黄曲霉毒素是由黄曲霉和寄生曲霉产生的次级代谢产物[1-2],常见的有黄曲霉毒素B1、B2、M1、G1、G2。其中黄曲霉毒素B1(aflatoxins,AFB1)和黄曲霉毒素M1(aflatoxins,AFM1)是迄今发现毒性最强的霉菌毒素之二,具有强致癌性、致突变性和致畸性,占动物和食品饲料中所有黄曲霉毒素污染的75%[3-4],早已被国际癌症研究机构(International Agency for Researchon Cancer,IARC)列为人类致癌物(第I类)[5]。因此,大多数国家都对黄曲霉毒素有限量要求。例如,我国在GB 2761—2017《食品安全国家标准食品中真菌毒素限量》规定粮食及粮食制品的AFB1含量不超过5 μg/kg,奶制品AFM1含量不超过0.5 μg/kg[6-8],欧盟委员会将食品和牛奶中AFB1和AFM1的最大残留量定为0.5~50 ng/mL[9-10]。所以,对食品中黄曲霉毒素的及时检测对保障人体健康具有重要意义。常见的检测方法以大型仪器检测为主,包括薄层色谱法、质谱法[11]、高效液相色谱法[12]、液相色谱-质谱法[13]等,这些检测方法具有特异性强,灵敏度高的特点,但仪器价格高昂且需要专业技术人才操作、待检样品需要复杂的前处理等弊端,大大限制了其在样品现场快速分析中的应用[14]。此外,基于抗体为识别分子的免疫方法如酶联免疫吸附测定法[15]、免疫层析法[16]等,由于抗体价格高和稳定性差等不足,也限制了其进一步的应用。因此,迫切需要开发简便、快速、特异性好、精准度高的检测方法用于黄曲霉毒素的检测。
适配体(aptamer,Apt)是一种单链寡核苷酸序列,通过指数富集的配基系统进化技术(systematic evolution of ligands by exponential enrichment,SELEX)在体外筛选[17],具有成本较低、稳定性高、特异性强等优势。适配体传感器利用适配体作为识别元件,通过信号转换元件将特异性识别结合作用转化为电信号、颜色、荧光或拉曼信号。量子点[18]、金纳米颗粒[19-20]、石墨烯[21]等具有光/电稳定性的纳米材料可作为信号转换元件,根据信号转化方式的不同,将适配体传感器分为电化学适配体传感器、比色适配体传感器、荧光适配体传感器和表面增强拉曼散射适配体传感器,与传统检测方法相比,这些传感器具有简单、快捷、易于操作等优点,已经广泛应用于食品分析中。基于适配体传感器的独特优势,本文综述了近年来基于电化学法、比色法、荧光法、拉曼光谱法等开发的适配体传感器在检测黄曲霉毒素中的研究进展,探讨了其在检测过程中的优势及局限性,并进一步对其在未来发展中的前景进行展望,为新型适配体传感器的开发提供参考。
1 电化学适配体传感器在黄曲霉毒素检测中的应用
电化学适配体传感器检测黄曲霉毒素主要通过黄曲霉毒素与适配体结合后引起的电化学信号的变化来实现。电化学法使用的电极主要以玻碳电极(glassy carbon electrode,GCE)和金电极为主,其中,GCE在与适配体组装之前一般需要进行活化或修饰处理。CUI等[22]在裸GCE上修饰羧化氧化石墨烯(graphene oxide,GO)和聚(4-乙烯基吡啶)[poly(4-vinyl pyridine),P4VP]组成的复合膜,建立了新型电化学适配体传感器用于准确、灵敏的检测AFB1。如图1-a所示,当导电性弱的Apt与羧基化GO连接后,复合膜表面电流下降,AFB1被Apt捕获后,由于电子转移进一步受到阻碍,电流信号不断下降,从而实现了对AFB1的检测。
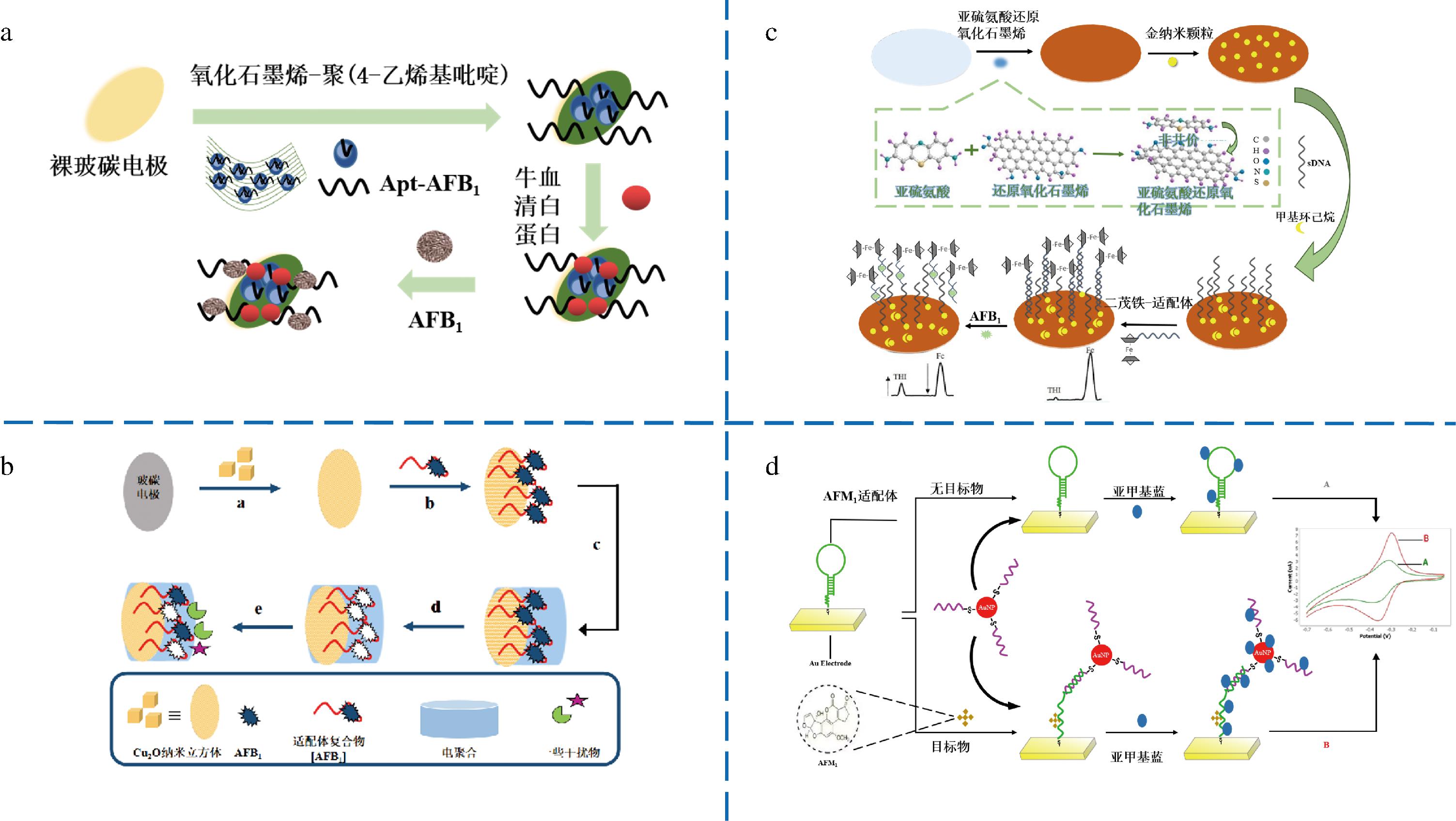
a-ON/OFF电化学适配体传感器检测AFB1[22];b-Apt-MIP纳米杂交体电化学适配体传感器检测AFB1[23];c-可编程适配体传感器检测AFB1[24];d-发夹状结构适配体传感器检测AFM1[28]
图1 电化学适配体传感器的传感示意图
Fig.1 Sensing diagram of electrochemical aptasensor
