寄生虫病是指人们生食寄生虫污染的生鲜食品,或食用未进行彻底热加工的食品,导致人们身体出现多种不适的疾病总称[1]。近年来,我国饮食习惯发生明显变化,生鱼或半生海鲜类产品以其肉质清鲜甘甜、营养价值丰富,受到广大消费者喜爱,市场需求量逐年增加,现已成为人们餐桌上必不可少的美食[2]。然而,生食海鲜类产品易增加感染和过敏风险[3],随之而来的寄生虫导致水产品质量安全问题频频发生,引发的寄生虫疾病更成为世界范围内影响人们健康的关键[4-5]。据估计,全球人类寄生虫病的每年患病人数约为4.07亿例,其中9 110万例由于生食生鲜类食品导致患病的患者中,有5.2万死亡病例[6]。
水产品中寄生虫的检测技术主要有传统的病原学检测、免疫学与分子生物学等技术,但前述方法往往存在耗时、专业性强、操作繁琐、易出现错漏检等问题,已不能满足人们对水产品中寄生虫快速检测的要求。因此,开发简便快速、准确灵敏的寄生虫检测技术已成为保障水产品质量安全的需求。为促进寄生虫检测技术在水产品中的发展和应用,同时减少或消除误检和漏检的发生,实现快速检测,现已有环介导等温扩增(loop-mediated isothermal amplification, LAMP)与重组酶聚合酶扩增(recombinase polymerase amplification, RPA)等多种等温扩增技术被应用于食品安全检测。其突出优势是操作简单,可实现快速高效检测。基于此,本文介绍了部分等温扩增技术原理、优缺点及在吸虫、线虫等水产品寄生虫检测领域的应用研究进展,提出其存在的问题,如引物设计繁琐、扩增产物纯化与假阳性等。同时,介绍了可通过研发新型等温扩增技术引物设计软件来简化引物设计步骤、扩增产物纯化等办法予以解决,并对其在水产品寄生虫检测中的未来发展前景进行分析总结,以期为早期寄生虫疾病的预防和诊断提供保障。
1 等温扩增技术的作用原理及特点
等温扩增技术的作用原理主要是使用便携设备,在恒温下用更短的时间,实现目标核酸的放大。该技术与传统检测技术相比的主要特点如表1所示。
表1 水产品主要寄生虫检测方法
Table 1 Detection technology of parasites in aquatic products
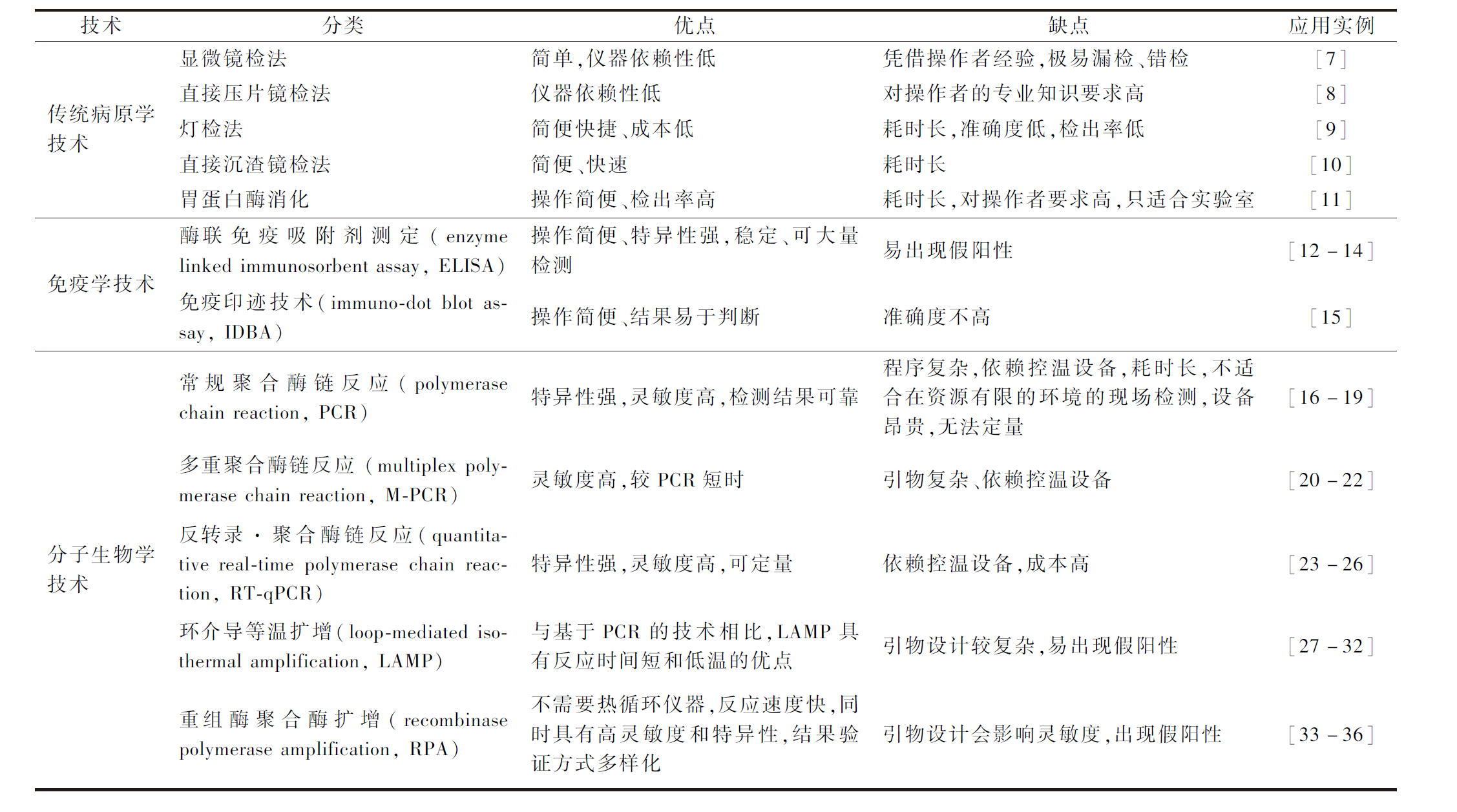
技术分类优点缺点应用实例传统病原学技术显微镜检法简单,仪器依赖性低凭借操作者经验,极易漏检、错检[7]直接压片镜检法仪器依赖性低对操作者的专业知识要求高[8]灯检法简便快捷、成本低耗时长,准确度低,检出率低[9]直接沉渣镜检法简便、快速耗时长[10]胃蛋白酶消化操作简便、检出率高耗时长,对操作者要求高,只适合实验室[11]免疫学技术酶联免疫吸附剂测定(enzyme linked immunosorbent assay, ELISA)操作简便、特异性强,稳定、可大量检测易出现假阳性[12-14]免疫印迹技术(immuno-dot blot assay, IDBA)操作简便、结果易于判断准确度不高[15] 分子生物学技术常规聚合酶链反应(polymerase