蜂蜜含有丰富的营养物质和生物活性成分,具有抗氧化、抑菌、抗炎等多种作用[1-2],是天然的抗氧化剂和抑菌剂。目前研究表明蜂蜜中约含有200种物质[3],其中80%的物质是大多数蜂蜜共有的,其他物质则取决于蜜源植物、蜂种、环境气候、地理来源和贮存条件等[4]。蜜源植物种类丰富,因此形成了丰富的蜂蜜品种。
近几十年来,不同植物和地理来源蜂蜜的抗氧化能力以及对临床和食源性病菌的抑菌活性受到广泛关注[5-11],而且抑菌活性被认为是决定蜂蜜品质的重要评价指标[12]。麦卢卡蜜被誉为新西兰“国宝级”蜂蜜,是蜜蜂采集麦卢卡树(Leptospermum scoparium)酿造而成。其中,甲基乙二醛(methylglyoxal,MGO)被认为是麦卢卡蜜中主要的抑菌成分。独麦素(unique manuka factor,UMF)等级用来评估麦卢卡蜜的抑菌活性,该等级是以麦卢卡蜜杀菌活性的苯酚当量表示,等级越高,表明抑菌活性越强。麦卢卡蜜因其含有的独特抑菌物质和强大的抗氧化能力成为国内外学者研究的热点对象[13],并且目前其已被开发成医疗级蜂蜜,作为治疗伤口感染的局部药物制剂。DENG等[14]比较了荞麦蜜和麦卢卡蜜的抗菌和细胞抗氧化能力,结果表明,荞麦蜜对金黄色葡萄球菌和铜绿假单胞菌的抑菌活性与麦卢卡蜜相当,而且荞麦蜜的细胞抗氧化能力强于麦卢卡蜜。有研究比较了麦卢卡蜜、荞麦蜜、石楠蜜、蜜露蜜和多花蜜的抗氧化能力,结果表明麦卢卡蜜的总酚含量和抗氧化能力显著高于其他蜂蜜[6]。ZHANG等[15]研究指出茴香蜜、藿香蜜和石榴蜜对金黄色葡萄球菌(Staphylococcus aureus)的抑菌活性高于麦卢卡蜜(UMF 20+),但是其抗氧化能力低于麦卢卡蜜。GKOUTZOUVELIDOU等[6]比较了利姆诺斯岛蜂蜜和麦卢卡蜂蜜对10种临床相关细菌的抑菌活性,结果发现所有的利姆诺斯岛蜂蜜均有抑菌活性,甚至一些蜂蜜样品的抑菌活性与麦卢卡蜜(UMF 30+)相当。目前研究逐渐发现细菌对蜂蜜的敏感性与细菌的种类及蜂蜜的来源有关[16],所以对不同来源蜂蜜抑制多种细菌的研究很有必要,可以充分表征蜂蜜的抑菌活性。
植物的药用特性可以通过蜂蜜传递[17-18],所以也有人将来源于药用植物的蜂蜜作为研究目标[19-21]。基于本实验室前期的中国蜂蜜的抑菌实验筛查并结合蜜源植物的药用特性,本研究最后选择了3种抑菌活性较好的药用植物来源的蜂蜜(茴香蜜、枸杞蜜和玄参蜜)进行抗氧化和抑菌能力的深入分析,以期发掘和开发具有抗氧化和抑菌活性较好的蜂蜜,并为蜂蜜在医学方面研究和应用提供帮助。
1 材料与方法
1.1 材料与试剂
蜂蜜样品的来源信息见表1,其中茴香蜜、枸杞蜜、玄参蜜由当地蜂农提供,麦卢卡蜜(UMF 20+)购自新西兰海恩斯蜂业有限公司。所有蜂蜜样品避光保存在-20 ℃冰箱直至分析。
表1 蜂蜜样品的来源信息
Table 1 The origin of honey samples

蜂蜜样品植物来源蜂种来源地理来源采样时间麦卢卡蜜Leptospermum scoparium意大利蜜蜂新西兰 2018茴香蜜 Foeniculum vulgare Mill.意大利蜜蜂甘肃省 2020.7枸杞蜜 Lycium chinense Miller意大利蜜蜂宁夏回族自治区2020.7玄参蜜 Scrophularia ningpoensis Hemsl.意大利蜜蜂重庆市 2020.7
实验中使用的所有菌株:金黄色葡萄球菌(S.aureus ATCC 29213)、表皮葡萄球菌(Staphylococcus epidermidis ATCC 12228)、大肠杆菌(Escherichia coli ATCC 25922)、鲍曼不动杆菌(Acinetobacter baumannii ATCC 19606)、铜绿假单胞菌(Pseudomonas aeruginosa ATCC 27853)和白色念珠菌(Candida albicans ATCC 90028),上海北诺生物科技有限公司;福林酚,上海麦克林生化科技有限公司;总抗氧化能力检测试剂盒[FRAP(ferric ion reducing/antioxidant power)法],上海碧云天生物技术有限公司;其他试剂均为国产分析纯。
1.2 仪器与设备
EL20 pH计,梅特勒-托利多仪器(上海)有限公司;UV-2550岛津分光光度计,日本岛津有限公司;Synergy HTX多功能酶标仪,美国伯腾仪器有限公司。
1.3 实验方法
1.3.1 蜂蜜孢粉学鉴定
蜂蜜的植物来源鉴定参考BELAY等[22]的孢粉学分析方法并稍作修改。取10 g蜂蜜充分溶解在20 mL蒸馏水中,4 000 r/min离心10 min后去掉上清液,沉淀部分加20 mL蒸馏水再次离心,去掉上清液,最后底部留约0.5 mL 溶液,振荡混匀。取20 μL样液滴在载玻片上,盖上盖玻片,用滤纸吸去多余样液。先在低倍镜下找到花粉,然后换到高倍镜下观察花粉形态,随机选取10个视野,且花粉总数不少于200个,统计计算花粉比例。计算如公式(1)所示:
某种花粉比例![]()
(1)
1.3.2 基本理化指标
蜂蜜的含水量用手持式折射仪测定。将10 g蜂蜜样品溶解在75 mL蒸馏水中,用pH计在25 ℃测定样品的pH值[23]。电导率参照GB/T 18932.15—2003蜂蜜电导率测定方法。配制500 g/L蜂蜜水溶液,在635 nm处测定吸光度(absorbance,Abs)。利用公式mm Pfund=38.70+371.39×Abs将吸光度转化为Pfund单位(mm)[24]。参考ZHANG等[25]的方法测定蜂蜜中蛋白质含量。淀粉酶活性参照GB/T 183932.16—2003《蜂蜜中淀粉酶值的测定方法 分光光度法》。
1.3.3 总酚含量
采用福林酚(Folin-Ciocalteu)比色法测定总酚含量[26]。以没食子酸含量(0.04~0.24 mg/mL)绘制标准曲线,回归方程为y=3.045x+0.022(R2=0.999 6)。结果表示为100 g蜂蜜中没食子酸当量(gallic acid equivalent,GAE),单位为mg GAE/100 g。
1.3.4 抗氧化能力测定
