甜菜(Beta vulgaris L.)是一种重要的糖料作物,占世界上约三分之一的蔗糖来源[1]。我国种植甜菜的历史已有100多年,存在“南蔗北菜”的说法,即甜菜是我国除甘蔗外最大的制糖来源,主要分布在新疆、黑龙江、内蒙古等北部地区[2]。制糖作为甜菜最大的用途,其工艺流程对提取产出以及提取投入的影响则至关重要。传统甜菜制糖采用热水浸提法,存在诸多现实问题,如费时耗能,副反应多,产品纯度低、色泽差等[3]。因此,为了降低甜菜制糖的能耗,提高其产品质量,许多研究者开发新型提取技术应用于甜菜制糖。
脉冲电场(pulsed electric field, PEF)处理是一种高效的绿色非热加工技术,通过在物料介质间施加一定电压的直流电以达到对物料细胞造成不可逆破坏等效果[4]。PEF技术因其加工时间短、能耗低、处理效果显著,近年来受到广泛的研究,尤其在美、日、德等发达国家,该技术已经在杀菌钝酶、农产品加工预处理、物料提取等方面取得了进展并应用于实际生产[5]。其中,脉冲电场预处理技术在处理植物源物料进而提取其目标组分方面表现出巨大应用潜力和庞大的市场需求[6]。
早在本世纪初,就有研究者将PEF技术应用于甜菜制糖[7-9],近年来相关的研究报道已较为深入[10-11],然而目前使用传统水热提取法加工甜菜制糖的实际生产依然占据主要部分。因此,如能在实际生产中结合PEF等新兴技术,从而切实实现减少能耗、提高得率的目的,显然具有更好的经济环保效益。通过阅读国内外相关文献报道,本文概述了PEF技术预处理甜菜制糖的应用、提取机理、影响因素等并提出展望,以期为实际生产提供理论支撑与指导。
1 甜菜及其传统制糖工艺
甜菜,藜科甜菜属二年生草本植物,至今已有200余年的栽培历史[12]。目前,世界的甜菜种植面积占据了总产糖作物的48%,是重要的糖料作物,其主要栽培的品种有糖用甜菜、根用甜菜、叶用甜菜、以及饲用甜菜[13]。甜菜除了其最主要的制糖应用外,本身也具有较高的营养价值[14-15]。富含多种微量元素和人体必需氨基酸,如表1和表2所示。同时,其制糖副产物也具有较高的综合利用价值,1 t鲜甜菜平均可生产160 kg糖,以及500 kg甜菜粕和38 kg糖蜜[16]。糖蜜是甜菜制糖终结晶分离后剩余的深色液体,其中有50%的糖(蔗糖、葡萄糖、果糖)和50%非糖化合物,包括一系列重要的生物活性物质,如B族维生素、甜菜碱、胆碱、丙氨酸、嘌呤、胞嘧啶、鸟苷、胞苷、多酚和氨基酸等[17]。甜菜粕是甜菜制糖榨汁后剩余的固态副产物,其干基物料含有 20%~25%的纤维素,25%~36%的半纤维素,20%~25%的果胶,10%~15%的蛋白质和 1%~2%的木质素[18]。上述副产物具有极高的再利用价值,被广泛应用于生产沼气能源转换、果胶等活性物质提取、基础化工原料生产、饲料生产等领域[19]。因此,甜菜是一种综合利用度非常高的经济作物。
表1 甜菜的营养成分组成表[20]
Table 1 Nutritional composition of sugar beets[20]

注:表中数据为每100 g新鲜甜菜所含营养成分含量(表2同)。
营养成分含量宏量营养成分水分87.58 g/100 g能量43 kcal/100 g碳水化合物9.56~9.96 g/100 g蔗糖7.96 g/100 g膳食纤维2~2.8 g/100 g蛋白质1.61~1.68 g/100 g脂肪0.17~0.18 g/100 g维生素 维生素A2 μg/100 g硫胺素0.031 mg/100 g核黄素0.27 mg/100 g烟酸0.331 mg/100 g泛酸0.145 mg/100 g维生素B60.067 mg/100 g抗坏血酸3.6 mg/100 g叶酸80 μg/100 g矿物质元素 Na77 mg/100 gCa16 mg/100 gFe0.79 mg/100 gP38 mg/100 gK305 mg/100 gMg23 mg/100 gZn0.35 mg/100 gMn0.329 mg/100 g