chain reaction, PCR)特异性强,灵敏度高,检测结果可靠程序复杂,依赖控温设备,耗时长,不适合在资源有限的环境的现场检测,设备昂贵,无法定量 [16-19]多重聚合酶链反应 (multiplex pol-ymerase chain reaction, M-PCR)灵敏度高,较PCR短时引物复杂、依赖控温设备[20-22]反转录·聚合酶链反应(quantita-tive real-time polymerase chain re-action, RT-qPCR)特异性强,灵敏度高,可定量依赖控温设备,成本高[23-26]环介导等温扩增(loop-mediated i-sothermal amplification, LAMP)与基于PCR的技术相比,LAMP具有反应时间短和低温的优点引物设计较复杂,易出现假阳性[27-32]重组酶聚合酶扩增(recombinase polymerase amplification, RPA)不需要热循环仪器,反应速度快,同时具有高灵敏度和特异性,结果验证方式多样化引物设计会影响灵敏度,出现假阳性[33-36]
1.1 环介导等温扩增技术
环介导等温扩增技术最初由Eiken Chemical Company于2000年开发的,其可在60~65 ℃的恒温条件下实现核酸扩增,对热循环仪器无依赖性,且与传统PCR检测技术相比较,该技术具有扩增快速高效、实用性强等优点[37]。该技术已应用于线虫、血吸虫、病原微生物、转基因以及食源性致病菌的检测。其中,CAI等[27]使用环介导等温扩增法快速检测鱼肉中的华支睾吸虫囊蚴,比PCR法的检测灵敏度高100倍,为快速、灵敏检测鱼中华支睾吸虫提供了有效工具;张森等[28]实现了对棘颚口线虫的快速检测,检测限为2 fg棘颚口线虫质粒DNA,灵敏度较传统PCR低100倍;TONG等[29]以28S rDNA为靶点的环介导等温扩增试验,能快速有效地检测感染和早期感染钉螺中的日本血吸虫DNA,检测限为100 fg,日本血吸虫基因组DNA;CAMMILLERI等[30]建立的LAMP方法实现了对人工污染异尖线虫的加工鱼样品的阳性扩增,检测限比实时荧光定量PCR方法低100倍;乔艳等[31]建立的环介导等温扩增技术结合试纸条检测,能在50 min内准确鉴定出异尖线虫/派氏异尖线虫,且操作简便;CHEN等[32]报告的LAMP法可以在60 ℃的等温条件下在45 min内完成并殖吸虫检测,为淡水蟹、小龙虾等样本中并殖吸虫DNA的检测提供了快速灵敏工具,这对有效控制人类寄生虫疾病具有重要意义。
1.2 重组酶聚合酶等温扩增技术
2006年,有学者利用参与细胞DNA合成、重组和修复的蛋白质开发了RPA技术。该反应中,T4 噬菌体的重组酶在 ATP存在下与引物结合,形成重组酶-引物复合物,该复合物会寻找双链DNA同源序列,并促进同源位点与引物的结合,就会发生链交换反应启动DNA合成,单链结合蛋白将与另一条链结合防止被进一步替换。最后,重组酶分解, DNA聚合酶结合到引物的3′端,在dNTP存在下启动DNA合成,实现核酸扩增[38-40],如图1所示。
