佛手又名佛手柑、五指柑、福寿柑等,为芸香科柑橘属植物佛手的果实,具有药食两用价值,主要产于我国的广东、广西、四川、浙江、福建等地,其主要活性成分有多糖类、挥发油类、黄酮类、香豆素类等[1]。不同产地的佛手多糖在种类、单糖组成及结构等方面存在差异,这可能与土壤、气候和栽培条件等因素有关[2]。研究显示,川佛手多糖的单糖组分为鼠李糖、木糖、甘露糖、葡萄糖和半乳糖,而金佛手和建佛手多糖的单糖组分均为甘露糖、葡萄糖和半乳糖[3]。广佛手多糖由D-木糖、D-甘露糖、D-葡萄糖、D-半乳糖和L-鼠李糖组成[4]。佛手多糖具有免疫调节[5]、抗氧化[6]、抗肿瘤[7]等生物活性。
帕金森病(Parkinson′s disease,PD)是一种中枢神经系统退行性疾病,主要以中脑黑质致密部多巴胺能神经元变性、死亡为病理特征。帕金森病的发病机制尚未完全明确[8],研究表明,氧化应激、线粒体功能障碍、细胞自噬等因素都参与了帕金森病的致病过程[9]。帕金森病多发于中老年群体,我国老龄化不断加剧,预计到2030年,全国帕金森病人数高达500万[10]。目前还没有能完全根治帕金森病的药物。研究表明,很多中药活性成分对帕金森病有较好的预防治疗效果,而且具有低毒、副作用小等优点。因此,对中药活性成分预防和治疗帕金森病的效果进行深入研究,具有重要的实际意义[11]。
表1 干酪成熟过程中风味物质的变化
Table 1 Changes of flavor compounds during cheese ripening
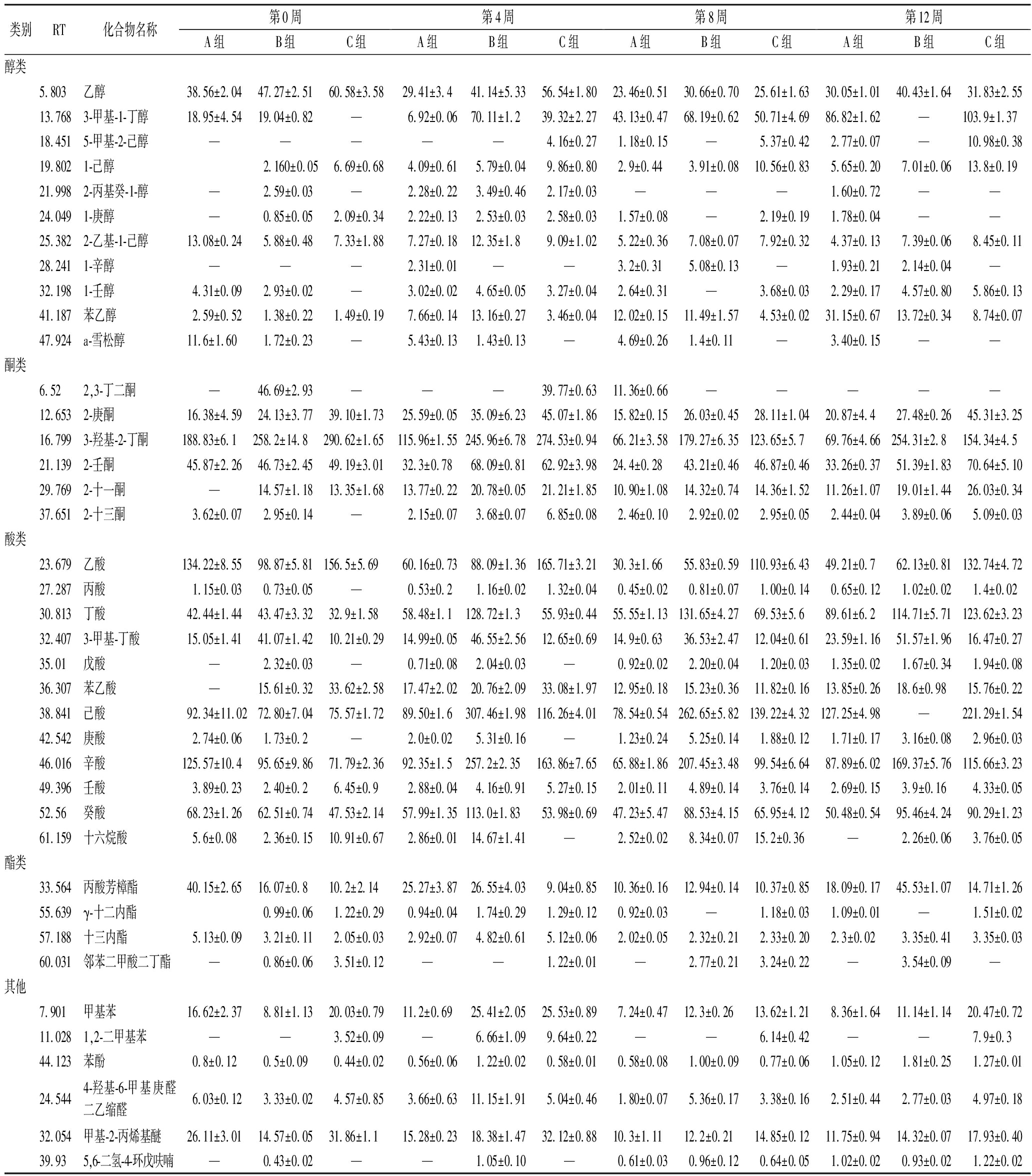
类别RT化合物名称第0周第4周第8周第12周A组B组C组A组B组C组A组B组C组A组B组C组醇类5.803乙醇38.56±2.0447.27±2.5160.58±3.5829.41±3.441.14±5.3356.54±1.8023.46±0.5130.66±0.7025.61±1.6330.05±1.0140.43±1.6431.83±2.5513.7683-甲基-1-丁醇18.95±4.5419.04±0.82—6.92±0.0670.11±1.239.32±2.2743.13±0.4768.19±0.6250.71±4.6986.82±1.62—103.9±1.3718.4515-甲基-2-己醇—————4.16±0.271.18±0.15—5.37±0.422.77±0.07—10.98±0.3819.8021-己醇2.160±0.056.69±0.684.09±0.615.79±0.049.86±0.802.9±0.443.91±0.0810.56±0.835.65±0.207.01±0.0613.8±0.1921.9982-丙基癸-1-醇—2.59±0.03—2.28±0.223.49±0.462.17±0.03———1.60±0.72——24.0491-庚醇—0.85±0.052.09±0.342.22±0.132.53±0.032.58±0.031.57±0.08—2.19±0.191.78±0.04——25.3822-乙基-1-己醇13.08±0.245.88±0.487.33±1.887.27±0.1812.35±1.89.09±1.025.22±0.367.08±0.077.92±0.324.37±0.137.39±0.068.45±0.1128.2411-辛醇———2.31±0.01——3.2±0.315.08±0.13—1.93±0.212.14±0.04—32.1981-壬醇4.31±0.092.93±0.02—3.02±0.024.65±0.053.27±0.042.64±0.31—3.68±0.032.29±0.174.57±0.805.86±0.1341.187苯乙醇2.59±0.521.38±0.221.49±0.197.66±0.1413.16±0.273.46±0.0412.02±0.1511.49±1.574.53±0.0231.15±0.6713.72±0.348.74±0.0747.924a-雪松醇11.6±1.601.72±0.23—5.43±0.131.43±0.13—4.69±0.261.4±0.11—3.40±0.15——酮类6.522,3-丁二酮—46.69±2.93———39.77±0.6311.36±0.66—————12.6532-庚酮16.38±4.5924.13±3.7739.10±1.7325.59±0.0535.09±6.2345.07±1.8615.82±0.1526.03±0.4528.11±1.0420.87±4.427.48±0.2645.31±3.2516.7993-羟基-2-丁酮188.83±6.1258.2±14.8290.62±1.65115