1.3.4.1 DPPH自由基清除能力测定
参考BUENO-COSTA等[12]方法,并作适当修改。取100 μL蜂蜜溶液(0.2 g/mL)与100 μL DPPH乙醇溶液(0.08 mg/mL,现配现用)混合,并在室温下避光反应 30 min,在517 nm 波长下测定吸光度。以维生素C含量(5~50 μg/mL)绘制标准曲线,回归方程为y=-28.539x+1.601(R2=0.998 8)。结果表示为每100 g蜂蜜中毫克维生素C当量(ascorbic acid equivalent,AAE),单位为mg AAE/100 g。
1.3.4.2 ABTS阳离子自由基清除能力测定
参考BICUDO DE ALMEIDA-MURADIAN等[27]的方法,并稍作修改。制备ABTS母液:将7.5 mL 7 mmol/L ABTS溶液和132 μL 140 mmol/L过硫酸钾溶液混合并在室温下避光反应16 h。在使用前,把ABTS母液稀释为在734 nm处吸光度值为0.7~0.8。在96孔板中加入5 μL蜂蜜溶液(0.2 g/mL)和200 μL ABTS工作液,混匀后室温避光孵育6 min,测定734 nm处的吸光度。Trolox溶液标准曲线为0.1~1.3 mmol/L,回归方程为y=-0.369x+0.669(R2=0.999 8)。结果用mmol TE/kg表示(TE:Trolox equivalent)。
1.3.4.3 铁离子还原/抗氧化能力(ferric ion reducing/antioxidant power,FRAP)测定
采用总抗氧化能力检测试剂盒(FRAP法)检测蜂蜜抗氧化能力。将5 μL 0.2 g/mL蜂蜜溶液和180 μL FRAP工作液混合均匀,然后在37 ℃孵育5 min,于593 nm处测定吸光度。以Trolox溶液(0.05~1.3 mmol/L)为横坐标,吸光度值为纵坐标绘制标准曲线,回归方程为y=0.673x+0.008(R2=0.999 6)。结果表示为mmol TE/kg。
1.3.5 过氧化氢含量测定
参考ZHANG等[15]的方法,并稍作修改。称量1 g蜂蜜样品用蒸馏水定容至30 mL,然后用过氧化氢定量分析试剂盒(水兼容性)测定蜂蜜溶液中过氧化氢的含量。
1.3.6 抑菌能力测定
1.3.6.1 琼脂扩散法
参考GUO等[28]的方法,并稍作修改。用无菌蒸馏水配制500 g/L蜂蜜溶液。取25 mL灭菌营养琼脂培养基平铺于75 mm的培养皿中使其冷却凝固。在培养皿上涂布100 μL菌悬液(1×106 CFU/mL),然后用打孔器在培养基上打直径为8 mm的孔。向孔中加入100 μL待测蜂蜜溶液,以无菌水和10%(体积分数)苯酚溶液分别作为阴性对照和阳性对照。将培养皿置于恒温培养箱中37 ℃培养18 h,最后使用游标卡尺测量抑菌圈的直径(mm)。
1.3.6.2 微量肉汤稀释法
参考GKOUTZOUVELIDOU等[6]和DENG等[14]的方法,并稍作修改。采用微量肉汤稀释法测定蜂蜜样品对微生物的最小抑菌浓度(minimum inhibitory concentration,MIC)和最小杀菌浓度(minimum bactericidal concentration,MBC)。
采用两倍稀释法用LB肉汤培养基制备500.00、250.00、200.00、125.00、100.00、62.50、50.00、31.25、25.00、12.50 g/L 10个不同浓度的蜂蜜样品溶液并将菌悬液稀释至1×107 CFU/mL。将180 μL被测蜂蜜溶液和20 μL菌悬液混合,加入96孔细菌培养板(未添加细菌的孔和未添加蜂蜜的孔分别作为阴性生长对照和阳性生长对照)。用酶标仪在600 nm处测量培养前和37 ℃培养18 h后的吸光度。蜂蜜抑制微生物生长的最低浓度为MIC。
取微量肉汤稀释试验中无明显微生物生长的各孔10 μL悬液在营养琼脂平板上培养,37 ℃孵育24 h,平板上无菌落出现的最低浓度为MBC。
1.4 数据处理
所有蜂蜜样品均测定3个平行,结果以平均值(mean)±标准差(SD)表示。蜂蜜样品之间的统计学差异(P<0.05)采用Tukey检验的方差分析。研究参数之间的相关性通过Pearson检验得到,在显著性P<0.05的水平上用双尾检验评估显著性。
2 结果与分析
2.1 蜂蜜理化性质分析
4种蜂蜜的孢粉学结果及基本理化参数见表2。单花蜂蜜要求主要花粉粒所占比例超过45%[29]。孢粉学结果表明麦卢卡蜜、茴香蜜、枸杞蜜和玄参蜜的相应蜜源花粉率为70.85%~82.65%,所以4种蜂蜜都为纯度较高的单花蜂蜜。蜂蜜的含水量与天气、季节、酿造时间等有关[30]。4种蜂蜜的含水量为16.50%~17.97%,都低于规定的蜂蜜最大含水量20%[31-32]。本研究中所有蜂蜜样品的pH值均在ALJOHAR等[33]研究所指出的范围内,即3.24~6.10。蜂蜜的电导率取决于蜂蜜的灰分(矿物质含量)和酸度[27],而且这一参数还被用作鉴定单花蜂蜜以及评价蜂蜜质量的一个重要指标[34]。本文中4种蜂蜜的电导率为247.67~453.67 μS/cm,均符合相关标准规定的最大值800 μS/cm[31-32]。蜂蜜的颜色受蜜源植物影响,此外其可能在贮存过程中发生变化(可能受贮存温度以及蜂蜜成分的影响)[35]。浅色蜂蜜的味道清淡,深色蜂蜜的味道较浓郁[36]。本研究中4种蜂蜜的色值具有显著差异(P<0.05),其中枸杞蜜的颜色最浅[(53.78±0.37) mm],麦卢卡蜜的颜色最深[(193.67±0.77) mm]。蜂蜜的蛋白质含量最低的是枸杞蜜[(4.63±0.26) mg/g],最高的则为茴香蜜[(9.49±0.12) mg/g],这与先前研究的意大利蜜蜂蜂蜜的蛋白质含量(2~16 mg/g)一致[37]。研究表明淀粉酶活性可以表征蜂蜜的新鲜度和加工程度[35]。麦卢卡蜜的淀粉酶值显著低于其他3种蜂蜜。过氧化氢通常被认为是许多蜂蜜发挥抗菌作用的主要物质[38-39]。本研究中茴香蜜的过氧化氢含量最高,而麦卢卡蜜的过氧化氢含量最低,这可能是由于麦卢卡蜜中的MGO通过抑制葡萄糖氧化酶的活性,从而影响麦卢卡蜜中过氧化氢的含量[40-41]。蜂蜜中过氧化氢水平受多种因素的影响,如葡萄糖氧化酶氧化葡萄糖和多酚自氧化过程可以产生过氧化氢,而过氧化氢酶、金属酶、维生素C和芬顿反应可以降解过氧化氢[42]。
