合生元由活微生物和能被宿主微生物选择性利用的底物组成,能够为宿主健康带来益处[1],如抑制致病菌、调节肠道微生态等[2-5]。合生元可以分为互补型合生元和协同型合生元[6]。互补型合生元是指具有独立益生作用的益生菌和益生元结合之后,它们独立工作展现出一种或多种益生功效[7]。协同型合生元由活微生物和能被该活微生物选择性利用的底物组成,它们组合后可以展现出更优的益生功效[8]。研究表明,协同型合生元相较于单一低聚糖,低聚糖组合对益生菌的生长和功能效果更好[9-10]。如LIU等[9]报道,低聚果糖(fructo-oligosaccharide,FOS)-低聚半乳糖(galacto-oligosaccharides,GOS)-菊粉-水苏糖-低聚木糖(xylo-oligosaccharide,XOS)组合更有利于发酵粘液乳杆菌(Limosilactobacillus fermentum) DALI02肠道定植。乳糖-母乳低聚糖组合、乳果糖-棉子糖-GOS组合等可以协同促进双歧杆菌生长[11-12]。壳寡糖-阿奇霉素组合协同抑制铜绿假单胞菌生长[13]。但是这些低聚糖的协同作用并没有通过多剂量的系统评估。不同低聚糖益生元如何组合,低聚糖益生元组合对益生菌生长的促进作用属于协同作用还是叠加作用尚不明确。
SynergyFinder 3.0是近年开发的用于交互式和可视化分析多种药物组合效应的工具包,适用于探究多种益生元的组合效应[14-15]。SynergyFinder 3.0整合零相互作用效力模型(zero interaction potency,ZIP)、最高单用药效模型(highest single-agent,HSA)等多种模型,用于多个剂量和样本的协同性分析,可以降低由于模型差异、实验误差等导致的低可信度协同效应风险。
因此,本文以益生动物双歧杆菌乳亚种(Bifidobacterium animals subsp.lactis)bb-12[16],和本研究室自主筛选分离的短双歧杆菌(Bifidobacterium breve)grx05[17]、植物乳植杆菌(Lactiplantibacillus plantarum)660为例,将不同浓度的低聚糖组合添加到无糖MRS培养基,以菌株生长的最大ΔOD600和最大比生长率为指标,基于SynergyFinder 3.0理性分析不同低聚糖组合对菌株生长的组合效应,以期为协同性合生元的理性设计和产品开发提供参考。
1 材料与方法
1.1 材料与试剂
B.breve grx05、B.animals bb-12和L.plantarum 660由扬州大学益生菌与乳品深加工江苏省高校重点实验室提供;FOS(>90%)、GOS(98%)、棉子糖(raffinose,RAF,98%)、水苏糖(stachyose,STA,80%),上海麦克林生化科技有限公司;MRS-Glc20无糖MRS培养基、HBYY001厌氧产气包,青岛海博生物技术有限公司。本实验中低聚糖组合如表1所示。MRS-Glc20+STA2.5+RAF2.5,在MRS-Glc20中添加2.5 g/L RAF和2.5 g/L STA。FOS2.5+GOS2.5等其他浓度与此类似。
表1 低聚糖组合
Table 1 Oligosaccharides combination
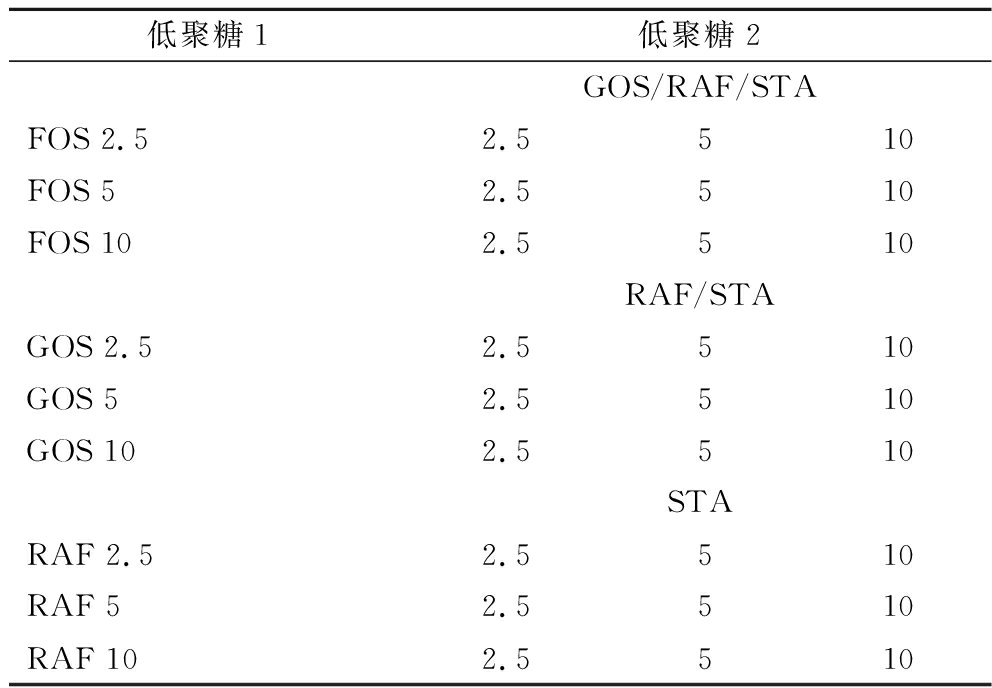
低聚糖1低聚糖2GOS/RAF/STAFOS 2.52.5510FOS 52.5510FOS 102.5510RAF/STAGOS 2.52.5510GOS 52.5510GOS 102.5510STARAF 2.52.5510RAF 52.5510RAF 102.5510
1.2 仪器与设备