ROUSHANI等[23]基于Cu2O纳米立方体修饰的GCE与适配体-分子印迹聚合物杂交,构建了一种新型电化学双识别策略检测AFB1,原理如图1-b所示。Cu2O纳米立方体通过增加表体积比来增加适配体的负载,由于适配体与Cu2O纳米立方体形成Cu-N键产生空间阻碍降低电子转移,电流信号急剧下降,当目标物存在时,Apt对AFB1特异性识别和捕获,电子转移和空穴增加使得电流增加,实现准确经济高效地检测AFB1。
此外,为了提高电流信号强度,可将化学探针如二茂铁(ferrocene,Fc)[24]、亚甲基蓝(methylene blue,MB)[25-26]和酶[27]等标记在黄曲霉毒素适配体上来建立电化学适配体传感器检测黄曲霉毒素。例如,最近LI等[24]提出了一种竞争形式(图1-c),使用亚硫氨酸和还原氧化石墨烯的纳米复合材料来修饰GCE,Fc标记的适配体为输出响应信号,而硫堇功能化氧化还原石墨烯(thionine functionalized reduced graphene oxide,THI-rGO)为参考信号。Fc-Apt-AFB1络合物的形成导致其从电极上剥离,使Fc的电流强度减弱,而THI的电流强度增强,实现对AFB1特异性检测。通过选择2种电活性化合物给出的比率作为分析信号,可以获得更好的稳定性和重现性。WANG等[25]开发了一种无试剂电化学传感器,使用与AFB1高度亲和的26-merDNA适配体,在特定位点连接MB,适配体与AFB1的结合导致MB电流信号显著增加,通过方波伏安法分析MB的电流检测AFB1。该传感器可以通过简单的去离子水清洗而再生,可以很好地重复使用。
有研究还通过循环信号放大的方法,进一步增加反应灵敏度,CUI等[27]使用核酸外切酶I(exonuclease I,Exo I)驱动的级联放大对AFB1进行高灵敏度检测,c-DNA表面的COO-能使带正电的MB显蓝色,产生一个电化学信号,Fc-Apt为探针检测AFB1为另一个信号,Exo I消化分离的适配体,释放的AFB1进入下一轮循环,形成靶循环,实现信号放大,该适配体传感器表现出较宽的检测范围(0.000 1~1 pg/mL)和低检出限。JALALIAN等[28]基于适配体信标和构象改变的电化学适配体传感器(图1-d)。在没有AFM1的情况下,Apt的发夹结构是完整的,得到了一个弱峰值电流。而AFM1的加入使Apt的发夹结构发生分解,加入MB作为氧化还原剂后,cDNA修饰的纳米金(gold nanoparticles,AuNPs)靠近电极表面,产生强烈的电流信号。该适配体传感器能以pg/mL水平检测牛奶中的AFM1。此外,RAHMANI等[29]构建了基于电纺碳纳米纤维的电化学适配体传感器,固定AFM1适配体,采用循环伏安法对AFM1进行检测,检出限为0.000 6 ng/mL。KORDASHT等[30]制造了一种用于适配体固定的新型生物相容性支架,首次合成氨基官能化的树枝状纤维纳米二氧化硅和壳聚糖负载的金纳米粒子,并通过计时电流法成功地将其电沉积在GCE表面,最后将MB标记的适配体固定化,实现AFM1检测。KHOLAFAZAD等[31]研制了一种新型的电化学界面,此界面由壳聚糖改性石墨烯量子点作为导电层、胺基官能化的树枝状纤维纳米二氧化硅作为高表面元素和特异性核酸适配体构成,将独特的寡核苷酸固定在工程电极的表面,使用循环伏安法和差分脉冲伏安法技术实现AFM1高灵敏度定量。由此可见,通过在工作电极表面修饰功能材料,不仅提升了工作电极的电子传导效率,还能提高适配体在界面的负载量,进而提高检测灵敏度。
电化学传感所需的电极及传感膜可以通过分布清洗降低除分析物以外的干扰,表现出更高的抗干扰性能。但是,电化学法具有需要对电极进行活化、材料修饰、复杂检测平台组装、信号输出不稳定及电极昂贵等不足,这对其批量生产和制备提出挑战。同时,样品制备仍然存在瓶颈,尤其在霉菌毒素方面,样品处理周期长、食品基质干扰及纯化不彻底等问题降低电化学法的检测效率和准确性。
2 比色适配体传感器在检测黄曲霉毒素中的应用