表2 蜂蜜样品的理化参数
Table 2 Physicochemical parameters of honey samples

检测指标麦卢卡蜜茴香蜜枸杞蜜玄参蜜蜜源植物花粉率/%70.85±2.42b82.65±4.36a71.25±3.24b72.88±2.55b含水量/%17.97±0.06a16.50±0.00c17.00±0.00b16.97±0.06bpH3.92±0.01c4.55±0.01a3.98±0.00b3.70±0.00d电导率/(μS/cm)432.00±3.00b431.67±1.15b247.67±1.53c453.67±0.58a色值/mm193.67±0.77a114.93±0.57c53.78±0.37d140.56±0.21b蛋白质含量/(mg/g)5.28±0.27b9.49±0.12a4.63±0.26c9.15±0.14a淀粉酶值/([mL/(g·h)])6.72±0.43d48.00±1.12a45.59±0.78b13.69±0.33c总酚含量/(mg GAE/kg)862.28±27.01a859.63±17.60a156.94±17.79c654.45±17.27bDPPH自由基清除能力/(mg AAE/100 g)24.20±0.30a19.62±0.36c4.98±0.66d21.93±0.29bABTS阳离子自由基清除能力/(mmol TE/kg)5.23±0.42ab6.01±0.64a1.23±0.31c4.87±0.28bFRAP/(mmol TE/kg)2.37±0.25a1.40±0.11b0.22±0.03c1.17±0.03b过氧化氢含量/(μmol/g)0.07±0.01c0.62±0.03a0.48±0.02b0.43±0.02b
注:每一行中不同小写字母表示差异显著(P<0.05)(下同)。
2.2 总酚含量和抗氧化能力分析
蜂蜜中的酚类物质来自于花蜜,是蜂蜜发挥抗氧化能力的主要成分[26]。总酚含量测定结果表明,麦卢卡蜜和茴香蜜的总酚含量相当(P>0.05),但显著高于玄参蜜和枸杞蜜(表2)。本研究中麦卢卡蜜的总酚含量(862.28±27.01) mg GAE/kg高于DENG等[14]的结果(561.00±2.82) mg GAE/kg,与LIN等[20]和ALZAHRANI等[43]测定结果相似。何亮亮等[44]测定枸杞蜜的总酚含量为401~532 mg GAE/kg,高于本研究测定结果。蜂蜜的总酚含量受蜂蜜的地理来源、植物来源和气候条件的影响[45]。由于蜂蜜氧化反应的复杂性,采用单一的抗氧化能力测定法评价蜂蜜抗氧化能力可能不准确[46],所以本研究采用清除DPPH自由基、ABTS阳离子自由基和总抗氧化能力(FRAP)来表征4种蜂蜜的抗氧化能力(表2)。结果显示,麦卢卡蜜的DPPH自由基清除能力[(24.20±0.30) mg AAE/100 g]和总抗氧化能力[(2.37±0.25) mmol TE/kg]显著高于其他3种蜂蜜(P<0.05),但是其ABTS阳离子自由基清除能力[(5.23±0.42) mmol TE/kg]和茴香蜜[(6.01±0.64) mmol TE/kg]及玄参蜜[(4.87±0.28) mmol TE/kg]没有显著差异。玄参蜜的DPPH自由基清除能力[(21.93±0.29)mg AAE/100 g]显著高于茴香蜜[(19.62±0.36) mg AAE/100 g],茴香蜜[(1.40±0.11) mmol TE/kg]的总抗氧化能力高于玄参蜜[(1.17±0.03) mmol TE/kg],但是无显著差异(P>0.05)。
2.3 理化参数相关性分析
本研究采用Pearson检验分析了蜂蜜样品理化参数之间的相关性,结果见表3。蜂蜜的含水量与色值和FRAP呈显著正相关,与蛋白质含量、淀粉酶活性和过氧化氢含量呈显著负相关。蜂蜜的含水量是表征蜂蜜成熟度的重要指标,在酿造过程中,蜂蜜的含水量下降,蛋白质含量和相关酶活(淀粉酶和葡萄糖氧化酶)升高[47]。TSAVEA等[48]研究也表明含水量与过氧化氢含量呈显著负相关。pH值与淀粉酶活性呈极显著相关(r=0.712,P<0.01)。电导率与多项参数之间具有显著相关性,如蛋白质含量(r=0.685,P<0.05)、总酚含量(r=0.918,P<0.01)和抗氧化能力(r>0.757,P<0.01)等。蜂蜜的电导率与色值相关[49]。本研究中色值与电导率呈极显著正相关(r=0.810,P<0.01),这是因为蜂蜜的颜色受矿物质含量的影响[35,50]。此外,色值与淀粉酶活性(r=-0.844,P<0.01)和过氧化氢含量(r=-0.730,P<0.01)呈极显著负相关,与总酚含量(r=0.822,P<0.01)和抗氧化能力(r>0.727,P<0.01)呈极显著正相关。总酚含量与DPPH自由基清除能力(r=0.932)、ABTS阳离子自由基清除能力(r=0.957)和FRAP(r=0.879),以及3种抗氧化方法之间(r>0.740)都存在很强的(P<0.01)线性相关关系。多项研究报道了蜂蜜的颜色与总酚含量和抗氧化能力有很强的相关性[51-53],并且总酚含量与抗氧化能力也密切相关[54-55]。蛋白质含量与ABTS阳离子自由基清除能力呈显著正相关(r=0.679,P<0.05),说明蜂蜜中一些蛋白质具有抗氧化活性[56-57]。淀粉酶活性与DPPH自由基清除能力(r=-0.691,P<0.05)、FRAP(r=-0.665,P<0.05)和过氧化氢含量(r=0.821,P<0.01),FRAP和过氧化氢含量(r=-0.669,P<0.05)也明显相关。
表3 蜂蜜理化参数之间的相关性分析
Table 3 Correlation analysis between physical and chemical parameters of honey samples
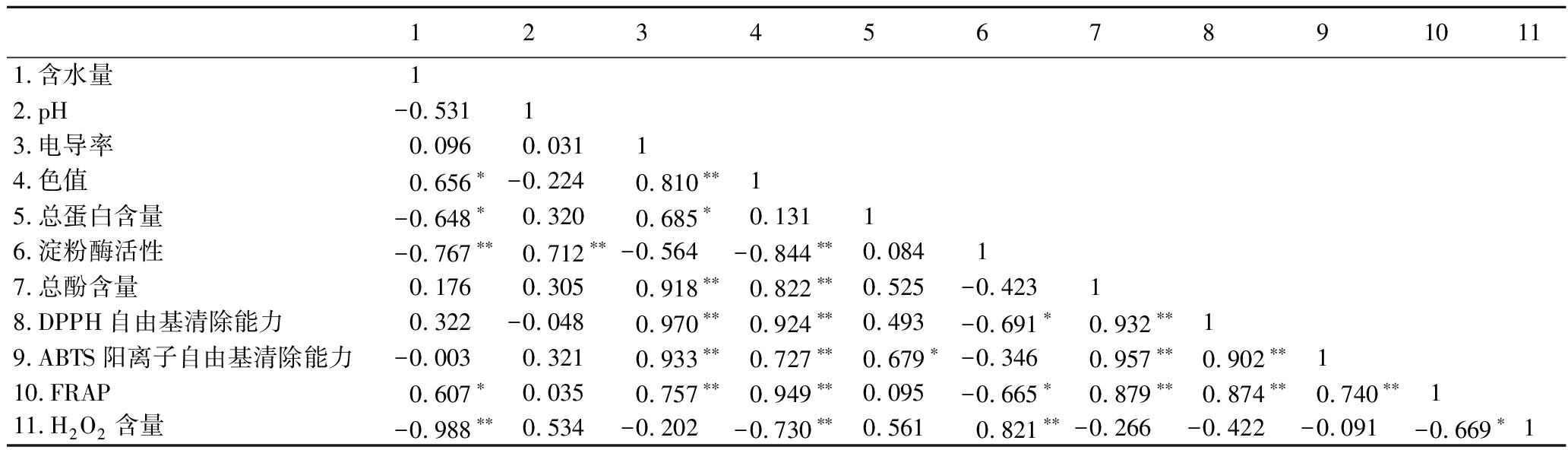