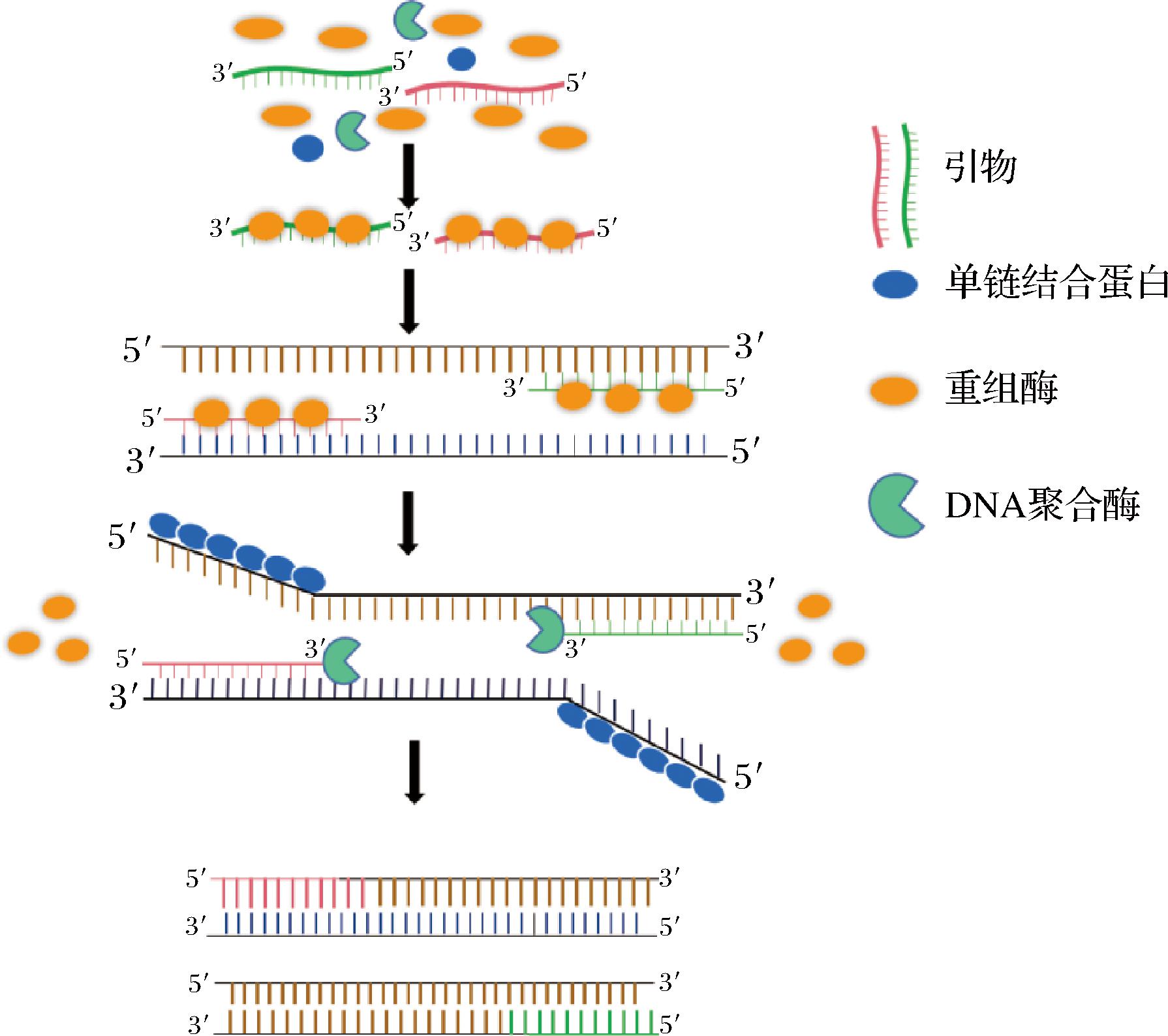
图1 重组酶聚合酶扩增原理
Fig.1 Amplification principle of recombinase polymerase
重组酶聚合酶等温扩增技术具有简单、高灵敏度、与多重检测的兼容性、快速扩增,以及在低温和恒定温度下操作,无需初始变性步骤与使用多个引物等优势,已成功应用于细菌、真菌、寄生虫等早期诊断[41]。其中,SUN等[33]通过RPA技术结合侧流层析试纸条(lateral flow dipstick, LFD)可从粪便样品中快速检测日本血吸虫,且RPA-LFD技术拥有较高灵敏度和特异性,在现场应用中具有良好潜力。XING等[34]以RPA扩增日本血吸虫SjR 2基因,该法检测限为0.9 fg的日本血吸虫DNA,结果表明,基于RPA的检测方法可用作血吸虫病诊断的有前景的即时检测。POULTON等[35]开发了一种针对曼式血吸虫28S rDNA区域的RPA侧流层析测定,检测限为10 pg DNA,该法对曼式血吸虫诊断检测有很高的潜力,几乎不需要设备和技术支持,结果可快速获得且易于判读。WU等[36]建立了针对B1基因检测弓形虫的LF-RPA方法,检测限为0.1个卵囊/反应。该技术在37~42 ℃(人体体温)下即可进行,灵敏度和特异性均高,结果读取多样化。
2 存在问题和解决办法
LAMP与RPA新型等温扩增技术,通过提供等温环境合成目的基因DNA,避免传统PCR的部分弊端,并结合免疫学检测技术、生物传感器等,使用多样化的判读方式,如通过LFD代替琼脂糖凝胶电泳(agarose gel electrophoresis, AGE)等,缩短检测时间。等温扩增技术虽然拥有较好特异性、选择性、灵敏度和效率,但仍有假阳性、引物设计繁琐与扩增产物需要纯化等问题,针对于此,有必要采取相应措施予以解决[42]。
2.1 引物设计繁琐
LAMP、RPA 已逐步应用于多重扩增,这不仅需要设计适宜的引物,也需对引物浓度进行优化。迄今为止,还未对这些新型等温扩增技术引物设计开发专属的软件[43],只有聚合酶链式反应软件可用于引物设计和筛选。但LAMP需设计多条引物,RPA的引物理想长度为30~35 bp,不论是引物数还是引物长度均不同于聚合酶链式反应软件所设计的引物。有时虽可用PCR引物扩增相应的片段,但可能无法达到最佳的扩增效果[43]。因此大多数情况下,需人工设计合成引物,这可能导致引物设计以及浓度优化时间过长、引物合成成本消耗很大的问题。引物浓度优化是由于引物竞争重组酶蛋白,一个靶标引物可抑制另一个靶标扩增,因此需要优化每种引物的比例。此外,不同的DNA靶标,即使有相同的GC含量、引物熔解温度和扩增子长度,也会以不同的效率进行扩增。若引物设计不够好,会导致RPA在琼脂糖凝胶电泳和侧向流动分析试纸的情况下出现拖尾[44]。在扩增过程中没有热循环会增加形成引物二聚体的风险,这也是许多目前等温检测中最具挑战性的问题,研发新型等温扩增技术专属的引物设计软件,该软件若在达到这些等温扩增技术引物本身要求的同时,又可自动规避引物二聚体形成,这将在一定程度上有效解决引物设计所带来的一系列技术问题。