JF-SX-500 全自动灭菌锅,日本TOMY公司;SW-CJ-1FD 超净工作台,苏州净化设备有限公司;DNP-9272 恒温培养箱,上海精宏实验设备有限公司;SPX-15085H-Ⅱ 恒温培养箱,上海新苗医疗器械制造有限公司;MX-S 混匀仪,大龙兴创实验仪器(北京)股份公司;Millipore Direct 8 超纯水仪,美国Millipore 公司;FP-110-C 全自动生长曲线分析仪,芬兰Bioscreen 公司;1510 酶标仪,美国赛默飞世尔科技有限公司。
1.3 方法
1.3.1 菌株的活化
L.plantarum 660:将冻存在70%甘油管中的菌株以3%接种量接到新鲜MRS培养基中,在37 ℃孵育16 h。将获得的培养物转到另一新鲜MRS培养基中,培养至活细胞数达到1×108CFU/mL以上[18-19]。
B.breve grx05、B.animals bb-12:传代与L.plantarum 660操作相同,需要注意的是在培养过程中应放入2.5 L厌氧产气包,并放置于厌氧袋中。
1.3.2 生长曲线测定
L.plantarum 660生长曲线的测定:将活化后的菌株以3%的接种量接种于MRS-Glc20培养基中,振荡均匀后,取200 μL加于生长曲线仪培养板中,做3个平行,用全自动生长曲线仪记录菌株的生长情况。以不添加低聚糖的MRS培养基作为阴性对照组。以培养时间为横坐标,吸光度(OD600值)为纵坐标,绘制生长曲线。
双歧杆菌生长曲线的测定:将菌悬液以3%的接种量分别接种于不同低聚糖组合的液体培养基中,放置厌氧袋中,于37 ℃恒温培养箱厌氧培养,分别于0、18、36 h使用酶标仪测定OD600值。以不添加葡萄糖的MRS培养基为阴性对照。以培养时间为横坐标,吸光度(OD600值)为纵坐标,绘制生长曲线[20]。
1.3.3 比生长速率分析
采用Origin 2018拟合s型模型,计算菌株的最大比生长率(μmax, h-1)。
1.3.4 基于SinergyFinder 3.0的组合效应分析
分别选取每一株菌在54种低聚糖组合培养基中生长的最大ΔOD600值和最大比生长率,利用SynergyFinder 3.0分析其组合效应。协同效应评分采用零相互作用效价ZIP模型[14]。若结果的Z>10,则表明分析的2种低聚糖之间存在协同效应;-10 所有实验重复3次。采用SPSS软件对试验数据进行统计分析。检验结果以“平均值±标准差”表示。Origin 2018用于绘图。P<0.05为差异有统计学意义。 如图1所示,在一定范围内随着低聚糖浓度的增加,菌株最大ΔOD600也在逐渐增大。当低聚糖浓度过高时,会抑制菌株的生长,OD600趋于平缓甚至下降。每株菌达到最大ΔOD600和最大比生长率对应的低聚糖组合不同,表现出了特定的低聚糖组合偏好性。 A-B.breve grx05;B-B.animals bb-12;C-L.plantarum 660 图1 菌株在不同低聚糖组合培养基中的生长曲线 如图2所示,B.breve grx05 在54种低聚糖组合培养基中的最大ΔOD600值为0.80,其次为0.79和0.78,最大比生长率为0.34,其次为0.31和0.28。B.animals bb-12 在54种低聚糖组合培养基中的最大ΔOD600值为0.81,其次为0.69和0.67,最大比生长率为0.34,其次为0.25和0.27。L.plantarum 660 在54种低聚糖组合培养基中的最大ΔOD600值为1.76,其次为1.73和1.67,最大比生长率为0.38,其次为0.32和0.30。 A-最大细胞生长量(ΔOD600);B-最大比生长速率 图2 菌株在不同低聚糖组合培养基中的生长情况 不同低聚糖组合促进菌株生长的能力不同。低聚糖作为菌株生长的底物,不仅影响菌株生长的最大OD600值也会影响菌株的生长速率。益生菌在低聚糖组合中的生长速率将影响它们在结肠中与其他肠道微生物的竞争能力。 分别以最大细胞生长量和最大比生长率为指标,基于SynergyFinder 3.0探究54组低聚糖组合对3株菌生长的组合效应。