12345678910111.含水量12.pH-0.53113.电导率0.0960.03114.色值0.656∗-0.2240.810∗∗15.总蛋白含量-0.648∗0.3200.685∗0.13116.淀粉酶活性-0.767∗∗0.712∗∗-0.564-0.844∗∗0.08417.总酚含量0.1760.3050.918∗∗0.822∗∗0.525-0.42318.DPPH自由基清除能力0.322-0.0480.970∗∗0.924∗∗0.493-0.691∗0.932∗∗19.ABTS阳离子自由基清除能力-0.0030.3210.933∗∗0.727∗∗0.679∗-0.3460.957∗∗0.902∗∗110.FRAP0.607∗0.0350.757∗∗0.949∗∗0.095-0.665∗0.879∗∗0.874∗∗0.740∗∗111.H2O2含量-0.988∗∗0.534-0.202-0.730∗∗0.5610.821∗∗-0.266-0.422-0.091-0.669∗1
注:*表示在0.05水平上显著;** 表示表示在0.01水平上显著。
2.4 抗菌活性分析
蜂蜜样品对微生物的抑菌圈直径见表4。所有蜂蜜样品均未对P.aeruginosa和C.albicans产生抑菌圈(图1)。茴香蜜对S.aureus和A.baumannii产生的抑菌圈直径分别为(20.32±0.32) mm和(11.53±0.17) mm,显著高于其他3种蜂蜜(P<0.05)。ZHANG等[15]研究同样表明茴香蜜对S.aureus的抑菌圈明显超过麦卢卡蜜。枸杞蜜对S.aureus的抑菌圈与麦卢卡蜜相当(P>0.05)。麦卢卡蜜与茴香蜜对S.epidermidis产生的抑菌圈无显著差异,但都显著高于枸杞蜜和玄参蜜。此外,麦卢卡蜜对E.coli的抑菌圈显著大于其他3种蜂蜜。

A-S.aureus;B-S.epidermidis;C-E.coli;D-A.baumannii;E-P.aeruginosa;F-C.albicans
图1 蜂蜜样品对S.aureus、S.epidermidis、E.coli、A.baumannii、P.aeruginosa和C.albicans的抑菌圈图片
Fig.1 Inhibition zones images of honey samples against S.aureus,S.epidermidis,E.coli,A.baumannii,P.aeruginosa,and C.albicans
表4 蜂蜜样品对微生物的抑菌圈直径(琼脂扩散法)
Table 4 The inhibition zone diameter of honey samples against microorganisms (agar diffusion assay)
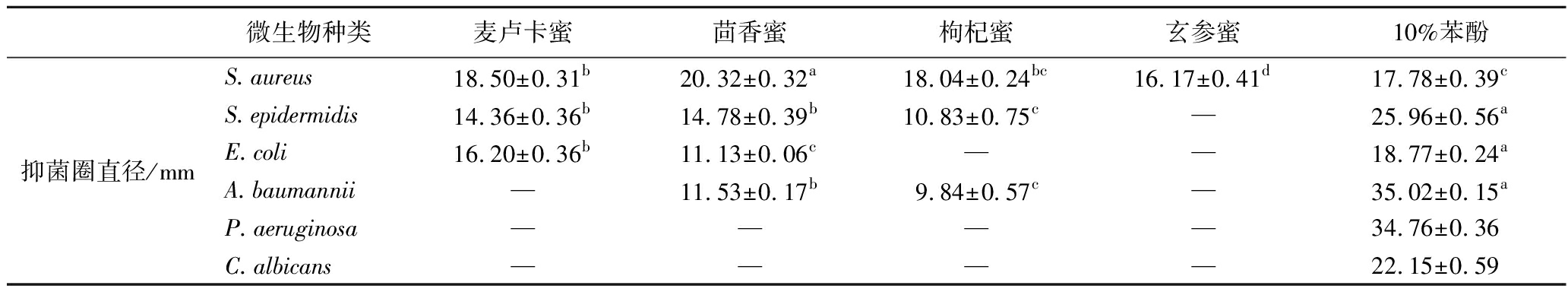
微生物种类麦卢卡蜜茴香蜜枸杞蜜玄参蜜10%苯酚抑菌圈直径/mmS.aureus18.50±0.31b20.32±0.32a18.04±0.24bc16.17±0.41d17.78±0.39cS.epidermidis14.36±0.36b14.78±0.39b10.83±0.75c—25.96±0.56aE.coli16.20±0.36b11.13±0.06c——18.77±0.24aA.baumannii—11.53±0.17b9.84±0.57c—35.02±0.15aP.aeruginosa————34.76±0.36C.albicans————22.15±0.59
注:—表示未检测到抑菌圈。
虽然琼脂扩散法具有简单和快速等优点,但是该方法依靠的是扩散作用,蜂蜜中的大分子质量物质的扩散(如蜜蜂防御素-1等)可能会受到阻碍。微量肉汤稀释法是抗菌剂与微生物直接接触,还可实现对抗菌剂的定量分析[58]。4种蜂蜜对微生物的MIC和MBC值见表5。所有蜂蜜样品对本研究中的微生物都有不同程度的抑菌活性。茴香蜜和枸杞蜜对S.aureus和A.baumannii的抑菌能力最好,MIC和MBC分别为50.00 g/L和100.00 g/L(对单一菌种的MIC和MBC相同),均低于麦卢卡蜜和玄参蜜。茴香蜜对S.epidermidis和P.aeruginosa的MIC和MBC均低于其他3种蜂蜜,表明茴香蜜对这3种细菌的抑菌能力最好。麦卢卡蜜对E.coli的抑菌能力最强,其在100.00 g/L浓度下就可抑制并杀死E.coli。4种蜂蜜对C.albicans的MIC均为500.00 g/L,但只有茴香蜜对C.albicans的MBC为500.00 g/L,其他3种均超过500.00 g/L。王晶波等[59]研究表明茴香蜂蜜对S.aureus的抑菌能力(MIC为75.00 g/L,MBC为15.00 g/L)比麦卢卡蜂蜜(UMF 15+)高,这与本研究结果相似。玄参蜜对S.aureus和P.aeruginosa的抑菌活性低于LIN等[20]报道的。ANTHIMIDOU等[60]研究发现麦卢卡蜜(UMF 25+)对S.aureus和P.aeruginosa的MIC分别为6.25%和12.5%(体积分数),这与本研究测定的结果基本相同。研究还发现S.aureus是对蜂蜜最敏感的菌,而C.albicans对包括麦卢卡蜜在内的蜂蜜敏感性较差,这一结果得到琼脂扩散试验的验证,并且与前人得出的结论一致[15,54,61]。
表5 蜂蜜样品对微生物的MIC和MBC
Table 5 The MIC and MBC values of honey samples against microorganisms

微生物种类麦卢卡蜜茴香蜜枸杞蜜玄参蜜MIC/(g/L)S.aureus62.5050.0050.00250.00S.epidermidis100.0062.50100.00500.00E.coli100.00200.00200.00500.00A.baumannii125.00100.00100.00500.00P.aeruginosa125.0062.50100.00500.00C.albicans500.00500.00500.00500.00MBC/(g/L)S.aureus62.5050.0050.00250.00S.epidermidis100.0062.50100.00>500.00E.coli100.00200.00200.00>500.00A.baumannii125.00100.00100.00>500.00P.aeruginosa125.00100.00100.00>500.00C.albicans>500.00500.00>500.00>500.00