.96±1.55245.96±6.78274.53±0.9466.21±3.58179.27±6.35123.65±5.769.76±4.66254.31±2.8154.34±4.521.1392-壬酮45.87±2.2646.73±2.4549.19±3.0132.3±0.7868.09±0.8162.92±3.9824.4±0.2843.21±0.4646.87±0.4633.26±0.3751.39±1.8370.64±5.1029.7692-十一酮—14.57±1.1813.35±1.6813.77±0.2220.78±0.0521.21±1.8510.90±1.0814.32±0.7414.36±1.5211.26±1.0719.01±1.4426.03±0.3437.6512-十三酮3.62±0.072.95±0.14—2.15±0.073.68±0.076.85±0.082.46±0.102.92±0.022.95±0.052.44±0.043.89±0.065.09±0.03酸类23.679乙酸134.22±8.5598.87±5.81156.5±5.6960.16±0.7388.09±1.36165.71±3.2130.3±1.6655.83±0.59110.93±6.4349.21±0.762.13±0.81132.74±4.7227.287丙酸1.15±0.030.73±0.05—0.53±0.21.16±0.021.32±0.040.45±0.020.81±0.071.00±0.140.65±0.121.02±0.021.4±0.0230.813丁酸42.44±1.4443.47±3.3232.9±1.5858.48±1.1128.72±1.355.93±0.4455.55±1.13131.65±4.2769.53±5.689.61±6.2114.71±5.71123.62±3.2332.4073-甲基-丁酸15.05±1.4141.07±1.4210.21±0.2914.99±0.0546.55±2.5612.65±0.6914.9±0.6336.53±2.4712.04±0.6123.59±1.1651.57±1.9616.47±0.2735.01戊酸—2.32±0.03—0.71±0.082.04±0.03—0.92±0.022.20±0.041.20±0.031.35±0.021.67±0.341.94±0.0836.307苯乙酸—15.61±0.3233.62±2.5817.47±2.0220.76±2.0933.08±1.9712.95±0.1815.23±0.3611.82±0.1613.85±0.2618.6±0.9815.76±0.2238.841己酸92.34±11.0272.80±7.0475.57±1.7289.50±1.6307.46±1.98116.26±4.0178.54±0.54262.65±5.82139.22±4.32127.25±4.98—221.29±1.5442.542庚酸2.74±0.061.73±0.2—2.0±0.025.31±0.16—1.23±0.245.25±0.141.88±0.121.71±0.173.16±0.082.96±0.0346.016辛酸125.57±10.495.65±9.8671.79±2.3692.35±1.5257.2±2.35163.86±7.6565.88±1.86207.45±3.4899.54±6.6487.89±6.02169.37±5.76115.66±3.2349.396壬酸3.89±0.232.40±0.26.45±0.92.88±0.044.16±0.915.27±0.152.01±0.114.89±0.143.76±0.142.69±0.153.9±0.164.33±0.0552.56癸酸68.23±1.2662.51±0.7447.53±2.1457.99±1.35113.0±1.8353.98±0.6947.23±5.4788.53±4.1565.95±4.1250.48±0.5495.46±4.2490.29±1.2361.159十六烷酸5.6±0.082.36±0.1510.91±0.672.86±0.0114.67±1.41—2.52±0.028.34±0.0715.2±0.36—2.26±0.063.76±0.05酯类33.564丙酸芳樟酯40.15±2.6516.07±0.810.2±2.1425.27±3.8726.55±4.039.04±0.8510.36±0.1612.94±0.1410.37±0.8518.09±0.1745.53±1.0714.71±1.2655.639γ-十二内酯0.99±0.061.22±0.290.94±0.041.74