiae DPP1 gene encoding diacylglycerol pyrophosphate phosphatase[J].The Journal of Biological Chemistry, 1998, 273(6):3278-3284.
[20] SCHUCHT R, WIRTH D, MAY T.Precise regulation of transgene expression level and control of cell physiology[J].Cell Biology and Toxicology, 2010, 26(1):29-42.