为了探究3种药用植物来源蜂蜜(茴香蜜、枸杞蜜和玄参蜜)的抑菌成分,本研究分析了总体抗菌活性(以MIC表示)与理化参数、抗氧化能力之间的相关性。由表6可知,蜂蜜的含水量、电导率、蛋白质含量、总酚含量、抗氧化能力与蜂蜜的抗菌活性无显著相关性。研究表明蜂蜜的高酚酸含量有助于蜂蜜发挥抗菌活性[62-63],且部分蜂蜜的酚类或黄酮类化合物与抗菌活性之间存在显著相关性[64]。但是TRUCHADO等[65]发现蜂蜜的抗菌活性与总酚和单个酚类化合物不呈线性相关。ULUSOY等[66]也表明总酚含量与蜂蜜的抗菌活性不相关。蜂蜜的pH为酸性,不适合大部分细菌生长,相关性分析显示pH值与蜂蜜的抗菌能力显著负相关(P<0.05),表明在一定pH值范围内,pH值越高,反而对蜂蜜抗菌能力有正向影响,这可能是由于pH值的升高促进了蜂蜜中其他化学反应的发生。色值与蜂蜜的抗菌能力呈显著正相关(r≥0.673,P<0.05)。值得注意的是淀粉酶活性与细菌的MIC极显著负相关(P<0.01),表明较高的酶活性与蜂蜜较高的抗菌能力相关。TSAVEA等[48]研究同样发现淀粉酶活性与蜂蜜对S.aureus的抗菌能力呈显著负相关。过氧化氢含量与5种细菌的MIC呈显著负相关,表明蜂蜜中过氧化氢含量越高,蜂蜜的抗菌活性越强。FARKASOVSKA等[67]研究同样表明蜂蜜的总体抗菌活性(MIC)与H2O2水平密切相关。目前研究表明,蜂蜜的抗菌活性是pH(酸度)、高糖、低水活度、蛋白质(蜜蜂防御素-1、王浆主蛋白、外泌体囊泡等)、过氧化氢、酚类化合物、胶体结构、MGO等共同作用下的结果[13,58,68]。
表6 抗菌活性与理化参数之间的相关性
Table 6 The correlation between antibacterial activity (MIC) and physicochemical parameters
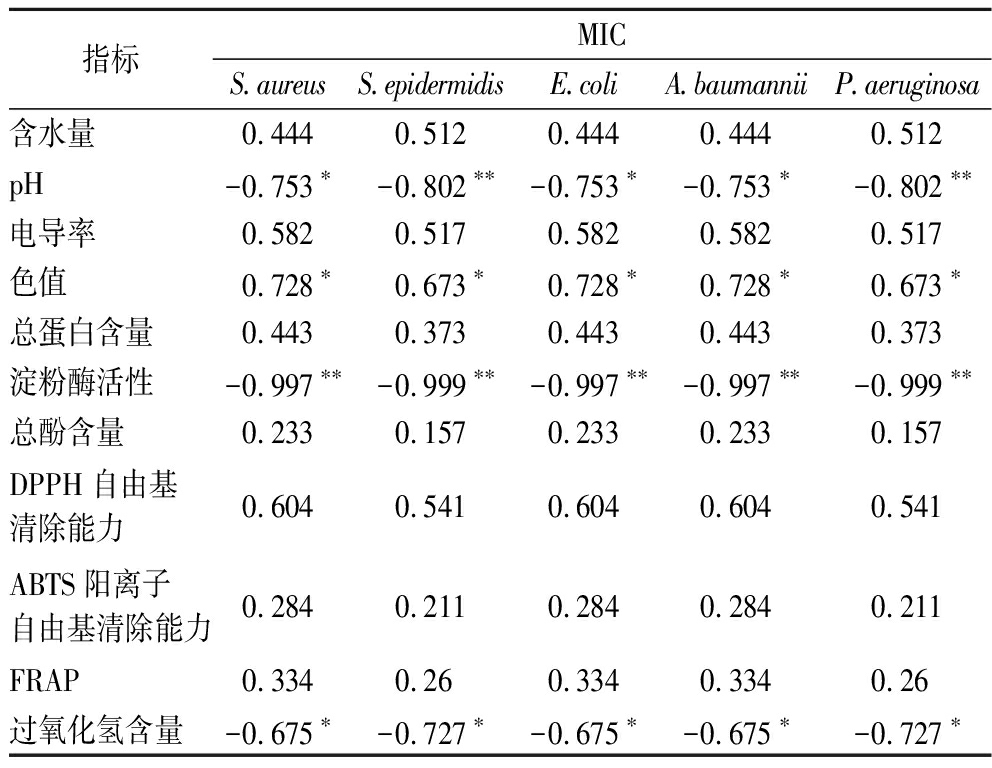
指标MICS.aureusS.epidermidisE.coliA.baumanniiP.aeruginosa含水量0.4440.5120.4440.4440.512pH-0.753∗-0.802∗∗-0.753∗-0.753∗-0.802∗∗电导率0.5820.5170.5820.5820.517色值0.728∗0.673∗0.728∗0.728∗0.673∗总蛋白含量0.4430.3730.4430.4430.373淀粉酶活性-0.997∗∗-0.999∗∗-0.997∗∗-0.997∗∗-0.999∗∗总酚含量0.2330.1570.2330.2330.157DPPH自由基清除能力0.6040.5410.6040.6040.541ABTS阳离子自由基清除能力0.2840.2110.2840.2840.211FRAP0.3340.260.3340.3340.26过氧化氢含量-0.675∗-0.727∗-0.675∗-0.675∗-0.727∗
注:*表示在0.05水平上显著;**表示表示在0.01水平上显著。
3 结论
在这项研究中,除麦卢卡蜜(UMF 20+)的淀粉酶活性外,4种蜂蜜样品的基本理化指标均符合相关标准规定。茴香蜜与麦卢卡蜜的总酚含量无显著差异,但总抗氧化能力低于麦卢卡蜜。茴香蜜和玄参蜜的蛋白质含量相当,均高于麦卢卡蜜。3种药蜜的过氧化氢含量显著高于麦卢卡蜜。此外,研究还发现了这些参数之间的各种相关性。抗菌试验表明蜂蜜对不同菌株的抗菌能力不同。茴香蜜对S.aureus和S.epidermidis的抗菌能力高于麦卢卡蜜,枸杞蜜对这2种细菌的抗菌能力总体与麦卢卡蜜相当。茴香蜜和枸杞蜜对A.baumannii和P.aeruginosa的抗菌活性也高于麦卢卡蜜。麦卢卡蜜对E.coli的抗菌能力高于其他3种蜂蜜(MIC和MBC为100.00 g/L),所有蜂蜜对C.albicans的抗菌能力都较差。3种药蜜的抗菌活性与pH、淀粉酶活性和过氧化氢含量显著相关。结果表明茴香蜜和枸杞蜜具有与麦卢卡蜜相当或者更高的抗菌活性,值得进一步开发和研究。
[1] CIANCIOSI D,FORBES-HERN NDEZ T Y,AFRIN S,et al.Phenolic compounds in honey and their associated health benefits:A review[J].Molecules,2018,23(9):2322.
NDEZ T Y,AFRIN S,et al.Phenolic compounds in honey and their associated health benefits:A review[J].Molecules,2018,23(9):2322.
[2] SERAGLIO S K T,SILVA B,BERGAMO G,et al.An overview of physicochemical characteristics and health-promoting properties of honeydew honey[J].Food Research International,2019,119:44-66.
[3] ESCUREDO O,M GUEZ M,FERN
GUEZ M,FERN NDEZ-GONZ
NDEZ-GONZ LEZ M,et al.Nutritional value and antioxidant activity of honeys produced in a European Atlantic area[J].Food Chemistry,2013,138(2-3):851-856.