比色法是将靶标与识别元件的响应信号转化为颜色信号,比较检测溶液对光选择性吸收产生的颜色变化,通过肉眼和简单的仪器来确定待测组分及其含量的方法。基于适配体的比色生物传感器已经证明了其在灵敏度、选择性及便捷性上具有显著优势[32-34]。
贵金属纳米材料,特别是AuNPs和纳米银(silver nanoparticles,AgNPs),是比色分析常用的信号探针,AuNPs和AgNPs固有的表面等离子体共振特性对比色信号的产生起关键性作用[35]。由于独特的光学、导热性和电子特性,AuNPs和AgNPs常用作纳米组装单元和固定适配体来构建比色适配体传感器。LIU等[36]采用磁性GO结合SELEX筛选出AFM1高亲和力适配体并作为特异性识别探针,适配体通过静电相互作用吸附到AuNPs表面,使其在盐溶液中保持分散而不改变其颜色,在AFM1存在下,适配体与其特异性结合,失去保护的AuNPs在盐溶液的作用下聚集,颜色由红色变为蓝色(图2-a),利用该传感器可实现对牛奶样品中AFM1的检测,检出限为0.078 ng/mL,检测范围为0.078~10 ng/mL。KASOJU等[37]构建了AuNPs-适配体-盐比色体系,根据AFB1与适配体特异性结合后高盐环境导致的AuNPs聚集,可在1 min内实现对AFB1的特异性、定性和定量检测。JALALIAN等[38]同样用该体系,基于链霉亲和素修饰的二氧化硅纳米粒子、AuNPs和适配体互补链构建适配体传感器,AFM1不存在时,盐诱导的AuNPs发生聚集,样品颜色变为紫色,反之为红色,可有效应用于牛奶中AFM1的检测。此类基于金属纳米材料的适配体传感器检测原理简单且实验易操作,但该检测体系易受到背景颜色干扰,有时也可能发生分子聚集,从而影响检测结果。LERDSRI等[39]基于AgNPs聚集和局域表面等离子体共振效应,在没有AFB1情况下,带正电荷的苝二酰亚胺(positively charged perylene diimide,PCPD)与适配体结合,产生PCPD-适配体复合物。由于PCPD不足,AgNPs在溶液中分散良好,保持黄色。相反,在存在AFB1时,适配体特异性的与AFB1结合,使得靶标响应结构变化并形成复杂结构,随后PCPD连接到AgNPs表面,导致AgNPs聚集,通过肉眼可以观察到AgNPs颜色从黄色到橙色到红色到深灰色的变化(图2-b),实现AFB1的可视化检测,检出限为0.09 ng/mL。
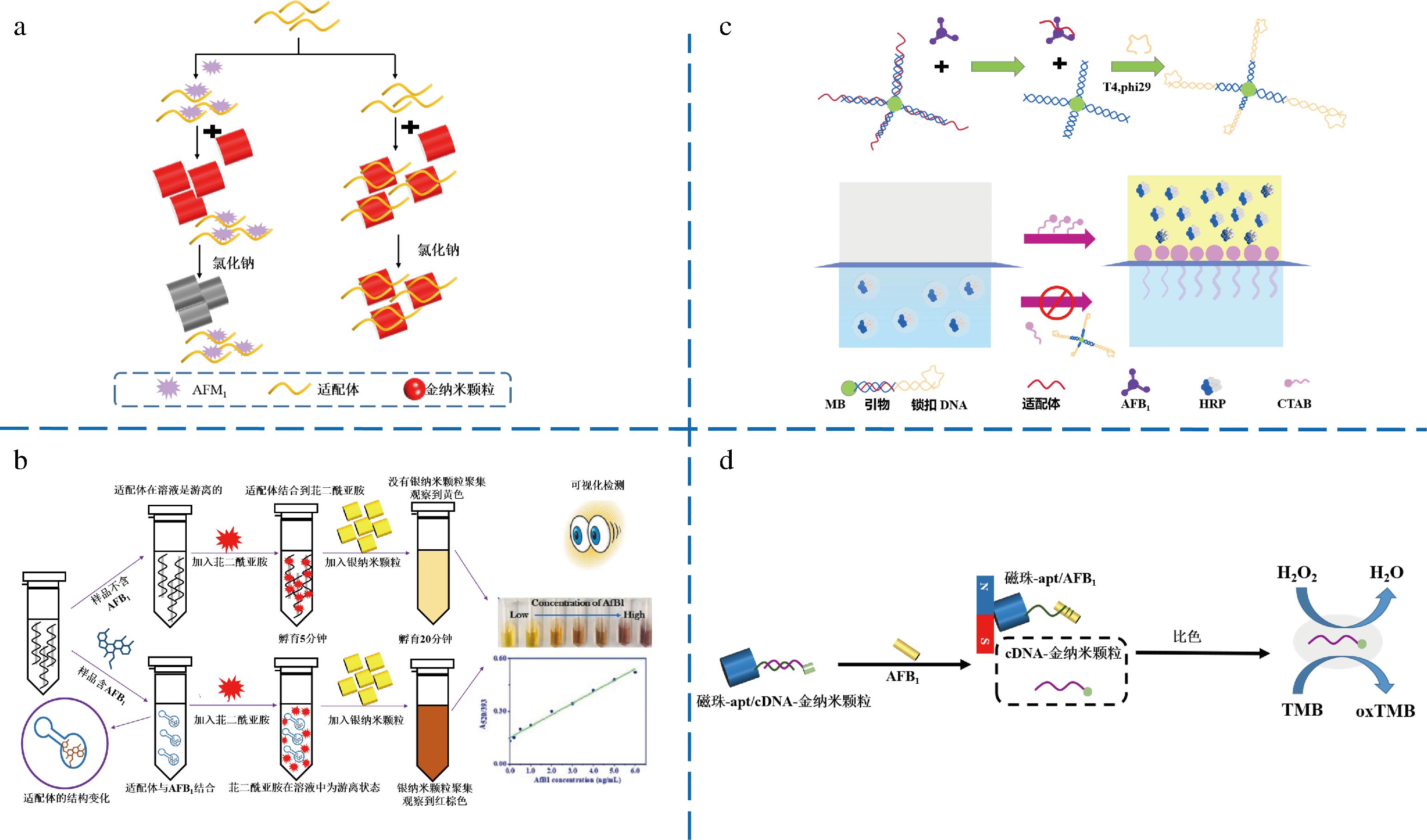
a-Apt-AuNP比色适配体传感器检测AFM1[36];b-Apt-PCPD-AgNPs比色适配体传感器检测AFB1[39];c-液晶比色适配体传感器检测AFB1[40];d-双通道比色适配体传感器检测AFB1[42]
图2 比色适配体传感器的传感示意图
Fig.2 Sensing diagram of colorimetric aptasensor
为提升比色法检测的灵敏度,WU等[40]构建了基于滚环扩增(roll ring amplification,RCA)的液晶(liquid crystal,LC)比色适配体传感器,原理如图2-c所示,使用分散有水性微滴的LC,在磁珠(magnetic beads,MBs)上触发适配体识别,AFB1结合的适配体诱导MBs上修饰的适配体/互补链双链体的解离,随后启动MBs上的RCA反应,生成长链ssDNA,辣根过氧化物酶(horse radish peroxidase,HRP)被包裹在十二烷基硫酸钠稳定的微滴中。由于界面电荷相互作用,HRP从LC喷射到覆盖的水溶液中,进而催化无色TMB转化为氧化TMB,实现肉眼检测,相反,由于扩增的ssDNA完全捕获十六烷基三甲基溴化铵(cetyl trimethyl ammonium bromide,CTAB),当CTAB与MBs上的RCA产物预孵育时,无法观察到颜色变化,表明存在AFB1,此方法具有较高的选择性和灵敏度,并成功实现对大米和花生油中AFB1的检测。除此之外,SEOK等[41]开发了超灵敏比色传感器,该传感器使用适配体和2个分裂DNA酶结合为探针,在AFB1存在下,适配体与AFB1特异性结合使得探针结构变形并解离,导致DNA酶分裂为两半、过氧化物酶催化活性降低,颜色由深绿色变为无色,可经肉眼识别,催化过程产生的比色信号会以浓度依赖性方式降低,检测范围为0.1~1.0×104 ng/mL,比色检出限为0.1 ng/mL。