±0.291.29±0.120.92±0.03—1.18±0.031.09±0.01—1.51±0.0257.188十三内酯5.13±0.093.21±0.112.05±0.032.92±0.074.82±0.615.12±0.062.02±0.052.32±0.212.33±0.202.3±0.023.35±0.413.35±0.0360.031邻苯二甲酸二丁酯—0.86±0.063.51±0.12——1.22±0.01—2.77±0.213.24±0.22—3.54±0.09—其他7.901甲基苯16.62±2.378.81±1.1320.03±0.7911.2±0.6925.41±2.0525.53±0.897.24±0.4712.3±0.2613.62±1.218.36±1.6411.14±1.1420.47±0.7211.0281,2-二甲基苯——3.52±0.09—6.66±1.099.64±0.22——6.14±0.42——7.9±0.344.123苯酚0.8±0.120.5±0.090.44±0.020.56±0.061.22±0.020.58±0.010.58±0.081.00±0.090.77±0.061.05±0.121.81±0.251.27±0.0124.5444-羟基-6-甲基庚醛二乙缩醛6.03±0.123.33±0.024.57±0.853.66±0.6311.15±1.915.04±0.461.80±0.075.36±0.173.38±0.162.51±0.442.77±0.034.97±0.1832.054甲基-2-丙烯基醚26.11±3.0114.57±0.0531.86±1.115.28±0.2318.38±1.4732.12±0.8810.3±1.1112.2±0.2114.85±0.1211.75±0.9414.32±0.0717.93±0.4039.935,6-二氢-4-环戊呋喃—0.43±0.02——1.05±0.10—0.61±0.030.96±0.120.64±0.051.02±0.020.93±0.021.22±0.02
目前,植物多糖对PD细胞模型和动物模型的保护作用已有不少报道[12-14],但未见有佛手多糖对PD细胞模型的保护作用方面的报道。因此,本实验采用被广泛使用的1-甲基-4-苯基-吡啶离子(1-methyl-4-phenyl-pyridine ion, MPP+)诱导人神经母细胞瘤(SH-SY5Y)细胞损伤为PD细胞模型,探究佛手多糖对神经细胞的保护作用及其机制,为缓解帕金森病的发生发展提供理论依据,同时也能更好地开发和利用佛手资源。
1 材料与方法
1.1 材料与试剂
SH-SY5Y细胞株,武汉大学保藏中心;佛手,广东省肇庆市德庆县莫村镇(2020年);无水乙醇,广东广试试剂科技有限公司;AB-8大孔树脂,郑州勤实科技有限公司;纤维素酶、果胶酶,上海源叶生物科技有限公司;木瓜蛋白酶、MPP+,上海麦克林生化科技股份有限公司;DMEM培养基、胎牛血清、0.25%胰酶,美国Gibco公司;磷酸盐缓冲溶液(PBS),大连美仑生物技术有限公司;二甲基亚砜(dimethyl sulfoxide,DMSO),天津市富宇精细化工有限公司;噻唑蓝(methye thiazdye telrazlium, MTT)、Hoechst33258染色液、活性氧(reactive oxygen species,ROS)检测试剂盒、线粒体膜电位检测试剂盒(JC-1)、BCA蛋白浓度测定试剂盒、ECL发光液,上海碧云天生物科技有限公司;蛋白激酶B(protein kinase B, Akt)抗体、磷酸化蛋白激酶B(phosphorylated protein kinase B, p-Akt)抗体、磷酸化细胞外调节蛋白激酶(phosphorylated extracellular regulated protein kinases1/2, p-ERK1/2)抗体、细胞色素c(cytochrome c, Cyt-c)抗体、美国Cell Signaling Technology公司;β-肌动蛋白(β-actin)抗体,美国Santa Cruz Biotechnology公司;Goat anti-Rabbit IgG二抗,美国Merck Millipore公司;其余试剂均为国产分析纯。
1.2 仪器与设备
DHG-9140A鼓风干燥箱、HWS-12恒温水浴锅,上海一恒科学仪器有限公司;800A多功能粉碎机,永康市红太阳机电有限公司;TDL-80-2C低速离心机,上海安亭科学仪器厂;SQP电子天平,赛多利斯科学仪器(北京)有限公司;RE-3000旋转蒸发仪,上海亚荣生化仪器厂;Smart-Q30纯水机,上海和泰仪器有限公司;ELx800酶标仪,美国Bio-Tek公司;FRESCO 21高速冷冻离心机、HEARACELL 150i CO2培养箱,美国Thermo Fisher公司;Eclipse Ts2 FL倒置荧光、Eclipse TS100F正置荧光显微镜,日本Nikon公司;ChemiDocXRS+化学发光成像分析系统,美国Bio-Rad公司;SW-CJ-2FD超净工作台,苏州安泰空气技术有限公司。