结果如图3所示,本实验条件下所有低聚糖组合的ZIP值都在10以下(-0.125~-2.945)。这表明在本实验的条件下,2种寡糖之间均不存在协同作用,而是存在叠加效应(-10 a-最大细胞生长量(ΔOD600);b-最大比生长率 图3 基于SynergyFinder 3.0的低聚糖组合促进菌株生长的组合效应分析 本结果与FAYED等[21]的报道不同,其采用Chou-Talalay方法分析壳寡糖-FOS、壳寡糖-乳果糖、壳寡糖-菊粉组合对B.animals bb-12生长的促进作用,发现多数都属于协同作用。而本研究采用的是零相互作用效力(zero interaction potency,ZIP)模型[14]。协同性分析模型的差异,可能是导致结果差异的原因。其次,FAYED等[21]采用的低聚糖组合中都含有壳寡糖,壳寡糖是自然界中唯一带正电荷阳离子碱性氨基低聚糖,不同低聚糖对菌株生长的影响不同,可能是导致结果差异的另一个原因[22-23]。 需要注意的是,有研究表明低聚糖与益生菌组合对促进体内营养物质的消化吸收、调节体内免疫应答等表现出潜在的协同效应[24-25]。另有研究指出,基于基因组尺度的代谢网络模型预测益生菌与低聚糖的最佳组合以及益生菌代谢之间的相互作用,以此构建协同性合生元,有助于降低随机试错实验,提高成功效率[26-27]。未来结合代谢网络预测模型,继续在体内探究益生菌-低聚糖组合的协同效应,对协同性合生元的理性设计和产品开发十分必要。 益生菌在不同低聚糖组合的生长具有菌株特异性。选择菌株生长量较高的前3种低聚糖组合,并比较对应的比生长速率,确定最有利于菌株生长的低聚糖组合。结果如表2所示,L.plantarum 660在前3种低聚糖组合中生长的ΔOD600值分别为1.758、1.729、1.672,无显著性差异。但是在FOS2.5+GOS5组合中的比生长速率最大,为本实验条件下最有利于L.plantarum 660生长的低聚糖组合。最有利于B.animals bb-12、B.breve grx05生长的低聚糖组合分别为GOS10+STA10、FOS10+GOS10。 表2 不同低聚糖组合培养基中菌株生长的最大生物量和最大比生长率 菌种低聚糖组合最大细胞产量(ΔOD600)最大比生长速率/h660FOS2.5+STA51.758a0.21bFOS2.5+GOS51.729a0.23aFOS5+RAF51.672a0.21bbb-12FOS2.5+GOS100.810a0.27bRAF10+STA100.686b0.25aGOS10+STA100.673b0.34agrx05FOS10+GOS100.799a0.31aFOS10+STA100.794a0.26bGOS10+RAF100.746a0.16c 注:不同小写字母表示差异显著(P<0.05)。 本研究以最大细胞生长量(ΔOD600)和最大比生长率为指标,基于SynergyFinder 3.0理性分析了54种低聚糖组合对3种益生菌生长的组合效应。结果表明菌株达到最大ΔOD600和最大比生长率对应的低聚糖组合不同,表现出了特定的低聚糖组合偏好性。采用零相互作用效力模型进行协同效应分析,本实验条件下所有低聚糖组合的ZIP值都在10以下,表明2种寡糖组合对菌株生长的促进作用不属于协同效应,而是叠加效应(-10 [1] MARCO M L, SANDERS M E, G [2] WU S Y, XU Y, CHEN Z Q, et al.Lactiplantibacillus plantarum ZJ316 reduces Helicobacter pylori adhesion and inflammation by inhibiting the expression of adhesin and urease genes[J].Molecular