由于单信号输出缺乏自校准能力,易受到操作条件和生物环境变化的干扰,因此将颜色信号和荧光信号集成在一个传感平台中不仅可以有效地提高分析效率,而且通过增大检测线性范围、增强精度和增加应用灵活性来提高分析性能。QIAN等[42]开发了双通道分析的新型多功能适配体传感器,具有类过氧化物酶活力和促进银沉积能力的AuNPs被用作比色和电化学技术的通用标记(图2-d),制备适配体修饰的Fe3O4@Au MBs和cDNA修饰的AuNPs作为捕获探针和信号探针,2个探针使用Apt和cDNA之间的杂交反应相互偶联,在暴露于AFB1后,由于Apt-AFB1复合物的形成,部分cDNA-AuNPs与MBs-Apt分离。AuNPs可以催化H2O2氧化TMB产生蓝色氧化TMB,释放的cDNA-AuNPs报告探针使用外部磁场进行分离和收集,用于AFB1的比色和电化学双通道检测,双通道方法相互验证,提高检测结果的准确性,进一步拓宽了比色传感器在食品安全领域中的应用范围。
比色适配体传感器适用于批量样品检测,目前比色法大多采用溶液均相体系,检测探针、目标物质均放置在同一溶液中,虽然在缓冲体系、标准品及加标样品检测时有较好的检测性能,但在实际样品检测时,会有一些局限性,如样品颜色的影响、环境因素的干扰及多靶标分析,检测结果的准确性和可靠性会受到影响。因此在构建传感器之前,应考虑待检样品中非靶标物质的影响,提升比色适配体传感器的抗干扰能力是研究重点和发展趋势。
3 荧光适配体传感器在检测黄曲霉毒素中的应用
荧光法具有信号响应快、灵敏度高、可选择性标记等优点,能适用于多种靶物质检测[43]。通过适配体与黄曲霉毒素强识别亲和力,实现对目标物的半定量/定量分析。
利用分子信标原理,适配体用荧光团或猝灭剂进行标记,通过两者产生荧光共振能量转移(fluorescence resonance energy transfer,FRET)效应[44]构建不同传感装置,实现“信号开启”或“信号关闭”的荧光响应差异。荧光材料如荧光染料和荧光纳米粒子,与适配体的集成可以实现高灵敏度、选择性及快速分析,使其成为荧光适配体传感器生物测定候选材料。PAN等[45]设计了基于双发射持久发光纳米粒子(persistent luminescent nanoparticles,PLNP)标记Apt和Cy5.5标记cDNA的比率适配体荧光传感器,原理如图3-a所示,双发射PLNP ZnGa2O4:Cr 0.000 1作为磷光光源,Cy5.5为能量转移的受体和猝灭基团,AFB1存在时,荧光信号增强。此荧光适配体传感器具有不需要原位激发、无自发荧光和外源干扰及高灵敏度和选择性等优点,在精确测定复杂基质中痕量AFB1中表现出巨大潜力,检出限为0.016 ng/mL。REN等[46]成功构建了基于自组装DNA四面体信号开关荧光适配体传感器,DNA四面体结构由4个特殊的单链DNA S1、S2、S3和S4组成,其中一个边缘延伸有发夹结构,与OTA适配体部分互补,另一个发夹结构嵌入在另一边,与AFB1适配体部分互补。OTA和AFB1的存在与否可以控制发夹结构的关闭和打开,从而调节Cy3与Cy5和BHQ2之间的距离,相应地改变荧光强度。可以通过Cy3和Cy5的荧光强度变化间接定量分析OTA和AFB1,检出限分别为0.005 ng/mL和0.01 ng/mL,该荧光传感器成功地应用于玉米和葡萄酒的OTA和AFB1的同时检测。
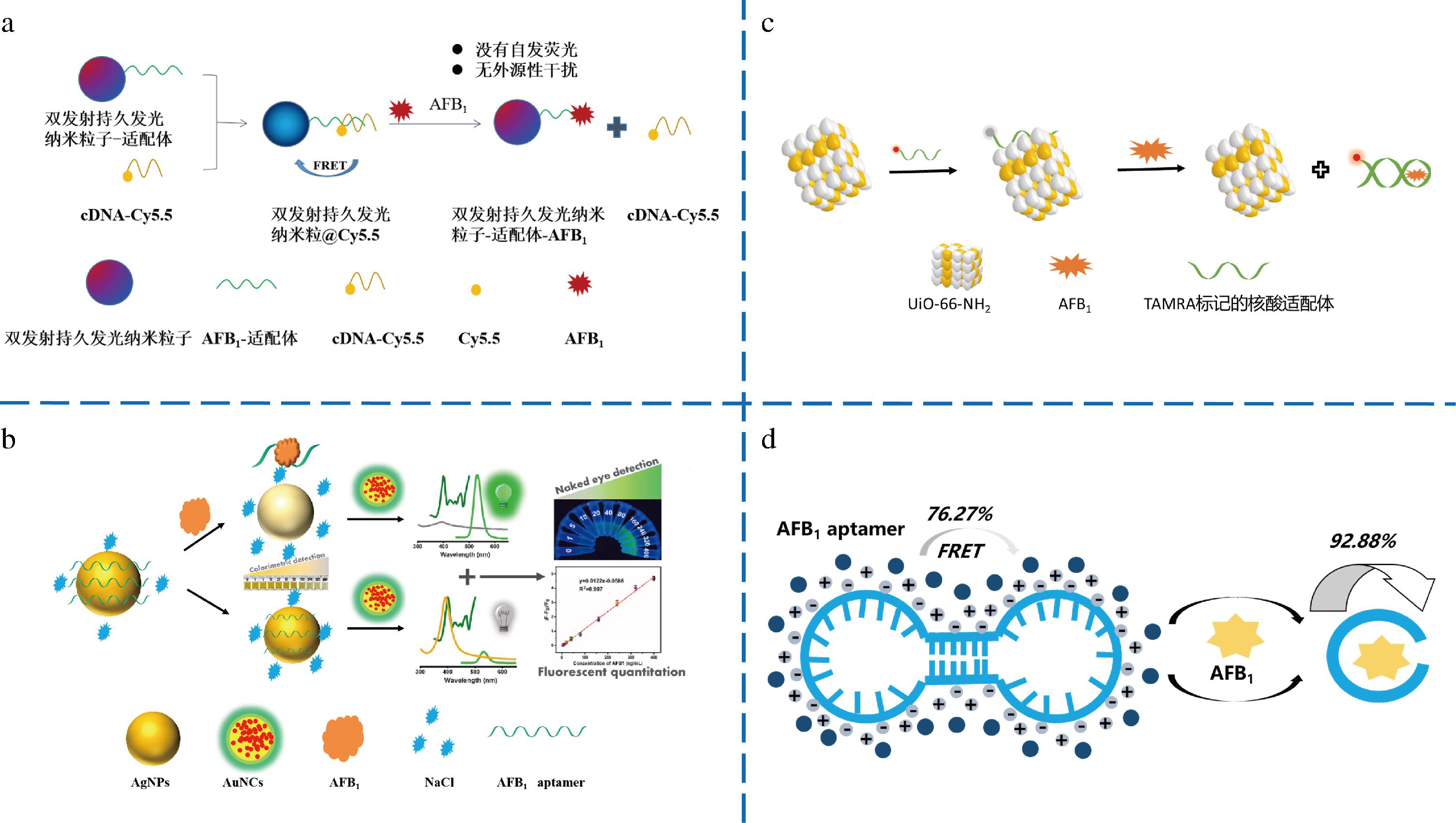
a-PLNP@Cy5.5荧光适配体传感器检测![]() 荧光适配体传感器检测
荧光适配体传感器检测![]() c-Uio-66-NH2-apt-TAMRA荧光适配体传感器检测
c-Uio-66-NH2-apt-TAMRA荧光适配体传感器检测![]() 荧光适配体传感器检测AFB1[49]
荧光适配体传感器检测AFB1[49]