LEZ M,et al.Nutritional value and antioxidant activity of honeys produced in a European Atlantic area[J].Food Chemistry,2013,138(2-3):851-856.
[4] RANNEH Y,AKIM A M,HAMID H A,et al.Honey and its nutritional and anti-inflammatory value[J].BMC Complementary Medicine and Therapies,2021,21(1):30.
[5] ALVAREZ-SUAREZ J M,GASPARRINI M,FORBES-HERN NDEZ T Y,et al.The composition and biological activity of honey:A focus on Manuka honey[J].Foods,2014,3(3):420-432.
NDEZ T Y,et al.The composition and biological activity of honey:A focus on Manuka honey[J].Foods,2014,3(3):420-432.
[6] GKOUTZOUVELIDOU M,PANOS G,XANTHOU M N,et al.Comparing the antimicrobial actions of Greek honeys from the island of Lemnos and Manuka honey from New Zealand against clinically important bacteria[J].Foods,2021,10(6):1042.
[7] ![]() K,
K, UCZYCKA D,
UCZYCKA D,![]() J,et al.Polish honey as a source of antioxidants—A comparison with Manuka honey[J].Journal of Apicultural Research,2020,59(5):939-945.
J,et al.Polish honey as a source of antioxidants—A comparison with Manuka honey[J].Journal of Apicultural Research,2020,59(5):939-945.
[8] OTMANI A,AMESSIS-OUCHEMOUKH N,BIRINCI C,et al.Phenolic compounds and antioxidant and antibacterial activities of Algerian honeys[J].Food Bioscience,2021,42:101070.
[9] ![]() M,NOWAK D,K
M,NOWAK D,K
![]() BUKOWSKA L.Antioxidant properties and antimicrobial activity of Manuka honey versus Polish honeys[J].Journal of Food Science and Technology,2020,57(4):1269-1277.
BUKOWSKA L.Antioxidant properties and antimicrobial activity of Manuka honey versus Polish honeys[J].Journal of Food Science and Technology,2020,57(4):1269-1277.
[10] HULEA A,OBI TIOIU D,COCAN I,et al.Diversity of monofloral honey based on the antimicrobial and antioxidant potential[J].Antibiotics,2022,11(5):595.
TIOIU D,COCAN I,et al.Diversity of monofloral honey based on the antimicrobial and antioxidant potential[J].Antibiotics,2022,11(5):595.
[11] ANAND S,DEIGHTON M,LIVANOS G,et al.Agastache honey has superior antifungal activity in comparison with important commercial honeys[J].Scientific Reports,2019,9(1):18197.
[12] BUENO-COSTA F M,ZAMBIAZI R C,BOHMER B W,et al.Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul,Brazil[J].LWT-Food Science and Technology,2016,65:333-340.
[13] MAJTAN J,BUCEKOVA M,KAFANTARIS I,et al.Honey antibacterial activity:A neglected aspect of honey quality assurance as functional food[J].Trends in Food Science &Technology,2021,118:870-886.
[14] DENG J L,LIU R,LU Q,et al.Biochemical properties,antibacterial and cellular antioxidant activities of buckwheat honey in comparison to Manuka honey[J].Food Chemistry,2018,252:243-249.