1.3 实验方法
1.3.1 标准曲线的制备
称取21 mg无水葡萄糖,定容于100 mL容量瓶,分别吸取0.2、0.4、0.6、0.8、1.0、1.5 mL于试管中,分别加入1.8、1.6、1.4、1.2、1.0、0.5 mL纯净水,另一个试管加入2.0 mL去离子水作为空白组,各试管中加入6%(质量分数)苯酚1.0 mL,摇匀,加入5.0 mL硫酸,摇匀,静置5 min,放入沸水浴加热15 min,立即冷却至室温,490 nm测吸光度,绘制标准曲线。得葡萄糖标准曲线方程y=11.669x+0.014 9,其中R2=0.999 1。
1.3.2 佛手多糖的提取
参考章斌等[15]的方法,并稍加修改:佛手片烘干,粉碎,过100目筛,5倍95%(体积分数)乙醇浸泡过夜(脱脂),过滤,烘干,酶提取[酶用量1.5%(质量分数),料液比1∶30,提取温度50 ℃,提取时间2 h],90 ℃灭酶10 min,抽滤浓缩,离心,取上清液,Sevage法除蛋白,活性炭去色素,80%乙醇沉淀,无水乙醇、丙酮、乙醚依次洗涤,烘干,去离子水重溶,离心,80%乙醇沉淀,无水乙醇、丙酮、乙醚依次洗涤,烘干,测定多糖得率。
1.3.3 佛手多糖的纯化
本研究使用AB-8大孔树脂对佛手粗多糖进行纯化,参考杨波等[16]的方法,并稍作修改。以大孔吸附树脂AB-8为填料,佛手粗多糖溶于去离子水,上样浓度为6 mg/L,先用300 mL去离子水进行洗脱,再用300 mL 40%乙醇洗脱,流速为1 mL/min,洗脱液体积为600 mL,50 mL/管进行收集,苯酚-硫酸法进行检测跟踪,浓缩,醇沉,烘干备用。
1.3.4 佛手多糖纯度测定
称取20 mg佛手粗多糖置于100 mL容量瓶中,用去离子水定容至刻度线,取1 mL多糖溶液按照1.3.1节的方法测定吸光度,计算纯度。
1.3.5 细胞培养与分组
将SH-SY5Y细胞接种于含10%(体积分数)血清的DMEM完全培养基中,在恒温37 ℃、5%(体积分数)CO2的培养箱中培养。每天观察细胞生长状况,待细胞长到80%以上即可传代,取对数生长期的细胞进行实验。将细胞分为对照组、模型组(加入1 mmol/L MPP+)和佛手多糖组(20、40、60、80、100 mg/L),佛手多糖(溶于PBS)预处理6 h,然后加入1 mmol/L MPP+处理细胞48 h。
1.3.6 细胞存活率的检测
以每孔细胞数1×104将细胞接种于96孔板中,每组5个重复,在恒温37 ℃、5% CO2的培养箱培养24 h后,各组给予相应的药物处理。加药48 h后,每孔加20 μL MTT(5 mg/mL),在培养箱继续孵育4 h,吸尽旧培养基,每孔加入DMSO 150 μL,放置37 ℃、200 r/min摇床下振荡30 min,用酶标仪在490 nm处测定吸光度值(A值)。细胞存活率计算如公式(1)所示:
细胞存活率![]()
(1)
1.3.7 Hoechst33258染色
将细胞接种于6孔板(接种前放入盖玻片),每孔细胞数约3×105,在恒温37 ℃、5% CO2的培养箱培养24 h后,佛手多糖组加入80 mg/L佛手多糖,对照组及模型组加入相同体积PBS,6 h后佛手多糖组和模型组加入1 mmol/L MPP+,对照组加入相同体积PBS。继续培养48 h后,每孔加入Hoechst33258染色液5 μL,放入培养箱孵育20 min,用PBS洗涤2次,取出盖玻片,置于载玻片中,在荧光显微镜下随机选取视野拍照。
1.3.8 细胞ROS水平的检测
按1.3.7节分组处理细胞,培养48 h后,吸去细胞培养基,每孔加入10 μmol/L DCFH-DA 荧光染色液1 mL,放入培养箱中孵育20 min,用DMEM完全培养基清洗细胞3次,最后加入1 mL DMEM完全培养基,在荧光显微镜下随机选取视野拍照,随后使用Image J软件计算荧光强度。
1.3.9 线粒体膜电位的检测
按1.3.7节项分组处理细胞,48 h后,吸去培养基,用1 mL PBS洗涤1次,各组分别加入1 mL完全培养基,避光条件下,加入1 mL的JC-1染色工作液,摇匀,放入培养箱继续培养20 min。孵育结束后,吸走培养基,用提前预冷的1× JC-1染色缓冲液,洗涤2次,最后加入1.5 mL基本培养基,置于倒置荧光显微镜,随机选取视野拍照,并使用Image J软件测定各组细胞的红绿荧光强度。