图3 荧光适配体传感器的传感示意图
Fig.3 Sensing diagram of fluorometric aptasensor
内滤效应(inner filter effect,IFE)不需要供体的表面修饰或供体和受体之间的任何共价连接来满足特定距离,效率与光谱重叠程度直接相关。XIONG等[47]开发了一种用于AFB1比色和荧光双模检测的无标记适配传感器,原理如图3-b所示。在没有AFB1的情况下,AgNPs由于适配体保护,溶液保持黄色。在AFB1存在下,Apt优先与AFB1结合,随后从AgNPs表面解吸,导致AgNPs聚集在盐溶液中,颜色变为灰色;之后将精氨酸修饰的发光增强金纳米团簇引入反应体系,通过光谱重叠介导的IFE,将颜色信号成功转化为更灵敏的荧光信号。荧光信号下检测AFB1的检测限为1.91 ng/mL,比颜色信号提高6.4倍。构建的适配体传感器集成了无标记、双信号、自校准、操作简便、成本效益高等多种优点,可作为定量AFB1控制食品样品中霉菌毒素的替代方法。
光诱导电子转移(photoinduced electron transfer,PET)是指在光照下不同状态的供电子基团和接收电子基团发生电子转移从而导致荧光猝灭。姚惠文等[48]利用金属有机框架氨基功能化的奥斯陆大学66(Amino-functionalized University of Oslo 66,UiO-66-NH2)和标记有四甲基罗丹明(tetramethyl rhodamine,TAMRA)荧光团的核酸适配体(TAMRA-aptamer)构建了荧光适配体传感器用于检测AFB1。TAMRA-aptamer通过π-π堆积作用吸附于UiO-66-NH2表面,由于PET使TAMRA-aptamer的荧光猝灭。加入目标物AFB1后,荧光恢复。所构建的荧光传感器的荧光强度与AFB1的浓度呈现良好的线性关系,检测限为0.5 ng/mL。
聚集诱导发射(aggregation-induced emission,AIE)是指水溶液中不发射或者弱发射荧光,而在聚集状态时由于分子内运动受限而表现出高度发光。ZHAO等[49]提出了一种基于AIE和FRET的内在构象响应杠杆适配体探针(iconAptamer)用于AFB1检测(原理示意图如图3-d所示)。AIE染料与末端标记的荧光团之间的FRET过程会在结构转换过程中引起距离效应,产生荧光信号响应。室温下1 min内即可实现食品和环境样品中AFB1污染的检测。
与比色法相似,荧光法主要在溶液均相体系中进行检测,即荧光探针和检测物在同一溶液中,所以在检测靶标分析时会受到一些因素的影响,如实际样品或检测体系中的重金属离子、pH、离子强度及其他干扰物,进而影响传感器检测可靠性基于适配体的荧光法能借鉴电化学法的检测原理,开发并构建荧光界面传感膜或其他可保护材料的壳,进而提升传感装置的抗干扰性能及对黄曲霉毒素分析的准确性,这也作为适配体荧光传感器的未来发展趋势。
4 表面增强拉曼散射适配体传感器在检测黄曲霉毒素中的应用
表面增强拉曼散射(surface enhanced Raman scattering,SERS)是一种用于检测贵金属(金或银)纳米结构为增强基底表面或附近的分子,可将分子微弱的拉曼信号增强至几个数量级的超灵敏振动光谱技术,具有窄带宽和特征分子指纹光谱的特点[50-51]。基于SERS适配体传感器具有检测速度快、特异性强、灵敏度高、多目标物检测等优点。
CHEN等[52]开发了基于氧化石墨烯的比率性SERS适配体传感器,如图4-a所示,4-巯基苯甲酸作为拉曼信号物嵌入Au@Ag核壳纳米粒子和与Ag壳偶联的AFB1适配体作为信号探针,AFB1-Apt通过π-π堆叠相互作用与拉曼信号修饰的AuNPs/铟锡氧化物玻璃连接到GO表面,促进信号的进一步放大,实现真实花生样品中AFB1检测,检出限低至0.000 1 ng/mL。HE等[53]构建了基于磁分离的SERS适配体传感器,AFB1适配体通过Au-S键标记在Au@Ag纳米球上,cDNA标记于Fe3O4@Au纳米花上,在没有AFB1的情况下,由于SERS效应获得高强度的拉曼信号。