国际食品法典委员会将膳食纤维(dietary fiber,DF)定义为食物中不易被人体中酶消化和吸收的部分,由3个及以上单体单元的碳水化合物聚合物组成,通常来源于植物[1-2]。DF包括低聚糖、非淀粉多糖、抗性淀粉,大多数来源于植物性食物,因成分结构和功能而异。根据在水中的溶解度,DF被分为水溶性膳食纤维(soluble dietary fiber,SDF)和不溶性膳食纤维(insoluble dietary fiber,IDF)两种。IDF是细胞壁的组成成分,主要作用于肠道产生机械蠕动作用,而SDF多来自半纤维素及细胞内、细胞间成分,如果胶及一些黏性水溶性多糖,则会更多发挥其在肠道中的代谢功能,影响可利用碳水化合物和酯类代谢,降低血脂胆固醇等[3-5]。
1 膳食纤维对肠道微生物群的作用
1.1 肠道微生物群的组成及作用
肠道微生物群由数万亿种不同的微生物、细菌、真菌、酵母等组成,约占人体内微生物总量的78%,具有1 500多种不同种类[6-8],从高到低依序分类成为界、门、纲、目、科、属、种。它可以代表一个实体器官,约重2 kg[9-10],能够影响宿主的代谢和免疫系统,被认为是人类重要的器官。其中含量较多的是厚壁菌门(约65%)、拟杆菌门(约25%)和变形菌门(约8%),而放线菌门(约5%)在细菌总含量中较少,拟杆菌门和厚壁菌门构成了人体重要的微生态系统[11-13]。
由于人类肠道微生物群进化和活动过程的复杂性和多种因素,很难确定人体最佳健康状态下的微生物群的组成。七岁以后,人体肠道内拟杆菌和厚壁菌之间的比例相对稳定,而比例紊乱可能会导致肥胖和糖尿病等代谢综合征[14];肠道中变形菌的长期富集可能代表不平衡微生物群落结构或宿主的疾病状态。越来越多的数据将变形菌确定为疾病的可能微生物特征[15]。目前主要涉及代谢紊乱和炎症甚至癌症。研究表明[16-17],哮喘和慢性阻塞性肺病等肺部疾病是因为变形菌不受控制扩张导致。此外,肥胖、糖尿病和过敏等各种疾病都与生命各个阶段的放线菌门数量减少有关;还有很多研究表明慢性疾病与肠道菌群失衡有关,如肥胖、糖尿病等。早期通过对比研究肥胖人群与瘦人群之间的肠道菌群[18],发现肥胖者体内具有较高的厚壁菌门丰度和较低的拟杆菌门丰度,在研究肥胖小鼠与消瘦小鼠中也观察到了类似的现象。在一项研究肠道菌群与糖尿病关系的研究中[19-20],盛产丁酸的肠道罗斯拜瑞氏菌(Roseburia intestinals)和普劳斯尼奇粪杆菌(Faecalibacterium prausnitzii)的相对丰度在Ⅱ型糖尿病患者中显著下降,而会造成肠道敏感性、肠漏或者腹痛等的脱硫弧菌(Desulfovibrio)则表现出明显升高的趋势。此外。肠道微生物组成的变化还被认为在帕金森病(Parkinson’s disease,PD)的病理生理学中发挥作用。
此外,微生物感染和肠道微生物群失衡与炎症性肠病和其他免疫相关疾病也有关系,机体在炎症的状态下[21-22],所表现出来的特征是厚壁菌门和拟杆菌门的数量增加,以及变形菌门的数量下降。不仅遗传和环境等方面因素会影响肠道微生物群的平衡,饮食习惯也间接地影响人体肠道中的微生物发酵和微生物比例的平衡[23-24]。
1.2 食物中DF来源及其对菌群的作用
DF的一些健康益处可能与肠道微生物群有关,并且纤维、脂肪、蛋白质和微量营养素可以塑造微生物的活性和结构[25],DF是良好的肠道微生物群底物,可产生有益分子,通过调节肠道中微生物群的组成和促进短链脂肪酸的产生来促进人体健康。有研究表明,全谷物DF可以显著改变肠道微生物组的多样性,增加阿克曼菌和乳杆菌含量,显著降低总胆固醇、低密度脂蛋白和非高密度脂蛋白胆固醇[26];通过评估22种市售纤维食品对3名健康志愿者肠道微生物组成和功能的影响[2],发现在短期孵育后,全谷物产品组与棕色扁豆、豌豆纤维、猕猴桃和苹果纤维组相比,在增加乙酸盐、乳酸盐和丙酸盐以及刺激双歧杆菌和乳酸杆菌方面具有一致的效果。有研究比较了拟杆菌和普雷沃菌在不同类型DF中的体外发酵情况,结果表明,不同类型的DF体外发酵后拟杆菌和普雷沃菌类群的多样性更高[27];有研究者通过体外发酵研究了铁皮石斛中的多糖对肠道微生物群的影响。结果表明,铁皮石斛多糖组的pH值随着发酵时间的增加而下降,而总短链脂肪酸、乙酸、丙酸和丁酸的产量显著增加。铁皮石斛多糖显示出潜在的益生元作用,通过增加有益细菌数量、减少病原体生长以及改善肠道微生物群氨基酸和脂肪酸来调节肠道微生物群组成[28]。
DF在到达结肠后进行细菌发酵,从而影响细菌群落的组成和功能,产生对宿主有不同影响的发酵代谢产物[29]。正常情况下,肠道各种细菌种群与宿主相互依存和相互制约,维持种动态的生态平衡,一旦受到宿主及外环境变化的影响,平衡状态就会被打破,生成病理性组合,从而造成肠道菌群失调并导致一些疾病发生。肠道微生态失调,分为关键分类群的丧失、多样性的丧失、代谢能力的改变或病原体过度生长,通常与自身免疫有关[30-31]。饮食是肠道微生物群结构和功能的主要调节剂之一;DF可以诱导肠道细菌群的特异性生长或活性,这是维持肠道微生物群稳态和预防非传染性疾病的一种很有前途的策略[32]。
