1817年,BERZELIUS首次发现了一种非金属元素并将其命名为硒[1]。在此后的很长一段时间里,硒一直被认为是有毒物质。直到20世纪50年代,SCHWARTZ等[2]在酵母中发现了含硒的活性因子,并证实了饮食中补充硒可以预防大鼠因营养不良导致的肝坏死。研究结果表明,摄入适量的硒对健康是有益的。由此,人们开始关注硒的重要生理功能。硒主要通过植物、海产品(藻类)和添加硒的动物饲料进入人类食物链,参与人体内的一系列细胞代谢过程[3]。当硒的摄入量低于40 μg/d或高于400 μg/d时会有缺乏或中毒的风险,对健康产生不利影响[4]。动物性食物摄入较少、居住在缺硒地区或有特殊健康需求的人群(表1)应关注硒的摄入量。
表1 特殊健康需求人群硒补充量
Table 1 Selenium supplementation for people with special health needs
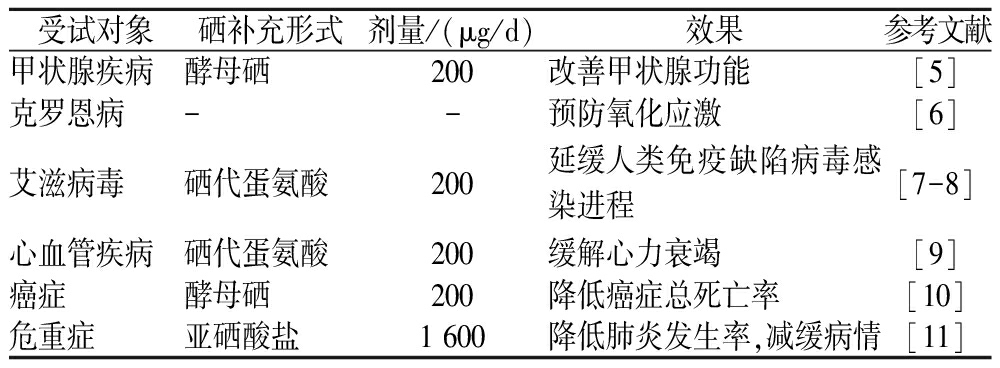
受试对象硒补充形式剂量/(μg/d)效果参考文献甲状腺疾病酵母硒200改善甲状腺功能[5]克罗恩病--预防氧化应激[6]艾滋病毒硒代蛋氨酸200延缓人类免疫缺陷病毒感染进程[7-8]心血管疾病硒代蛋氨酸200缓解心力衰竭[9]癌症酵母硒200降低癌症总死亡率[10]危重症亚硒酸盐1 600降低肺炎发生率,减缓病情[11]
硒的生物作用与其形态密切相关,硒在自然界中有3种存在形式:无机硒、有机硒和单质硒。无机硒,如硒酸盐和亚硒酸盐,毒性强且生物利用度低,目前已有部分国家和地区禁止在动物饲料及食品中使用无机硒。有机硒,如硒代蛋氨酸(SeMet)和甲基硒代半胱氨酸(SeMCys),生物活性高、毒性低、吸收性好,是被允许使用的硒源营养强化剂。开发高效、低毒的有机硒是当前国内外硒研究的热点领域之一。单质硒基本无毒,但难溶于水且生物利用度低,存在灰色、黑色和红色3种形式。
纳米硒(selenium nanoparticles, SeNPs)是一种红色单质硒,具有高吸收率、高生物活性和低毒性等优点[12]。尤其是来源于益生菌的生物合成纳米硒,被认为是安全且附加值高的产品,具有抗氧化、抗癌、调节肠道免疫和微生物群落等功能[13-14]。肠道是营养物质消化、吸收和转运的主要场所,同时也是人体最大的免疫器官。肠道中寄居着大量微生物群落,这些菌群在营养代谢和黏膜免疫等方面发挥着重要作用[15]。其中,肠道黏膜免疫系统包含大量免疫细胞,能够识别并清除病原微生物,有效阻止病原体(包括细菌、病毒等)穿过肠壁进入血液循环;此外,肠道黏膜免疫系统还能抑制有害菌群的过度定植,维持肠道微生物的稳态。本文总结了基于植物和微生物的纳米硒生物合成方法,详细介绍了生物合成纳米硒在调节肠道黏膜免疫和维持肠道稳态方面的作用,为未来生物合成纳米硒相关的基础研究及产业化应用提供理论和实践依据。
1 纳米硒的生物合成方法
纳米硒具有抗氧化、抗肿瘤、调节免疫等功能特性,在食品、医疗等领域受到广泛关注,其生物活性取决于粒径、形貌和稳定性等,因此纳米硒的绿色高效合成显得尤为重要[16]。目前,纳米硒的合成方法可分为物理法、化学法和生物法。物理法通常利用激光烧蚀、微波辐射、超声、升华冷凝等方式,将大块的固体硒“切割”为一定尺寸的纳米硒颗粒。化学法是将亚硒酸盐、硒酸盐或二氧化硒还原为单质纳米硒颗粒,而后在化学稳定剂聚山梨酯80、十二烷基硫酸或胰蛋白等作用下合成具有一定形貌、粒径的纳米硒,是目前最常用的纳米硒合成技术。生物法是以微生物(如细菌、真菌)或植物提取物为还原剂或稳定剂,在胞外蛋白、多糖等作用下将亚硒酸盐或硒酸盐转化成纳米硒,该方法通常使用可再生资源在室温下进行。相较于物理法和化学法,生物合成法绿色环保,经济安全,且合成的纳米硒粒径均匀、结构稳定、分散性好,是近年来的研究热点(表2)。根据原料不同,生物合成法可分为植物合成法和微生物合成法。
表2 生物合成纳米硒文献研究
Table 2 Literature reports on the biosynthesis of selenium nanoparticles