相反,适配体与AFB1结合,从而触发信号探针的释放,导致磁分离后SERS强度降低,实现AFB1的痕量检测。上述方法基于单个信号,易受到共存物质、仪器和非标准分析仪器的干扰,因此,HE等[54]进一步构建了SERS和荧光双信号适配体传感器,用于花生、核桃和杏仁样品中的AFB1检测(图4-b)。Cy5为荧光信号和拉曼信号,聚乙烯亚胺修饰的MNP@Ag微球通过静电相互作用吸附Apt,当检测出AFB1时,荧光信号增强,SERS信号减弱,结果表明,SERS法和荧光法在0.001~1 000 ng/mL和0.2~20 000 ng/mL范围内具有良好的线性关系。
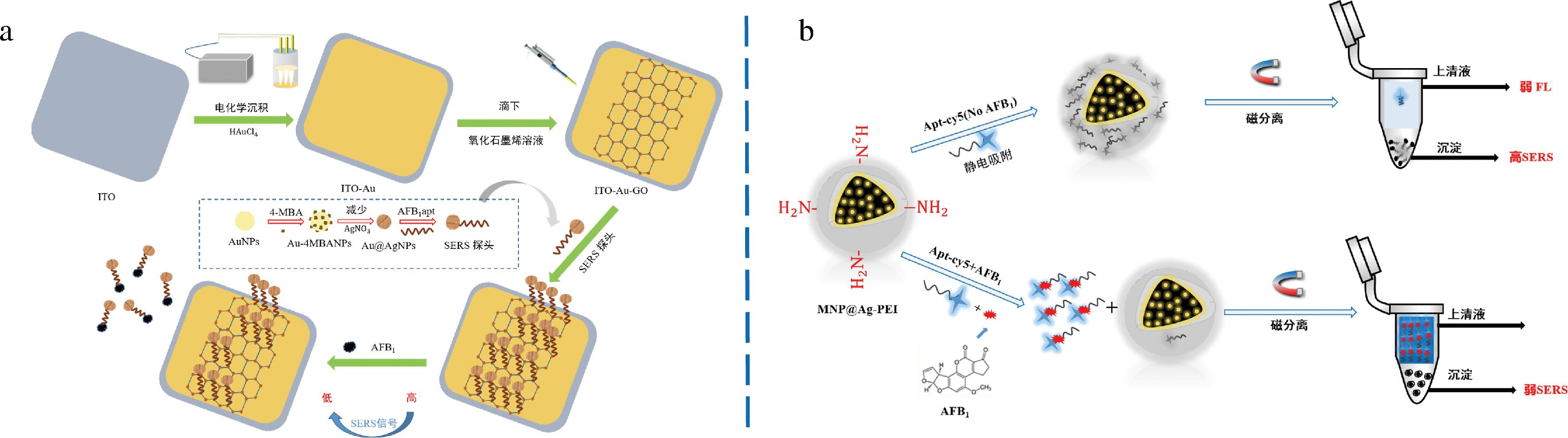
a-SERS适配体传感器检测![]() 荧光双信号适配体传感检测AFB1[54]
荧光双信号适配体传感检测AFB1[54]
图4 SERS适配体传感器的传感示意图
Fig.4 Sensing diagram of SERS aptasensor
SERS作为一种原位无损的检测技术,无需在检测前进行较复杂的前处理,即可进行检测,具有成本低、灵敏度高、多路复用性好等特点,在检测黄曲霉毒素分析应用中优势显著并取得较大研究进展。但SERS适配体传感器在检测黄曲霉毒素时也存在信号重现性差及样本在敞开体系中易被污染等问题,因此实现在微升甚至纳升的基质中目标物的快速灵敏检测需作出更多的努力。
5 结论与展望
本文综述了基于适配体的生物传感器在黄曲霉毒素检测中的应用研究。利用适配体的优良特性,构建高性能的适配体传感器,能够实现对黄曲霉毒素快速、灵敏及特异性检测。其中,电化学适配体传感灵敏度高、抗干扰能力强,但是由于受检测仪器的限制,高通量传感阵列适配体传感器难以制备。比色法适配体传感器制备时间短、可操作性强及信号易于采集,能实现可视化现场检测,但检测体系大多采用溶液相反应,使得容易受到待测系统中其他物质的干扰,进而影响检测的精密度和可靠性,因此仍需提高比色适配体传感器的抗干扰性,推动快检产品应用的发展。荧光法因其简单、快速和高灵敏度而备受关注,然而大多数荧光方法需要外部光源的连续激发,难以避免来自样品复杂基质的自体荧光干扰,仍需开发自体荧光和抗外源干扰的传感器,用于测定复杂样品中的黄曲霉毒素。SERS适配体传感器能满足食品现场检测分析的基本要求,但基底的稳定性和均一性对环境要求严格,导致SERS传感器使用受到限制,对黄曲霉毒素检测灵敏度显著下降,因此避免基质效应是保证方法准确性的重要措施。
综上所述,采用适配体传感技术可以实现目标物黄曲霉毒素快速、特异性检测,但随着科学技术的发展和应用的需要,为更好不断的展现该技术的广泛应用范围和发展潜力,适配体传感器的构建在未来的研究中仍需关注:a)传感探针的再生性和重复性有待改善,以便进一步提高检测黄曲霉毒素的灵敏性;b)目前适配体传感器大多仅针对单一目标物如AFB1或AFM1的检测,需增加多通道阵列、多目标物检测;c)多种检测技术是在溶液相均相体系中反应,因此会存在对黄曲霉毒素检测时存在干扰基质影响检测的可靠性,探索便携式、模块化、减少基质效应的生物传感方法;d)在能实现检测AFB1和AFM1要求的基础上,将预处理简单化,提高检测效率;e)目前大多数传感器所用的AFB1和AFM1适配体序列还为多年前筛选出的,有必要增加适配体种类。总之,今后传感技术的应用仍需要深入研究和探索,致力于研发快速、高效、现场、家庭化、商业化和便携式的定量检测霉菌毒素的适配体传感器,使得更多的家庭和欠发达地区能实现食品或环境中的AFB1和AFM1的检测,为食品安全、医药卫生、生命科学等领域创造实际价值,进而为人类和动物的生命健康安全提供必要保障。
[1] FAN Y Y,LI J,FAN L,et al.A label-free aptasensor based on a dual-emission fluorescent strategy for aflatoxin B1 detection[J].Sensors and Actuators B:Chemical,2021,346:130561.