2 DF体外发酵模型研究
发酵是大肠或结肠的一项重要功能,被认为是厌氧细菌将碳水化合物分解成短链脂肪酸(short-chain fatly acids,SCFA)、气体(H2、CH4和CO2)和其他代谢产物的过程[33]。大肠或结肠中的微生物经过发酵有可能产生有益和有害的产物,最终被机体吸收和利用或通过粪便排出。产生的有益产物,如SCFA,是碳水化合物发酵中最常见的微生物衍生最终产物,主要包括乙酸盐、丙酸盐和丁酸盐[34-35]。这些SCFA可被结肠细胞作为能量来源,并已被证明具有抗炎特性,同时具有调节肠道运动和改善渗漏的肠道屏障的作用。将SCFA修饰作为一种潜在的治疗策略,PD 动物模型表明,丁酸盐给药可改善运动缺陷,减少炎症和多巴胺缺乏症[36];MATT等[37]表明,腹膜内丁酸盐给药和高DF饮食都导致老年小鼠大脑中促炎基因表达的减少;高DF饮食可以通过结肠纤维发酵增加PD患者的丁酸盐产量,丁酸盐给药可改善运动缺陷,减少炎症和多巴胺缺乏;通过缓慢发酵的淀粉包埋微球可以提高丁酸盐的产生、维持远端结肠的低pH值和抑制潜在有害细菌的生长,诱导对炎症性肠病(inflammatory bowel disease,IBD)患者有利的结肠环境。
粪便样本通常被用作研究肠道微生物群的参考标准,因为它们方便且无创伤性,并且可以分析微生物和代谢产物[38]。研究表明,在体外发酵实验中,纯化DF具有明确的物理化学活性。新鲜粪便需要从3个月内未服用益生菌或抗生素的健康志愿者或者在取样前需要提供标准的半限定饮食动物(猪、老鼠)身上取样。收集的粪便样品用磷酸盐缓冲盐水/碳酸盐磷酸盐缓冲液/无菌厌氧盐水溶液稀释后充分混匀,缓冲溶液的pH值需保持在6.5~7,发酵期间样品需全程在无菌和37 ℃条件下培养,选取0~24 h的发酵时间点进行分析。离心后,收集上清液作为粪便浆液用于进一步实验[39-40]。图1为体外发酵实验流程图。表1是采用体外发酵模型研究DF改善肠道微生物群和SCFA的部分文献。很多学者通过体外粪便发酵研究了不同来源的DF对肠道微生物群和SCFA的影响,研究结果表明DF可以改变肠道微生物群的丰度以及SCFA的含量。
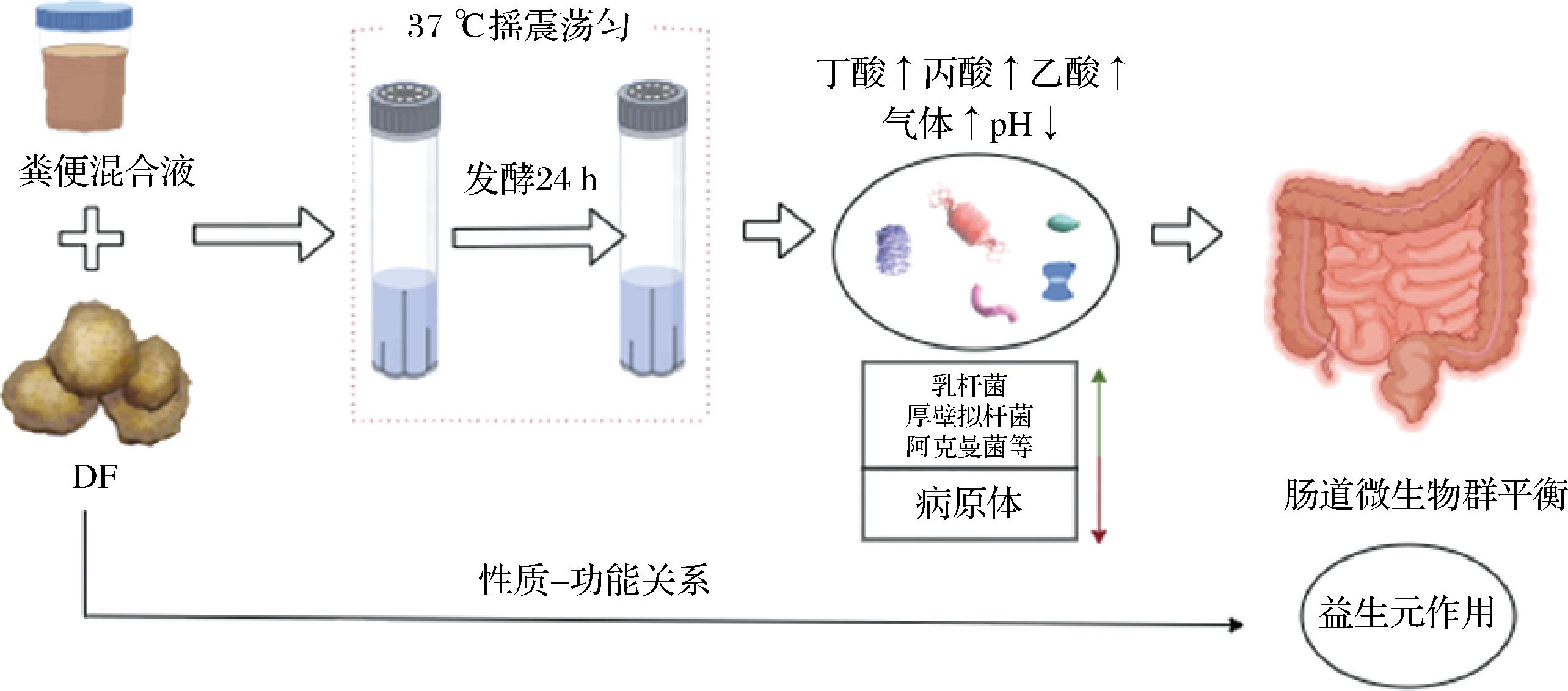
图1 体外发酵实验流程图
Fig.1 In vitro fermentation experiment flowchart
表1 不同来源的DF通过体外粪便法发酵,发酵后产生高含量的肠道微生物群和短链脂肪酸
Table 1 Dietary fiber from different sources is fermented through in vitro fecal methods, resulting in a high content of gut microbiota and short chain fatty acids after fermentation

原料体外发酵方法研究指标指标变化参考文献双胞菇DF粪便和碳酸盐-磷酸盐缓冲液混合过滤,粪便溶液添加到DF、磷酸盐缓冲液和空白对照中。摇晃,37 ℃培养24 hSCFA产生SCFA,提高有益菌的相对丰度[11]甘薯SDF粪便与体外发酵液混匀,粪便混合液加入DF、体外发酵液和空白对照中。于厌氧培养箱中37 ℃培养48 hpHNH3-NSCFA发酵液中氨浓度降低SCFA含量提升,pH、NH3-N显著降低[33]木聚糖、低聚木糖、β-葡聚糖、柑橘果胶、菊粉和抗性淀粉纤维溶解在发酵培养基中并在4 ℃下水合过夜。将混合液体于37 ℃厌氧室中培养。规定时间后引入液氮终止发酵pH微生物群落结构pH显著降低SCFA的生物合成途径与纤维多糖的链结构有关[41]红薯皮DF粪便与磷酸盐缓冲溶液混匀,静置过滤。抽取粪便滤液注入6种发酵瓶中,摇匀,37 ℃培养箱培养SCFApH氨含量有益细菌丰度增加,有害细菌丰度减少SCFA含量提高,肠道pH、氨含量降低[42]全谷物黑麦面包、全谷物燕麦面包、含有燕麦胚乳面粉的精制小麦面包粪便与缓冲液在CO2处理的瓶中混合。含有缓冲液和基质作空白和对照组。37 ℃孵育24 h,规定时间收集液体,分3等份试样SCFA微生物群的变化供体I粪便中类杆菌和埃希氏菌/志贺菌相对丰度较高,发酵8 h后SCFA较低供体Ⅱ发酵样品中丁酸盐水平较高[43]菠菜、胡萝卜、芹菜、苹果和香蕉中分离出的IDF粪便混合预热的无菌厌氧盐水溶液稀释粪便。接种在粪便收集后2 h内向每个血清瓶添加接种物产气量SCFA苹果IDF产气量最高,香蕉和菠菜IDF产气量最低每种底物的SCFA具有相似的等级顺序[44]青稞、大麦、荞麦、小米、藜麦、高粱、椰壳、谷子和燕麦将混合粪便滤液加到厌氧管中,37 ℃振荡混匀。