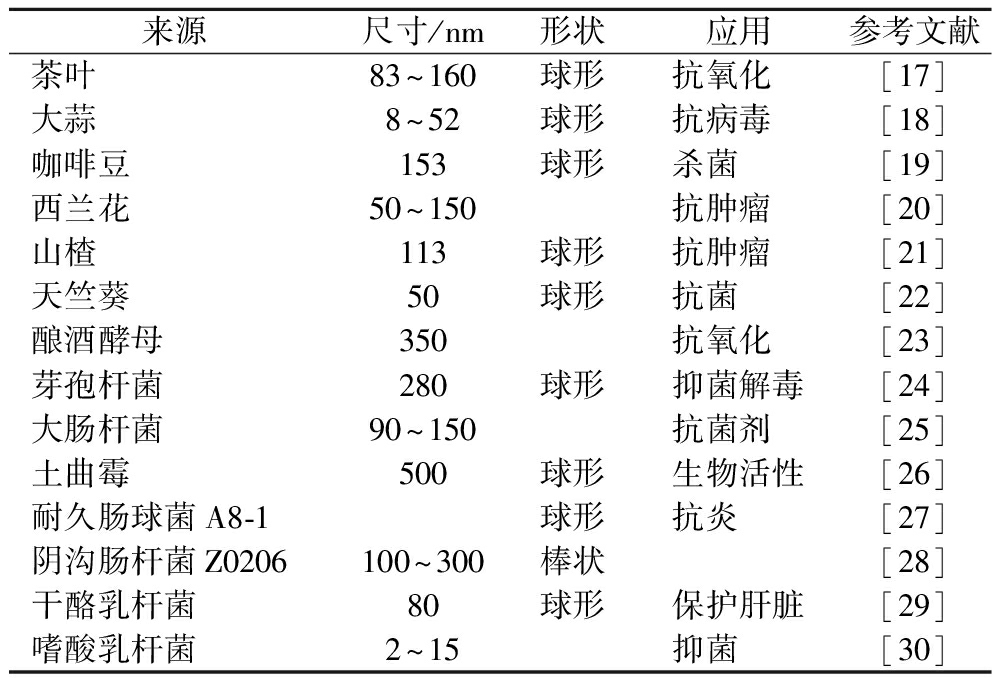
来源尺寸/nm形状应用参考文献茶叶83~160球形抗氧化[17]大蒜8~52球形抗病毒[18]咖啡豆153球形杀菌[19]西兰花50~150抗肿瘤[20]山楂113球形抗肿瘤[21]天竺葵50球形抗菌[22]酿酒酵母350抗氧化[23]芽孢杆菌280球形抑菌解毒[24]大肠杆菌90~150抗菌剂[25]土曲霉500球形生物活性[26]耐久肠球菌A8-1球形抗炎[27]阴沟肠杆菌Z0206100~300棒状[28]干酪乳杆菌80球形保护肝脏[29]嗜酸乳杆菌2~15抑菌[30]
注:空白处为无相关数据或资料。
1.1 植物合成法
植物提取物中富含的多羟基物质可将硒前体还原为零价硒,强络合物(即氨基和醛基)又可长期维持纳米硒的生物活性。HASHEM等[31]利用仙人果皮提取物合成纳米硒,并对纳米硒进行表征。结果表明,生物合成的纳米硒颗粒呈球形,具有高度结晶性,尺寸为10~87.4 nm,对革兰氏阴性菌(大肠杆菌和铜绿假单胞菌)和革兰氏阳性菌(枯草芽孢杆菌和金黄色葡萄球菌)以及单细胞真菌具有良好的抑菌效果。SUN等[32]利用山楂中分离纯化的山楂多糖(HP2)制备纳米硒颗粒,并对其结构和抗氧化活性进行研究。结果表明,该纳米硒平均粒径为137.85 nm,经过硒修饰后显著提高了山楂多糖的抗氧化活性。GHADERI等[33]首次报道了以秋葵提取物作为还原剂和稳定剂,利用Na2SeO3合成纳米硒的方法。该方法制得的球形纳米硒颗粒平均粒径约为30.7 nm,对革兰氏阳性菌具有较好的抗菌活性。柑橘类植物因富含精油、黄酮类活性成分,具有较好的抗菌、抗氧化和抗炎功效[34]。SALEM等[35]利用橘皮废弃物绿色合成纳米硒,该纳米硒对多重耐药菌具有良好的抗菌和抗生物膜活性,可用于耐药菌的防治。此外,番石榴叶[36]、柠檬[37]、决明子叶[38]等植物提取物都可用于纳米硒的合成。利用植物提取物合成的纳米硒作为一种绿色、可持续的纳米材料,在生物医学、食品工业和农业等领域显示出巨大的应用潜力。该方法不需要使用有毒化学品,对环境友好,且成本低廉,但由于植物生长条件、种类等因素的不同,通过植物合成纳米硒的可重复性较差,后期提取纯化难度高,合成周期长[39],在大规模生产和应用方面仍面临挑战。
1.2 微生物合成法
微生物可通过异化还原、同化还原和甲基化等代谢作用将无机硒转化为纳米硒,同时在纳米硒表面生成蛋白质、多糖、脂类等有机物,使其结构更加稳定且具有较好的分散性[40]。细菌、真菌、放线菌等均可用于合成纳米硒,其中细菌因其代谢旺盛、繁殖能力强、营养要求低等特点,是合成纳米硒的主要微生物。SI等[41]研究发现,利用植物乳植杆菌KNF-5生物合成的纳米硒通过下调变异链球菌生物被膜形成相关基因(如GtfB、GtfC、BrpA和GbpB)的表达,可以有效抑制变异链球菌的生长,这一发现为预防和治疗龋齿提供了新的研究方向。KORA等[42]从受污染的湖泊中分离出一株蜡样芽胞杆菌,具有较强的亚硒酸盐还原能力,制备的纳米颗粒呈球形,分散性好,平均粒径为93 nm,该研究可应用于污染环境中亚硒酸盐的生物修复。ULLAH等[43]利用枯草芽孢杆菌BSN313制备纳米硒,其纳米硒的产量达200 μg/mL,平均粒径为530 nm。当纳米硒的质量浓度为200 μg/mL时,对大肠杆菌、金黄色葡萄球菌和铜绿假单胞菌具有抗菌活性。SPYRIDOPOULOU等[44]使用益生菌菌株干酪乳杆菌ATCC393合成纳米硒颗粒,发现益生菌合成的纳米硒颗粒及富硒干酪乳杆菌在体外的肿瘤特异性抗增殖活性均优于口服干酪乳杆菌,且富硒干酪乳杆菌比分离的纳米硒颗粒或干酪乳杆菌能更有效地抑制小鼠结肠癌生长。这表明,纳米颗粒和益生菌可能具有加成效应。真菌还原硒前体的效率较细菌低,但也常被用于合成纳米硒。FARAMARZI等[45]使用酿酒酵母制备纳米硒,紫外-可见光结果表明,合成的纳米硒在350 nm作用的波长处有较宽的发射峰,表明纳米硒是在细胞内形成的。利用动态光散射粒度分析仪对合成的纳米硒进行理化表征,发现纳米硒粒径为75~709 nm。将培养基中亚硒酸钠的添加量从5.0 μg增加到25 μg,其抗氧化活性从48.4降低到20.8。除酵母菌外,土曲霉、香菇等真菌也具有合成纳米硒的能力[46-47]。微生物合成的纳米硒培养条件温和,稳定性强,生物利用度高,具有广阔的应用前景。