[2] XIANG X R,YE Q H,SHANG Y T,et al.Quantitative detection of aflatoxin B1 using quantum dots-based immunoassay in a recyclable gravity-driven microfluidic chip[J].Biosensors and Bioelectronics,2021,190:113394.
[3] SHARMA A,GOUD K,HAYAT A,et al.Recent advances in electrochemical-based sensing platforms for aflatoxins detection[J].Chemosensors,2016,5(1):1-15.
[4] MUHARREMI H,RAKA L,SPAHIU J,et al.Investigation of aflatoxin M1 in baby milk and aflatoxin B1 in infant cereals marketed in Kosovo[J].Journal of Food Processing and Preservation,2022,46(6):1-15.
[5] YAN C L,WANG Q L,YANG Q,et al.Recent advances in aflatoxins detection based on nanomaterials[J].Nanomaterials (Basel),2020,10(9):1626.
[6] FORCADA S,SáNCHEZ-VISEDO A,MELENDRERAS C,et al.Design and evaluation of a competitive phosphorescent Immunosensor for aflatoxin M1 quantification in milk samples using Mn:ZnS quantum dots as antibody tags[J].Chemosensors,2022,10(2):41.
[7] LI M,WANG H M,SUN J D,et al.Rapid,on-site,and sensitive detection of aflatoxin M1 in milk products by using time-resolved fluorescence microsphere test strip[J].Food Control,2021,121:107616.
[8] DANESH N M,BOSTAN H B,ABNOUS K,et al.Ultrasensitive detection of aflatoxin B1 and its major metabolite aflatoxin M1 using aptasensors:A review[J].TrAC Trends in Analytical Chemistry,2018,99:117-128.
[9] DAI H R,LIANG S H,SHAN D D,et al.Efficient and simple simultaneous adsorption removal of multiple aflatoxins from various liquid foods[J].Food Chemistry,2022,380:132176.
[10] JIA B Y,LIAO X F,SUN C N,et al.Development of a quantum dot nanobead-based fluorescent strip immunosensor for on-site detection of aflatoxin B1 in lotus seeds[J].Food Chemistry,2021,356:129614.
[11] ZARESHAHRABADI Z,BAHMYARI R,NOURAEI H,et al.Detection of aflatoxin and ochratoxin A in spices by high-performance liquid chromatography[J].Journal of Food Quality,2020,2020:8858889.
[12] ZHANG B,YU L T,LIU Z J,et al.Rapid determination of aflatoxin B1 by an automated immunomagnetic bead purification sample pretreatment method combined with high-performance liquid chromatography[J].Journal of Separation Science,2020,43(17):3509-3519.
[13] PANARA A,KATSA M,KOSTAKIS M,et al.Monitoring of aflatoxin M1 in various origins greek milk samples using liquid chromatography tandem mass spectrometry[J].Separations,2022,9(3):58.
[14] SERGEYEVA T,YARYNKA D,PILETSKA E,et al.Development of a smartphone-based biomimetic sensor for aflatoxin B1 detection using molecularly imprinted polymer membranes[J].Talanta,2019,201:204-210.
[15] WU Y X,YU J Z,LI F,et al.A calibration curve implanted enzyme-linked immunosorbent assay for simultaneously quantitative determination of multiplex mycotoxins in cereal samples,soybean and peanut[J].Toxins (Basel),2020,12(11):718.
[16] WANG Q,LI S J,ZHANG Y,et al.A highly sensitive photothermal immunochromatographic sensor for detection of aflatoxin B1 based on Cu2-xSe-Au nanoparticles[J].Food Chemistry,2023,401:134065.
[17] BOZOKALFA G,AKBULUT H,DEMIR B,et al.Polypeptide functional surface for the aptamer immobilization:Electrochemical cocaine biosensing[J].Analytical Chemistry,2016,88(7):4161-4167.
[18] SINGH H,SINGH S,BHARDWAJ S.K,et al.Development of carbon quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin M1 in milk[J].Food Chemistry,2022,393:133374.
[19] HAMAMI M,MARS A,RAOUAFI N.Biosensor based on antifouling PEG/Gold nanoparticles composite for sensitive detection of aflatoxin M1 in milk[J].Microchemical Journal,2021,165:106102.
[20] MAVRIKOU S,FLAMPOURI E,ICONOMOU D,et al.Development of a cellular biosensor for the detection of aflatoxin B1,based on the interaction of membrane engineered Vero cells with anti-AFB1 antibodies on the surface of gold nanoparticle screen printed electrodes[J].Food Control,2017,73:64-70.
[21] ZHENG S,WANG C G,LI J X,et al.Graphene oxide-based three-dimensional Au nanofilm with high-density and controllable hotspots:A powerful film-type SERS tag for immunochromatographic analysis of multiple mycotoxins in complex samples[J].Chemical Engineering Journal,2022,448:137760.
[22] CUI K L,LI G H,WANG L Y,et al.An on/off aptasensor for detection of AFB1 based on pH-sensitive polymer and GO composite[J].Journal of The Electrochemical Society,2020,167(2):027508.
[23] ROUSHANI M,FAROKHI S,RAHMATI Z.Development of a dual-recognition strategy for the aflatoxin B1 detection based on a hybrid of aptamer-MIP using a Cu2O NCs/GCE[J].Microchemical Journal,2022,178:107328.
[24] LI Y Y,LIU D,ZHU C X,et al.Sensitivity programmable ratiometric electrochemical aptasensor based on signal engineering for the detection of aflatoxin B1 in peanut[J].Journal of Hazardous Materials,2020,387:122001.
[25] WANG C,ZHAO Q.A reagentless electrochemical sensor for aflatoxin B1 with sensitive signal-on responses using aptamer with methylene blue label at specific internal thymine[J].Biosensors and Bioelectronics,2020,167:112478.
[26] JIA F,LIU D,DONG N,et al.Interaction between the functionalized probes:The depressed efficiency of dual-amplification strategy on ratiometric electrochemical aptasensor for aflatoxin B1[J].Biosensors and Bioelectronics,2021,182:113169.
[27] CUI H N,AN K Q,WANG C Q,et al.A disposable ratiometric electrochemical aptasensor with exonuclease I-powered target recycling amplification for highly sensitive detection of aflatoxin B1[J].Sensors and Actuators B:Chemical,2022,355:311238.