规定时间后取出。菊粉和无菌水分别作为阳性和阴性对照,每个时间点设3个平行pHSCFA小米、大麦、椰壳和高粱(蒸制样品)发酵过程中产生的SCFA≥相应的煮制样品燕麦、藜麦、青稞和荞麦pH值呈下降趋势[45]橙子、芒果和刺梨皮粪便与碳酸-磷酸盐缓冲液混匀过滤。将浆液加入试管并水浴连续搅拌。规定发酵时间点分析产气量SCFA刺梨皮的产气量最低发酵24 h后,未挤压橙皮和挤压橙皮的SCFA最高[46]蘑菇粉粪便与磷酸盐缓冲盐水混匀。含基础培养基的水套发酵罐和抗性水解产物为处理和对照组。37 ℃下搅拌,pH保持在6.65~6.95微生物群的变化SCFA抗性水解产物使厚壁菌门/拟杆菌门比率降低抗性水解产物富集拟杆菌、双歧杆菌等相对丰度和SCFA的浓度[47]豆渣IDF粪便与磷酸盐缓冲液混匀过滤。无菌混合DF,37 ℃厌氧培养16 h,规定发酵时间收集样品分析微生物群结构IDF发酵后的结构厚壁菌门、拟杆菌门、乳杆菌丰度高发酵后的IDF颗粒不光滑,撕裂感低、结构混乱且不均匀[48]黄小米、燕麦粪便与磷酸盐缓冲液混匀过滤。粪便溶液添加到含DF、缓冲液和空白对照中。37 ℃连续振荡厌氧培养SCFASCFA随发酵时间的增加而增加,也随小米、燕麦DF含量增加而增加[49]胡萝卜DF粪便与磷酸钠缓冲液混匀,加入含DF的培养基中。37 ℃震荡培养发酵后取出样品分析pHSCFApH值随着发酵时间的增加SCFA随发酵时间的增加而逐渐增加[50]
3 不同类型DF的体外发酵性质研究
DF在体外的溶解度可能不足以反映其在肠道中的溶解度,DF的某些α-糖苷键可以在小肠中被胰淀粉酶或葡萄糖淀粉酶部分水解,而其他一些糖苷键不能被消化,从而转移到大肠,由肠道微生物群发酵[51-52]。
3.1 SDF对肠道菌群的调节作用
3.1.1 SDF种类/结构对肠道菌群丰度/多样性的影响
SDF比IDF更易于结肠发酵,经过粪便发酵后肠道微生物丰富度和多样性增加[53]。通过比较多种SDF与未添加SDF的空白组,结果表明,添加不同的SDF可以显著影响SCFA结果和有机酸谱,可以不同程度地改变肠道微生物中SCFA形成的生物合成途径,导致不同的微生物谱[54]。ZHENG等[55]采用复合酶提取油菜蜂花粉中的SDF,与人类粪便微生物群发酵后,增加了有益微生物群落的微生物丰富度和多样性。有研究者从进口羊肚菌中提取SDF进行体外发酵,有益细菌相对丰度显著增加,呈现出剂量依赖性,而有害细菌相对丰度显著减少[56]。李文远[57]采用食用菌SDF进行体外发酵,发现食用菌SDF增加了厚壁菌门和拟杆菌门等肠道有益菌的相对丰度,促进不同的优势菌群的增殖。CAO等[58]研究了甘薯皮SDF体外发酵性质,结果表明甘薯皮SDF增加肠道中有益菌(如双歧杆菌门、粪杆菌门和普雷沃氏菌)的丰度,降低有害菌(如变形菌门、Romboutsia)的丰度,提高SCFA产菌的相对丰度,促进SCFA的产生。
3.1.2 SDF种类/结构对肠道SCFA的影响
SDF经发酵之后可以促进SCFA的合成。通过添加甘薯渣SDF进行体外发酵,结果表明,添加甘薯渣SDF有利于增加体外发酵的产气量,并且能促进SCFA的合成[59]。还有研究采用发酵的大豆残渣的SDF研究其对大鼠肠道菌群组成和多样性的影响,研究发现,在发酵液中发酵24 h后,大豆残渣SDF提高了产气量和SCFA的水平[60]。 AHIN等[61]研究坚果(杏仁、腰果、榛子、开心果和核桃)的DF对肠道微生物群的影响,结果发现,腰果DF在体外发酵过程中能产生较高的丁酸盐,主要原因是腰果DF具有较高的SDF/总DF比例和显著不同的单糖组成。JANG等[62] 通过向基础培养基中分别添加1%低聚半乳糖(galactooligosaccharides,GOS)以及1%和2%甘露寡糖(mannose oligo saccharides,MOS)作为碳源来评估人类粪便微生物的发酵特性。与添加1%的MOS和GOS相比,当添加2%的MOS时,SCFA含量增加。刘心仪等[63]通过体外发酵实验探究了藜麦IDF和外源添加乳酸菌对肠道菌群及SCFA的影响,研究发现,随着发酵时间的增加,乙酸、丙酸、异丁酸、丁酸、异戊酸、戊酸的产量均呈现增加趋势。
AHIN等[61]研究坚果(杏仁、腰果、榛子、开心果和核桃)的DF对肠道微生物群的影响,结果发现,腰果DF在体外发酵过程中能产生较高的丁酸盐,主要原因是腰果DF具有较高的SDF/总DF比例和显著不同的单糖组成。JANG等[62] 通过向基础培养基中分别添加1%低聚半乳糖(galactooligosaccharides,GOS)以及1%和2%甘露寡糖(mannose oligo saccharides,MOS)作为碳源来评估人类粪便微生物的发酵特性。与添加1%的MOS和GOS相比,当添加2%的MOS时,SCFA含量增加。刘心仪等[63]通过体外发酵实验探究了藜麦IDF和外源添加乳酸菌对肠道菌群及SCFA的影响,研究发现,随着发酵时间的增加,乙酸、丙酸、异丁酸、丁酸、异戊酸、戊酸的产量均呈现增加趋势。