2 生物合成纳米硒调节肠道黏膜免疫功能
2.1 缓解氧化应激诱导的肠道损伤
硒是一种具有高代谢活性的必需微量元素,在维持氧化还原稳态、抗炎和抗肿瘤中起着至关重要的作用[48]。氧化应激是指体内氧化与抗氧化作用失衡的一种状态。当抗氧化防御系统不能清除体内多余的活性氧(reactive oxygen species, ROS)时,就会发生氧化应激损伤[49]。肠道与外界直接相通,是活性氧的重要来源,更易受到氧化应激的影响。研究表明,氧化应激可引起肠道黏膜屏障损伤,促进炎症发生,进而导致炎症性肠病(inflammatory bowel disease,IBD)、直肠癌、结肠癌等疾病的发生发展[50-51]。
核因子-E2相关因子2(nuclear factor erythroid 2-related factor 2, Nrf2)是氧化应激的主要细胞传感器,在未受激活状态下Nrf2与kelch样环氧氯丙烷相关蛋白1(Kelch-like ECH-associated protein 1, Keap1)结合并存在于细胞质中,Nrf2处于抑制状态[52-53]。当细胞遭受氧化应激时,Keap1对Nrf2的抑制作用减弱,Nrf2移动到细胞核中,与特定的DNA序列——抗氧化应答元件(antioxidant response element, ARE)结合,激活下游抗氧化应答基因的表达。这些基因编码一系列抗氧化酶及蛋白,如超氧化物歧化酶(superoxide dismutase,SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase,GPx)、谷胱甘肽S-转移酶(glutathione S-transferase,GST)等[54-55]。有效激活Nrf2通路对维持氧化还原稳态和肠道细胞功能起着重要作用(图1)。
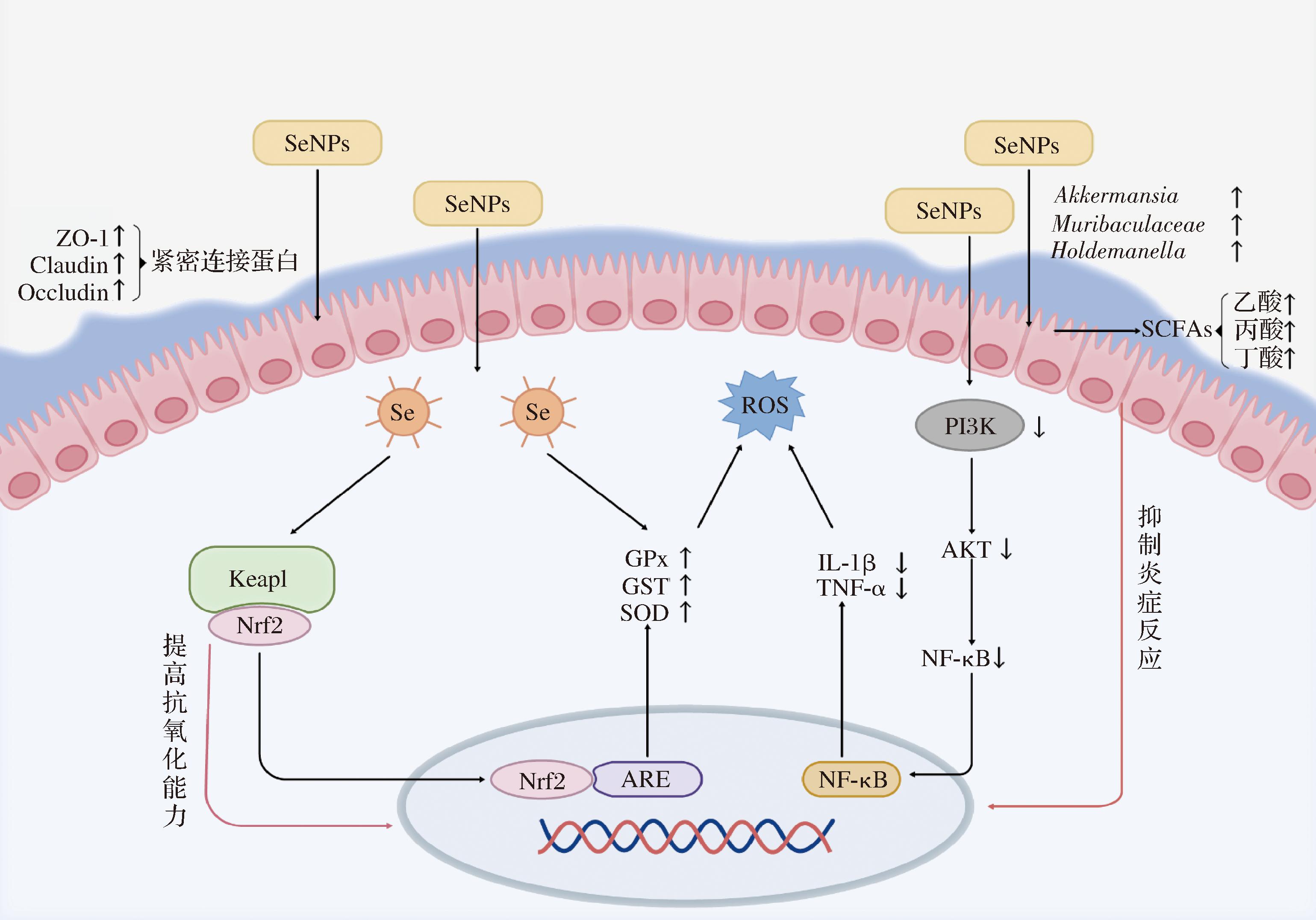
图1 纳米硒对肠道上皮细胞稳态和微生物群落功能的影响
Fig.1 Effects of SeNPs on the homeostasis of intestinal epithelial cells and microbial community function
注:紧密连接蛋白是维持肠道上皮细胞之间紧密连接、保障肠道屏障完整性的重要结构,主要由ZO-1(Zonula Occludens-1,闭锁小带蛋白-1)、Claudin(封闭蛋白)和Occludin(闭合蛋白)等构成;SCFAs:short-chain fatty acids,短链脂肪酸;PI3K:phosphatidylinositide 3-kinases,磷脂酰肌醇3-激酶;AKT:protein klinase B,蛋白激酶B;↑表示表达量或含量上升,↓表示表达量或含量下降。
SONG等[28]利用阴沟肠杆菌Z0206生物合成纳米硒。研究发现,生物活性纳米硒通过激活Nrf2通路及其下游基因,促进抗氧化酶的表达,从而抑制活性氧的产生。在小鼠肠道氧化应激模型中,与硒蛋白和化学合成的纳米硒颗粒相比,生物活性纳米硒可以更有效地保护小鼠肠道屏障功能、维持肠道氧化还原稳态。XU等[48]利用干酪乳杆菌ATCC 393合成的生物源纳米硒可通过Nrf2信号通路减轻活性氧介导的线粒体功能障碍,从而保护肠上皮屏障功能免受氧化损伤,靶向预防氧化应激相关肠道疾病。QIAO等[56]探究了ATCC 393合成的纳米硒对除草剂敌草快诱导的C57BL/6小鼠肠道屏障功能障碍的保护作用及其内在机制。结果表明,口服纳米硒可通过Nrf2介导的信号通路保护肠道屏障功能免受氧化损伤。