Nutrition &Food Research, 2023, 67(18):2300241. [3] XU J, TU M L, FAN X K, et al.A novel strain of Levilactobacillus brevis PDD-5 isolated from salty vegetables has beneficial effects on hyperuricemia through anti-inflammation and improvement of kidney damage[J].Food Science and Human Wellness, 2024, 13(2):898-908. [4] LI M Y, XU X X, JIA Y F, et al.Transformation of mulberry polyphenols by Lactobacillus plantarum SC-5:Increasing phenolic acids and enhancement of anti-aging effect[J].Food Research International, 2024, 192:114778. [5] WANG H Y, DAI J X, HAN Y F, et al.Screening and evaluation of a novel nucleotide-degrading Levilactobacillus brevis grx821 with anti-hyperuricemia ability[J].Food Bioscience, 2024, 60:104337. [6] WOLTER M, GRANT E T, BOUDAUD M, et al.Leveraging diet to engineer the gut microbiome[J].Nature Reviews Gastroenterology &Hepatology, 2021, 18(12):885-902. [7] HILL C, GUARNER F, REID G, et al.The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic[J].Nature Reviews Gastroenterology &Hepatology, 2014, 11(8):506-514. [8] HAN D, ZULEWSKA J, XIONG K, et al.Synergy between oligosaccharides and probiotics:From metabolic properties to beneficial effects[J].Critical Reviews in Food Science and Nutrition, 2024, 64(13):4078-4100. [9] LIU X X, CHEN D W, LI Q M, et al.Effect of complex prebiotics on the intestinal colonization ability of limosilactobacillus fermentum DALI02[J].Fermentation, 2023, 9(1):25. [10] [11] JAKOBSEN L M A, SUNDEKILDE U K, ANDERSEN H J, et al.Lactose and bovine milk oligosaccharides synergistically stimulate B.longum subsp.longum growth in a simplified model of the infant gut microbiome[J].Journal