3.2 IDF对肠道菌群的调节作用
3.2.1 IDF种类/结构对肠道菌群丰度/多样性的影响
通常认为IDF在结肠(模型)中的发酵速度要慢于SDF,并且在结肠中的发酵程度较小,IDF经过不同方式处理(超高压、挤压、微波等)后可不同程度的改变肠道菌群的组成。通过研究超声波提取的刺梨IDF体外发酵,发现超高压改性显著提高了刺梨IDF的保水性、保油性和溶胀能力,虽表现出最慢的发酵速率,但产生了最高的丁酸盐产量,并且富集了产丁酸盐瘤胃球菌属和粪球菌物种[64]。SELBER-HNATIW等[65]研究微波处理后的水不溶性麸皮纤维的发酵能力,与天然纤维相比,促进了厚壁菌门/拟杆菌门比例的显著增加,同时布劳特氏菌属(Blautia)和铜球菌属(Copprococcus)的相对丰度增加,而拟杆菌门的相对丰度减少。有研究采用长链复合阿拉伯木聚糖研究其对超重和I级肥胖的成人肠道微生物群组成影响,发现长链复合阿拉伯木聚糖导致了粪便细菌群落组成的转变,减少了α多样性,并促进了特定菌群的发展[66]。在采用黑米IDF进行体外发酵实验时,发现IDF的添加调节了微生物群结构并促进拟杆菌门和普雷沃特拉科菌群的增长[67]。刘东杰等[68]采用麻竹笋壳IDF进行体外发酵实验,研究结果表明,麻竹笋IDF能有效改善肠道菌群种类和丰度,有助于有益菌如拟杆菌、考拉杆菌相对丰度的增加。
3.2.2 IDF种类/结构对肠道SCFA的影响
IDF不仅可以不同程度调节肠道菌群的组成同时也提高了SCFA的产生。通过研究探讨3种竹IDF(碱性H2O2处理竹纤维、酶水解处理竹纤维和商用竹纤维)的发酵特性,结果表明,添加3种IDF提高了人体肠道菌群的产酸能力,碱性H2O2处理过的竹IDF,显著提高了SCFA的产量,并在不同程度上调节肠道菌群的组成[69]。有研究采用高压均质处理后的刺梨果渣IDF研究体外发酵特性,结果表明,高压均质处理的刺梨果渣IDF促进了SCFA的产生,其中乙酸占总酸含量较高[70]。通过比较超高压(90 MPa)处理和超声波提取的苹果渣IDF,结果显示超高压处理的苹果渣IDF表现出最慢的发酵速率,但有最高的丁酸盐产量[71]。胡杰[72]研究了雷笋IDF在体外发酵后SCFA含量的变化,结果表明,IDF在肠道菌群发酵后戊酸、丁酸、异戊酸、乙酸、异丁酸和丙酸含量均显著高于对照组。
3.3 IDF对肠道菌群的调节作用
体外发酵特性根据膳食纤维的来源以及性质的不同而改变,与IDF相比,SDF更多地刺激丁酸盐的产生[73]。LIANG等[74]研究发现菊粉SDF不仅大大增加了丁酸盐的产量,而且富含β-葡聚糖的牡蛎蘑菇茎SDF也显著增加了PD患者和健康人群丁酸盐的产生。从库尔勒香梨中提取的SDF和IDF进行结肠体外发酵模型评估发酵特性,研究结果表明SDF组的SCFA含量高于IDF,SDF组的乳酸含量也与IDF组存在显著性差异(P<0.05)。研究纯化后麸皮中的水溶性纤维和水不溶性纤维的发酵特性,结果表明,IDF产生的气体总量比SDF少[75]。有学者研究了米糠水不溶性和水溶性部分对菌群调节作用的差异及相互作用,发现IDF主要促进了拟杆菌属 (Bacteroides)和肠道罗斯拜瑞氏菌 (Roseburia)的增殖,产生更高比例的丙酸;SDF主要促进了普劳斯尼奇粪杆菌(Faecalibacterium)、革兰氏阳性菌 (Butyricicoccus)以及瘤胃球菌(unclassified_Ruminococcacea)的增殖,产生了更高比例的乙酸和丁酸[76]。
4 结论和未来展望
使用粪便作为接种物进行体外发酵的策略被认为是具有成本效益的方法,可以从机制上了解粪便微生物群落动态。然而,所有体外发酵方法由于固有的差异和方法的局限性,致使结果产生偏差。DF为肠道微生物生长提供能量和营养,是平衡结肠生态系统所必需的物质。DF的不同结构特征和不同来源影响其在结肠中的发酵特性,并且也会对肠道微生物群产生影响。目前,DF的体外发酵研究提供了有关DF与人类健康关系的信息,这篇综述总结了DF和肠道微生物群的相互作用以及不同DF对肠道微生物群的影响。然而,肠道微生物群非常复杂,目前对肠道微生物群落内部相互作用的了解有限。大多数研究都是微生物组景观的快照,需要对DF与人体作用的机制更多研究。目前体外发酵实验也被用作临床前的研究步骤,以经济高效的方式评估药物对肠道微生物群的影响,有助于在未来实现“精确营养、治疗”的目的。
[1] TUNCIL Y E, THAKKAR R D, MARCIA A D R, et al.Divergent short-chain fatty acid production and succession of colonic microbiota arise in fermentation of variously-sized wheat bran fractions[J].Scientific Reports, 2018, 8(1):16655.