1.3.10 Western blot检测p-Akt、Akt、p-ERK和Cyt-c蛋白表达水平
将细胞接种于6 cm培养皿,每皿细胞数约1×106,按1.3.7节分组处理细胞,培养24 h后,吸去细胞培养液,PBS清洗1次,每皿加入细胞裂解液300 μL,放于冰上,置于4 ℃摇床振荡30 min,将细胞转移至EP管,13 000 r/min离心10 min后,吸取上清液。取少量上清液,用BCA蛋白浓度试剂盒测定总蛋白含量。余下样品加适量5×loading buffer,沸水浴5 min,冷却后,将蛋白样品加入泳道,进行电泳,先恒压80 V电泳 1 h,再恒压100 V电泳至底部,随后将蛋白转至聚偏二氟乙烯膜(250 mA,100 min)。转膜完成后将膜置于5%奶粉或1×Tris缓冲盐吐温混合液(tris buffered saline tween,TBST)中封闭1 h,加一抗(p-Akt,Akt,p-ERK1/2,Cyt-c,β-actin,体积比1∶1 000) 4 ℃孵育过夜。1×TBST洗膜3次,每次10 min,然后加二抗(1∶3 000)孵育1 h,1×TBST洗膜3次后加ECL发光液显影,于化学发光成像系统成像及分析,使用Image J软件分析条带灰度值。
1.4 统计学分析
采用SPSS 25.0软件进行统计学分析,单因素方差分析(ANOVA),LSD检验以及t检验,实验数据以![]() 形式表示,以P<0.05为差异显著,P<0.01为差异极显著。
形式表示,以P<0.05为差异显著,P<0.01为差异极显著。
2 结果与分析
2.1 佛手多糖的提取、纯化
通过复合酶提法对佛手粗多糖进行提取,由苯酚-硫酸法测定得佛手粗多糖得率为(4.86±0.50)%,纯度为(44.46±4.64)%。与王琴等[17]的文献相比,多糖得率提高了1.68%,但纯度降低了21.4%,这可能是蛋白质等杂质去除不够干净[17]。经过AB-8大孔吸附树脂对佛手粗多糖的纯化,佛手多糖纯度达到(60.81±1.41)%,纯度提高了16.35%。
2.2 佛手多糖对MPP+损伤的细胞存活率的影响
如图1所示,SH-SY5Y细胞经MPP+作用后,细胞存活率明显下降(P<0.01)。经佛手多糖预处理后,细胞的存活率显著上升(P<0.01),在20~100 mg/L浓度内呈先上升后下降的趋势,佛手多糖组细胞存活率均高于模型组(50.58%),最高达62.24%。故选择80 mg/L的佛手多糖浓度进行后续实验。
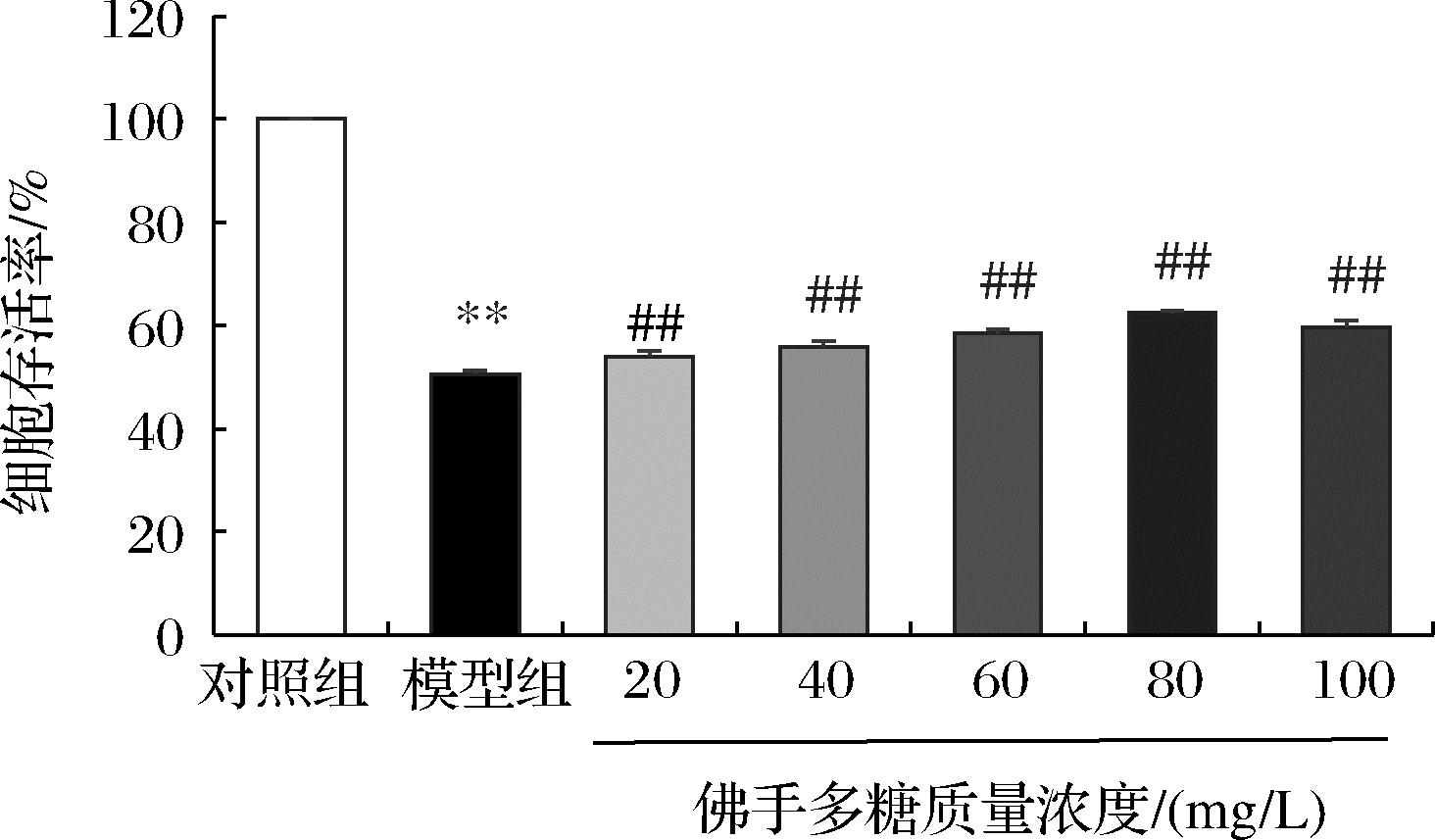
图1 佛手多糖对MPP+诱导SH-SY5Y细胞损伤的 保护作用![]()
Fig.1 Protective effect of bergamot polysaccharide on damaged SH-SY5Y cells induced by ![]()
注:与对照组比较,**P<0.001,与模型组比较,** P<0.01。
2.3 佛手多糖对MPP+诱导的细胞形态的影响
通过Hoechst33258染色后可见(图2),对照组细胞呈现出均匀的低强度荧光,模型组中部分细胞呈现出高强度的颗粒状蓝色荧光,出现细胞破碎、细胞核皱缩等细胞凋亡现象,佛手多糖组细胞的形态和对照组接近,荧光强度较模型组的低。
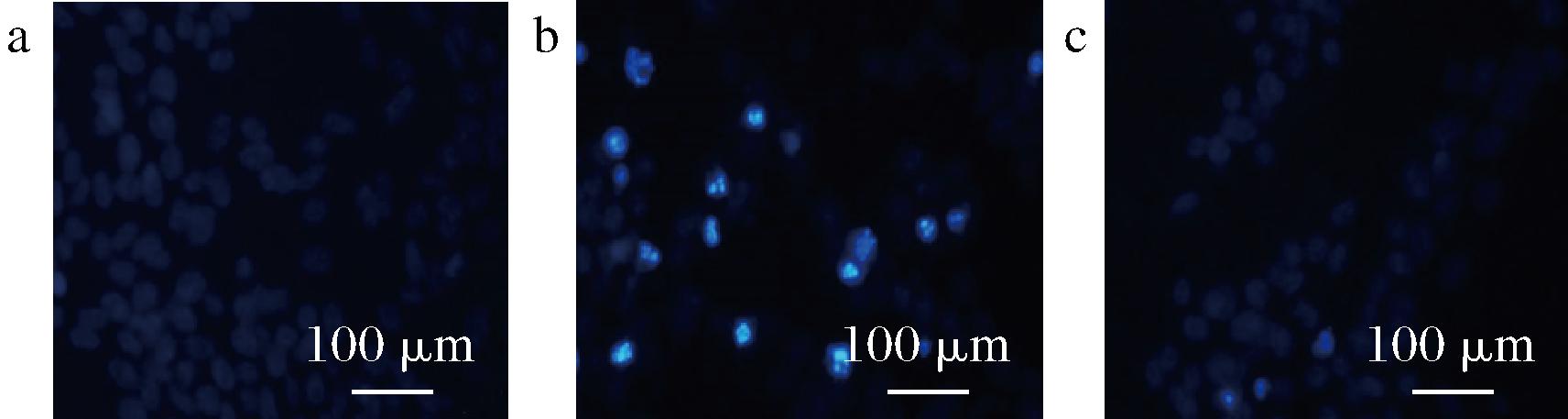
a-对照组;b-模型组;c-佛手多糖组(80 mg/L)
图2 佛手多糖对SH-SY5Y细胞形态的影响 (Hoechst33258,×400)
Fig.2 Effects of bergamot polysaccharide on the morphology of SH-SY5Y cells(Hoechst33258,×400)
2.4 佛手多糖对MPP+诱导的细胞活性氧水平的影响
如图3所示,与对照组比较,MPP+组细胞内二氯荧光素信号显著增强,绿色荧光强度大,表明细胞内活性氧水平显著增加(P<0.01)。与模型组比较,佛手多糖组的荧光强度明显降低,细胞内活性氧水平显著减少(P<0.01)。以上结果表明,佛手多糖能够抑制MPP+所导致的SH-SY5Y细胞活性氧水平增加。

图3 佛手多糖对MPP+诱导SH-SY5Y细胞ROS水平的影响 (荧光显微镜,![]()
Fig.3 Effect of bergamot polysaccharide on ROS level in SH-SY5Y cells induced by MPP+(fluorescence ![]()
注:与对照组比较,**P<0.01,与模型组比较,##P<0.01。
2.5 佛手多糖对MPP+诱导的细胞线粒体膜电位的影响