[15] ZHANG Y Z,SI J J,LI S S,et al.Chemical analyses and antimicrobial activity of nine kinds of unifloral Chinese honeys compared to Manuka honey (12+ and 20+)[J].Molecules,2021,26(9):2778.
[16] ![]() P M,SZWEDA P,
P M,SZWEDA P,![]() I,et al.Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour,phenolic content,antioxidant capacity and other parameters[J].Letters in Applied Microbiology,2016,62(3):269-276.
I,et al.Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour,phenolic content,antioxidant capacity and other parameters[J].Letters in Applied Microbiology,2016,62(3):269-276.
[17] ALVAREZ-SUAREZ J M,TULIPANI S,ROMANDINI S,et al.Contribution of honey in nutrition and human health:A review[J].Mediterranean Journal of Nutrition and Metabolism,2010,3(1):15-23.
[18] WANG K,WAN Z R,OU A Q,et al.Monofloral honey from a medical plant,Prunella vulgaris,protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats[J].Food &Function,2019,10(7):3828-3838.
[19] BOBIS O,MOISE A R,BALLESTEROS I,et al.Eucalyptus honey:Quality parameters,chemical composition and health-promoting properties[J].Food Chemistry,2020,325:126870.
[20] LIN T X,HUANG L,CHENG N N,et al.The in vitro and in vivo antibacterial activities of uniflorous honey from a medicinal plant,Scrophularia ningpoensis Hemsl.,and characterization of its chemical profile with UPLC-MS/MS.[J].Journal of Ethnopharmacology,2022,296:115499.
[21] KADRI S M,ZALUSKI R,PEREIRA LIMA G P,et al.Characterization of Coffea arabica monofloral honey from Espírito Santo,Brazil[J].Food Chemistry,2016,203:252-257.
[22] BELAY A,SOLOMON W K,BULTOSSA G,et al.Botanical origin,colour,granulation,and sensory properties of the Harenna forest honey,Bale,Ethiopia[J].Food Chemistry,2015,167:213-219.
[23] BENTABOL MANZANARES A,HERN NDEZ GARC
NDEZ GARC A Z,RODR
A Z,RODR GUEZ GALD
GUEZ GALD N B,et al.Physicochemical characteristics of minor monofloral honeys from Tenerife,Spain[J].LWT-Food Science and Technology,2014,55(2):572-578.
N B,et al.Physicochemical characteristics of minor monofloral honeys from Tenerife,Spain[J].LWT-Food Science and Technology,2014,55(2):572-578.
[24] WHITE J W,COLLABORATORS,BEATY M R,et al.Instrumental color classification of honey:Collaborative study[J].Journal of AOAC INTERNATIONAL,1984,67(6):1129-1131.
[25] ZHANG Y,WANG Y X,ZHAO H A,et al.Characterization of novel protein component as marker for floral origin of jujube (Ziziphus jujuba Mill.) honey[J].Journal of Agricultural and Food Chemistry,2019,67(44):12255-12263.
[26] ![]() A.Effect of filtration on colour,antioxidant activity and total phenolics of honey[J].LWT-Food Science and Technology,2014,57(2):767-774.
A.Effect of filtration on colour,antioxidant activity and total phenolics of honey[J].LWT-Food Science and Technology,2014,57(2):767-774.
[27] BICUDO DE ALMEIDA-MURADIAN L,MONIKA BARTH O,DIETEMANN V,et al.Standard methods for Apis mellifera honey research[J].Journal of Apicultural Research,2020,59(3):1-62.
[28] GUO N N,ZHAO L W,ZHAO Y Z,et al.Comparison of the chemical composition and biological activity of mature and immature honey:An HPLC/QTOF/MS-based metabolomic approach[J].Journal of Agricultural and Food Chemistry,2020,68(13):4062-4071.
[29] CORVUCCI F,NOBILI L,MELUCCI D,et al.The discrimination of honey origin using melissopalynology and Raman spectroscopy techniques coupled with multivariate analysis[J].Food Chemistry,2015,169:297-304.
[30] MOHAMMED M E A.Factors affecting the physicochemical properties and chemical composition of bee′s honey[J].Food Reviews International,2022,38(6):1330-1341.
[31] EUROPEAN COUNCIL.Council directive 2001/110/EC of 20 December 2001 relating honey[J].Official Journal of the European Communities,2002,45:47-52.
[32] CODEX ALIMENTARIUS COMMISSION.Revised codex standard for honey[S].Codex Standard,2001,12:1982.
[33] ALJOHAR H I,MAHER H M,ALBAQAMI J,et al.Physical and chemical screening of honey samples available in the Saudi market:An important aspect in the authentication process and quality assessment[J].Saudi Pharmaceutical Journal,2018,26(7):932-942.
[34] BOGDANOV S.Harmonised methods of the international honey commission[EB/OL].International Honey Commission,2009.Retrieved from http://www.ihc-platform.net/ihcmethods2009.pdf.
[35] DA SILVA P M,GAUCHE C,GONZAGA L V,et al.Honey:Chemical composition,stability and authenticity[J].Food Chemistry,2016,196:309-323.
[36] BOUSSAID A,CHOUAIBI M,REZIG L,et al.Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia[J].Arabian Journal of Chemistry,2018,11(2):265-274.
[37] WON S R,LI C Y,KIM J W,et al.Immunological characterization of honey major protein and its application[J].Food Chemistry,2009,113(4):1334-1338.
[38] BRUDZYNSKI K,ABUBAKER K,ST-MARTIN L,et al.Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey[J].Frontiers in Microbiology,2011,2:213.
[39] BUCEKOVA M,BUGAROVA V,GODOCIKOVA J,et al.Demanding new honey qualitative standard based on antibacterial activity[J].Foods,2020,9(9):1263.
[40] MAJTAN J,KLAUDINY J,BOHOVA J,et al.Methylglyoxal-induced modifications of significant honeybee proteinous components in Manuka honey:Possible therapeutic implications[J].Fitoterapia,2012,83(4):671-677.
[41] MAJTAN J,BOHOVA J,PROCHAZKA E,et al.Methylglyoxal may affect hydrogen peroxide accumulation in Manuka honey through the inhibition of glucose oxidase[J].Journal of Medicinal Food,2014,17(2):290-293.
[42] BRUDZYNSKI K.A current perspective on hydrogen peroxide production in honey.A review[J].Food Chemistry,2020,332:127229.
[43] ALZAHRANI H A,ALSABEHI R,BOUKRA L,et al.Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins[J].Molecules,2012,17(9):10540-10549.
L,et al.Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins[J].Molecules,2012,17(9):10540-10549.
[44] 何亮亮,赵浩安,刘新艳,等.枸杞蜜的抗氧化活性及其对DNA氧化损伤的保护作用[J].食品工业科技,2019,40(12):105-111.