[2] CALATAYUD M, VAN DEN ABBEELE P, GHYSELINCK J, et al.Comparative effect of 22 dietary sources of fiber on gut microbiota of healthy humans in vitro[J].Frontiers in Nutrition, 2021, 8:700571.
[3] THOMSON C, GARCIA A L, EDWARDS C A.Interactions between dietary fibre and the gut microbiota[J].Proceedings of the Nutrition Society, 2021,80(4):398-408.
[4] TAO S Y, BAI Y, ZHOU X J, et al.In vitro fermentation characteristics for different ratios of soluble to insoluble dietary fiber by fresh fecal microbiota from growing pigs[J].ACS Omega, 2019, 4(12):15158-15167.
[5] LI P D, LI C, FU X, et al.Physicochemical, functional and biological properties of soluble dietary fibers obtained from Rosa roxburghii Tratt pomace using different extraction methods[J].Process Biochemistry, 2023, 128:40-48.
[6] EVANS C E L.Dietary fibre and cardiovascular health:A review of current evidence and policy[J].Proceedings of the Nutrition Society, 2020, 79(1):61-67.
[7] STEPHEN A M, CHAMP M M J, CLORAN S J, et al.Dietary fibre in Europe:Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health[J].Nutrition Research Reviews, 2017, 30(2):149-190.
[8] TRUMBO P, SCHLICKER S, YATES A A, et al.Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids[J].Journal of the American Dietetic Association, 2002, 102(11):1621-1630.
[9] GIANFREDI V, SALVATORI T, VILLARINI M, et al.Is dietary fibre truly protective against colon cancer? A systematic review and meta-analysis[J].International Journal of Food Sciences and Nutrition, 2018, 69(8):904-915.
[10] QUAGLIANI D, FELT-GUNDERSON P.Closing America’s fiber intake gap:Communication strategies from a food and fiber summit[J].American Journal of Lifestyle Medicine, 2016, 11(1):80-85.
[11] 向情儒, 李文远, 冯涛.基于体外发酵的双孢菇膳食纤维及双孢菇粉对人体肠道菌群的调节作用[J].食品工业科技, 2023, 44(10):130-137.
XIANG Q R, LI W Y, FENG T.Regulating effects of dietary fiber and powder of Agaricus bisporus based on in vitro fermentation on human gut microbiota[J].Science and Technology of Food Industry, 2023, 44(10):130-137.