2.2 扩增产物需纯化
重组酶等温扩增技术扩增时需与重组酶、DNA聚合酶与单链DNA结合蛋白等多种酶充分接触,共同作用,达到短时成倍扩大目的基因的效果。检测结果判断方式多样,常见的有琼脂糖凝胶电泳[45]、实时荧光定量[46]、侧流层析试纸条[47]与CRISPR/Cas联动系统[48]等。但反应结束后,大量酶残留在反应体系中,在对RPA产物采用AGE检测时,会导致电泳条带出现抹带现象,影响结果判别,因此需要进行产物纯化[44],同时,通过加入酚-氯仿-异戊醇(25∶24∶1,体积比)进行纯化处理,以除去蛋白杂质,吸取上清液进行后续检测[49],同时还需要注意操作过程中气溶胶的产生与扩散,防止交叉污染。
2.3 假阳性
近年来,分子生物学发展迅速,有着不需要控温设备、检测时间短等优势的等温扩增技术现已开始逐步应用于寄生虫虫体鉴定或病原检测,并显示出非常广阔的应用前景。需要注意的是,新型的等温扩增技术克服了传统检测技术的缺点,但任何一项新兴技术总有其相应的不足之处。LAMP反应受自身原理所限,需使用多个引物进行扩增,而这会增加引物与引物的非特异性扩增的风险,导致假阳性结果[50]。多年来,其已与各种分子方法(如实时和多重检测方法)相结合,并结合各种比色和视觉检测方法,轻松识别阳性样品[51]。目前较新颖的RPA亦不例外,如引物长度需控制在30 bp左右,若长度过短则会导致引物之间的非特异性结合,使结果出现假阳性结果。因此,在以后发展中需不断优化技术反应条件,避免假阳性结果的产生。
3 结论和展望
寄生虫疾病仍是当今社会关注的重点感染性疾病之一,传统检测技术的不成熟是导致寄生虫病原从鱼体到水源性食品,再到餐桌的重要原因。由于寄生虫本身复杂的生物学特性限制,传统病原学检测技术目前仍是检测寄生虫的金标准,其不仅依赖检测人员丰富的经验积累,有关的便携式仪器也少之又少,无法实现真正意义上的快速检测。基于分子生物学的新型等温扩增技术是对寄生虫疾病的早期预防与诊断重要保障,不仅可限制食源性寄生虫类疾病的传播,更能促进相关食品工业的发展。
随着经济快速发展,人们健康意识不断提高,对寄生虫病诊断要求也不断提高,研发快速新型检测技术的重要性也愈加突显。高灵敏度、准确、简便及快速、高通量、低成本等温扩增技术是水产品中寄生虫检测技术研发的趋势。因此,可通过多种技术组合,优势互补,如将等温扩增技术与生物传感器、侧流分析和微流体设备联合使用将有望成为检测寄生虫的新方法,这有助于实现更加快速的分子诊断,但核酸提取等前处理步骤仍难以在实验室外实施,仍需一个包含现场检测所有必要工具的便携式手提包,里面可装有迷你离心机、移液管、手套、操作说明书等。这将为寄生虫检测领域的发展开辟一个新的局面,为我国寄生虫检测方法和技术的创新以及寄生虫检测能力的提高提供参考。
[1] BAO M, PIERCE G J, STRACHAN N J C, et al.Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis:Challenges in risk assessment[J].Trends in Food Science &Technology, 2019, 86:298-310.