HE L L,ZHAO H A,LIU X Y,et al.Antioxidant activity and protective effect on DNA oxidative damage of medlar honey[J].Science and Technology of Food Industry,2019,40(12):105-111.
[45] LACHMAN J,ORS K M,HEJTM
K M,HEJTM NKOV
NKOV A,et al.Evaluation of antioxidant activity and total phenolics of selected Czech honeys[J].LWT-Food Science and Technology,2010,43(1):52-58.
A,et al.Evaluation of antioxidant activity and total phenolics of selected Czech honeys[J].LWT-Food Science and Technology,2010,43(1):52-58.
[46] GHELDOF N,ENGESETH N J.Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples[J].Journal of Agricultural and Food Chemistry,2002,50(10):3050-3055.
[47] ZHANG G Z,TIAN J,ZHANG Y Z,et al.Investigation of the maturity evaluation indicator of honey in natural ripening process:The case of rape honey[J].Foods,2021,10(11):2882.
[48] TSAVEA E,VARDAKA F P,SAVVIDAKI E,et al.Physicochemical characterization and biological properties of pine honey produced across Greece[J].Foods,2022,11(7):943.
[49] HEMPATTARASUWAN P,SETTACHAIMONGKON S,DUANGMAL K.Impact of botanical source and processing conditions on physicochemical properties and antioxidant activity of honey in the northern part of Thailand[J].International Journal of Food Science & Technology,2019,54(12):3185-3195.
[50] K![]() DZIERSKA-MATYSEK M,TETER A,STRYJECKA M,et al.Relationships linking the colour and elemental concentrations of blossom honeys with their antioxidant activity:A chemometric approach[J].Agriculture,2021,11(8):702.
DZIERSKA-MATYSEK M,TETER A,STRYJECKA M,et al.Relationships linking the colour and elemental concentrations of blossom honeys with their antioxidant activity:A chemometric approach[J].Agriculture,2021,11(8):702.
[51] HALAGARDA M,GROTH S,POPEK S,et al.Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland[J].Antioxidants,2020,9(1):44.
[52] ANAND S,PANG E,LIVANOS G,et al.Characterization of physico-chemical properties and antioxidant capacities of bioactive honey produced from Australian grown Agastache rugosa and its correlation with colour and poly-phenol content[J].Molecules,2018,23(1):108.
[53] SAXENA S,GAUTAM S,SHARMA A.Physical,biochemical and antioxidant properties of some Indian honeys[J].Food Chemistry,2010,118(2):391-397.
[54] ALVAREZ-SUAREZ J M,TULIPANI S,D AZ D,et al.Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color,polyphenol content and other chemical compounds[J].Food and Chemical Toxicology,2010,48(8-9):2490-2499.
AZ D,et al.Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color,polyphenol content and other chemical compounds[J].Food and Chemical Toxicology,2010,48(8-9):2490-2499.
[55] AVILA S,HORNUNG P S,TEIXEIRA G L,et al.Bioactive compounds and biological properties of Brazilian stingless bee honey have a strong relationship with the pollen floral origin[J].Food Research International,2019,123:1-10.
[56] GHELDOF N,WANG X H,ENGESETH N J.Identification and quantification of antioxidant components of honeys from various floral sources[J].Journal of Agricultural and Food Chemistry,2002,50(21):5870-5877.
[57] RAM N-SIERRA J M,VILLANUEVA M A,YAM-PUC A,et al.Antimicrobial and antioxidant activity of proteins isolated from Melipona beecheii honey[J].Food Chemistry-X,2022,13:100177.
N-SIERRA J M,VILLANUEVA M A,YAM-PUC A,et al.Antimicrobial and antioxidant activity of proteins isolated from Melipona beecheii honey[J].Food Chemistry-X,2022,13:100177.
[58] HOSSAIN M L,LIM L Y,HAMMER K,et al.A review of commonly used methodologies for assessing the antibacterial activity of honey and honey products[J].Antibiotics,2022,11(7):975.
[59] 王晶波,秦文,杨倬,等.微孔板改良法评价多种国内外单花蜜的体外抑菌活性[J].中国食物与营养,2019,25(6):29-33.
WANG J B,QIN W,YANG Z,et al.Antibacterial activities of various domestic and foreign monofloral honeys in vitro by microplates modified method[J].Food and Nutrition in China,2019,25(6):29-33.
[60] ANTHIMIDOU E,MOSSIALOS D.Antibacterial activity of Greek and Cypriot honeys against Staphylococcus aureus and Pseudomonas aeruginosa in comparison to Manuka honey[J].Journal of Medicinal Food,2013,16(1):42-47.
[61] HERMANNS R,CREMERS N A J,LEEMING J P,et al.Sweet relief:Determining the antimicrobial activity of medical grade honey against vaginal isolates of Candida albicans[J].Journal of Fungi,2019,5(3):85.
[62] ALVAREZ-SUAREZ J M,GIAMPIERI F,BRENCIANI A,et al.Apis mellifera vs Melipona beecheii Cuban polifloral honeys:A comparison based on their physicochemical parameters,chemical composition and biological properties[J].LWT-Food Science and Technology,2018,87:272-279.
[63] JAVIER LEYVA-JIMENEZ F J,LOZANO-SANCHEZ J,BORRAS-LINARES I,et al.Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria[J].LWT-Food Science and Technology,2019,101:236-245.
[64] PEL EZ-ACERO A,COBOS-VELASCO J E,GONZ
EZ-ACERO A,COBOS-VELASCO J E,GONZ LEZ-LEMUS U,et al.Bioactive compounds and antibacterial activities in crystallized honey liquefied with ultrasound[J].Ultrasonics Sonochemistry,2021,76:105619.
LEZ-LEMUS U,et al.Bioactive compounds and antibacterial activities in crystallized honey liquefied with ultrasound[J].Ultrasonics Sonochemistry,2021,76:105619.
[65] TRUCHADO P,L PEZ-G
PEZ-G LVEZ F,GIL M I,et al.Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics[J].Food Chemistry,2009,115(4):1337-1344.
LVEZ F,GIL M I,et al.Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics[J].Food Chemistry,2009,115(4):1337-1344.
[66] ULUSOY E,KOLAYLI S,SARIKAYA A O.Antioxidant and antimicrobial activity of different floral origin honeys from Turkiye[J].Journal of Food Biochemistry,2010,34:321-335.
[67] FARKASOVSKA J,BUGAROVA V,GODOCIKOVA J,et al.The role of hydrogen peroxide in the antibacterial activity of different floral honeys[J].European Food Research and Technology,2019,245(12):2739-2744.
[68] BRUDZYNSKI K,SJAARDA C P.Colloidal structure of honey and its influence on antibacterial activity[J].Comprehensive Reviews in Food Science and Food Safety,2021,20(2):2063-2080.