[28] JALALIAN S H,RAMEZANI M,DANESH N M,et al.A novel electrochemical aptasensor for detection of aflatoxin M1 based on target-induced immobilization of gold nanoparticles on the surface of electrode[J].Biosensors and Bioelectronics,2018,117:487-492.
[29] RAHMANI H R,ADABI M,BAGHERI K P,et al.Development of electrochemical aptasensor based on gold nanoparticles and electrospun carbon nanofibers for the detection of aflatoxin M1 in milk[J].Journal of Food Measurement and Characterization,2021,15(2):1826-1833.
[30] KORDASHT H K,HASANZADEH M.Specific monitoring of aflatoxin M1 in real samples using aptamer binding to DNFS based on turn-on method:A novel biosensor[J].Journal of Molecular Recognition,2020,33(6):e2832.
[31] KHOLAFAZAD KORDASHT H,MOOSAVY M H,HASANZADEH M,et al.Correction:Determination of aflatoxin M1 using an aptamer-based biosensor immobilized on the surface of dendritic fibrous nano-silica functionalized by amine groups[J].Analytical Methods,2022,14(12):1291.
[32] YANG T,LUO Z W,TIAN Y H,et al.Design strategies of AuNPs-based nucleic acid colorimetric biosensors[J].Trends in Analytical Chemistry,2020,124:115795.
[33] JIANG Y,SHI M,LIU Y,et al.Aptamer/AuNP biosensor for colorimetric profiling of exosomal proteins[J].Angewandte Chemie International Edition,2017,56(39):11916-11920.
[34] SHEN Z,XU D Y,WANG G X,et al.Novel colorimetric aptasensor based on MOF-derived materials and its applications for organophosphorus pesticides determination[J].Journal of Hazardous Materials,2022,440:129707.
[35] CHANG D R,ZAKARIA S,DENG M M,et al.Integrating deoxyribozymes into colorimetric sensing platforms[J].Sensors (Basel),2016,16(12):2061.
[36] LIU R B,ZHANG F Y,SANG Y X,et al.Selection and characterization of DNA aptamers for constructing aptamer-AuNPs colorimetric method for detection of AFM1[J].Foods,2022,11(12):1802.
[37] KASOJU A,SHRIKRISHNA N S,SHAHDEO D,et al.Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay[J].RSC Advances,2020,10(20):11843-11850.
[38] JALALIAN S H,LAVAEE P,RAMEZANI M,et al.An optical aptasensor for aflatoxin M1 detection based on target-induced protection of gold nanoparticles against salt-induced aggregation and silica nanoparticles[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2021,246:119062.
[39] LERDSRI J,THUNKHAMRAK C,JAKMUNEE J.Development of a colorimetric aptasensor for aflatoxin B1 detection based on silver nanoparticle aggregation induced by positively charged perylene diimide[J].Food Control,2021,130:108323.
[40] WU W L,XIA S,ZHAO M,et al.Colorimetric liquid crystal-based assay for the ultrasensitive detection of AFB1 assisted with rolling circle amplification[J].Analytica Chimica Acta,2022,1220:340065.
[41] SEOK Y,BYUN J Y,SHIM W B,et al.A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme[J].Analytica Chimica Acta,2015,886:182-187.
[42] QIAN J,REN C C,WANG C Q,et al.Gold nanoparticles mediated designing of versatile aptasensor for colorimetric/electrochemical dual-channel detection of aflatoxin B1[J].Biosensors and Bioelectronics,2020,166:112443.
[43] YU Y X,LI G L.Design of terbium (III)-functionalized covalent organic framework as a selective and sensitive turn-on fluorescent switch for ochratoxin A monitoring[J].Journal of Hazardous Materials,2022,422:126927.
[44] NGUYEN T B,VU T B,PHAM H M,et al.Detection of aflatoxins B1 in maize grains using fluorescence resonance energy transfer[J].Applied Sciences,2020,10(5):1578.
[45] PAN L M,ZHAO X,WEI X,et al.Ratiometric luminescence aptasensor based on dual-emissive persistent luminescent nanoparticles for autofluorescence-and exogenous interference-free determination of trace aflatoxin B1 in food samples[J].Analytical Chemistry,2022,94(16):6387-6393.
[46] REN W J,PANG J R,MA R R,et al.A signal on-off fluorescence sensor based on the self-assembly DNA tetrahedron for simultaneous detection of ochratoxin A and aflatoxin B1[J].Analytica Chimica Acta,2022,1198:339566.
[47] XIONG J C,HE S,ZHANG S,et al.A label-free aptasensor for dual-mode detection of aflatoxin B1 based on inner filter effect using silver nanoparticles and arginine-modified gold nanoclusters[J].Food Control,2023,144:109397.
[48] 姚惠文,王馨悦,雷欣莹.基于金属有机框架的荧光适配体传感器检测红酒中黄曲霉毒素B1[J].现代食品科技,2023,36(9):306-312.
YAO H W,WANG X Y,LEI X Y.A metal organic framework-based fluorescent aptasensor for determination of aflatoxin B1 in red wine[J].Modern Food Science and Technology,2023,36(9):306-312.
[49] ZHAO Z F,YANG H,DENG S,et al.Intrinsic conformation response-leveraged aptamer probe based on aggregation-induced emission dyes for aflatoxin B1 detection[J].Dyes and Pigments,2019,171:107767.
[50] WU Z H,PU H B,SUN D W.Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy:Principles and recent applications[J].Trends in Food Science &Technology,2021,110:393-404.
[51] 胡奕津,范申,黄丽珊,等.光学适配体传感器检测赭曲霉毒素A的研究进展[J].化学通报,2022,85(10):9.
HU Y J,FAN S,HUANG L S,et al.Research progress in optical aptamer sensors for the detection of ochratoxin A[J].Chemistry.2022,85(10):9.
[52] CHEN P F,LI C B,MA X Y,et al.A surface-enhanced Raman scattering aptasensor for ratiometric detection of aflatoxin B1 based on graphene oxide-Au@Ag core-shell nanoparticles complex[J].Food Control,2022,134:108748.
[53] HE H R,SUN D W,PU H B,et al.Bridging Fe3O4 @Au nanoflowers and Au@Ag nanospheres with aptamer for ultrasensitive SERS detection of aflatoxin B1[J].Food Chemistry,2020,324:126832.
[54] HE H Y,SUN D W,PU H B.et al.A SERS-Fluorescence dual-signal aptasensor for sensitive and robust determination of AFB1 in nut samples based on Apt-Cy5 and MNP@Ag-PEI[J].Talanta,2023,253:123962.