表2 甜菜的氨基酸含量[20]
Table 2 Amino acid content of sugar beets[20]
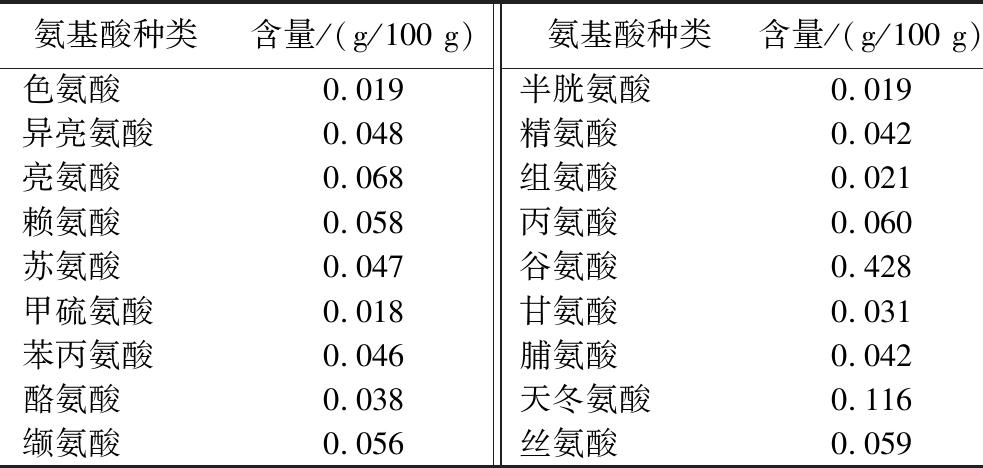
氨基酸种类含量/(g/100 g)氨基酸种类含量/(g/100 g)色氨酸0.019 半胱氨酸0.019异亮氨酸0.048 精氨酸0.042亮氨酸0.068 组氨酸0.021赖氨酸0.058 丙氨酸0.060苏氨酸0.047 谷氨酸0.428甲硫氨酸0.018 甘氨酸0.031苯丙氨酸0.046 脯氨酸0.042酪氨酸0.038 天冬氨酸0.116缬氨酸0.056 丝氨酸0.059
传统甜菜制糖工艺流程如下描述,首先对甜菜进行预处理和切块切丝,将其送入渗出器加水于70~75 ℃下提取60~90 min,分离甜菜粕得到渗出汁,即粗糖液;然后立刻进行糖液清净,主要使用双碳酸法,通过两次加石灰和碳酸气,去除如果胶、固体杂质等非糖组分;为了得到高品质白糖,蒸发前会进行SO2漂白脱色,蒸发结晶分蜜干燥[21]。这种传统提取技术主要通过不断通入溶剂提高甜菜细胞内外渗透压差,以渗透作用使得溶剂进入胞内,目标物料扩散出胞外,同时以较高温度破坏组织便于挤压出汁。该方法虽能提炼出白糖,但伴随着诸多缺点和局限性,如提取温度过高,高温不仅能耗高,还伴随热敏物质氧化分解、有害的副反应以及大量果胶溶出,渗出汁中果胶的存在对后续结晶分离有极大的影响[22];以及提取时间长、溶剂物料消耗严重、提取效率差等[23]。因此,传统甜菜制糖的方法与绿色环保可持续发展的原则相背离,进而结合新兴科技改善传统技术是该行业发展的必然趋势。
2 脉冲电场预处理甜菜
在农产品加工物料提取领域,大多传统手段均具有上述缺点和局限。目前,已有许多新型提取技术被开发并应用于物质提取,如微波辅助提取、高压溶剂提取、超临界流体萃取、超声辅助提取以及脉冲电场辅助提取等[24-28]。下文将介绍脉冲电场预处理提取甜菜糖的应用,并概述其主要机制和影响因素。
2.1 脉冲电场辅助甜菜制糖的应用
脉冲电场技术最早在食品领域的应用是食品原料的灭菌,而用于食品物料以及活性组分的提取则在20世纪末就已被证明[29]。经过学者大量的研究,脉冲电场逐渐被公认为是一种可以有效辅助提取的技术[30-31]。该技术可提高细胞膜的通透性,导致细胞膜可逆或不可逆的损伤,进而促进了胞内目标物化合物的释放[32]。短时处理、低能耗、高效率的特点使其能够实现连续化生产,十分适合农作物的工业提取。
近年来,许多关于PEF技术用于提取甜菜糖的研究已被报道[33],如图1所示为GRIMI[34]设计的PEF预处理提取甜菜糖的设备。NAKTHONG等[35]研究了PEF预处理提取甜菜多糖,在中低温度(35 ℃/50 ℃)下PEF处理30 min,甜菜提取蔗糖效果就能达到传统水热浸提法70 ℃下提取90 min的效果,提糖率最高可达到99.8%。MHEMDI等[36]同样对比了20 ℃下PEF处理和80 ℃下传统水提法,结果表明PEF处理组的糖(93.5%)纯度高于热水提取组(92.3%),并且浸出液的色泽显著降低(5 680~7 820 IU);同时,PEF处理组浸出液的蛋白质和胶体的含量低于传统水热提取组,通过能量计算,与高温水提(≈138~194 Wh/kg)相比,冷脉冲电场处理(≈2~3 Wh/kg)的能耗低。