of Proteome Research, 2019, 18(8):3086-3098. [12] EHARA T, IZUMI H, TSUDA M, et al.Combinational effects of prebiotic oligosaccharides on bifidobacterial growth and host gene expression in a simplified mixed culture model and neonatal mice[J].British Journal of Nutrition, 2016, 116(2):270-278. [13] HE X J, HWANG H M, AKER W G, et al.Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa[J].Microbiological Research, 2014, 169(9-10):759-767. [14] IANEVSKI A, GIRI A K, AITTOKALLIO T.SynergyFinder 3.0:An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples[J].Nucleic Acids Research, 2022, 50(W1):W739-W743. [15] ZHENG S Y, WANG W Y, ALDAHDOOH J, et al.SynergyFinder plus:Toward better interpretation and annotation of drug combination screening DatasetsOpen access[J].Genomics, Proteomics &Bioinformatics, 2022, 20(3):587-596. [16] UTTARWAR R G, MEKONNEN S A, VAN BEECK W, et al.Effects of Bifidobacterium animalis subsp.lactis BB-12 and yogurt on mice during oral antibiotic administration[J].Microbiological Research, 2024, 286:127794. [17] 顾瑞霞, 潘丽娜, 李丹丹, 等.短双歧杆菌菌株grx05在缓解便秘及调节肠道菌群中的应用:中国, CN117617506A[P].2024-03-01.GU R X, PAN L N, LI D D,et al.Application of Bifidobacterium breve strain grx05 in alleviating constipation and regulating intestinal flora:China, CN117617506A[P].2024-03-01. [18] MA W L, LI Y H, KANG W L, et al.Synergistic combination of cryoprotectants improved freeze-dried survival rate and viable counts of Lactiplantibacillus plantarum[J].International Journal of Dairy Technology, 2024, 77(2):348-357. [19] ZHOU T, KANG W L, HAN Y F, et al.Combinedly increased viability of Lactiplantibacillus plantarum grx16 by