2.2 减少肠道上皮屏障损伤诱导的肠道炎症
肠道上皮细胞是动物机体抵御微生物的第一道防线,在保护宿主免受病原体感染方面起着至关重要的作用。核转录因子NF-κB是一种蛋白质复合物,与免疫细胞的活化、淋巴细胞的发育、应激性反应和细胞凋亡等多种细胞活动有关。NF-κB在细胞质中无活性,但在细胞受到特定信号刺激后可进入细胞核,激活目标基因的转录[57]。Toll样受体4 (Toll-like receptor 4,TLR4)作为一种模式识别受体,在肠道抗病原体防御中起着关键作用,TLR4介导的炎症相关肠道损伤可加速肠道炎症发展。抑制TLR-4/NF-κB信号通路是抗炎作用的有效途径[58]。
YE等[59]首次采用杜仲多糖(Eucommia ulmoides polysaccharide,EUP)对纳米硒进行改性得EUP-SeNP,并探究其对葡聚糖硫酸钠(dextran sulphate sodium,DSS)诱导的结肠炎的影响。结果表明,脂多糖 (lipopolysaccharide, LPS)诱导TLR-4/NF-κB信号通路的激活,口服EUP-SeNP可有效抑制该通路的激活,调节肠上皮细胞的凋亡和增殖以及炎症因子的表达来减缓DSS诱导的结肠损伤。MI等[60]通过绿色合成技术,利用水飞蓟素和硒纳米粒子制备了一种新型纳米粒子Si-SeNPs,并研究其在RAW264.7细胞中由LPS诱导的炎症反应方面的机制。研究表明,Si-SeNPss能够降低LPS诱导的RAW264.7细胞中PI3K和Akt的磷酸化水平,下调PI3K/Akt信号通路;研究者还评估了Si-SeNPs对NF-κB激活的抑制效果,结果显示,添加Si-SeNPs后,LPS处理的RAW264.7细胞中NF-κB从细胞质转移到细胞核的转位和累积明显减少,这表明Si-SeNPs能够抑制NF-κB的核转位。研究还探讨了Si-SeNPs对PI3K/Akt/NF-κB信号通路的影响,发现使用PI3K激活剂740 Y-P能够部分逆转Si-SeNPs对RAW264.7细胞中TNF-α和IL-1β mRNA表达的抑制作用,进一步证实了PI3K/Akt/NF-κB信号通路在Si-SeNPs的抗炎效应中扮演了重要角色。五味子多糖(Schisandra chinensis polysaccharide,SCP)具有治疗肠道损伤的潜在作用。DU等[61]通过合成SCP-Se纳米粒子,并研究其对肠道损伤的影响。结果表明,与SCP相比,SCP-Se NPs能更有效地缓解LPS诱导的小鼠腹泻、肠道组织损伤和紧密连接蛋白破坏作用,并能降低小鼠模型中炎症因子的表达水平。双酚A (bisphenol A, BPA)可对包括肠道在内的多个器官造成严重的毒性[62]。PAN等[63]制备壳聚糖包被的纳米硒粒子,并探讨比较其与无机硒(Na2SeO3)对BPA诱导的猪肠上皮细胞(IPEC-J2)毒性的作用机制。结果表明,纳米硒和无机硒处理均可减轻BPA引起的肠道损伤,且纳米硒的效果优于无机硒。纳米硒通过抑制内质网应激,减弱促炎反应和氧化应激,抑制细胞凋亡,从而增强肠上皮屏障功能。呕吐毒素(deoxynivalenol,DON)是一种真菌毒素,给世界各地的谷物食品造成了严重污染,威胁着人和动物的健康。DON暴露可通过内质网应激引起小鼠肠道屏障功能障碍。SONG等[64]探讨了干酪乳杆菌ATCC 393合成的纳米硒对DON诱导的肠上皮屏障功能障碍的保护作用。结果发现,纳米硒可以有效缓解DON暴露导致的IPEC-J2细胞氧化应激和炎症反应,上调紧密连接蛋白的表达水平,维持细胞钙离子稳态和肠上皮屏障的完整性,该研究为预防和治疗DON诱导的肠屏障功能障碍提供了新的营养调控策略。
3 生物合成纳米硒维持肠道菌群稳态
肠道菌群与身体健康密切相关,肠道菌群参与营养吸收、能量调节、黏膜屏障和免疫调节等[65]。当肠道微生物群的组成发生变化时,其功能可能发生变化,从而影响宿主健康。硒会影响肠道菌群的组成和功能,从而干扰肠道菌群的多样性,对微生物组成产生独特的影响。