[2] CAI J N, LEUNG P.Unlocking the potential of aquatic foods in global food security and nutrition:A missing piece under the lens of seafood liking index[J].Global Food Security, 2022, 33:100641.
[3] RAMOS P.Parasites in fishery products-Laboratorial and educational strategies to control[J].Experimental Parasitology, 2020, 211:107865.
[4] STRYI SKI R,
SKI R,  OPIE
OPIE SKA-BIERNAT E, CARRERA M.Proteomic insights into the biology of the most important foodborne parasites in Europe[J].Foods, 2020, 9(10):1403.
SKA-BIERNAT E, CARRERA M.Proteomic insights into the biology of the most important foodborne parasites in Europe[J].Foods, 2020, 9(10):1403.
[5] YU C P, CHOU Y C, WU D C, et al.Surveillance of foodborne diseases in Taiwan A retrospective study[J].Medicine, 2021, 100(5):e24424.
[6] TORGERSON P R, DEVLEESSCHAUWER B, PRAET N, et al.World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010:A data synthesis[J].PLoS Medicine, 2015, 12(12):e1001920.
[7] GUARDONE L, MALANDRA R, COSTANZO F, et al.Assessment of a sampling plan based on visual inspection for the detection of anisakid larvae in fresh anchovies (Engraulis encrasicolus).A first step towards official validation?[J].Food Analytical Methods, 2016, 9(5):1418-1427.