co-encapsulation of cryoprotectants and porous starch within calcium alginate capsules[J].International Journal of Food Science &Technology, 2023, 58(10):5291-5298. [20] ZHANG C C, KANG W L, HAN Y F, et al.Combined effect of oligosaccharides combination on the growth of probiotics:Synergistic or superposable?[J].International Journal of Food Science &Technology, 2024, 59(7):4970-4978. [21] FAYED B, EL-SAYED H, ISMAIL S.Novel assessment of synergistic stimulatory effect of prebiotic chitooligosaccharide and some commercial prebiotics on the probiotic growth:A preliminary study[J].Journal of Microbiology, Biotechnology and Food Sciences, 2021, 11(1):e3341. [22] 张聪春, 康文丽, 韩一凤, 等.模拟肠液培养基中壳寡糖对益生菌生长的影响[J].中国乳品工业, 2024, 52(4):5-9.ZHANG C C, KANG W L, HAN Y F, et al.Effects of Chito-oligosaccharides on the growth of probiotics in simulated intestinal medium[J].China Dairy Industry, 2024, 52(4):5-9. [23] CHEN Y L, LING Z M, WANG X, et al.The beneficial mechanism of chitosan and chitooligosaccharides in the intestine on different health status[J].Journal of Functional Foods, 2022, 97:105232. [24] WANG J, WANG S X, LIU H, et al.Effects of oligosaccharides on the growth and stress tolerance of Lactobacillus plantarum ZLP001 in vitro, and the potential synbiotic effects of L.plantarum ZLP001 and fructo-oligosaccharide in post-weaning piglets1[J].Journal of Animal Science, 2019, 97(11):4588-4597. [25] ZHANG H N, ZHOU Y F, XU H, et al.Bacillus amyloliquefaciens BLCC1-0238 alone or in combination with mannan-oligosaccharides alleviates subclinical necrotic enteritis in broilers[J].Probiotics and Antimicrobial Proteins, 2022, 14(1):158-168. [26] RUAN