这2种方法的能耗差异也被其他研究所证实,MASKOOKI等[37]优化了PEF处理条件,最优条件下的提取平均能耗为8 kJ/kg,而对照组则高达156 kJ/kg;BARBA等[38]报道了不同处理方式提取的能耗,PEF辅助(1~15 kJ/kg)、机械加工(20~40 kJ/kg)、酶法辅助(60~100 kJ/kg)、热处理(>100 kJ/kg),同时对比了不同作物的平均提取能耗,其中甜菜的能耗仅在4 kJ/kg左右。LOGINOVA等[39]也发现PEF处理提取的甜菜糖不仅处理温度更低,而且产品品质更高;以及JEMAI等[40]扩大试验规模,在中试规模的甜菜制糖中加入PEF,发现PEF处理得到的糖液色泽浅、纯度高。

图1 PEF辅助提取甜菜糖试验性带式压榨机
Fig.1 A pilot belt press used for PEF-assisted expression from the sugar beets
甜菜制糖过程中,浸出液的清净环节至关重要,清净的彻底程度与后续结晶分离密切相关。传统制糖方法由于提取温度过高,甜菜中的果胶等易溶出进入浸出液,果胶含量过高会导致后续蒸发溶液黏度上升,于滤膜发生极性吸附,造成过滤分离糖结晶的难度显著上升。有研究者发现PEF处理可减少果胶的浸出,并减少后续清净的投入。ALMOHAMMED等[41]结合PEF优化了甜菜制糖的提取清净环节,提出四段法PEF碱压浸泡,显著提高了蔗糖提取率,损失在甜菜粕中的蔗糖含量仅为0.23%;该团队进而提出了PEF预处理结合碱性压榨的方法,该方法提取甜菜糖出汁率提高了12%,浸出液糖纯度高色泽浅,胶体蛋白含量低[42],同时处理组的石灰用量(6 kg/m3)低于对照组(10 kg/m3);进一步评估了浸出液结晶后的比阻和超滤动力学,发现PEF预处理提取能够降低后续结晶分离的难度[43]。这一现象同样被其他研究者报道,探究了PEF对甜菜制糖清净环节的物料用量,证实了石灰用量可以从15 kg/m3(70 ℃热水提取)降至约8 kg/m3(PEF辅助30 ℃提取)。有研究者关注甜菜制糖的终端超滤,研究了吸附于滤膜上的聚合物对过滤通量和渗透质量的影响,结果表明,甜菜糖分离过程中,极性吸附于膜上的果胶、蛋白等大分子会严重影响过滤效率,而PEF技术能够大大减少果胶提取量,降低滤膜污染情况[44-45]。因此,PEF预处理不仅能够增大糖提取效率,还能够为后续结晶分离降低难度,节省成本。
为了追求更高的提取效率,研究者尝试PEF协同其他手段共同处理,PRAPORSCIC等[46]研究了欧姆加热与PEF联合处理甜菜切片的效果,结果表明这两种技术联合处理具有一定的协同效应,提取率提高了85%~87%;EL-BELGHITI等[47]研究了PEF协同高速离心处理甜菜组织,通过双指数动力学模型,正确描述了甜菜组织的离心萃取过程,发现PEF协同离心场可以显著提高甜菜糖的提取动力学。也有研究者发现PEF处理协同固液两相结合发酵后糖液的糖含量更高,蒸馏液中乙醇含量更高,CO2失重率也高于未处理组织[48]。POIESZ等[49]通过PEF技术辅助提取甜菜糖申请了一项国际专利,甜菜糖的提取效率能够高达86%,其中优质甜菜糖浆的占比67%~71%。综上所述,PEF预处理是一种提取甜菜糖的节能省时手段,并且能够提升产品质量。
2.2 脉冲电场提取的机理