[8] 缪峰, 张本光, 刘新, 等.山东黄海海域水产品异尖线虫感染情况调查[J].寄生虫病与感染性疾病, 2014, 12(4):189-190.
MIU F, ZHANG B G, LIU X, et al.Investigation on the infection of Anisakis in aquatic products in the Yellow Sea of Shandong Province[J].Parasitic Diseases and Infectious Diseases, 2014, 12(4):189-190.
[9] LEVSEN A, LUNESTAD B T, BERLAND B.Low detection efficiency of candling as a commonly recommended inspection method for nematode larvae in the flesh of pelagic fish[J].Journal of Food Protection, 2005, 68(4):828-832.
[10] 马雪莲, 谭峰, 潘长旺.广州管圆线虫中间宿主福寿螺感染检测方法的比较[J].中国病原生物学杂志, 2008, 3(2):130-132.
MA X L, TAN F, PAN C W.Comparison of detection methods for the third larvae of Angiostrongylus cantonensis in Ampullaria gigas[J].Journal of Pathogen Biology, 2008, 3(2):130-132.
[11] LLARENA-REINO M, PI EIRO C, ANTONIO J, et al.Optimization of the pepsin digestion method for anisakids inspection in the fishing industry[J].Veterinary Parasitology, 2013, 191(3-4):276-283.
EIRO C, ANTONIO J, et al.Optimization of the pepsin digestion method for anisakids inspection in the fishing industry[J].Veterinary Parasitology, 2013, 191(3-4):276-283.
[12] MAZIDUR RAHMAN S M, CHOI M H, BAE Y M, et al.Coproantigen capture ELISA for detection of Clonorchis sinensis infection in experimentally infected rats[J].Parasitology International, 2012, 61(1):203-207.
[13] XU X, SUI J X, CAO L M, et al.Direct competitive enzyme-linked immunosorbent assay (ELISA) for rapid screening of anisakid larvae in seafood[J].Journal of the Science of Food and Agriculture, 2010, 90(5):877-881.
[14] KOCHANOWSKI M, ![]() M, D
M, D![]() BROWSKA J, et al.Development and application of novel chemiluminescence immunoassays for highly sensitive detection of Anisakis simplex proteins in thermally processed seafood[J].Pathogens, 2020, 9(10):777.
BROWSKA J, et al.Development and application of novel chemiluminescence immunoassays for highly sensitive detection of Anisakis simplex proteins in thermally processed seafood[J].Pathogens, 2020, 9(10):777.
[15] THIRUPPATHIRAJA C, KAMATCHIAMMAL S, ADAIKKAPPAN P, et al.An advanced dual labeled gold nanoparticles probe to detect Cryptosporidium parvum using rapid immuno-dot blot assay[J].Biosensors and Bioelectronics, 2011, 26(11):4624-4627.
[16] MÜLLER B, SCHMIDT J, MEHLHORN H.Sensitive and species-specific detection of Clonorchis sinensis by PCR in infected snails and fishes[J].Parasitology Research, 2007, 100(4):911-914.
[17] MATTIUCCI S, CIPRIANI P, WEBB S C, et al.Genetic and morphological approaches distinguish the three sibling species of the Anisakis simplex species complex, with a species designation as Anisakis berlandi n.sp.for A.simplex sp.C (Nematoda:Anisakidae)[J].The Journal of Parasitology, 2014, 100(2):199-214.
[18] TIMI J T, PAOLETTI M, CIMMARUTA R, et al.Molecular identification, morphological characterization and new insights into the ecology of larval Pseudoterranova cattani in fishes from the Argentine coast with its differentiation from the Antarctic species, P.decipiens sp.E (Nematoda:Anisakidae)[J].Veterinary Parasitology, 2014, 199(1-2):59-72.
[19] 魏纪玲, 周卫川, 邵碧英, 等.PCR检测螺类感染广州管圆线虫方法的建立与应用[J].中国人兽共患病学报, 2008, 24(12):1136-1140.
WEI J L, ZHOU W C, SHAO B Y, et al.Establishment of a PCR assay for detection of snails infected with Angiostrongylus cantonensis[J].Chinese Journal of Zoonoses, 2008, 24(12):1136-1140.
[20] LE T H, VAN DE N, BLAIR D, et al.Clonorchis sinensis and Opisthorchis viverrini:Development of a mitochondrial-based multiplex PCR for their identification and discrimination[J].Experimental Parasitology, 2006, 112(2):109-114.