[12] 武明月, 孔祥丽, 张天阳, 等.菊粉和大豆膳食纤维对牛肉饮食的小鼠肠道菌群及其代谢产物的改善作用[J].食品科学, 2022, 43(5):158-167.
WU M Y, KONG X L, ZHANG T Y, et al.Inulin and soybean dietary fiber improved the intestinal flora and metabolites in mice fed a beef-containing diet[J].Food Science, 2022, 43(5):158-167.
[13] 王海松, 任鹏飞.不同单糖组成的低聚糖对人肠道菌群的调节作用[J].中国食品学报, 2020, 20(7):44-52.
WANG H S, REN P F.Modulation of oligosaccharides with different monosaccharide composition on the human gut microbiota[J].Journal of Chinese Institute of Food Science and Technology, 2020, 20(7):44-52.
[14] LEY R E, TURNBAUGH P J, KLEIN S, et al.Human gut microbes associated with obesity[J].Nature, 2006, 444:1022-1023.
[15] LEY R E, B CKHED F, TURNBAUGH P, et al.Obesity alters gut microbial ecology[J].Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(31):11070-11075.
CKHED F, TURNBAUGH P, et al.Obesity alters gut microbial ecology[J].Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(31):11070-11075.
[16] QIN J J, LI Y R, CAI Z M, et al.A metagenome-wide association study of gut microbiota in type 2 diabetes[J].Nature, 2012, 490(7418):55-60.
[17] WU L, TANG C H, CHEN L L, et al.Modified dietary fiber from soybean dregs by fermentation alleviated constipation in mice[J].Food Chemistry:X, 2023, 19:100810.
[18] LATERZA L, RIZZATTI G, GAETANI E, et al.The gut microbiota and immune system relationship in human graft-versus-host disease[J].Mediterranean Journal of Hematology and Infectious Diseases, 2016, 8(1):e2016025.
[19] ARUMUGAM M, RAES J, PELLETIER E, et al.Enterotypes of the human gut microbiome[J].Nature, 2011, 473(7346):174-180.
[20] GILL S R, POP M, DEBOY R T, et al.Metagenomic analysis of the human distal gut microbiome[J].Science, 2006, 312(5778):1355-1359.
[21] DA TAN E, ÇELIK Ö F, BA
TAN E, ÇELIK Ö F, BA O, et al.Sex-dependent colonic microbiota modulation by hazelnut (Corylus avellana L.) dietary fiber[J].Food &Function, 2023, 14(6):2896-2907.
O, et al.Sex-dependent colonic microbiota modulation by hazelnut (Corylus avellana L.) dietary fiber[J].Food &Function, 2023, 14(6):2896-2907.
[22] 尚玮璇, 刘璐, 雷素珍, 等.功能性碳水化合物通过调节肠道菌群和代谢物改善非酒精性脂肪肝的作用机制[J].食品与发酵工业, 2022, 48(14):311-318.
SHANG W X, LIU L, LEI S Z, et al.Mechanism of functional carbohydrates alleviating non-alcoholic fatty liver disease by regulating intestinal flora and metabolites[J].Food and Fermentation Industries, 2022, 48(14):311-318.
[23] VAN DEN ABBEELE P, VENEMA K, VAN DE WIELE T, et al.Different human gut models reveal the distinct fermentation patterns of Arabinoxylan versus inulin[J].Journal of Agricultural and Food Chemistry, 2013, 61(41):9819-9827.
[24] 王绍康.抗性淀粉发酵特性及其对肠道菌群的调控机制研究[D].广东:华南理工大学, 2021:196.
WANG S K.Study on the fermentation characteristics of resistant starches and their regulation mechanism on gut microbiota[D].Guangdong:South China University of Technology, 2021:196.
[25] SARBINI S R, KOLIDA S, DEAVILLE E R, et al.Potential of novel dextran oligosaccharides as prebiotics for obesity management through in vitro experimentation[J].British Journal of Nutrition, 2014, 112(8):1303-1314.
[26] COOPER D N, KABLE M E, MARCO M L, et al.The effects of moderate whole grain consumption on fasting glucose and lipids, gastrointestinal symptoms, and microbiota[J].Nutrients, 2017, 9(2):173.
[27] CHEN T T, LONG W M, ZHANG C H, et al.Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota[J].Scientific Reports, 2017, 7(1):2594.
[28] FU Y S, ZHANG J N, CHEN K N, et al.An in vitro fermentation study on the effects of Dendrobium officinale polysaccharides on human intestinal microbiota from fecal microbiota transplantation donors[J].Journal of Functional Foods, 2019, 53:44-53.
[29] WANG M M, WICHIENCHOT S, HE X W, et al.In vitro colonic fermentation of dietary fibers:Fermentation rate, short-chain fatty acid production and changes in microbiota[J].Trends in Food Science &Technology, 2019, 88:1-9.
[30] MOON J S, LI L, BANG J, et al.Application of in vitro gut fermentation models to food components:A review[J].Food Science and Biotechnology, 2016, 25(1):1-7.
[31] COSTABILE A, KLINDER A, FAVA F, et al.Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota:A double-blind, placebo-controlled, crossover study[J].British Journal of Nutrition, 2008, 99(1):110-120.
[32] LI L, ZHAO Y F, LI J Q, et al.The adhesion of the gut microbiota to insoluble dietary fiber from soy hulls promoted the proliferation of probiotics in vitro[J].LWT, 2022, 153:112560.
[33] 高美玲, 余诚玮, 范亚苇, 等.甘薯渣中可溶性膳食纤维对肠道菌群代谢产物的影响[J].中国食品学报, 2020, 20(12):56-61.