国内外对于PEF辅助提取加工技术的理论研究,存在多种假说,如黏弹极性形成模型、臭氧效应、电崩解以及电穿孔[50-53]。其中电穿孔的模型被多数研究者认可[53-54],即在处理物料两端施加瞬时、高频、高强的电压差,使组织细胞膜内外的跨膜电压差超过膜的承受极限,产生细胞电穿孔效应。脉冲电场通过将高压电脉冲输送给新鲜的甜菜组织,使其产生电穿孔现象来增加磷脂膜的渗透性,进而达到高效溶出甜菜糖的目的。通过处理室内的电极将电能传送给待处理的甜菜组织,随着脉冲电场的场强增大,甜菜组织细胞膜内外两侧的电压差随之增加,当跨膜电压超过细胞膜的承受极限(0.5~1.5 V),组织细胞就会形成可逆或不可逆的损伤,当电孔重新闭合时为可逆电穿孔,而当细胞平衡被完全破坏,电孔不再重新闭合时为不可逆电穿孔[55]。但更多研究者相信多种假说的同时存在,即脉冲电场引发的一系列极化效应,综合导致了组织细胞的受损,细胞半渗透功能丧失,胞内物质能够轻易地与外部介质交换,达到辅助提取的效果。REZAEE等[56] 通过Maxwell模型描述甜菜组织的黏弹性行为,结果表明甜菜的黏弹特性随脉冲电场处理强度的变化而变化,同时扫描电子显微镜图像显示,PEF处理的甜菜细胞变形增加,细胞间隙变大。VISOCKIS等[57]使用2.0 kV/cm电场强度的脉冲电场处理甜菜组织,通过测量上清液电导率比渗透指数发现甜菜根组织的崩解程度显著提高,进而实现对目标物质提取效率的提高。NOWACKA等[58]使用PEF处理甜菜,与未处理的样品相比,用PEF处理的甜菜物料表现出了导电性的增加,研究表明这是由于细胞膜渗透性的增加,与能够导电的物质(包括矿物盐)渗漏到细胞间隙有关。
2.3 影响提取效率的主要因素
在实际生产中使用PEF辅助提取的加工技术,势必需要清楚该技术在提取过程中影响提取率的关键因素,包括脉冲电场相关参数(电场强度、脉冲时间、脉冲波形等)、提取介质的相关参数(介质种类、介质温度、料液比等)以及提取原料的性质(组织尺寸、形貌结构)等。
电场强度是影响提取效果的决定性因素之一。如图2-a所示,研究者探究了PEF处理电场强度对甜菜浸出液中糖浓度的影响,随着电场强度增大,糖浓度先平缓上升,当电场强度在100~200 V/cm时,糖度迅速上升,随后趋于平缓[59]。当场强超过甜菜组织细胞的临界承受场强时,随着场强继续增加,细胞发生不可逆损伤增多,进而呈现糖浓度随场强增加迅速上升的现象。图2-b中也显示电场强度增加会导致甜菜细胞的裂解程度增加[56]。
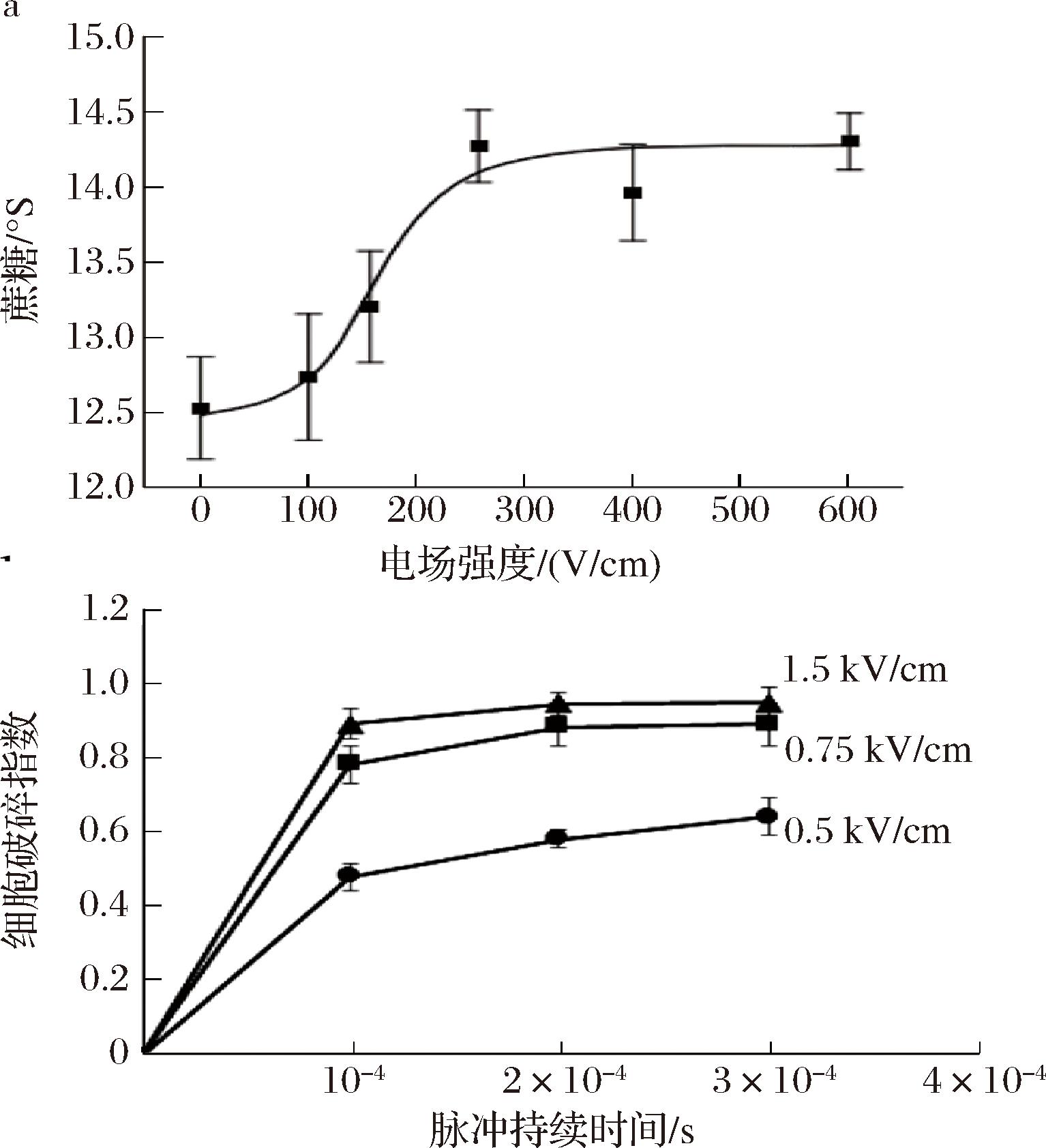
a-脉冲电场强度对甜菜渗出液糖度的影响;b-脉冲处理时间对甜菜组织细胞裂解程度的影响
图2 脉冲电场对甜菜提取效率的影响[56, 59]
Fig.2 Effect of pulsed electric fields on the extraction efficiency of sugar beets[56, 59]
脉冲时间为脉宽和脉冲数的乘积,也是影响提取效率的主要因素。如图2-b和图3所示,在各电场强度下脉冲处理时间延长,甜菜组织细胞裂解程度呈上升趋势[56,60]。在一定场强下,脉冲处理时间越长,组织细胞发生电穿孔越严重,且穿孔不可逆程度越高。
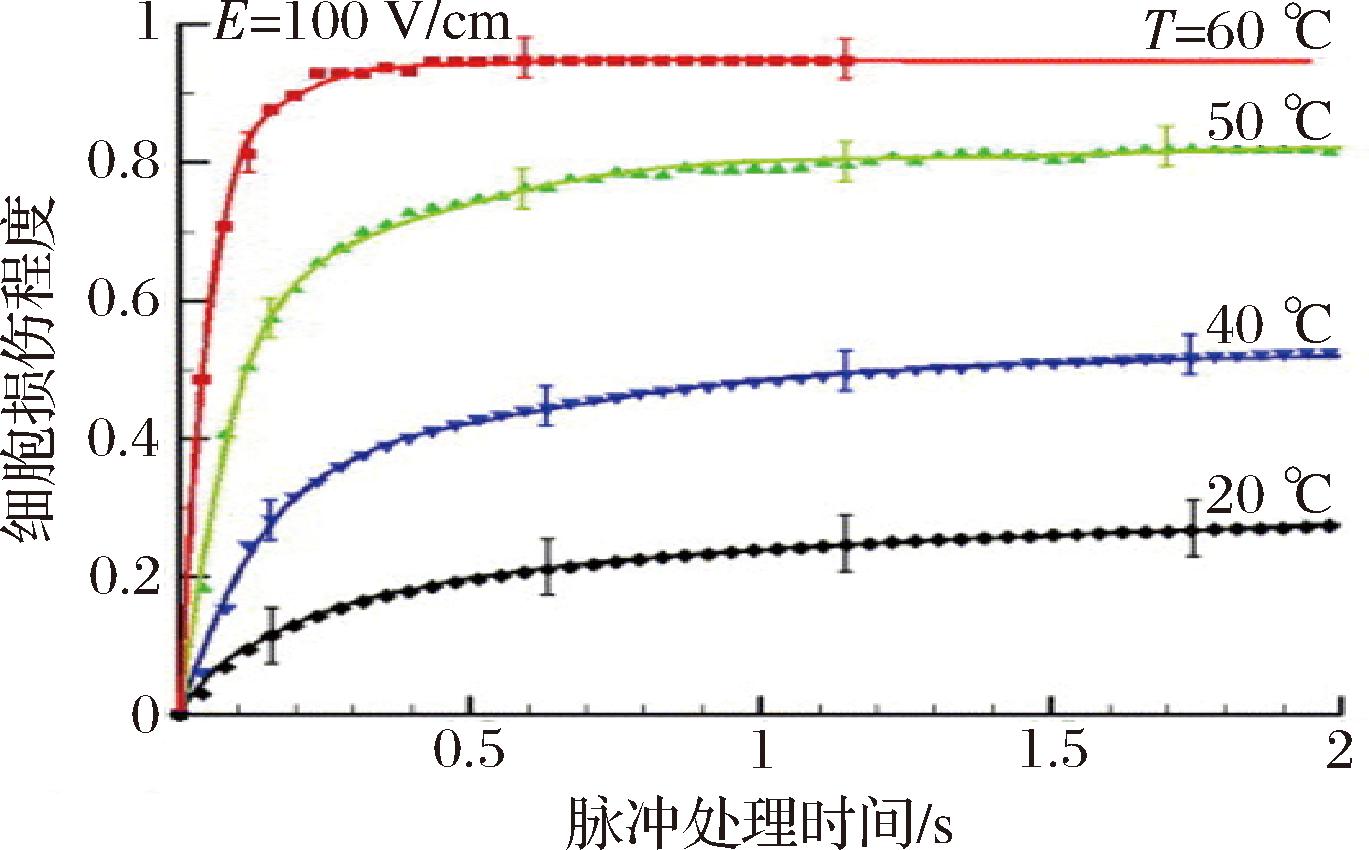
图3 不同温度下脉冲处理时间对甜菜细胞损伤程度的影响[60]
Fig.3 Effect of the treatment time on the cell damage degree of sugar beet at different temperatures
脉冲波形是指PEF实施过程中的脉冲形式,有指数衰减波、震荡波、平方波等。研究表明,平方波比震荡波和指数衰减波提取效果更好,同时双极脉冲波比单极脉冲波效果更好[61]。因此,在PEF辅助提取中,一般采用双极平方波脉冲。然而,也有研究结果表明,在7 kV/cm的场强下,甜菜糖提取的固液萃取效率并不受施加电场波形影响[62]。