3.1 影响肠道微生物群落多样性及其代谢
ZHANG等[66]研究了膳食中不同硒摄入量对肠道菌群的影响,结果发现,老年人群硒摄入量的差异改变了肠道菌群的组成,且显著影响肠道微生物的功能和代谢。OJEDA等[67]研究了硒对雄性青春期大鼠的结肠微生物群的影响,结果表明,补充低浓度的纳米硒使Akkermansia和Muribaculaceae的相对丰度升高,厚壁菌门/拟杆菌门的比例下降;中等剂量的纳米硒导致严重的菌群失衡,并增加了致病菌的丰度,对机体是有毒的。短链脂肪酸是肠道菌群发酵膳食纤维的主要产物,是结肠细胞和其他身体重要功能的能量来源[68]。将纳米硒添加至鸡饲料中,发现纳米硒(0.9 mg/kg)可以增加有益细菌(如乳酸杆菌和粪杆菌)的丰度及短链脂肪酸,特别是丁酸的含量[69]。QIAO等[70]为了验证纳米硒在仔猪饲粮中的应用效果,比较了饲粮中添加干酪乳杆菌ATCC 393合成的纳米硒替代Na2SeO3对早期断奶仔猪生长性能和肠道健康的影响。结果表明,与Na2SeO3对比,纳米硒提高了平均日增重和日采食量,降低了腹泻发生率,提高了饲料系数。此外,纳米硒通过上调黏蛋白2和紧密连接蛋白的表达水平,提高硒利用率,维持线粒体结构和功能,从而提高小鼠抗氧化及免疫力,缓解肠道屏障功能障碍。组学结果表明,饲粮中添加纳米硒导致Holdemanella的相对丰度增加,短链脂肪酸,特别是乙酸、丙酸浓度升高。GANGADOO等[71]采用体外培养和16S rRNA基因测序方法研究了纳米硒对成熟肉鸡盲肠微生物群的影响,结果表明,纳米硒显著降低了家禽病原体-盲肠球菌的丰度,且可以在不显著干扰总微生物群落的情况下靶向控制盲肠球菌。
3.2 促进肠道有益微生物的定植
纳米硒因其良好的生物可溶性和低毒性被广泛用作膳食补充剂。硒缺乏会导致动物生长减慢、采食量减少、细胞氧化受损和免疫力下降。LIU等[72]评价了纳米硒对饲喂高脂饲料的草鱼幼鱼肠道形态和肠道完整性的保护作用。结果表明,添加纳米硒可以减轻高脂饲料对肠道的损伤,维持肠道完整性。此外,纳米硒可显著上调草鱼幼鱼肠道紧密连接相关基因(ZO-1、Claudin-3和Occludin),抗氧化相关基因(GPx4a和GPx4b)和ZO-1蛋白的表达。添加纳米硒可显著抑制高脂饲料诱导的草鱼幼鱼炎症相关基因的表达,包括炎症因子(IL-8、IL-1β、IFN-γ、TNF-α和IL-6)、信号分子(TLR4、p38 MAPK和NF-κB p65)以及NF-κB p65和TNF-α蛋白的表达。饲料中添加纳米硒可以增加有益菌(如梭杆菌)的丰度,缓解高脂饲料引起的肠道菌群失调。硒参与GPx和硫氧还蛋白还原酶的合成,这些酶在镉解毒中发挥作用[73]。SHANG等[74]通过在饲粮中添加富硒植物乳杆菌研究其对暴露于水中镉的大鳞肥鱼的生物积累、氧化应激和肠道菌群的保护作用。结果表明,富硒植物乳杆菌可以减少镉在组织中的积累,调节正常肠道菌群,提高因镉暴露而降低的抗氧化活性。
4 生物合成纳米硒的发展前景
细菌合成的生物源性纳米硒具有抗氧化、抗菌、抗病毒、抗癌等功能活性,是目前合成纳米硒的主要途径之一,并在医疗健康、农业、食品工业和环境保护等多个领域展现出广泛的应用潜力。然而,微生物合成纳米硒也面临一些挑战和问题,提高效率与降低成本是推动其广泛应用的关键。此外,益生菌来源的纳米硒不仅具有硒元素的生物与功效,还可以发挥益生菌本身的益生作用。但目前尚不清楚硒元素与微生物之间是如何相互协同的,其协同机制仍需进一步研究,只有充分了解益生菌生物合成纳米硒的协同机制,才能实现“1+1>2”的协同效应,从而达到更高效、更安全的健康促进效果。
[1] RAYMAN M P.Food-chain selenium and human health:Emphasis on intake[J].British Journal of Nutrition, 2008, 100(2):254-268.