GAO M L, YU C W, FAN Y W, et al.The effect of soluble dietary fiber in sweet potato residues on intestinal flora metabolites[J].Journal of Chinese Institute of Food Science and Technology, 2020, 20(12):56-61.
[34] BRAHMA S, WEIER S A, ROSE D J.Moisture content during extrusion of oats impacts the initial fermentation metabolites and probiotic bacteria during extended fermentation by human fecal microbiota[J].Food Research International, 2017, 97:209-214.
[35] YAO T, CHEN M H, LINDEMANN S R.Carbohydrate complexity structures stable diversity in gut-derived microbial consortia under high dilution pressure[J].Cold Spring Harbor Laboratory, 2020.DOI:10.1101/2020.01.31.929760.
[36] ST LAURENT R, O’BRIEN L M, AHMAD S T.Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease[J].Neuroscience, 2013, 246:382-390.
[37] MATT S M , ALLEN J M , LAWSON M A ,et al.Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice[J].Other, 2018, 9.DOI:10.3389/fimmu.2018.01832.
[38] CANTU-JUNGLES T M,HAMAKER B R.Erratum for cantu-jungles and hamaker, “new view on dietary fiber selection for predictable shifts in gut microbiota”[J].mBio,2020,11(3).DOI:10.1128/mBio.00747-20.
[39] KANG J, YIN S J, LIU J, et al.Fermentation models of dietary fibre in vitro and in vivo-A review[J].Food Hydrocolloids, 2022, 131:107685.
[40] GIBSON G R, PROBERT H M, LOO J V, et al.Dietary modulation of the human colonic microbiota:Updating the concept of prebiotics[J].Nutrition Research Reviews, 2004, 17(2):259-275.
[41] BAI J Y, LI Y, LI T T, et al.Comparison of different soluble dietary fibers during the in vitro fermentation process[J].Journal of Agricultural and Food Chemistry, 2021, 69(26):7446-7457.
[42] DE ALBUQUERQUE T M R, MAGNANI M, LIMA M D S, et al.Effects of digested flours from four different sweet potato (Ipomoea batatas L.) root varieties on the composition and metabolic activity of human colonic microbiota in vitro[J].Journal of Food Science, 2021, 86(8):3707-3719.
[43] PIRKOLA L, DICKSVED J, LOPONEN J, et al.Fecal microbiota composition affects in vitro fermentation of rye, oat, and wheat bread[J].Scientific Reports, 2023, 13(1):99.
[44] WIDANINGRUM, FLANAGAN B M, WILLIAMS B A, et al.Fruit and vegetable insoluble dietary fibre in vitro fermentation characteristics depend on cell wall type[J].Bioactive Carbohydrates and Dietary Fibre, 2020, 23:100223.
[45] FANG F, HE Y X, ZHAO J T, et al.Effects of boiling and steaming process on dietary fiber components and in vitro fermentation characteristics of 9 kinds of whole grains[J].Food Research International, 2023, 164:112328.
[46] TEJADA-ORTIGOZA V, GARCIA-AMEZQUITA L E, CAMPANELLA O H, et al.Extrusion effect on fecal fermentation of fruit peels used as dietary fiber sources[J].LWT-Food Science and Technology, 2022, 153:112569.
[47] AYIMBILA F, SIRIWONG S, NAKPHAICHIT M, et al.In vitro gastrointestinal digestion of Lentinus squarrosulus powder and impact on human fecal microbiota[J].Scientific Reports, 2022, 12(1):2655.
[48] LYU B, WANG Y, ZHANG X, et al.Changes of high-purity insoluble fiber from soybean dregs (okara) after being fermented by colonic flora and its adsorption capacity[J].Foods, 2021, 10(10):2485.
[49] GUO W K, ZHAO F.In vitro Fermentation characteristics of dietary fibers from yellow millet and oats[J].Journal of Food and Nutrition Research, 2019, 7(1):6-11.
[50] LIU S, YU Q, HUANG H R, et al.The effect of bound polyphenols on the fermentation and antioxidant properties of carrot dietary fiber in vivo and in vitro[J].Food &Function, 2020, 11(1):748-758.
[51] SU A X, MA G X, XIE M H, et al.Characteristic of polysaccharides from Flammulina velutipes in vitro digestion under salivary, simulated gastric and small intestinal conditions and fermentation by human gut microbiota[J].International Journal of Food Science &Technology, 2019, 54(6):2277-2287.
[52] WANG Y D, CHEN G J, PENG Y J, et al.Simulated digestion and fermentation in vitro with human gut microbiota of polysaccharides from Coralline pilulifera[J].Lwt, 2019, 100:167-174.
[53] CRONIN P, JOYCE S A, O’TOOLE P W, et al.Dietary fibre modulates the gut microbiota[J].Nutrients, 2021, 13(5):1655.
[54] LIU J, JOHNSON R, DILLON S, et al.Among older adults, age-related changes in the stool microbiome differ by HIV-1 serostatus[J].EBioMedicine, 2019, 40:583-594.
[55] ZHENG H, SUN Y, ZENG Y Q, et al.Effects of four extraction methods on structure and in vitro fermentation characteristics of soluble dietary fiber from rape bee pollen[J].Molecules, 2023, 28(12):4800.
[56] LEI J Y, ZHANG Y T, GUO D D, et al.Extraction optimization, structural characterization of soluble dietary fiber from Morchella importuna, and its in vitro fermentation impact on gut microbiota and short-chain fatty acids[J].CyTA-Journal of Food, 2022, 20(1):128-142.
[57] 李文远.食用菌可溶性膳食纤维对人体肠道菌群的影响[D].上海:上海应用技术大学,2021.
LI W Y.Effects of Soluble Dietary Fiber fromEdible Fungi on Human Intestinal Microflora[D].Shanghai:Shanghai Institute of Technology,2021.