甜菜制糖的介质多选择水,因为蔗糖能够较好的分散溶于水,且水是成本最低的溶剂,对于提取效率影响较大的介质因素主要是温度。如图3所示,温度从20 ℃升至60 ℃,甜菜组织细胞的损伤水平显著上升[60]。这是由于温度升高,一方面可以降低介质黏度,增强基体颗粒的渗透作用,另一方面则是加速分子热运动,增强糖的扩散作用。但PEF加工技术为了突出其非热加工的特性,多在室温条件下进行提取。
通常情况下,更高的电场强度以及更长的脉冲时间能够更有效地提取甜菜糖,然而现实生产受制于提取设备,以及过高场强过长时间的脉冲处理会引起介质升温,导致甜菜果胶等杂质溶出,同时也会造成一定的能量浪费。因此,许多学者通过单因素、正交试验、构建模型等方法优化PEF辅助提取甜菜糖的工艺参数,MHEMDI等[63]在恒定的压力下,改变电场条件和温度构建甜菜样品在不同处理下的过滤-固结行为模型,得出在600 V/cm的电场下处理10 ms可以有效提升甜菜糖的提取效率;MASKOOKI等[34]通过单因素试验优化了PEF处理甜菜的参数,结果表明,电场强度为1 kV/cm或2 kV/cm,脉冲数为20个,电容为8 F时,甜菜组织细胞的解体率和糖产量最高;L PEZ等[62]系统研究了电场强度(1~7 kV/cm)、脉冲数(5~40个)、脉冲频率(1~10 Hz)、脉冲宽度(2~5 ms)、脉冲波形以及介质温度,结果表明甜菜糖提取效率更依赖电场强度、介质温度以及处理时间;EL BELGHITI等[64]通过双指数动力学模型优化出了最佳工艺参数为电场强度940 V/cm,脉冲250次,搅拌速率250 r/min,达到低能耗高产出的目的。
PEZ等[62]系统研究了电场强度(1~7 kV/cm)、脉冲数(5~40个)、脉冲频率(1~10 Hz)、脉冲宽度(2~5 ms)、脉冲波形以及介质温度,结果表明甜菜糖提取效率更依赖电场强度、介质温度以及处理时间;EL BELGHITI等[64]通过双指数动力学模型优化出了最佳工艺参数为电场强度940 V/cm,脉冲250次,搅拌速率250 r/min,达到低能耗高产出的目的。
3 展望
随着绿色食品和可持续发展的观念深入人心,传统热水浸提甜菜制糖已然无法满足现实生产的需求,将脉冲电场技术用于提取甜菜糖是可靠的发展道路。脉冲电场辅助提取作为一种非热加工技术,在甜菜制糖过程中表现出提取率高、产品品质优、处理时间短、能耗低、后续成本低,容易实现连续生产或批量产生等优点。本文综述了PEF预处理提取甜菜糖的相关研究报道,介绍了其影响因素与作用机制,对于实际生产应关注电场强度、脉冲处理时间和介质温度三大主要因素,并结合能耗、提取率、经济效益等综合考虑。
PEF辅助技术用于甜菜糖提取具有以上优点,但仍然存在一些现实问题,有待进一步深入研究解决,如PEF预处理甜菜制糖最主要受限制的部分是PEF处理器的容积,据报道目前的PEF设备最多仅能处理2 500 t/d的甜菜[65],且设备造价昂贵。同时,PEF预处理甜菜过程中,糖组分的传质理论缺乏精确描述,细胞电穿孔和物料扩散的复杂机制依然停留在猜测层面。
因此,PEF提取技术的未来发展可首先致力于低成本、大容量的设备开发,以早日实现PEF辅助提取技术取代传统热水提法;进而,将研究目光聚焦于PEF的处理机制,深入了解机理以建立精确的传质模型,对指导实际生产有重大意义。
[1] 刘乃新. 甜菜SSR分子标记的开发应用及差异代谢产物分析[D]. 哈尔滨: 东北林业大学, 2020.LIU N X. Development and application of SSR molecular markers in sugarbeet and analysis of differential metabolites[D]. Harbin: Northeast Forestry University, 2020.