[2] SCHWARZ K, FOLTZ C M.Selenium as an integral part of factor 3 against dietary necrotic liver degeneration[J].Journal of the American Chemical Society, 1957, 79(12):3292-3293.
[3] WINTHER K H, RAYMAN M P, BONNEMA S J, et al.Selenium in thyroid disorders - essential knowledge for clinicians[J].Nature Reviews Endocrinology, 2020, 16(3):165-176.
[4] WINKEL L H E, ANNETTE JOHNSON C, LENZ M, et al.Environmental selenium research:From microscopic processes to global understanding[J].Environmental Science &Technology, 2012, 46(2):571-579.
[5] HU Y F, FENG W W, CHEN H H, et al.Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis:A prospective randomized-controlled trial[J].CTS-Clinical and Translational Science, 2021, 14(4):1390-1402.
[6] DE LIMA BARROS S E, DIAS M D S, BESERRA DE MOURA M S, et al.Relationship between selenium status and biomarkers of oxidative stress in Crohn's disease[J].Nutrition, 2020, 74:110762.
[7] KAMWESIGA J, MUTABAZI V, KAYUMBA J, et al.Effect of selenium supplementation on CD4+ T-cell recovery, viral suppression and morbidity of HIV-infected patients in Rwanda:A randomized controlled trial[J].AIDS.2015, 29(9):1045-1052.
[8] MUZEMBO B A, NGATU N R, JANUKA K, et al.Selenium supplementation in HIV-infected individuals:A systematic review of randomized controlled trials[J].Clinical Nutrition ESPEN, 2019, 34:1-7.
[9] TANGUY S, RAKOTOVAO A, JOUAN M G, et al.Dietary selenium intake influences Cx43 dephosphorylation, TNF-α expression and cardiac remodeling after reperfused infarction[J].Molecular Nutrition &Food Research, 2011, 55(4):522-529.
[10] CLARK L C, COMBS G F Jr, TURNBULL B W, et al.Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin[J].JAMA, 1996, 276(24):1957-1963.
[11] MANZANARES W, BIESTRO A, TORRE M H, et al.High-dose selenium reduces ventilator-associated pneumonia and illness severity in critically ill patients with systemic inflammation[J].Intensive Care Medicine, 2011, 37(7):1120-1127.
[12] RAYMAN M P.Selenium and human health[J].The Lancet, 2012, 379:1256-1268.
[13] ULLAH A, MU J, WANG F H, et al.Biogenic selenium nanoparticles and their anticancer effects pertaining to probiotic bacteria-a review[J].Antioxidants, 2022, 11(10):1916.
[14] 何羿文, 黄乐, 周锡红, 等.硒和硒蛋白与宿主肠道健康的互作调节机制[J].中国科学:生命科学, 2023, 53:1055-1066.HE Y W, HUANG L, ZHOU X H, et al.Role of selenium and selenoprotein in gut health[J].Scientia Sinica Vitae, 2023, 53:1055-1066.
[15] LI N, ZUO B, HUANG S M, et al.Spatial heterogeneity of bacterial colonization across different gut segments following inter-species microbiota transplantation[J].Microbiome, 2020, 8(1):161.
[16] ZHANG T, QI M, WU Q, et al.Recent research progress on the synthesis and biological effects of selenium nanoparticles[J].Frontiers in Nutrition, 2023, 10:1183487.
[17] ZHANG W J, ZHANG J, DING D J, et al.Synthesis and antioxidant properties of Lycium barbarum polysaccharides capped selenium nanoparticles using tea extract[J].Artificial Cells Nanomedicine Biotechnology, 2018, 46(7):1463-1470.
[18] ANU K, SINGARAVELU G, MURUGAN K, et al.Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum):Biophysical characterization and cytotoxicity on vero cells[J].Journal of Cluster Science, 2016, 28(1):1-13.
[19] ABBASIAN R, JAFARIZADEH-MALMIRI H.Green approach in gold, silver and selenium nanoparticles using coffee bean extract[J].Open Agriculture, 2020, 5(1):761-767.
[20] KAPUR M, SONI K, KOHLI K.Green synthesis of selenium nanoparticles from broccoli, characterization, application and toxicity[J].Advanced Techniques in Biology &Medicine, 2017, 5(1):1000198.
[21] CUI D X, LIANG T T, SUN L Q, et al.Green synthesis of selenium nanoparticles with extract of hawthorn fruit induced HepG2 cells apoptosis[J].Pharmaceutical Biology, 2018, 56(1):528-534.
[22] FARDSADEGH B, VAGHARI H, MOHAMMAD-JAFARI R, et al.Biosynthesis, characterization and antimicrobial activities assessment of fabricated selenium nanoparticles using Pelargonium zonale leaf extract[J].Green Processing and Synthesis, 2019, 8(1):191-198.
[23] BHATTACHARYYA A, CHATTOPADHYAY R, MITRA S, et al.Oxidative stress:An essential factor in the pathogenesis of gastrointestinal mucosal diseases[J].Physiological Reviews, 2014, 94(2):329-354.