[21] 张子群, 韩彩霞, 谢晓峰, 等.多重PCR检测三种食源性寄生虫方法的建立[C].中国动物学会寄生虫学专业委员会第十三次全国学术会议暨第四次国际寄生虫学学术研讨会.中国广西壮族自治区南宁, 2011.
ZHANG Z Q, HAN C X, XIE X F, et al.Establishment of multiple PCR detection methods for three foodborne parasites[C].The 13th National Academic Conference and the 4th International Symposium on Parasitology of the Professional Committee of Parasitology of the Chinese Zoological Society.Nanning, Guangxi Zhuang Autonomous Region, China, 2011.
[22] 李树清, 李雯雯, 张鸿满, 等.多重PCR鉴定三种颚口线虫方法的建立[J].中国动物传染病学报, 2014, 22(6):38-45.
LI S Q, LI W W, ZHANG H M, et al.Development of a multiplex pcr assay to identify Gnathostoma spinigerum, Gnathostoa nipponicum and Gnathostoma doloresi[J].Chinese Journal of Animal Infectious Diseases, 2014, 22(6):38-45.
[23] CAI X Q, YU H Q, BAI J S, et al.Development of a TaqMan based real-time PCR assay for detection of Clonorchis sinensis DNA in human stool samples and fishes[J].Parasitology International, 2012, 61(1):183-186.
[24] KIM E M, VERWEIJ J J, JALILI A, et al.Detection of Clonorchis sinensis in stool samples using real-time PCR[J].Annals of Tropical Medicine and Parasitology, 2009, 103(6):513-518.
[25] PAOLETTI M, MATTIUCCI S, COLANTONI A, et al.Species-specific Real Time-PCR primers/probe systems to identify fish parasites of the Genera Anisakis, Pseudoterranova and Hysterothylacium (Nematoda:Ascaridoidea)[J].Fisheries Research, 2018, 202:38-48.
[26] FANG W Z, LIU F, ZHANG S L, et al.Anisakis pegreffii:A quantitative fluorescence PCR assay for detection in situ[J].Experimental Parasitology, 2011, 127(2):587-592.
[27] CAI X Q, XU M J, WANG Y H, et al.Sensitive and rapid detection of Clonorchis sinensis infection in fish by loop-mediated isothermal amplification (LAMP)[J].Parasitology Research, 2010, 106(6):1379-1383.
[28] 张森, 邓艳, 黄燕琼, 等.环介导等温扩增法检测棘颚口线虫方法的建立[J].现代食品科技, 2016, 32(12):308-313;297.
ZHANG S, DENG Y, HUANG Y Q, et al.Sensitive and rapid detection of Gnathostoma spinigerum by loop-mediated isothermal amplification(LAMP)[J].Modern Food Science and Technology, 2016, 32(12):308-313;297.
[29] TONG Q B, CHEN R, ZHANG Y, et al.A new surveillance and response tool:Risk map of infected Oncomelania hupensis detected by loop-mediated isothermal amplification (LAMP) from pooled samples[J].Acta Tropica, 2015, 141:170-177.
[30] CAMMILLERI G, FERRANTELLI V, PULVIRENTI A, et al.Validation of a commercial loop-mediated isothermal amplification (LAMP) assay for the rapid detection of Anisakis spp.DNA in processed fish products[J].Foods, 2020, 9(1):92.
[31] 乔艳, 周前进, 李孝军, 等.环介导等温扩增联合横向流动试纸条检测简单异尖线虫/派氏异尖线虫方法的建立[J].海洋与湖沼, 2019, 50(2):324-335.
QIAO Y, ZHOU Q J, LI X J, et al.A loop-mediated isothermal amplification technique combined with a lateral flow dipstick for the detection of Anisakis simplex sensu stricto/Anisakis pegreffii in commercial fish[J].Oceanologia et Limnologia Sinica, 2019, 50(2):324-335.
[32] CHEN M X, AI L, ZHANG R L, et al.Sensitive and rapid detection of Paragonimus westermani infection in humans and animals by loop-mediated isothermal amplification (LAMP)[J].Parasitology Research, 2011, 108(5):1193-1198.
[33] SUN K, XING W W, YU X L, et al.Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum[J].Parasites &Vectors, 2016, 9(1):476.
[34] XING W W, YU X L, FENG J T, et al.Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in Hunan Province of China[J].BMC Infectious Diseases, 2017, 17(1):164.
[35] POULTON K, WEBSTER B.Development of a lateral flow recombinase polymerase assay for the diagnosis of Schistosoma mansoni infections[J].Analytical Biochemistry, 2018, 546:65-71.