[2] 陈艺文, 李用财, 余凌羿, 等. 中国三大主产区甜菜糖业发展分析[J]. 中国糖料, 2017, 39(4):74-76; 80.CHEN Y W, LI Y C, YU L Y, et al. Analysis on the development of beet sugar industry in three main producing areas of China[J]. Sugar Crops of China, 2017, 39(4):74-76; 80.
[3] LI Y R, YANG L T. Sugarcane agriculture and sugar industry in China[J]. Sugar Tech, 2015, 17(1):1-8.
[4] G MEZ B, MUNEKATA P E S, GAVAHIAN M, et al. Application of pulsed electric fields in meat and fish processing industries: An overview[J]. Food Research International, 2019, 123:95-105.
MEZ B, MUNEKATA P E S, GAVAHIAN M, et al. Application of pulsed electric fields in meat and fish processing industries: An overview[J]. Food Research International, 2019, 123:95-105.
[5] KNORR D, FROEHLING A, JAEGER H, et al. Emerging technologies in food processing[J]. Annual Review of Food Science and Technology, 2011, 2:203-235.
[6] NALIYADHARA N, KUMAR A, GIRISA S, et al. Pulsed electric field (PEF): Avant-garde extraction escalation technology in food industry[J]. Trends in Food Science &Technology, 2022, 122:238-255.
[7] JHA A K, SIT N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review[J]. Trends in Food Science &Technology, 2022, 119:579-591.
[8] JEMAI A B, VOROBIEV E. Enhanced leaching from sugar beet cossettes by pulsed electric field[J]. Journal of Food Engineering, 2003, 59(4):405-412.
[9] AMMELT D, LAMMERSKITTEN A, WIKTOR A, et al. The impact of pulsed electric fields on quality parameters of freeze-dried red beets and pineapples[J]. International Journal of Food Science &Technology, 2021, 56(4):1777-1787.
[10] KHUBBER S, KAZEMI M, AMIRI SAMANI S, et al. Structural-functional variability in pectin and effect of innovative extraction methods: An integrated analysis for tailored applications[J]. Food Reviews International, 2023, 39(4):2352-2377.
[11] VOROBIEV E, LEBOVKA N. Processing of sugar beets assisted by pulsed electric fields[J]. Research in Agricultural Engineering, 2022, 68(2):63-79.
[12] 董宇飞, 吴玉梅, 刘乃新. 甜菜综合利用研究进展[J]. 轻工科技, 2019, 35(11):5-6.DONG Y F, WU Y M, LIU N X. Research progress on comprehensive utilization of sugar beet[J]. Light Industry Science and Technology, 2019, 35(11):5-6.
[13] DE OLIVEIRA S P A, DO NASCIMENTO H M A, SAMPAIO K B, et al. A review on bioactive compounds of beet (Beta vulgaris L. subsp. vulgaris) with special emphasis on their beneficial effects on gut microbiota and gastrointestinal health[J]. Critical Reviews in Food Science and Nutrition, 2021, 61(12):2022-2033.
[14] BIANCHI F, PÜNSCH M, VENIR E. Effect of processing and storage on the quality of beetroot and apple mixed juice[J]. Foods, 2021, 10(5):1052.
[15] CHHIKARA N, KUSHWAHA K, SHARMA P, et al. Bioactive compounds of beetroot and utilization in food processing industry: A critical review[J]. Food Chemistry, 2019, 272:192-200.
[16] GHARIB-BIBALAN S. High value-added products recovery from sugar processing by-products and residuals by green technologies: Opportunities, challenges, and prospects[J]. Food Engineering Reviews, 2018, 10(2):95-111.
[17] GUAN Y G, TANG Q, FU X, et al. Preparation of antioxidants from sugarcane molasses[J]. Food Chemistry, 2014, 152:552-557.
[18] GHARIB-BIBALAN S, KERAMAT J, HAMDAMI N. Better lime purification of raw sugar beet juice by advanced Fenton oxidation process[J]. Ozone: Science &Engineering, 2018, 40(1):54-63.
[19] ![]() D, et al. The influence of extruded sugar beet pulp on cookies’ nutritional, physical and sensory characteristics[J]. Sustainability, 2021, 13(9):5317.
D, et al. The influence of extruded sugar beet pulp on cookies’ nutritional, physical and sensory characteristics[J]. Sustainability, 2021, 13(9):5317.
[20] DHIMAN A, SUHAG R, CHAUHAN D S, et al. Status of beetroot processing and processed products: Thermal and emerging technologies intervention[J]. Trends in Food Science &Technology, 2021, 114:443-458.
[21] GARCIA GONZALEZ M N, BJÖRNSSON L. Life cycle assessment of the production of beet sugar and its by-products[J]. Journal of Cleaner Production, 2022, 346:131211.
[22] ALI RAJAEIFAR M, SADEGHZADEH HEMAYATI S, TABATABAEI M, et al. A review on beet sugar industry with a focus on implementation of waste-to-energy strategy for power supply[J]. Renewable and Sustainable Energy Reviews, 2019, 103:423-442.