如图4所示,对照组中的红绿荧光比值为4.44,与对照组相比,模型组中红绿荧光比值降低至0.72(P<0.01),而佛手多糖组的红绿荧光比值显著高于模型组,佛手多糖组为1.98(P<0.05),以上结果表明,佛手多糖能抑制MPP+导致的膜电位下降,从而减少线粒体损伤。
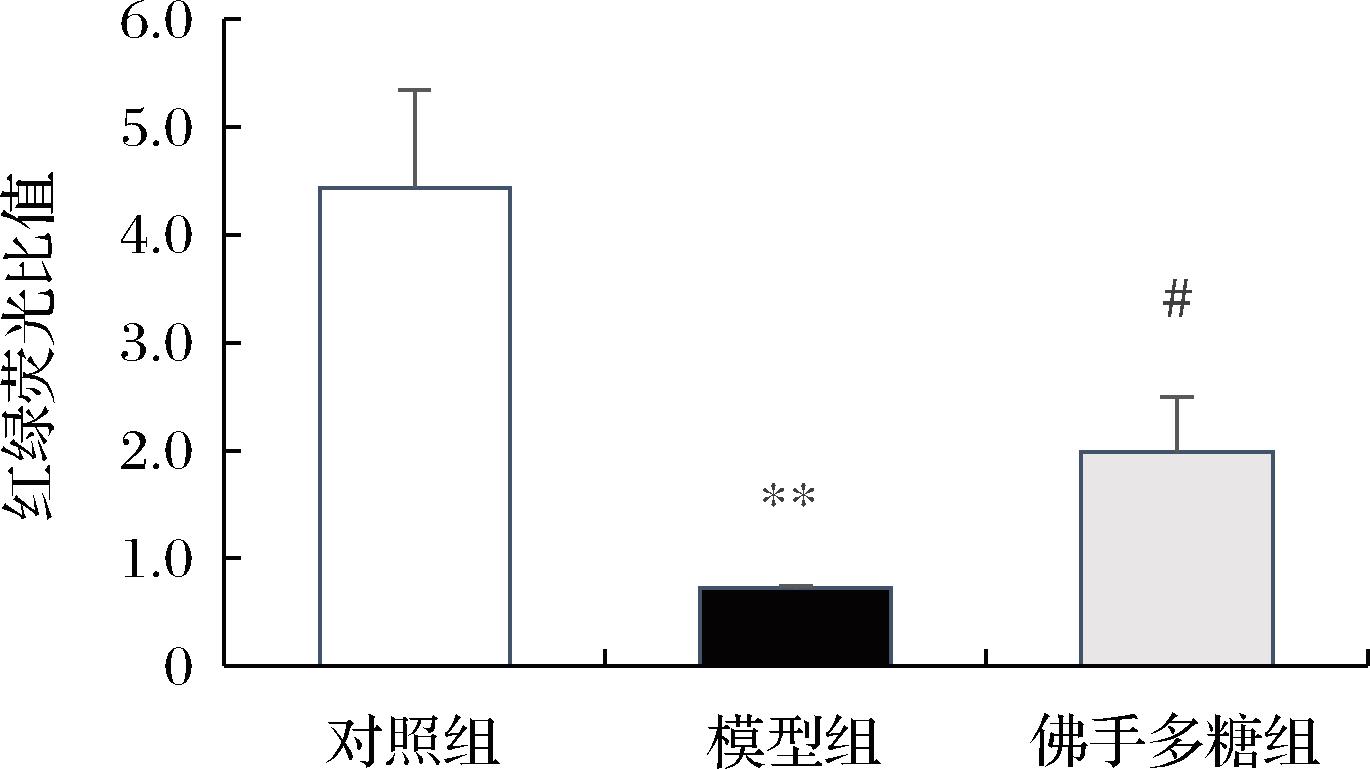
图4 佛手多糖对MPP+诱导SH-SY5Y细胞线粒体膜电位的 影响(荧光显微镜,![]()
Fig.4 Effect of bergamot polysaccharide on mitochondrial membrane potential of SH-SY5Y cells injured by MPP+ (fluorescence microscope,×200, ![]()
注:与对照组比较,**P<0.01,与模型组比较,#P<0.05。
2.6 佛手多糖对p-Akt、p-ERK和Cyt-c表达水平的影响
如图5所示,与对照组比较,模型组p-ERK1/2和p-Akt的表达水平均极显著减少(P<0.01),Cyt-c蛋白表达水平显著增加(P<0.05)。佛手多糖能显著抑制MPP+引起p-ERK1/2和p-Akt的下降以及Cyt-c水平的增加。
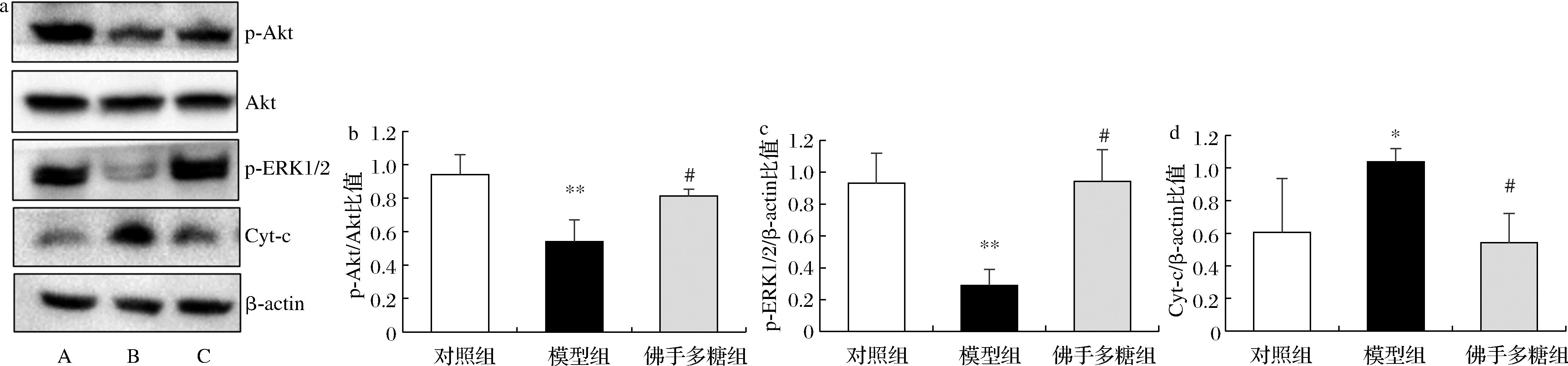
a-蛋白免疫印迹中的蛋白表达水平(A-对照组;B-模型组;C-佛手多糖组(80 mg/L));b-p-Akt/Akt;c-p-ERK1/2/β-actin;d-Cyt-c/β-actin
图5 佛手多糖对SH-SY5Y细胞p-Akt、p-ERK1/2和Cyt-c蛋白表达水平的影响![]()
Fig.5 Effects of bergamot polysaccharide on the expression of p-Akt, p-ERK1/2 and Cyt-c proteins in SH-SY5Y ![]()
注:与对照组比较,*P<0.05,**P<0.01,与模型组比较,#P<0.05。
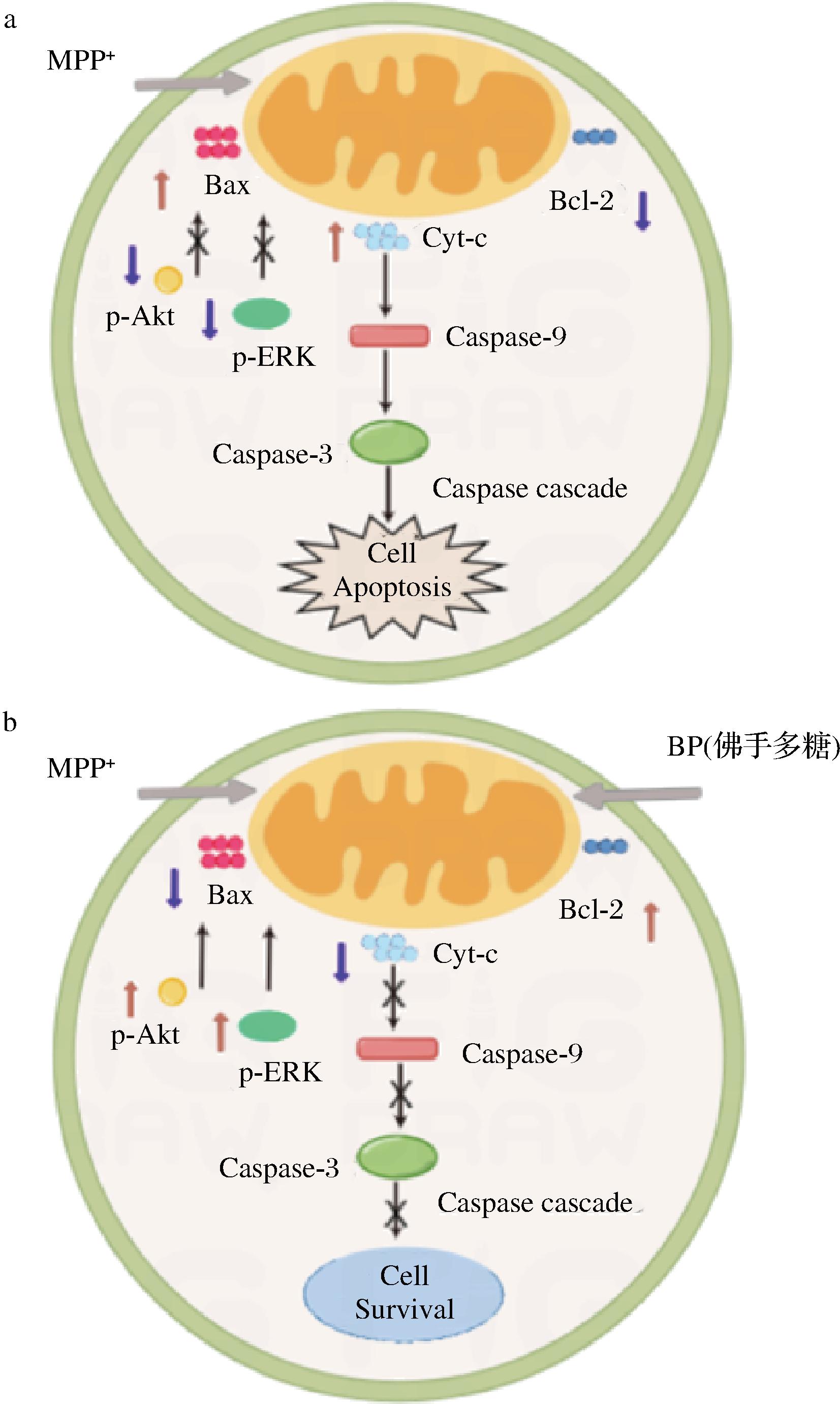
a-模型组;b-佛手多糖组(80 mg/L)
图6 佛手多糖细胞保护作用机制图
Fig.6 The diagram of cytoprotective mechanism of bergamot polysaccharide
2.7 作用机制
佛手多糖细胞保护作用机制如图6所示。
3 结果与讨论
MPP+是一种神经毒素,是PD模型常用的诱导剂,可经多巴胺转运体转运到多巴胺能神经元后,在线粒体中积累并产生大量ROS[18],随后诱导Cyt-c从线粒体释放到细胞质中,从而触发Caspase级联反应和细胞凋亡[19]。本研究结果显示,MPP+使得细胞内ROS增加,Cyt-c从线粒体释放到细胞质,经过佛手多糖处理后,细胞内ROS减少,Cyt-c释放减少,说明佛手多糖可通过调节线粒体中ROS的产生和Cyt-c的释放来抑制MPP+诱导的细胞凋亡。
ERK1/2是指细胞外调节蛋白激酶,是丝裂原活化蛋白激酶家族重要成员之一,参与细胞生长发育,细胞形态维持,细胞死亡等生理过程。ROS能激活ERK信号通路,可进一步激活蛋白激酶、转录因子等多种下游效应因子,调控基因表达,共同作用于神经系统,反过来对细胞起到保护作用[20-21]。ERK信号通路可通过三级酶促级联反应激活,促使ERK转变成p-ERK,从而发挥神经保护作用[22]。用1 mmol/L MPP+处理SH-SY5Y细胞,24 h/48 h后,细胞内p-ERK1/2表达水平均明显下降[23-24]。本实验得到相似的结果,经1 mmol/LMPP+作用后,细胞内p-ERK1/2水平明显下降,而经佛手多糖处理后,p-ERK1/2的下降得到抑制,提示佛手多糖可能通过激活ERK信号通路,进而抑制细胞凋亡。
研究表明,Akt参与神经细胞存活、凋亡、自噬以及细胞增殖等过程[25]。另外的研究还表明,PI3K/Akt通路是细胞抗凋亡信号转导机制的重要途径,能够避免细胞受多种凋亡信号刺激[26]。当PI3K被激活后,能够催化PIP2转化为PIP3,促进Akt发生磷酸化,磷酸化的Akt可以抑制多种凋亡蛋白的表达,进而发挥抗凋亡作用[27]。CAO等[23]研究显示,MPP+能够抑制Akt信号通路,使SH-SY5Y细胞中的p-Akt表达降低从而使细胞凋亡。李鹏等[13]研究发现,枸杞多糖能通过激活PI3K/Akt通路,抑制MPP+诱导的帕金森病模型细胞凋亡。本实验结果显示,MPP+处理SH-SY5Y细胞后,p-Akt表达水平显著下降,经佛手多糖处理后,能够显著抑制p-Akt的下降,提示佛手多糖可能通过激活Akt信号通路,进而抑制细胞凋亡。