[24] BHARATHI S, KUMARAN S, SURESH G, et al.Extracellular synthesis of nanoselenium from fresh water bacteria Bacillus sp.and its validation of antibacterial and cytotoxic potential[J].Biocatalysis and Agricultural Biotechnology, 2020, 27:101655.
[25] CRUZ D M, MI G J, WEBSTER T J.Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa[J].Journal of Biomedical Materials Research Part A, 2018, 106(5):1400-1412.
[26] RAJA C P, JACOB J M, BALAKRISHNAN R M.Selenium biosorption and recovery by marine Aspergillus terreus in an upflow bioreactor[J].Journal of Environmental Engineering, 2015, 142(9):C4015008.
[27] LIU J, SHI L, TUO X H, et al.Preparation, characteristic and anti-inflammatory effect of selenium nanoparticle-enriched probiotic strain Enterococcus durans A8-1[J].Journal of Trace elements in Medicine and Biology, 2022, 74:127056.
[28] SONG D G, LI X X, CHENG Y Z, et al.Aerobic biogenesis of selenium nanoparticles by Enterobacter cloacae Z0206 as a consequence of fumarate reductase mediated selenite reduction[J].Scientific Reports, 2017, 7(1):3239.
[29] VICAS S I, LASLO V, TIMAR A V, et al.Nano selenium-enriched probiotics as functional food products against cadmium liver toxicity[J].Materials, 2021, 14(9):2257.
[30] ALAM H, KHATOON N, KHAN M A, et al.Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria[J].Journal of Cluster Science, 2020, 31:1003-1011.
[31] HASHEM A H, SELIM T A, ALRUHAILI M H, et al.Unveiling antimicrobial and insecticidal activities of biosynthesized selenium nanoparticles using prickly pear peel waste[J].Journal of Functional Biomaterials, 2022, 13(3):112.
[32] SUN J R, LI J L, YAO L L, et al.Synthesis, characterization and antioxidant activity of selenium nanoparticle decorated with polysaccharide from hawthorn[J].Journal of Food Measurement and Characterization, 2023, 17:6125-6134.
[33] GHADERI R S, ADIBIAN F, SABOURI Z, et al.Green synthesis of selenium nanoparticle by Abelmoschus esculentus extract and assessment of its antibacterial activity[J].Materials Technology, 2022, 37(10): 1289-1297.
[34] NAJIMU NISHA S, AYSHA O S, SYED NASAR RAHAMAN J, et al.Lemon peels mediated synthesis of silver nanoparticles and its antidermatophytic activity[J].Spectrochimica Acta Part A Molecular &Biomolecular Spectroscopy, 2014, 124:194-198.
[35] SALEM S S, BADAWY M S E M, AL-ASKAR A A, et al.Green biosynthesis of selenium nanoparticles using orange peel waste:Characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria[J].Life, 2022, 12(6):893.
[36] ALAM H, KHATOON N, RAZA M, et al.Synthesis and characterization of nano selenium using plant biomolecules and their potential applications[J].Bionanoscience, 2019, 9(1):96-104.
[37] ANU K, DEVANESAN S, PRASANTH R, et al.Biogenesis of selenium nanoparticles and their anti-leukemia activity[J].Journal of King Saud University-Science, 2020, 32(4):2520-2526.
[38] PRASAD K S, PATEL H, PATEL T, et al.Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage[J].Colloids and Surfaces B:Biointerfaces, 2013, 103:261-266.
[39] VERSTEGEN J, GÜNTHER K.Biosynthesis of nano selenium in plants[J].Artificial Cells, Nanomedicine, and Biotechnology, 2023, 51(1):13-21.
[40] HU S Q, HU W C, LI Y R, et al.Construction and structure-activity mechanism of polysaccharide nano-selenium carrier[J].Carbohydrate Polymers, 2020, 236:116052.
[41] SI B B, YANG Y, NAVEED M, et al.Characterizations of biogenic selenium nanoparticles and their anti-biofilm potential against Streptococcus mutans ATCC 25175[J].Journal of Trace Elements in Medicine and Biology, 2024, 84:127448.
[42] KORA A J.Bacillus cereus, selenite-reducing bacterium from contaminated lake of an industrial area:A renewable nanofactory for the synthesis of selenium nanoparticles[J].Bioresources and Bioprocessing, 2018, 5(1):30.
[43] ULLAH A, YIN X, WANG F H, et al.Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities[J].Molecules, 2021, 26(18):5559.
[44] SPYRIDOPOULOU K, TRYFONOPOULOU E, AINDELIS G, et al.Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo[J].Nanoscale Advances, 2021, 3(9):2516-2528.
[45] FARAMARZI S, ANZABI Y, JAFARIZADEH-MALMIRI H.Nanobiotechnology approach in intracellular selenium nanoparticle synthesis using Saccharomyces cerevisiae-fabrication and characterization[J].Archives of Microbiology, 2020, 202(5):1203-1209.
[46] SAIED E, MEKKY A E, AL-ASKAR A A, et al.Aspergillus terreus-mediated selenium nanoparticles and their antimicrobial and photocatalytic activities[J].Crystals, 2023, 13(3):450.
[47] VETCHINKINA E, LOSHCHININA E, KURSKY V, et al.Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium[J].Journal of Microbiology, 2013, 51(6):829-835.
[48] XU C L, QIAO L, GUO Y, et al.Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393[J].Carbohydrate Polymers, 2018, 195:576-585.
[49] HUSSAIN T, TAN B, YIN Y L, et al.Oxidative stress and inflammation:What polyphenols can do for us?[J].Oxidative Medicine and Cellular Longevity, 2016, 2016:7432797.