[23] DE BRUIJN J M. Impact of beet quality on sugar manufacture Part 2. Impact of invert sugar on beet processing[J]. Sugar Industry, 2020:154-160.
[24] DHIMAN A, SUHAG R, CHAUHAN D S, et al. Status of beetroot processing and processed products: Thermal and emerging technologies intervention[J]. Trends in Food Science &Technology, 2021, 114:443-458.
[25] ![]() M, GRASSINO A N, ZHU Z Z, et al. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction[J]. Trends in Food Science &Technology, 2018, 76:28-37.
M, GRASSINO A N, ZHU Z Z, et al. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction[J]. Trends in Food Science &Technology, 2018, 76:28-37.
[26] LASUNON P, SENGKHAMPARN N. Effect of ultrasound-assisted, microwave-assisted and ultrasound-microwave-assisted extraction on pectin extraction from industrial tomato waste[J]. Molecules, 2022, 27(4):1157.
[27] HEWAGE A, OLATUNDE O O, NIMALARATNE C, et al. Novel Extraction technologies for developing plant protein ingredients with improved functionality[J]. Trends in Food Science &Technology, 2022, 129:492-511.
[28] MOHAMMADPOUR H, SADRAMELI S M, ESLAMI F, et al. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method[J]. Industrial Crops and Products, 2019, 131:106-116.
[29] K FERBÖCK A, SMETANA S, DE VOS R, et al. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology[J]. Algal Research, 2020, 48:101914.
FERBÖCK A, SMETANA S, DE VOS R, et al. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology[J]. Algal Research, 2020, 48:101914.
[30] MHEMDI H, BALS O, VOROBIEV E. Combined pressing-diffusion technology for sugar beets pretreated by pulsed electric field[J]. Journal of Food Engineering, 2016, 168:166-172.
[31] OKAMOTO S, MURAKAMI Y, URABE G, et al. Non-distractive extraction of intracellular molecules from yeast using PEF-assisted autolysis[J]. IEEJ Transactions on Fundamentals and Materials, 2021, 141(11):615-621.
[32] 苏文龙, 王谦鑫宏, 邱智东, 等. 脉冲电场技术在食品干燥加工中的研究进展[J]. 食品科技, 2023, 48(12):83-90.SU W L, WANG Q, QIU Z D, et al. Research progress of pulsed electric field technology in food drying and processing[J]. Food Science and Technology, 2023, 48(12):83-90.
[33] NIRMAL N P, MEREDDY R, MAQSOOD S. Recent developments in emerging technologies for beetroot pigment extraction and its food applications[J]. Food Chemistry, 2021, 356:129611.
[34] GRIMI N. Vers l’intensification du pressage industriel des agroressources par champs électriques pulsés: Étude multi-échelles [D]. Compiègne: Université de Technologie de Compiègne, 2009.
[35] NAKTHONG N, ESHTIAGHI M N. Pulsed electric field treatment of sugar beet[J]. IOP Conference Series: Earth and Environmental Science, 2020, 505(1):012055.
[36] MHEMDI H, BALS O, GRIMI N, et al. Alternative pressing/ultrafiltration process for sugar beet valorization: Impact of pulsed electric field and cossettes preheating on the qualitative characteristics of juices[J]. Food and Bioprocess Technology, 2014, 7(3):795-805.
[37] MASKOOKI A, ESHTIAGHI M N. Impact of pulsed electric field on cell disintegration and mass transfer in sugar beet[J]. Food and Bioproducts Processing, 2012, 90(3):377-384.
[38] BARBA F J, PARNIAKOV O, PEREIRA S A, et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry[J]. Food Research International, 2015, 77:773-798.
[39] LOGINOVA K, LOGINOV M, VOROBIEV E, et al. Quality and filtration characteristics of sugar beet juice obtained by “cold” extraction assisted by pulsed electric field[J]. Journal of Food Engineering, 2011, 106(2):144-151.
[40] JEMAI A B, VOROBIEV E. Pulsed electric field assisted pressing of sugar beet slices: Towards a novel process of cold juice extraction[J]. Biosystems Engineering, 2006, 93(1):57-68.
[41] ALMOHAMMED F, MHEMDI H, VOROBIEV E. Several-staged alkaline pressing-soaking of electroporated sugar beet slices for minimization of sucrose loss[J]. Innovative Food Science &Emerging Technologies, 2016, 36:18-25.
[42] ALMOHAMMED F, MHEMDI H, GRIMI N, et al. Alkaline pressing of electroporated sugar beet tissue: Process behavior and qualitative characteristics of raw juice[J]. Food and Bioprocess Technology, 2015, 8(9):1947-1957.
[43] ALMOHAMMED F, MHEMDI H, VOROBIEV E. Purification of juices obtained with innovative pulsed electric field and alkaline pressing of sugar beet tissue[J]. Separation and Purification Technology, 2017, 173:156-164.
[44] ZHU Z Z, MHEMDI H. Dead end ultra-filtration of sugar beet juice expressed from cold electrically pre-treated slices: Effect of membrane polymer on fouling mechanism and permeate quality[J]. Innovative Food Science &Emerging Technologies, 2016, 36:75-82.
[45] LOGINOV M, LOGINOVA K, LEBOVKA N, et al. Comparison of dead-end ultrafiltration behaviour and filtrate quality of sugar beet juices obtained by conventional and “cold” PEF-assisted diffusion[J]. Journal of Membrane Science, 2011, 377(1-2):273-283.
[46] PRAPORSCIC I, GHNIMI S, VOROBIEV E. Enhancement of pressing of sugar beet cuts by combined ohmic heating and pulsed electric field treatment[J]. Journal of Food Processing and Preservation, 2005, 29(5-6):378-389.