综上所述,佛手多糖对MPP+诱导的SH-SY5Y细胞损伤具有保护作用,其机制可能是通过调节线粒体ROS的产生和Cyt-c的释放,进而维持线粒体稳态,激活Akt信号通路和ERK信号通路,抑制细胞的凋亡,从而起到保护作用。本研究有助于阐明佛手多糖对细胞损伤的保护作用机制,为缓解帕金森病的发生发展提供理论依据。
[1] 岳玲, 程轩轩, 唐晓敏, 等.佛手的传统应用[J].中国实验方剂学杂志, 2019, 25(4):206-211. YUE L, CHENG X X, TANG X M, et al.Traditional application of citri sarcodactylis fructus[J].Chinese Journal of Experimental Traditional Medical Formulae, 2019, 25(4):206-211.
[2] 彭宝, 文瑶, 于荣敏, 等.佛手多糖提取、结构表征及生物活性研究进展[J].食品与药品, 2018, 20(3):236-241. PENG B, WEN Y, YU R M, et al.Advances in extraction, structure characterization and bioactivities of Citrus medica polysaccharide[J].Food and Drug, 2018, 20(3):236-241.
[3] 曹诣斌, 朱海玲, 王晓艳.不同产地佛手水溶性多糖的分离纯化及初步分析[J].浙江师范大学学报(自然科学版), 2008, 31(2):190-194. CAO Y B, ZHU H L, WANG X Y.Preliminary analysis on purification of the water-soluble polysaccharides in bergamots from different areas[J].Journal of Zhejiang Normal University (Natural Sciences), 2008, 31(2):190-194.
[4] 李小凤, 蔡春, 程荷凤, 等.广佛手多糖的分离纯化与相关成分的气相色谱分析[J].广东医学院学报, 2005(3):240-241;259. LI X F, CAI C, CHENG H F, et al.Isolation and purification of polysaccharide from fructus of Citri medica and analysis of its chemical composition by gas chromatography[J].Journal of Guangdong Medical College, 2005(3):240-241;259.
[5] 王淑惠, 杨玉洁, 周爱梅, 等.两种方法提取佛手渣多糖及其对巨噬细胞RAW264.7免疫调节活性的研究[J].食品工业科技, 2020, 41(15):179-187. WANG S H, YANG Y J, ZHOU A M, et al.Study on the polysaccharide extracted from bergamot (Citrus medica L.var.sarcodactylis) residue by two methods and its immunomodulatory function in RAW264.7 cells[J].Science and Technology of Food Industry, 2020, 41(15):179-187.
[6] 邹胜. 佛手多糖的分离纯化及抗氧化活性研究[D].重庆:重庆大学, 2015. ZOU S.Extraction and separation of polysaccharides from bergamot and their antioxidant activities in vitro[D].Chongqing:Chongqing University, 2015.
[7] 黄玲, 邝枣园. 佛手多糖对小鼠移植性肝肿瘤HAC22的抑制作用[J]. 江西中医学院学报, 2000, 12(1):41-47. HUANG L, KUANG Z Y. Inhibitory effect of bergamot polysaccharide on transplanted liver tumor HAC22 in mice[J]. Journal of Jiangxi College of Traditional Chinese Medicine, 2000, 12(1):41-47.