[50] REZAIE A, PARKER R D, ABDOLLAHI M.Oxidative stress and pathogenesis of inflammatory bowel disease:An epiphenomenon or the cause?[J].Digestive Diseases and Sciences, 2007, 52(9):2015-2021.
[51] BHAGAT S S, GHONE R A, SURYAKAR A N, et al.Lipid peroxidation and antioxidant vitamin status in colorectal cancer patients[J].Indian Journal of Physiology and Pharmacology, 2011, 55(1):72-76.
[52] SUZUKI T, MOTOHASHI H, YAMAMOTO M.Toward clinical application of the Keap1-Nrf2 pathway[J].Trends in Pharmacology Sciences, 2013, 34(6):340-346.
[53] BELLEZZA I, GIAMBANCO I, MINELLI A, et al.Nrf2-Keap1 signaling in oxidative and reductive stress[J].Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 2018, 1865(5):721-733.
[54] MA Q.Role of Nrf2 in oxidative stress and toxicity[J].Annual Review of Pharmacology of Toxicology, 2013, 53:401-426.
[55] CEBULA M, SCHMIDT E E J, ARNéR E S J.TrxR1 as a potent regulator of the Nrf2-Keap1 response system[J].Antioxidants &Redox Signaling, 2015, 23(10):823-853.
[56] QIAO L, DOU X N, YAN S Q, et al.Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity[J].Food &Function, 2020, 11(4):3020-3031.
[57] PENG L, GAO X Y, NIE L, et al.Astragalin attenuates dextran sulfate sodium (DSS)-induced acute experimental colitis by alleviating gut microbiota dysbiosis and inhibiting NF-κB activation in mice[J].Frontiers in Immunology, 2020, 11:2058.
[58] MEI Z W, HUANG X X, ZHANG H, et al.Chitin derivatives ameliorate DSS-induced ulcerative colitis by changing gut microbiota and restoring intestinal barrier function[J].International Journal of Biological Macromolecules, 2022, 202:375-387.
[59] YE R H, GUO Q Y, HUANG J Q, et al.Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity[J].Journal of Nanobiotechnology, 2023, 21(1):222.
[60] MI X J, LE H M, LEE S, et al.Silymarin-functionalized selenium nanoparticles prevent LPS-induced inflammatory response in RAW264.7 cells through downregulation of the PI3K/Akt/NF-κB pathway[J].ACS Omega, 2022, 7(47):42723-42732.
[61] DU H X, TAN X Y, LI Z X, et al.Effects of Schisandra chinensis polysaccharide-conjugated selenium nanoparticles on intestinal injury in mice[J].Animals, 2023, 13(5):930.
[62] MA Y, LIU H H, WU J X, et al.The adverse health effects of bisphenol A and related toxicity mechanisms[J].Environmental Research, 2019, 176:108575.
[63] PAN Z Z, HUANG J Q, HU T, et al.Protective effects of selenium nanoparticles against bisphenol a-induced toxicity in porcine intestinal epithelial cells[J].International Journal of Molecular Sciences, 2023, 24(8):7242.
[64] SONG X F, QIAO L, DOU X N, et al.Selenium nanoparticles alleviate deoxynivalenol-induced intestinal epithelial barrier dysfunction by regulating endoplasmic reticulum stress in IPEC-J2 cells[J].Toxicology, 2023, 494:153593.
[65] LIN C S, CHANG C J, LU C C, et al.Impact of the gut microbiota, prebiotics, and probiotics on human health and disease[J].Biomedical Journal, 2014, 37(5):259-268.
[66] ZHANG Z X, XIANG H, SUN G G, et al.Effect of dietary selenium intake on gut microbiota in older population in Enshi region[J].Genes and Environment, 2021, 43(1):56.
[67] OJEDA M L, NOGALES F, CARRASCO L PEZ J A, et al.Microbiota-liver-bile salts axis, a novel mechanism involved in the contrasting effects of sodium selenite and selenium-nanoparticle supplementation on adipose tissue development in adolescent rats[J].Antioxidants, 2023, 12(5):1123.
PEZ J A, et al.Microbiota-liver-bile salts axis, a novel mechanism involved in the contrasting effects of sodium selenite and selenium-nanoparticle supplementation on adipose tissue development in adolescent rats[J].Antioxidants, 2023, 12(5):1123.
[68] CHEN T T, KIM C Y, KAUR A, et al.Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model[J].Food &Function, 2017, 8(3):1166-1173.
[69] GANGADOO S, DINEV I, CHAPMAN J, et al.Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii[J].Applied in Microbiology and Biotechnology, 2018, 102(3):1455-1466.
[70] QIAO L, DOU X N, SONG X F, et al.Replacing dietary sodium selenite with biogenic selenium nanoparticles improves the growth performance and gut health of early-weaned piglets[J].Animal Nutrition, 2023, 15:99-113.
[71] GANGADOO S, BAUER B W, BAJAGAI Y S, et al.In vitro growth of gut microbiota with selenium nanoparticles[J].Animal Nutrition, 2019, 5(4):424-431.
[72] LIU S, YU H B, LI P J, et al.Dietary nano-selenium alleviated intestinal damage of juvenile grass carp (Ctenopharyngodon idella) induced by high-fat diet:Insight from intestinal morphology, tight junction, inflammation, anti-oxidization and intestinal microbiota[J].Animal Nutrition, 2022, 8(1):235-248.
[73] ZWOLAK I.The role of selenium in arsenic and cadmium toxicity:An updated review of scientific literature[J].Biological Trace Element Research, 2020, 193(1):44-63.
[74] SHANG X C, XU W, ZHAO Z G, et al.Effects of exposure to cadmium (Cd) and selenium-enriched Lactobacillus plantarum in Luciobarbus capito:Bioaccumulation, antioxidant responses and intestinal microflora[J].Comparative Biochemistry and Physiology Part C:Toxicology &Pharmacology, 2022, 257:109352.