[36] WU Y D, XU M J, WANG Q Q, et al.Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for detection of Toxoplasma gondii in the environment[J].Veterinary Parasitology, 2017, 243:199-203.
[37] 朱志伟, 赵金红.环介导等温扩增技术在寄生虫检测中的应用进展[J].热带病与寄生虫学, 2020, 18(4):234-238;233.
ZHU Z W, ZHAO J H.Application progress of loop-mediated isothermal amplification technology in the diagnosis of human parasitic diseases[J].Journal of Tropical Diseases and Parasitology, 2020, 18(4):234-238;233.
[38] CASTELLANOS-GONZALEZ A, WHITE A C, MELBY P, et al.Molecular diagnosis of protozoan parasites by Recombinase Polymerase Amplification[J].Acta Tropica, 2018, 182:4-11.
[39] LI J, MACDONALD J, VON STETTEN F.Review:A comprehensive summary of a decade development of the recombinase polymerase amplification[J].The Analyst, 2018, 144(1):31-67.
[40] PIEPENBURG O, WILLIAMS C H, STEMPLE D L, et al.DNA detection using recombination proteins[J].PLoS Biology, 2006, 4(7):e204.
[41] 梁芳芳, 张浩, 王帅, 等.重组酶聚合酶扩增技术在寄生虫快速检测中的应用[J].中国热带医学, 2021, 21(9):894-899.
LIANG F F, ZHANG H, WANG S, et al.Application of recombinase polymerase amplification technology in the rapid detection of parasites[J].China Tropical Medicine, 2021, 21(9):894-899.
[42] ASADI R, MOLLASALEHI H.The mechanism and improvements to the isothermal amplification of nucleic acids, at a glance[J].Analytical Biochemistry, 2021, 631:114260.
[43] TAN M Y, LIAO C, LIANG L N, et al.Recent advances in recombinase polymerase amplification:Principle, advantages, disadvantages and applications[J].Frontiers in Cellular and Infection Microbiology, 2022, 12:1019071.
[44] LOBATO I M, O’SULLIVAN C K.Recombinase polymerase amplification:Basics, applications and recent advances[J].TrAC Trends in Analytical Chemistry, 2018, 98:19-35.
[45] 韩利. 牛丸无牛肉虾丸中掺鸭 成都:公司掺假掺杂被罚40万[J].广西质量监督导报, 2016(12):35-36.
HAN L.Duck adulteration in beef balls and shrimp balls without beef Chengdu-the company was fined 400, 000 Yuan for adulteration[J].Guangxi Quality Supervision Guide Periodical, 2016(12):35-36.
[46] 刘爽, 黄广涛, 龚雅利, 等.基于重组酶聚合酶扩增技术建立实时荧光法快速检测鲍曼不动杆菌的研究[J].中国病原生物学杂志, 2019, 14(3):311-314.
LIU S, HUANG G T, GONG Y L, et al.Rapid detection of Acinetobacter baumannii by real-time fluorescence method based on recombinase polymerase amplification[J].Journal of Pathogen Biology, 2019, 14(3):311-314.
[47] MA B, FANG J H, LIN W, et al.A simple and efficient method for potential point-of-care diagnosis of human papillomavirus genotypes:Combination of isothermal recombinase polymerase amplification with lateral flow dipstick and reverse dot blot[J].Analytical and Bioanalytical Chemistry, 2019, 411(28):7451-7460.
[48] CHEN J S, MA E B, HARRINGTON L B, et al.CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity[J].Science, 2018, 360(6387):436-439.
[49] LIU H B, ZANG Y X, DU X J, et al.Development of an isothermal amplification-based assay for the rapid visual detection of Salmonella bacteria[J].Journal of Dairy Science, 2017, 100(9):7016-7025.
[50] AOKI M N, DE OLIVEIRA COELHO B, G ES L G B, et al.Colorimetric RT-LAMP SARS-CoV-2 diagnostic sensitivity relies on color interpretation and viral load[J].Scientific Reports, 2021, 11:9026.
ES L G B, et al.Colorimetric RT-LAMP SARS-CoV-2 diagnostic sensitivity relies on color interpretation and viral load[J].Scientific Reports, 2021, 11:9026.
[51] GARG N, AHMAD F J, KAR S.Recent advances in loop-mediated isothermal amplification (LAMP) for rapid and efficient detection of pathogens[J].Current Research in Microbial Sciences, 2022, 3:100120.