[47] EL-BELGHITI K, RABHI Z, VOROBIEV E. Effect of centrifugal force on the aqueous extraction of solute from sugar beet tissue pretreated by a pulsed electric field[J]. Journal of Food Process Engineering, 2005, 28(4):346-358.
[48] ALMOHAMMED F, MHEMDI H, VOROBIEV E. Pulsed electric field treatment of sugar beet tails as a sustainable feedstock for bioethanol production[J]. Applied Energy, 2016, 162:49-57.
[49] POIESZ E G, VREEKER R, VAN DER VAART J M. Sugar beet pulp capable of absorbing, and/or holding amount of water i.e. at least 14 times dry weight of sugar beet pulp used as ingredient in food product or carrier for further food-grade ingredients, preferably e.g. proteins, and salts: Nederland, WO2023217403A1[P]. 2023-11-16.
[50] 王辉, 薛淑花, 刘小花, 等. 高压电场在葡萄与葡萄酒加工中的应用及研究进展[J].中外葡萄与葡萄酒,2022(5):100-105.WANG H, XUE S H, LIU X H, et al. Application and research progress of high voltage electric field in grape and wine processing[J]. Sino-Overseas Grapevine &Wine, 2022(5):100-105.
[51] 孙炳新, 王月华, 冯叙桥, 等. 高压脉冲电场技术在果蔬汁加工及贮藏中的研究进展[J]. 食品与发酵工业, 2014, 40(4):147-154.SUN B X, WANG Y H, FENG X Q, et al. Advances on application of high voltage pulsed electric field in fruit and vegetable juice processing and storage[J]. Food and Fermentation Industries, 2014, 40(4):147-154.
[52] 李坚, 孟君, 曾新安, 等. 脉冲电场辅助浸提对红枣酒品质的影响[J]. 酿酒科技, 2019(5):61-64.LI J, MENG J, ZENG X A,et al. Effects of pulsed electric field-assisted extraction on the quality of jujube wine[J]. Liquor-Making Science &Technology, 2019(5):61-64.
[53] 李晓娟. 脉冲电场在天然产物提取中的应用研究[J]. 现代食品, 2020, 26(17):108-113.LI X J. Application research of pulsed electric field in natural product extraction[J]. Modern Food, 2020, 26(17):108-113.
[54] ![]() D, VOROBIEV E. Dual-porosity model of mass transport in electroporated biological tissue: Simulations and experimental work for model validation[J]. Innovative Food Science &Emerging Technologies, 2015, 29:41-54.
D, VOROBIEV E. Dual-porosity model of mass transport in electroporated biological tissue: Simulations and experimental work for model validation[J]. Innovative Food Science &Emerging Technologies, 2015, 29:41-54.
[55] ![]() D. Electroporation in food processing and biorefinery[J]. The Journal of Membrane Biology, 2014, 247(12):1279-1304.
D. Electroporation in food processing and biorefinery[J]. The Journal of Membrane Biology, 2014, 247(12):1279-1304.
[56] REZAEE K, NOGHABI M S, BEHZAD K, et al. Effect of moderate pulsed electric field treatment on viscoelastic properties of sugar beet[J]. Food Science and Technology Research, 2019, 25(2):157-166.
[57] VISOCKIS M, ![]() R, RUZGYS P, et al. Assessment of plant tissue disintegration degree and its related implications in the pulsed electric field (PEF)-assisted aqueous extraction of betalains from the fresh red beetroot[J]. Innovative Food Science &Emerging Technologies, 2021, 73:102761.
R, RUZGYS P, et al. Assessment of plant tissue disintegration degree and its related implications in the pulsed electric field (PEF)-assisted aqueous extraction of betalains from the fresh red beetroot[J]. Innovative Food Science &Emerging Technologies, 2021, 73:102761.
[58] NOWACKA M, TAPPI S, WIKTOR A, et al. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot[J]. Foods, 2019, 8(7):244.
[59] LOGINOVA K V, VOROBIEV E, BALS O, et al. Pilot study of countercurrent cold and mild heat extraction of sugar from sugar beets, assisted by pulsed electric fields[J]. Journal of Food Engineering, 2011, 102(4):340-347.
[60] LEBOVKA N I, SHYNKARYK M V, EL-BELGHITI K, et al. Plasmolysis of sugarbeet: Pulsed electric fields and thermal treatment[J]. Journal of Food Engineering, 2007, 80(2):639-644.
[61] DASTANGOO S, HAMED MOSAVIAN M T, YEGANEHZAD S. Optimization of pulsed electric field conditions for sugar extraction from carrots[J]. Food Science &Nutrition, 2020, 8(4):2025-2034.
[62] L PEZ N, PUÉRTOLAS E, COND
PEZ N, PUÉRTOLAS E, COND N S, et al. Enhancement of the solid-liquid extraction of sucrose from sugar beet (Beta vulgaris) by pulsed electric fields[J]. LWT-Food Science and Technology, 2009, 42(10):1674-1680.
N S, et al. Enhancement of the solid-liquid extraction of sucrose from sugar beet (Beta vulgaris) by pulsed electric fields[J]. LWT-Food Science and Technology, 2009, 42(10):1674-1680.
[63] MHEMDI H, BALS O, GRIMI N, et al. Filtration diffusivity and expression behaviour of thermally and electrically pretreated sugar beet tissue and press-cake[J]. Separation and Purification Technology, 2012, 95:118-125.
[64] EL BELGHITI K, VOROBIEV E. Mass transfer of sugar from beets enhanced by pulsed electric field[J]. Food and Bioproducts Processing, 2004, 82(3):226-230.
[65] POOJARY M M, BARBA F J, ALIAKBARIAN B, et al. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds[J]. Marine Drugs, 2016, 14(11):214.