[8] 袁惠莉, 汪璇, 张丽娟, 等.中药在防治帕金森病中的作用及研究进展[J].中国药理学通报, 2010, 26(7):850-854. YUAN H L, WANG X, ZHANG L J, et al.Mechanism and research progress of Chinese traditional medicine in the prevention and treatment of Parkinson′s disease[J].Chinese Pharmacological Bulletin, 2010, 26(7):850-854.
[9] 辛陈琦. 帕金森病发病机制与治疗研究进展[J].医学研究生学报, 2019, 32(6)646-651. XIN C Q.The mechanism underlying pathogenesis of Parkinson’s disease and the progress in its therapeutics[J].Journal of Medical Postgraduates, 2019, 32(6)646-651.
[9] 辛陈琦, 张承武, 李林.帕金森病发病机制与治疗研究进展[J].医学研究生学报, 2019, 32(6):646-651. XIN C Q, ZHANG C W, LI L.The mechanism underlying pathogenesis of Parkinson′s disease and the progress in its therapeutics[J].Journal of Medical Postgraduates, 2019, 32(6):646-651.
[10] 庞文渊, 翟利杰, 刘依琳, 等.全球帕金森病综合治疗指南的分析[J].中国临床药理学杂志, 2022, 38(21):2638-2643. PANG W Y, ZHAI L J, LIU Y L, et al.Analysis of global guidelines for comprehensive treatment of Parkinson′s disease[J].The Chinese Journal of Clinical Pharmacology, 2022, 38(21):2638-2643.
[11] 苏燕, 周亚莉, 田琳琳, 等.中药活性成分防治帕金森病的研究进展[J].神经解剖学杂志, 2020, 36(1):111-115. SU Y, ZHOU Y L, TIAN L L, et al.Research progress on prevention and treatment of Parkinson′s disease with active components of traditional Chinese medicine[J].Chinese Journal of Neuroanatomy, 2020, 36(1):111-115.
[12] 陈浩, 张皓洁, 师亮, 等.枸杞多糖对帕金森病小鼠的抗氧化作用和神经保护效应[J].中国神经精神疾病杂志, 2018, 44(10):613-618. CHEN H, ZHANG H J, SHI L, et al.Antioxidative and Neuroprotective effects of Lycium barbarum polysaccharide on Parkinson′s disease mice[J].Chinese Journal of Nervous and Mental Diseases, 2018, 44(10):613-618.
[13] 李鹏, 杜园园, 文杰, 等.枸杞多糖通过激活PI3K/Akt通路调节MPP+诱导的帕金森病模型细胞凋亡[J].中国药物与临床, 2020, 20(1):28-30. LI P, DU Y Y, WEN J, et al.Lycium barbarum polysaccharide regulates MPP+-induced apoptosis in Parkinson′s disease model by activating PI3K/Akt pathway[J].Chinese Remedies &Clinics, 2020, 20(1):28-30.
[14] 尹帅领, 王海波, 杨硕.肉苁蓉多糖通过激活Wnt/β-catenin信号通路对6-HODA致帕金森病大鼠的神经保护作用[J].中西医结合心脑血管病杂志, 2020, 18(8):1227-1230. YIN S L, WANG H B, YANG S.Neuroprotective effects of Cistanche deserticola polysaccharide on Parkinson′s rats induced by 6-HODA caused by activating the Wnt/β-catenin signaling pathway[J].Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease, 2020, 18(8):1227-1230.
[15] 章斌, 李远志, 陈宇, 等.复合酶法提取广佛手多糖的工艺研究[J].安徽农业科学, 2010, 38(15):7833-7835;7873. ZHANG B, LI Y Z, CHEN Y, et al.Extraction of polysaccharides from bergamot by compound enzymolysis method[J].Journal of Anhui Agricultural Sciences, 2010, 38(15):7833-7835;7873.
[16] 杨波, 韩凤波, 杨波.D301和LSA-700B大孔吸附树脂分离纯化玉竹多糖[J].食品研究与开发, 2014, 35(24):109-112. YANG B, HAN F B, YANG B.Isolation and purification of polysaccharide from Polygonatum odoratum by D301 and LSA-700B macroporous adsorption resins[J].Food Research and Development, 2014, 35(24):109-112.
[17] 王琴, 蒋林, 张爵玉.广佛手多糖分离提取的工艺优化[J].食品工业科技, 2008, 29(4):199-202. WANG Q, JIANG L, ZHANG J Y.Optimized technique of extraction of Bergamot polysaccharide[J].Science and Technology of Food Industry, 2008, 29(4):199-202.
[18] ZAWADA W M, BANNINGER G P, THORNTON J, et al.Generation of reactive oxygen species in 1-methyl-4-phenylpyridinium (MPP+) treated dopaminergic neurons occurs as an NADPH oxidase-dependent two-wave cascade[J].Journal of Neuroinflammation, 2011, 8:129.
[19] REDZA-DUTORDOIR M, AVERILL-BATES D A.Activation of apoptosis signalling pathways by reactive oxygen species[J].Biochimica et Biophysica Acta, 2016, 1863(12):2977-2992.
[20] LIN E, CAVANAUGH J E, LEAK R K, et al.Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells[J].Journal of Neuroscience Research, 2008, 86(1):108-117.
[21] ALBERT-GASC H, ROS-BERNAL F, CASTILLO-G
H, ROS-BERNAL F, CASTILLO-G MEZ E, et al.MAP/ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes[J].International Journal of Molecular Sciences, 2020, 21(12):4471.
MEZ E, et al.MAP/ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes[J].International Journal of Molecular Sciences, 2020, 21(12):4471.
[22] SINGH J, SHARMA K, FROST E E, et al.Role of PDGF-A-activated ERK signaling mediated FAK-paxillin interaction in oligodendrocyte progenitor cell migration[J].Journal of Molecular Neuroscience, 2019, 67(4):564-573.
[23] CAO Q, QIN L Y, HUANG F, et al.Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways[J].Toxicology and Applied Pharmacology, 2017, 319:80-90.
[24] 袁倩倩, 赵海洲, 马延红, 等.芦丁对MPP+诱导的SH-SY5Y细胞损伤的保护作用[J].中国实验方剂学杂志, 2018, 24(16):109-114. YUAN Q Q, ZHAO H Z, MA Y H, et al.Protective effect of rutin on SH-SY5Y cells injured by MPP+[J].Chinese Journal of Experimental Traditional Medical Formulae, 2018, 24(16):109-114.
[25] MANNING B D, TOKER A.AKT/PKB signaling:Navigating the network[J].Cell, 2017, 169(3):381-405.
[26] SONG G, OUYANG G L, BAO S D.The activation of Akt/PKB signaling pathway and cell survival[J].Journal of Cellular and Molecular Medicine, 2005, 9(1):59-71.
[27] CARPENTER R L, SIRKISOON S, ZHU D Q, et al.Combined inhibition of AKT and HSF1 suppresses breast cancer stem cells and tumor growth[J].Oncotarget, 2017, 8(43):73947-73963.