Z P, CHEN K, CAO W M, et al.Engineering natural microbiomes toward enhanced bioremediation by microbiome modeling[J].Nature Communications, 2024, 15:4694. [27] LAWSON C E, HARCOMBE W R, HATZENPICHLER R, et al.Common principles and best practices for engineering microbiomes[J].Nature Reviews Microbiology, 2019, 17(12):725-741.1.4 统计分析
2 结果与分析
2.1 低聚糖组合对菌株生长的影响

Fig.1 Growth curves of strains in medium supplemented with different combinations of oligosaccharides
Fig.2 Growth of strains in medium supplemented with different combinations of oligosaccharides2.2 低聚糖组合促进菌株生长的组合效应分析
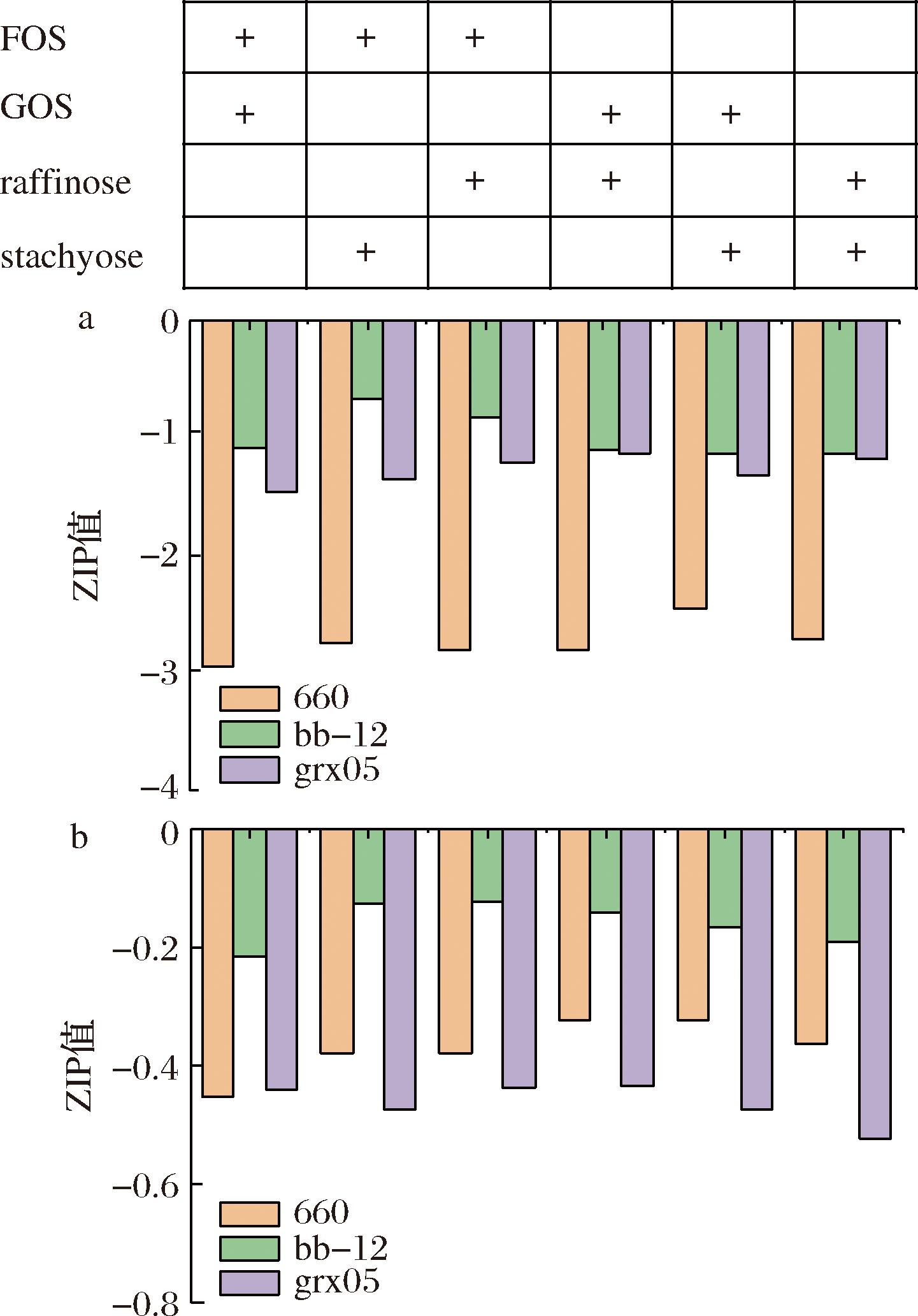
Fig.3 Analysis of the combined effect of oligosaccharides combination on promoting bacteria growth based on SynergyFinder2.3 适宜特定菌株的低聚糖组合的选择
Table 2 The maximum biomass and maximum specific growth rate of strains in medium supplemented with different oligosaccharide combinations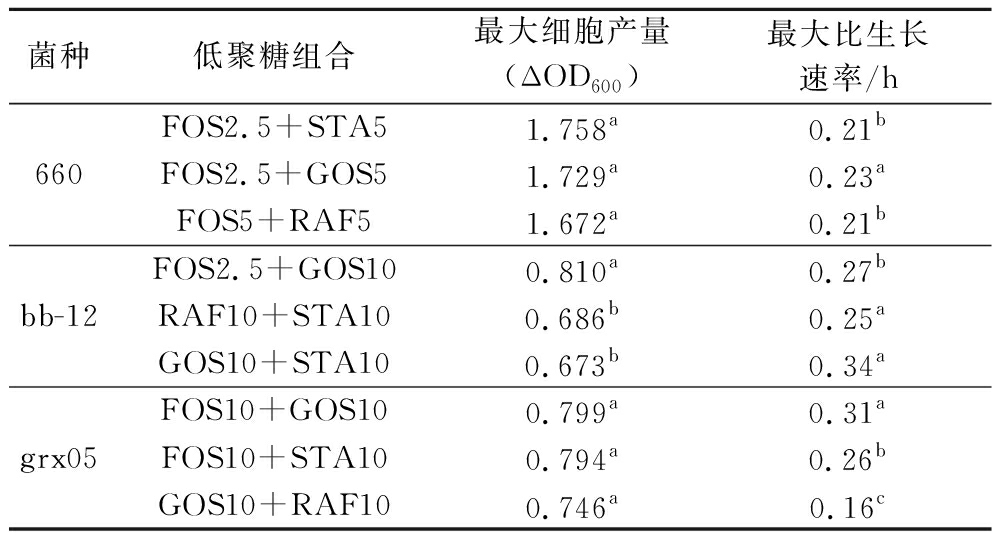
3 结论与讨论
 NZLE M, et al.The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods[J].Nature Reviews Gastroenterology &Hepatology, 2021, 18(3):196-208.
NZLE M, et al.The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods[J].Nature Reviews Gastroenterology &Hepatology, 2021, 18(3):196-208.![]() R, KAUNIETIS A,
R, KAUNIETIS A, ![]() N, et al.Combining prebiotics with probiotic bacteria can enhance bacterial growth and secretion of bacteriocins[J].International Journal of Biological Macromolecules, 2016, 89:669-676.
N, et al.Combining prebiotics with probiotic bacteria can enhance bacterial growth and secretion of bacteriocins[J].International Journal of Biological Macromolecules, 2016, 89:669-676.