[58] CAO Y, TIAN B M, ZHANG Z G, et al.Positive effects of dietary fiber from sweet potato[Ipomoea batatas (L.) Lam.]peels by different extraction methods on human fecal microbiota in vitro fermentation[J].Frontiers in Nutrition, 2022, 9:986667.
[59] 高美玲.甘薯渣中可溶性膳食纤维对肠道菌群的影响[D].江西:南昌大学,2019:44.
GAO M L.Effect of soluble dietary fiber sweet potato on intestinal microflora[D].Jiangxi:Nanchang University,2019:44.
[60] YU J W, FU Y X, DENG Z Y, et al.Effects of soluble dietary fiber from soybean residue fermented by Neurospora crassa on the intestinal flora in rats[J].Food &Function, 2020, 11(9):7433-7445.
[61]  AHIN M, ARIOGLU-TUNCIL S, ÜNVER A, et al.Dietary fibers of tree nuts differ in composition and distinctly impact the fecal microbiota and metabolic outcomes in vitro[J].Journal of Agricultural and Food Chemistry, 2023, 71(25):9762-9771.
AHIN M, ARIOGLU-TUNCIL S, ÜNVER A, et al.Dietary fibers of tree nuts differ in composition and distinctly impact the fecal microbiota and metabolic outcomes in vitro[J].Journal of Agricultural and Food Chemistry, 2023, 71(25):9762-9771.
[62] JANG E Y, HONG K B, CHANG Y B, et al.In vitro prebiotic effects of malto-oligosaccharides containing water-soluble dietary fiber[J].Molecules, 2020, 25(21):5201.
[63] 刘心仪, 陈孟涵, 李培实, 等.植物乳杆菌体外发酵藜麦水不溶性膳食纤维对肠道菌群及产短链脂肪酸的影响[J].食品科学, 2024, 45(13):138-145.
LIU X Y, CHEN M H, LI P S, et al.Effect of in vitro fermentation of quinoa water insoluble dietary fiber with Lactobacillus plantarum on intestinal flora and short-chain fatty acid production[J].Food Science, 2024, 45(13):138-145.
[64] WANG L, LI C, HUANG Q, et al.In vitro digestibility and prebiotic potential of a novel polysaccharide from Rosa roxburghii Tratt fruit[J].Journal of Functional Foods, 2019, 52:408-417.
[65] SELBER-HNATIW S, RUKUNDO B, AHMADI M, et al.Human gut microbiota:Toward an ecology of disease[J].Frontiers in Microbiology, 2017, 8:1265.
[66] NGUYEN N K, DEEHAN E C, ZHANG Z X, et al.Gut microbiota modulation with long-chain corn bran Arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate[J].Microbiome, 2020, 8(1):118.
[67] ZHANG S, DENG M, ZHANG R F, et al.Modulation effect of black rice dietary fiber on the metabolism and fermentation of cyanidin-3-glucoside in an in vitro human colonic model[J].Food &Function, 2023, 14(14):6707-6717.
[68] 刘东杰, 周心雨, 王锋, 等.麻竹笋壳不溶性膳食纤维理化特性及其体外酵解对肠道微生物的影响[J].食品研究与开发, 2023, 44(24):23-29;45.
LIU D J, ZHOU X Y, WANG F, et al.Physicochemical properties of insoluble dietary fiber from bamboo shoot shells and effects of its in vitro fermentation on intestinal microorganism[J].Food Research and Development, 2023, 44(24):23-29;45.
[69] GE Q, LI H Q, ZHENG Z Y, et al.In vitro fecal fermentation characteristics of bamboo insoluble dietary fiber and its impacts on human gut microbiota[J].Food Research International, 2022, 156:111173.
[70] 夏洁.刺梨果渣水不溶性膳食纤维的制备、结构表征及其体外发酵特性研究[D].广州:华南理工大学,2020.
XIA J.Study on extraction, structural characterization and fermentation of insoluble dietary fiber from Rosa roxburghii Tratt fruit[D].Guangzhou:South China University of Technology,2020.
[71] WANG S K, XIA J, DE PAEPE K, et al.Ultra-high pressure treatment controls in vitro fecal fermentation rate of insoluble dietary fiber from Rosa roxburghii tratt pomace and induces butyrogenic shifts in microbiota composition[J].Journal of Agricultural and Food Chemistry, 2021, 69(36):10638-10647.
[72] 胡杰. 竹笋膳食纤维体外活性及其组分变化规律研究[D].金华:浙江师范大学,2021.
HU J.Study on the invitro activity of dietary fiber in bamboo shoots and composition changes[D].Jinhua:Zhejiang Normal University,2021.
[73] BAERT F, MATTHYS C, MASELYNE J, et al.Parkinson’s disease patients’ short chain fatty acids production capacity after in vitro fecal fiber fermentation[J].NPJ Parkinson’s Disease, 2021, 7(1):72.
[74] LIANG X Y, LIU H C, WEI Z N, et al.Modulation of gut flore by dietary fibers from Pyrus bretschneideri rehd:Evaluation of fermentation characteristics using a colonic in vitro fermentation model[J].Journal of Functional Foods, 2023, 102:105466.
[75] COMINO P, WILLIAMS B A, GIDLEY M J.In vitro fermentation gas kinetics and end-products of soluble and insoluble cereal flour dietary fibres are similar[J].Food &Function, 2018, 9(2):898-905.
[76] 侯雅琴. 不溶性膳食纤维的结构变化对其肠道菌群调节作用的影响及机制初探[D].南昌:南昌大学,2023.
HOU Y Q.The influence and mechanism of structural changes in insoluble dietary fiber on its gut microbiota regulation[D].Nanchang:Nanchang University,2023.